Abstract
Introduction:
Females usually suffer from bone health problems, particularly with aging. Aerobic exercise has been shown to have health benefits for females.
Aim:
The main objective of this study was to investigate the impact of aerobic exercise on female bone health by measuring serum trace elements and bone metabolism markers.
Methods:
Prospective interventional study was conducted at rehabilitation clinics in Royal Medical Services, Jordan. A total of 65 female participants were included. Participants were assigned into three groups: control group (N = 20), osteopenic group (N = 22), and osteoporotic group (N = 23). A standard aerobic exercise protocol was followed for 12 weeks. Endurance exercise protocol involved three sessions weekly for 60 minutes each. At basal level and after the experiment, the following parameters were assessed: body mass index (BMI), bone-specific alkaline phosphatase (BAP), T-score, bone mineral density (BMD), and calcium. The analysis of data was carried out using SPSS version 21. The difference in means was computed based on t-test. Significance was considered at p < 0.05.
Results:
Aerobic training exercise improved the levels of all parameters in all groups for both sexes significantly, including BMI, BAP, T-score, BMD, and calcium (p < 0.05).
Conclusion:
Aerobic training exercise improves bone health and restores the hemostasis of bone tissue by restoring bone biomarkers, including BAP and calcium.
Keywords: Aerobic training exercise, osteopenic, osteoporosis, calcium, T-score, BAP
1. INTRODUCTION
Bone remodeling is a dynamic process that is highly regulated and responds to mechanical efforts as exercise. However, in modern medicine, bone is considered as an endocrine organ due to the synthesis and release of some hormones osteocalcin and FGF23. These proteins work to increase the sensitivity of insulin and lower the volume of fat mass (1).
Osteoporosis has been associated with low bone mineral density (BMD), and reduced bone geometry and microarchitecture, which leads to a more likely occurrence of minimal-trauma fractures (2). It has been reported that about 20% of mortality is associated with hip fractures alone (3, 4).
Osteoporosis influences more than 10 million people in the United States and this number is expected to exceed more than 14 million people by 2020 (5).
Several studies have confirmed that physical activity has the ability to lower the possibility of developing osteoporosis (6-8).
According to the study of McMillan et al. (9), physical activity has a great therapeutic potential against osteoporosis. In another study, Aldahr (10) investigated the variations of bone mineral status after training for 6 months between two groups of postmenopausal women: aerobic and resistance exercise. The results showed that both types of training significantly increased the mean levels of BMD, serum calcium (Ca), and parathyroid hormone (PTH).
2. AIM
The objective of this study was to investigate the impact of aerobic exercise on female bone health through measuring serum trace elements and bone metabolism markers on a sample of participants with osteopenia and osteoporosis in comparison with control subjects.
3. METHODS
Prospective interventional study was conducted. The study was conducted at rehabilitation clinics in Royal Medical Services, Jordan.
A total of 65 female participants were included.
The study was approved by the ethical committee at Royal Medical Services. Participants were assigned into three groups: control group (N = 20), osteopenic group (N = 22), and osteoporotic group (N = 23).
At basal level, body mass index (BMI) was measured as well other physiological parameters, such as blood pressure, hip and waist ratio. A blood sample was withdrawn from each participant to test for calcium, and bone-specific alkaline phosphatase (BAP). At the end of the experiment, all measurements were repeated.
Patients were previously diagnosed as osteopenic or osteoporotic. Control subjects were recruited from the general population to participate in this study.
A standard aerobic exercise protocol was described by the study of Aldahr (10). We followed this protocol with some modifications. The aerobic exercise was applied to all participants for 12 weeks. Endurance exercise protocol involved three sessions weekly. The time of each session was 60 minutes. Appropriate music was involved to make a feeling of relaxation. Exercise training protocol involved stretching and warm-up (10-15 minutes), aerobic activities such as stepping and graded walking (35-40 minutes), and cool-down/relaxation (10 minutes).
Data were analyzed using SPSS version 21. Descriptive statistical analysis was conducted to describe the general characteristics of the participants. Frequency was used to describe categorized variables such as gender. Means were used to describe continuous variables, such as age, T-scores, calcium level, and BAP. The differences in means were used to determine using a paired T test. Significance was considered at p < 0.05.
4. RESULTS
General characteristics of study participant
As shown in Figure 1, the study included 65 female participants assigned to normal group (N = 20), osteopenic group (N = 23), or osteoporotic group (N = 22).
Figure 1. Distribution of participants either normal or to disease.
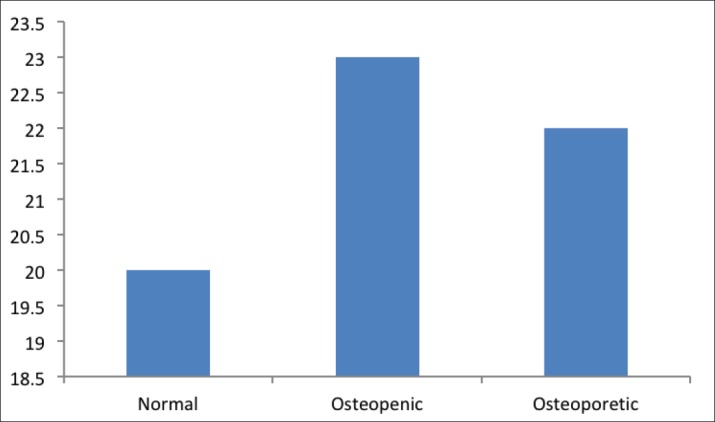
As indicated by Figure 2, the mean age of participants in normal group was 43.3 years, and the mean age for those in the osteopenic group was 45.7 years, and those in the osteopenic group had the mean age as 50.2 years.
Figure 2. Frequency of age by study groups.
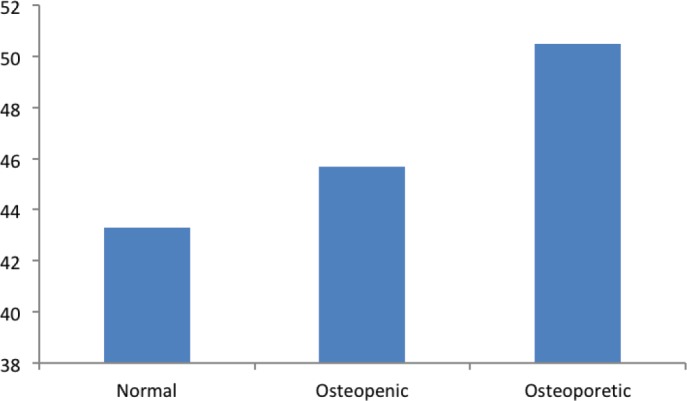
The mean BMI was 25.4 in the normal group and 26.8 in the osteopenic group, and increased further in the osteoporotic group (29.2). The differences in mean of BMI between the osteopenic group and osteoporotic group compared with the normal group were statistically significant (p < 0.05) (Figure 3).
Figure 3. The level of BMI in study groups.
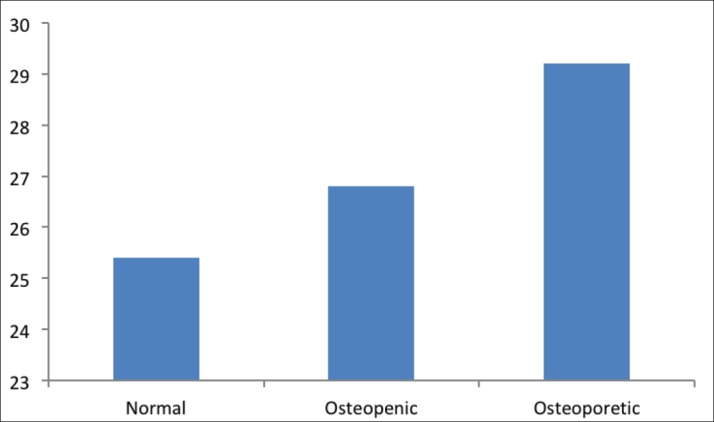
The mean level of calcium was the highest among normal participants (91.4 mg/l), and significantly decreased in the osteopenia group (66.7 mg/l) (p < 0.001) and osteoporotic group (59.2 mg/l) (p < 0.001) (Figure 4).
Figure 4. The level of calcium (mg/l) in study groups.
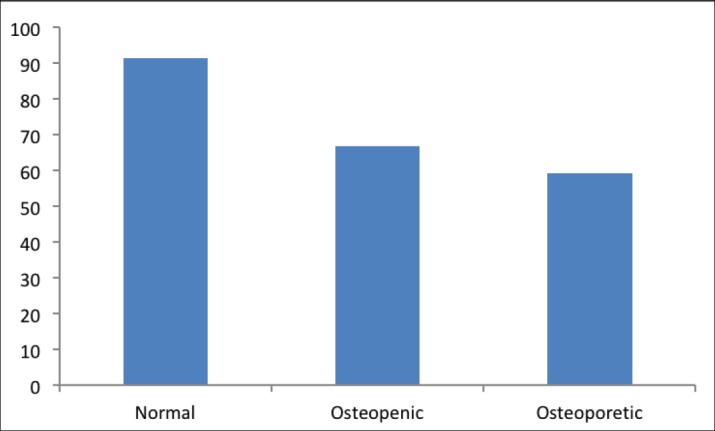
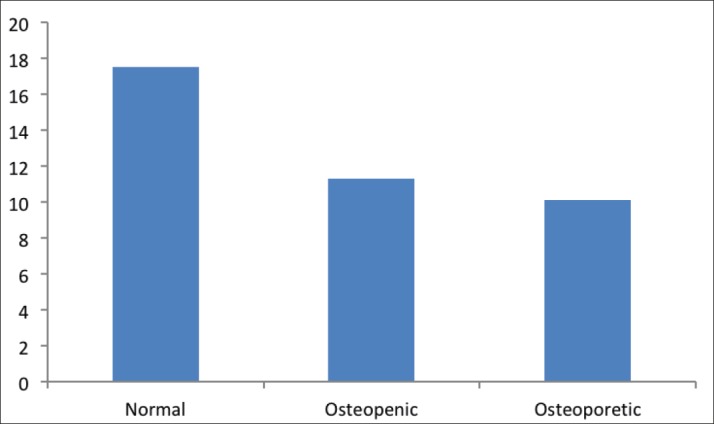
The mean level of BAP was the highest in the normal group (17.5), and decreased significantly in the osteopenic group (11.3, p < 0.001), osteoporotic group (10.1, p < 0.001) (Figure 5).
Figure 5. The level of BAP in study groups.
The impact of aerobic exercise on female trace elements and bone metabolism markers
The results in Table 1 showed that in the control group, there were significant relationships for all variables listed in Table 1 due to the aerobic exercise (p < 0.05 for all variables). In both groups, osteopenic and osteoporotic groups, there were strong impacts of aerobic exercise on the outcome of all listed variables (p < 0.001).
Table 1. Comparison of serum trace element (mg/l) and the bone metabolism markers for female subjects (N = 65) before and after 12-week aerobic exercise training (mean ± SD). *p < 0.05 (pretest vs. posttest of each group), **p < 0.001 (pretest vs. posttest of each group).
| Parameters | Normal control (N = 20) (T-score = 0 to −0.99) | Osteopenic (N = 23) (T-score = 1 to −2.49) | Osteoporotic (N = 22) (T-score ≤ −2.5) | |||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| BMI (kg/m2) | 25.6 | 25* | 27.3 | 26.4* | 29.4 | 28.5* |
| BAP | 17.4 | 24.5* | 11.1 | 23.3** | 9.9 | 17.1** |
| T-score | −0.61 | −0.4* | −1.6 | −1.1** | −3 | −2.2** |
| BMD hip (g/cm2) | 0.85 ± 0.1 | 0.95* | 0.81 | 0.92** | 0.7 | 0.94** |
| BMD spine (g/cm2) | 0.86 ± 0.1 | 0.96* | 0.99 | 1.4** | 0.91 | 1.7** |
| Calcium (mg/l) | 91.1 | 120* | 66.5 | 101** | 58.4 | 110.6** |
5. DISCUSSION
The results of this study revealed that aerobic exercise for 12 weeks had positive influences on bone health among three groups involved in this study: control group, osteopenic group, and osteoporotic group for males and females (p < 0.05 for all variables). These results are in agreement with other studies that showed positive impacts of aerobic exercise on bone health (9-12). Due to the nature of aerobic exercise training protocols involved in this study, such as stretching and warm-up, stepping and graded walking, and cool-down/relaxation, it is plausible to think of improved bone health due to such mechanisms involving mechanic transduction effects on bone (13, 14), and extracellular fluid shear stress within bone matrix (15).
BMI levels were significantly lowered in all groups (p < 0.05). This means that applying aerobic exercise protocol for 12 weeks was effective in lowering weight as reflected by lowered BMI. This finding is in line with other studies (19, 16).
The results of this study showed that aerobic exercise training protocols significantly increased the levels of BAP in all groups. Actually, this finding indicated that aerobic training for 12 weeks improved bone formation leading to better outcomes of bone health. These findings are consistent with previous studies that reported similar results (17-19).
The results of the present study showed significant improvement of T-scores and BMD due to aerobic exercise in all study groups (p < 0.05). These findings revealed that aerobic training protocol we applied was efficient and helped to restore the structure and function of the bone tissue in all groups, particularly in osteopenic and osteoporotic groups. These results are in agreement with other studies that reported significant improvement of T-score and BMD as a result of a moderate aerobic training exercise (10, 19, 20). The results of the present study showed that the levels of calcium at a basal level were significantly lowered in osteopenic and osteoporotic groups than the control group (p < 0.001). Aerobic exercise improved the calcium levels significantly in all study groups (p < 0.05). These results suggested that aerobic exercise can restore the hemostasis of calcium which is crucial for numerous biological mechanisms, such as bone metabolism (21). These findings agree with other studies that revealed the improvement of calcium levels due to exercise training (10, 19). It is plausible to explain the increased levels of calcium as a consequence of aerobic training exercise through stimulation of parathyroid glands to produce and release more PTH which, in turn, leads to mobilization of calcium from its stores into blood (22-24).
The results of this study showed the effects of aerobic exercise on improving the bone health as previously mentioned in both sexes, and this finding agrees with the study of Alghadir et al (19). (2016).
6. CONCLUSION
Aerobic training exercise improves bone health and restores the hemostasis of bone tissue by restoring bone biomarkers, including BAP and calcium.
Author’s contribution:
Zaid Al Dahamsheh, Kareem Al Rashdan, Awni Al Hadid, Ra’d Jaradat, Mohammad Al Bakheet, Zeyad S. Bataineh. Each author gave substantial contributions to the conception or design of the work in acquisition, analysis, or interpretation of data for the work. Each author had a part in article preparing for drafting or revising it critically for important intellectual content, and each author gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent forms.
Conflicts of interest:
There are no conflicts of interest.
Financial support and sponsorship:
None.
REFERENCES
- 1.Qi Z, Liu W, Lu J. The mechanisms underlying the beneficial effects of exercise on bone remodeling: Roles of bone-derived cytokines and microRNAs. Prog Biophys Mol Biol. 2016;122:131–139. doi: 10.1016/j.pbiomolbio.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Kanis JA. London, UK: Royal College of Physicians of London; 1990. Osteoporosis. [Google Scholar]
- 3.Harvey NC, Dennison E, Cooper C. Osteoporosis: impact on health and economics. Nat Rev Rheumatol. 2010;6:99–105. doi: 10.1038/nrrheum.2009.260. [DOI] [PubMed] [Google Scholar]
- 4.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81:646–656. [PMC free article] [PubMed] [Google Scholar]
- 5.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 6.Gunter KB, Almstedt HC, Janz KF. Physical activity in childhood may be the key to optimizing lifespan skeletal health. Exerc Sport Sci Rev. 2012;40(1):3–21. doi: 10.1097/JES.0b013e318236e5ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karlsson MK, Nordqvist A, Karlsson C. Physical activity increases bonemass during growth. Food Nutr Res. 2008;52 doi: 10.3402/fnr.v52i0.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizzoli R, Bianchi ML, Garabedian M, McKay HA, Moreno LA. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. 2010;46:294–305. doi: 10.1016/j.bone.2009.10. [DOI] [PubMed] [Google Scholar]
- 9.McMillan LB, Zengin A, Ebeling PR, Scott D. Prescribing physical activity for the prevention and treatment of osteoporosis in older adults. Healthcare. 2017;5:85. doi: 10.3390/healthcare5040085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aldahr MHS. Bone mineral status response to aerobic versus resistance exercise training in postmenopausal women. World Appl Sci J. 2012;16(6):806–813. [Google Scholar]
- 11.Norton K, Norton L, Lewis N. Effects of short-term physical activity interventions on simple and choice response times. BioMed Res Int. 2016;5613767 doi: 10.1155/2016/5613767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puciato D, Rozpara M, Borysiuk Z. Physical activity as a determinant of quality of life in working-age people in Wrocław, Poland. Int J Environ Res Public Health. 2018;15:623. doi: 10.3390/ijerph15040623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan VPS, Macdonald HM, Kim S, Nettlefold L, Gabel L, Ashe MC, et al. Influence of physical activity on bone strength in children and adolescents: a systematic review and narrative synthesis. J Bone Miner Res. 2014;29:2161–2181. doi: 10.1002/jbmr.2254. [DOI] [PubMed] [Google Scholar]
- 14.Wippert PM, Rector M, Kuhn G, Wuertz-Kozak K. Stress and alterations in bones: an interdisciplinary perspective. Front Endocrinol. 2017;8:96. doi: 10.3389/fendo.2017.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boers HE, Haroon M, Grand FL, Bakker AD, Klein-Nulend J, Jaspers RT. Mechanosensitivity of aged muscle stem cells. J Orthopae Res. 2018;36:632–641. doi: 10.1002/jor.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langsetmo L, Hitchcock CL, Kingwell EJ, Davison KS, Berger C, Forsmo S, et al. Physical activity, body mass index and bone mineral density-associations in a prospective population-based cohort of women and men: The Canadian Multicentre Osteoporosis Study (CaMos) Bone. 2012;50:401–408. doi: 10.1016/j.bone.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maimoun L, Mariano-Goulart D, Couret I, Manetta J, Peruchon E, Micallef JP, et al. Effects of physical activities that induce moderate external loading on bone metabolism in male athletes. J Sports Sci. 2004;22:875–883. doi: 10.1080/02640410410001716698. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Aguero A, Vicente-Rodriguez G, Gomez-Cabello A, Ara I, Moreno LA, Casajus JA. A 21 week bone deposition promoting exercise programme increases bone mass in youths with Down syndrome. Dev Med Child Neurol. 2012;54:552–556. doi: 10.1111/j.1469-8749.2012.04262.x. [DOI] [PubMed] [Google Scholar]
- 19.Alghadir AH, Aly FA, Gabr SA. Effect of moderate aerobic training on bone metabolism indices among adult humans. Pak J Med Sci. 2014;30(4):840–844. doi: 10.12669/pjms.304.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trivitayaratana W, Trivitayaratana P. Peripheral BMD T-scores in the diagnosis of osteoporosis. J Med Assoc Thai. 2005;88:S8–12. [PubMed] [Google Scholar]
- 21.Narattaphol C. Physical activity and exercise affect intestinal calcium absorption: a perspective review. J Sports Sci Technol. 2007;7(1):171–181. [Google Scholar]
- 22.Alghadir AH, Gabr SA, Al-Eisa ES, Alghad MH. Correlation between bone mineral density and serum trace elements in response to supervised aerobic training in older adults. Clin Interv Aging. 2016;11:265–273. doi: 10.2147/CIA.S100566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kara E. Effect of a three-month football training program on trace element metabolism of boys in the eight to twelve age groups. Afr J Biotechnol. 2011;11(1):169–172. [Google Scholar]
- 24.Kelly PJ, Eisman JA, Sambrook PN. Interaction of genetic and environmental influences on peak bone density. Osteoporosis Int. 1990;1:56–60. doi: 10.1007/BF01880417. [DOI] [PubMed] [Google Scholar]


