Abstract
Researchers in a recent multicenter study developed and validated a novel prognostic index, Risk Estimation of Tumor Recurrence After Transplant (RETREAT), which incorporates α-fetoprotein (AFP) at liver transplantation (LT), microvascular invasion, and the sum of the largest viable tumor and number of tumors on explant. We now aim to evaluate RETREAT in the United Network for Organ Sharing (UNOS) database in patients with hepatocellular carcinoma (HCC) who meet Milan criteria by imaging and underwent LT between 2012 and −2014. On explantation (n = 3276), 13% had microvascular invasion, 30% had no viable tumor, and 15% exceeded Milan criteria. Post-LT survival at 3 years decreased with increasing RETREAT score: 91% for a score of 0, 80% for a score of 3, and 58% for a score ≥5 (P < .001). Post-LT HCC recurrence probability within 3 years increased from 1.6% with RETREAT score of 0% to 29% for a score ≥5 (P < .001). Increasing RETREAT score was also associated with a shorter time to HCC recurrence. RETREAT was superior to Milan criteria (explant) in predicting HCC recurrence by the net reclassification index (P < .001). This study validates the prognostic power of RETREAT, which may help standardize post-LT surveillance, provide a framework for tumor staging and risk stratification, and select candidates for adjuvant therapies.
Keywords: classification systems: Milan criteria, clinical research/practice, health services and outcomes research, liver disease: malignant, liver transplantation/hepatology, organ procurement and allocation, Organ Procurement and Transplantation Network (OPTN), recurrent disease, United Network for Organ Sharing (UNOS)
1 |. INTRODUCTION
During the past 2 decades, liver transplantation (LT) has secured its place as a curative treatment for selected patients with hepatocellular carcinoma (HCC). Despite adherence to the Milan criteria1 (1 lesion ≤5 cm, 2–3 lesions ≤3 cm) under the Model of End-stage Liver Disease (MELD) allocation policy for priority listing since 2002, tumor recurrence still occurs in up to 15% after LT.2–4 The downstream effect of HCC recurrence is the significantly lower survival compared with those without recurrence.5 Furthermore, tumor recurrence is largely the reason for the slightly inferior post-LT survival for patients with HCC compared with those without HCC.6,7 This is a particularly important problem facing the transplant community as HCC now accounts for more than 20% of all LTs performed in the United States, compared with less than 5% in the early 2000s.8 Only 10% to 30% of recurrent HCCs are eligible for curative therapies including resection or ablation.5,9 While the median survival after HCC recurrence was less than 1 year,4,5 those with recurrent HCC amenable to resection or ablation may achieve a 50% survival at 3 to 5 years after recurrence.9,10
Until recently, there has been no validated prognostic score for HCC recurrence that includes both pre-LT factors and explant tumor characteristics to accurately quantify an individual’s risk for recurrence. Such a prognostic index, while not intended to improve candidate selection before LT, would help identify candidates for adjuvant therapies and determine appropriate post-LT HCC surveillance, which is currently not standardized and varies widely in practice across LT centers.2,4
Researchers in a large multicenter study recently developed and validated the Risk Estimation of Tumor Recurrence After Transplant (RETREAT) score incorporating 3 variables that independently predicted post-LT HCC recurrence: microvascular invasion, α-fetoprotein (AFP) at LT, and the sum of the largest viable tumor diameter and number of viable tumors on explant11 (Table 1). Among patients with HCC meeting Milan criteria before LT, RETREAT was able to stratify 5-year post-LT tumor recurrence risk from less than 3% for those with a score of 0 to greater than 75% for a score of 5 or greater. A potential limitation of this study is the significantly higher HCC recurrence rate in the validation cohort compared with the development cohort, despite including only patients with HCC meeting Milan criteria prior to LT. Since the RETREAT score was derived from high-volume LT centers, the validity of the RETREAT score when applied on a broader scale has not been tested.
TABLE 1.
RETREAT score points table created based on predictors of HCC recurrence
| Predictor | RETREAT pointsb |
|---|---|
| AFP at LT, ng/mL | |
| 0–20 | 0 |
| 21–99 | 1 |
| 100–999 | 2 |
| ≥1000 | 3 |
| Presence of microvascular invasion | 2 |
| Explant largest viable tumor diameter + number of viable tumors | |
| 0a | 0 |
| 1–4.9 | 1 |
| 5–9.9 | 2 |
| ≥10 | 3 |
AFP, α-fetoprotein; HCC, hepatocellular carcinoma; LT, liver transplantation; Ref, reference.
Explant largest viable tumor diameter + number = 0 if no viable tumor is identified.
The RETREAT score is obtained by adding the total number of points scored in each of the 3 variables (range 0–8). RETREAT score = 0 if a patient has AFP 0–20 ng/mL at LT, no microvascular invasion, and no viable tumor in the explant.
Since April 2012, United Network for Organ Sharing (UNOS) has captured explant pathology data in patients with HCC undergoing LT with MELD exception. This invaluable addition allows examination of histopathologic tumor staging as well as clinical-pathologic correlations in the largest database available. Another advantage of the UNOS database is the comprehensive collection of data across the country so that the overall reported outcome should not be significantly affected by center-specific differences in clinical practice. The aim of the present study is to evaluate the performance of RETREAT in patients transplanted for HCC who meet Milan criteria by imaging in the UNOS database since the initiation of the explant pathology file.
2 |. PATIENTS AND METHODS
2.1 |. Study design and patient population
This study included patients in the UNOS database (Standard Transplant Analysis and Research files released in March 2016) aged 18 years and older who received MELD exception for HCC and underwent LT between April 2012 and December 2014. This study start date was chosen as it was when the UNOS/Organ Procurement and Transplantation Network (OPTN) explant pathology form became available. LT centers were required to enter the size, location, and percent necrosis of each of the 5 largest tumors along with the worst tumor differentiation and the presence of microvascular or macrovascular invasion. Additional data, including lymph node involvement, extrahepatic spread, and presence of satellite lesions, were also captured on this form. Patients were excluded from the study if their radiographic tumor burden ever exceeded Milan criteria on any exception petition. Additionally, those without evidence of HCC on explantation who had not received locoregional therapy (LRT) before LT (ie, HCC misdiagnosis) and patients with either intrahepatic cholangiocarcinoma or mixed HCC/cholangiocarcinoma on explant were also excluded. Patients in whom the RETREAT score could not be calculated due to either lack of AFP within 90 days of LT or unknown explant tumor burden were also excluded.
Study variables collected from the UNOS database at the time of listing with MELD exception included age, sex, race/ethnicity, etiology of liver disease, MELD score, size and number of HCC lesions, and AFP. The percentage of patients who underwent LRT while on the waitlist and the time from listing to LT were also collected, as was the number of LTs performed at each center for HCC during the study period. Per UNOS listing policy, patients underwent contrast-enhanced computed tomography or magnetic resonance imaging at a minimum of once every 3 months after listing for LT.
2.2 |. LT-related variables and RETREAT score calculation
The 3 components of the RETREAT score (AFP, microvascular invasion, and largest viable tumor plus number of viable tumors) were collected from the UNOS exception and explant data files. The AFP closest to the date of LT (within 90 days of LT) was obtained from the liver exception data. Presence of microvascular invasion, size of the largest viable tumor (in centimeters) plus number of viable lesions (to calculate the explant pathology score), and tumor necrosis data were obtained from the explant data. Explant pathology was also reviewed to determine histologic grade based on the modified Edmondson criteria (grade 1: well differentiated; grade 2: moderately differentiated; and grade 3: poorly differentiated).12
The methods used to create the RETREAT score have previously been described.11 An individual’s RETREAT score (range 0–8) is calculated by adding the assigned points for each of the 3 components (Table 1). The sum of the size of the largest viable tumor (in centimeters) on explantation and the number of viable lesions on explantation were calculated, and completely necrotic tumors were excluded from the calculation. For example, if there were 3 lesions on explantation—2 viable lesions measuring 4 cm and 3 cm and a single completely necrotic lesion measuring 5 cm, the completely necrotic lesion would not be counted and the sum of the largest diameter of viable tumor and number of viable tumors would be 6 (4 = diameter of the largest lesion + 2 = number of viable tumors). If no viable tumors were identified, the sum of the largest tumor diameter plus the number of viable tumors would equal 0. A patient who has an AFP at LT of 0 to 20 and on explantation no microvascular invasion and only completely necrotic tumor(s) has a RETREAT score of 0.
2.3 |. HCC recurrence
To identify patients with post-LT HCC recurrence, liver malignancy follow-up data and cause of death variables underwent physician review (N.M.). Records indicating posttransplantation recurrence of pretransplantation malignancy or a cause of death indicating HCC or metastatic malignancy were classified as having HCC recurrence.
2.4 |. Statistical analysis and validation of the RETREAT score
Clinical and tumor characteristics were described with medians and interquartile ranges (IQRs) or frequency and percentages. Characteristics were compared by RETREAT score with the use of Kruskal–Wallis or χ2 tests to assess statistical differences. Observed HCC recurrence and post-LT survival probabilities and 95% confidence intervals (CIs) were estimated at 1 and 3 years by using the Kaplan–Meier method and compared by RETREAT score by using the log-rank test. The ability of the RETREAT score model to discriminate between events and nonevents, for the outcome of HCC recurrence, was assessed by using the overall C-index13 with bootstrap 95% CIs. For the primary outcome, HCC recurrence, net reclassification index14 evaluated improvement in model performance by quantifying the proportion of correct risk reclassification when using RETREAT score versus explant-based Milan criteria to predict risk. Net reclassification improvement was evaluated at 1 and 3 years post-LT by using a priori recurrence risk categories (<5%, 5% to <10%, 10% to <20%, and ≥20%). Correct risk reclassification occurred when RETREAT score–predicted probabilities reclassified patients with recurrence into higher risk categories and patients without recurrence into lower risk categories compared with estimates by explant Milan criteria.
3 |. RESULTS
3.1 |. Patient and center characteristics
Among the 4519 patients initially identified, 439 patients exceeded Milan criteria (by imaging information) on at least 1 MELD exception petition and therefore were excluded. Of the remaining 4080 patients with HCC always within Milan criteria who underwent LT, 68 were excluded due to explant demonstrating either cholangiocarcinoma (n = 13), unknown tumor burden (n = 20), or no HCC despite lack of LRT before LT (n = 35). Of the 4012 patients with available explant information, 736 patients did not have information on AFP within 90 days of LT and were also excluded. The remaining 3276 patients formed the final cohort (Figure 1).
FIGURE 1.
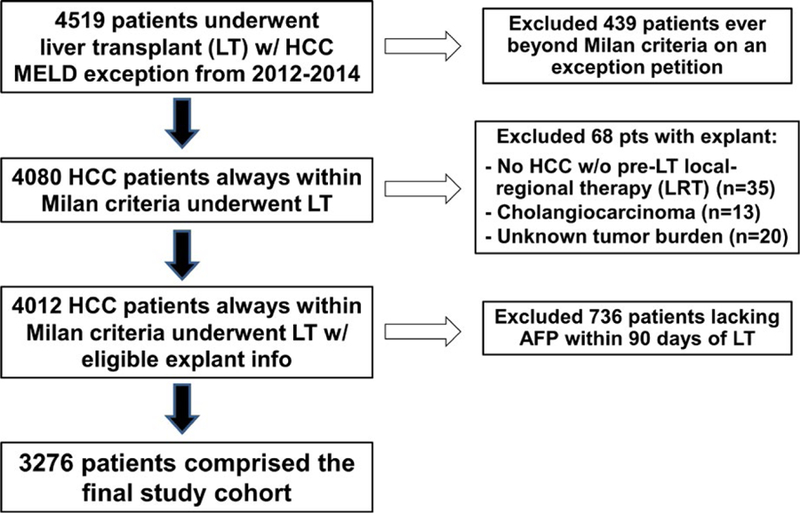
Flow diagram with exclusion criteria describing the formation of the study cohort [Color figure can be viewed at wileyonlinelibrary.com]
The baseline characteristics of the final study cohort are summarized in Table 2. The median age at listing with MELD exception was 60 years (IQR 56–63) and 76.7% were men. The most common race/ethnicities were white (69.0%), Hispanic (13.4%), African American (9.3%), and Asian (7.1%). Hepatitis C was the most common cause of liver disease (62.8%), followed by nonalcoholic fatty liver disease (8.0%), alcoholic liver disease (7.3%), and hepatitis B (5.5%). At the time of listing with MELD exception, the actual median MELD score was 11 (IQR 8–15). Median AFP level at listing was 14 ng/mL (IQR 6.3–84). The majority (77.6%) of patients had a single lesion, and only 5.2% had 3 lesions at listing.
TABLE 2.
Clinical characteristics of the study cohort (n = 3276) at listing with MELD exception and while on the waitlist
| Variables at listing with MELD exception | |
|---|---|
| Median age (IQR) | 60 (56–63) |
| Male sex, % | 2512 (76.7) |
| Race/ethnicity, % | |
| White | 2259 (69.0) |
| Hispanic | 438 (13.4) |
| African American | 305 (9.3) |
| Asian | 231 (7.1) |
| Others | 43 (1.3) |
| Etiology of liver disease, % | |
| Hepatitis C | 2056 (62.8) |
| Nonalcoholic fatty liver disease | 261 (8.0) |
| Alcoholic liver disease | 239 (7.3) |
| Hepatitis B | 180 (5.5) |
| Autoimmune liver diseasea | 67 (2.0) |
| Others | 491 (14.4) |
| Median MELD (IQR) | 11 (8–15) |
| HCC number,b % | |
| 1 | 2528 (77.6) |
| 2 | 560 (17.2) |
| 3 | 169 (5.2) |
| Median AFP, ng/mL (IQR) | 14.0 (6.3–84) |
| Variables on waitlist | |
| Received LRT, % | 2984 (91.1) |
| Median wait time (mo) to LT (IQR) | 5.0 (2.3–10.0) |
MELD, Model for End-stage Liver Disease; IQR, interquartile range; HCC, hepatocellular carcinoma; AFP, α-fetoprotein; LRT, local regional therapy; LT, liver transplantation.
Includes autoimmune hepatitis, primary biliary cholangitis, and primary sclerosing cholangitis.
n = 3257.
While on the waitlist, 91.1% received at least 1 LRT, and the median wait time from listing with MELD exception to LT was 5.0 months (IQR 2.3–10.0). LT was performed at 113 centers with a median of 25 (IQR 11–41) LTs performed per center. Just over half of the patients (52.0%) underwent LT at 1 of the 29 centers (25.7%) that performed more than 40 LTs for HCC during the study period.
3.2 |. Tumor characteristics at transplantation and RETREAT score distribution
In the explant, the tumors showed complete necrosis with no viable tumor as a result of LRT in 30.4%, were within Milan criteria in 55.4%, and were understaged to beyond Milan criteria in 14.2%. Macrovascular invasion was found in 1.7%. Among patients with viable tumors in the explant, most had either well-differentiated (31.8%) or moderately differentiated HCC (59.0%), while only 9.2% had poorly differentiated tumor grade.
Tumor characteristics at the time of LT used to calculate the RETREAT score are shown in Table 3. Median AFP at the time of LT was 9.0 ng/mL (IQR 4.0–26). AFP was 20 or less in 69.5%, 21 to 99 in 19.8%, 100 to 999 in 9.7%, and 1000 or greater in 0.9%, for which patients received 0, 1, 2, or 3 RETREAT points, respectively. Microvascular invasion was found in 12.7%, and these patients received an additional 2 points. The sum of the largest viable tumor size (in centimeters) plus number of viable lesions was 0 in the 30.4% with completely necrotic tumor(s), 1 to 4.9 in 48.0%, 5 to 9.9 in 20.8%, and greater than 10 in 0.8% giving these patients an additional 0, 1, 2, or 3 points, respectively (Table 3). Total RETREAT score was 0 in 23.2%, 1 in 33.8%, 2 in 19.7%, 3 in 11.6%, 4 in 7.1%, and 5 or higher in 4.7%.
TABLE 3.
Tumor characteristics at LT used to calculate a patient’s RETREAT score (n = 3276)
| Variable | N (%) | RETREAT points |
|---|---|---|
| AFP at LT, ng/mL | ||
| 0–20 | 2278 (69.5) | 0 |
| 21–99 | 650 (19.8) | 1 |
| 100–999 | 318 (9.7) | 2 |
| ≥1000 | 30 (0.9) | 3 |
| Microvascular Invasion | 392 (12.7) | 2 |
| Largest viable tumor diameter + number | ||
| 0a | 997 (30.4) | 0 |
| 1–4.9 | 1571 (48.0) | 1 |
| 5–9.9 | 681 (20.8) | 2 |
| ≥10 | 27 (0.8) | 3 |
AFP, α-fetoprotein; LT, liver transplantation.
No viable tumor identified on explant.
3.3 |. Posttransplant outcomes stratified by RETREAT score
Median post-LT follow-up was 1.9 years (IQR 1.0–2.2). Overall post-LT survival was 93.1% (95% CI 92.1–93.9) at 1 year and 83.2% (95% CI 81.3–85.0) at 3 years. Post-LT survival decreased with increasing RETREAT score. For example, at 3 years post-LT, observed survival was 90.7% for a score of 0, 79.9% for a score of 3, and only 58.3% for a score of 5 or higher (P < .001) (Figure 2).
FIGURE 2.
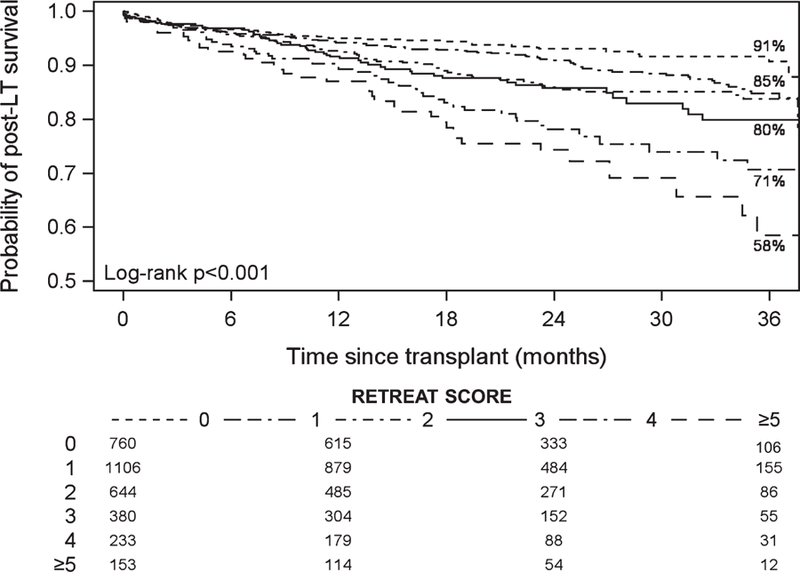
Observed probability of post–liver transplantation survival stratified by RETREAT score
HCC recurrence was found in 4.4% (145/3276) at a median of 11.3 months (IQR 6.3–18.8) after LT. Overall, the rate of post-LT HCC recurrence was 2.5% (95% CI 2.0–3.1) at 1 year and 6.9% (95% CI 5.7–8.2) at 3 years. Similar to the results for post-LT survival, recurrence increased with increasing RETREAT score. Observed probability of post-LT HCC recurrence at 3 years was 1.6% in patients with a RETREAT score of 0, 8.4% with score of 3, and 29.0% for a score of 5 or higher (P < .001) (Figure 3).
FIGURE 3.
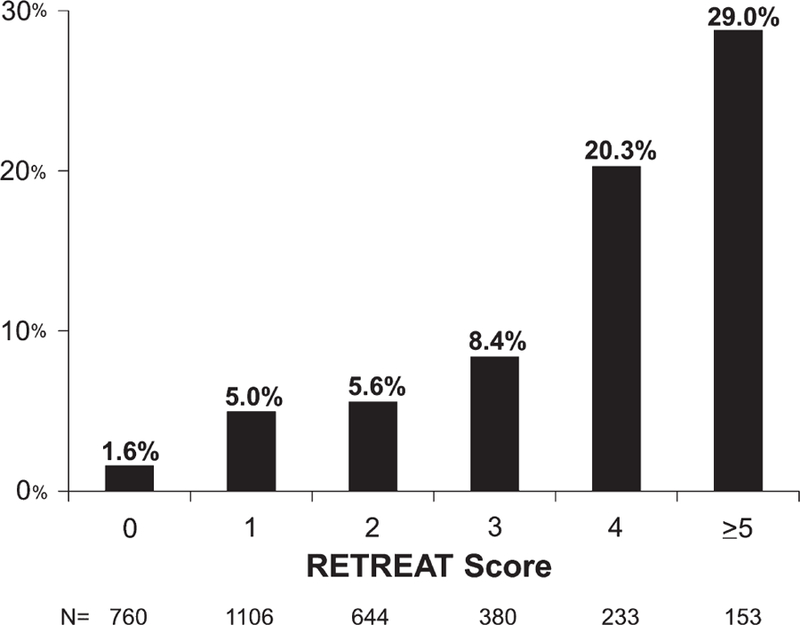
Observed 3-year post–liver transplantation hepatocellular carcinoma recurrence stratified by RETREAT score. The C-index was 0.75 for this validation cohort
We also sought to determine if RETREAT score was associated with either time from LT to HCC recurrence or time from HCC recurrence to death among patients with events. Patients with a high RETREAT score of 4 or higher had a median time from LT to recurrence of 10.9 months (IQR 5.1–17.9, n = 60), which was significantly shorter than for patients with a low RETREAT score of 0 to 1 whose time from LT to recurrence was 14.0 months (IQR 8.8–23.0, n = 38) (P = .03). Median time from HCC recurrence to death was 4.5 months (IQR 0.5–9.9, n = 99) and was similar across all RETREAT scores (P = .98). We also found no statistically significant differences in etiology of liver disease among the low- and high-risk HCC recurrence groups.
3.4 |. RETREAT score vs Milan criteria
For HCC recurrence prediction, RETREAT performed well in the study cohort with a C-index of 0.75 (95% CI 0.71–0.79) and appeared superior to the Milan criteria by explant (C-index 0.63, 95% CI 0.59–0.67). RETREAT performed well regardless of center volume with a C-index of 0.77 (95% CI 0.71–0.83) for centers that performed 40 or fewer LTs for HCC during the study period and 0.73 (95% CI 0.68–0.78) for centers that performed more than 40 LTs.
The overall net reclassification index at 1 year post-LT was 0.17 (P = .001), showing statistically significant improvement in risk classification for RETREAT compared with Milan criteria at explantation. Improvement was primarily among patients with HCC recurrence, where 27% were correctly assigned to a higher risk category and 10% were incorrectly assigned to a lower risk category (net relative improvement 17%, P = .001). Among nonevents, 8% were correctly reclassified to a lower risk category and 7% were incorrectly assigned to a higher risk category, resulting in no change in risk classification among nonevents (net relative improvement 1%, P = .32). RETREAT also improved risk reclassification at 3 years post-LT (overall net reclassification index 0.28, P < .001).
4 |. DISCUSSION
The present study using the UNOS database has provided further evidence supporting the RETREAT score as a powerful prognostic index for patients undergoing LT for HCC within Milan criteria. The present study included 3 times the number of patients compared with the original study.11 We found that increasing RETREAT scores in the UNOS database not only predicted increased post-LT recurrence but also worse survival. The RETREAT score is strongly associated with the risk of HCC recurrence after LT, from 1.6% at 3 years for a score of 0, to 29% for a score of 5 or higher. Patients with a RETREAT score of 0 had excellent observed 3 year post-LT survival of 91%, whereas a RETREAT score of 5 or higher was associated with a significantly poorer 3 year post-LT survival of 58%. Additionally, RETREAT demonstrated improved prognostication for HCC recurrence compared with explant Milan criteria by both Harrell’s C-Index and the net reclassification index. To our knowledge, RETREAT represents the first HCC prognostic index for LT that has been validated. The present study only focused on validation of the RETREAT score for HCC always within Milan criteria before LT. Further studies should be undertaken to evaluate the prognostic power of RETREAT in patients transplanted for expanded HCC criteria or after tumor downstaging.
Another important observation of the present study is that increasing RETREAT score is associated with a shorter time to HCC recurrence. Patients with a high RETREAT score of 4 or higher recurred 11 months after LT and on average 3 months faster than patients with a low RETREAT score of 0 to 1. Early HCC recurrence has been shown to be a poor prognostic factor. In the study by Sapisochin et al,9 the 1 year survival (postrecurrence) was only 30% in patients with early HCC recurrence compared with 60% in those who had recurrence more than 1 year after LT. Similarly, Bodzin et al10 showed the worst survival in patients with HCC recurrence less than 7 months after LT, followed by those who had HCC recurrence between 7 and 15 months after LT. Our finding that higher RETREAT score is associated with early tumor recurrence continues to strengthen the notion that early tumor recurrence is an important signal of poor tumor biology. While time from HCC recurrence to death was similar across RETREAT scores in the present study, this may be due to short follow-up after HCC recurrence, because less than two-thirds with HCC recurrence in UNOS had died at the end of study follow-up compared with 82% in the Bodzin et al study10 and 80% in the Sapisochin et al study.9
The distribution of RETREAT scores in UNOS was similar to those scores seen in the original development cohort,11 with 23% having a RETREAT score of 0 (AFP ≤20 and no viable tumor or microvascular invasion on explant), 33% having a score of 1, and 12% having a score of 4 or higher. Given that some patients with HCC recurrence after LT are eligible for resection or ablation and can achieve long-term survival after these treatments,9 tumor surveillance after LT remains useful. We believe that the RETREAT score helps determine whether HCC surveillance after LT is warranted. We have previously proposed guidelines for post-LT HCC surveillance strategy11 that have been implemented at our institution (Table 4). We believe that patients with a RETREAT score of 0 do not require surveillance, given their very low predicted HCC recurrence risk. This would be cost-saving and avoid unnecessary exposure to radiation and contrast media. Further studies addressing the cost-effectiveness and the survival benefit of surveillance for HCC recurrence should be undertaken within the context of the RETREAT score. A multicenter study is currently under way to prospectively evaluate the application of RETREAT in post-LT HCC surveillance.
TABLE 4.
Proposed post- LT HCC surveillance regimen stratified by patient’s RETREAT score
| RETREAT score | Percentage of cohort |
Proposed post- LT HCC surveillance regimena |
|---|---|---|
| 0 | 23% | No surveillance |
| 1–3 | 65% | Every 6 mo for 2 y |
| 4 | 7% | Every 6 mo for 5 y |
| >5 | 5% | Every 3– 4 mo for 2 y; then every 6 mo for y 2– 5 |
Surveillance should be performed with multiphasic abdominal computed tomography or magnetic resonance imaging, chest computed tomography, and AFP at the recommended interval.
In addition to guiding post-LT surveillance strategies, the RETREAT score could also potentially impact post-LT immunosuppression and adjuvant therapies. For patients with HCC undergoing LT, there has been a shift toward using mammalian target of rapamycin–based immunosuppression at many centers because of possible antineoplastic properties.15,16 While there are currently no proven posttransplantation adjuvant therapies, the list of potential future therapies is extensive and include liver allograft–derived natural killer cells17,18 and other T cells (ClinicalTrials.gov NCT02686372), radioimmunologic agents,19 and immune checkpoint inhibitors (programmed cell death protein 1, programmed cell death protein 1 ligand, and cytotoxic T-lymphocyte–associated protein 4)20,21 as well as a host of epigenetic mechanisms.22 The RETREAT score not only identifies candidates at high risk for HCC recurrence but also provides a reference for the expected incidence of HCC recurrence, both of which are extremely important to properly design and to select appropriate candidates for future clinical trials. Those with a high risk for HCC recurrence based on a RETREAT score of at least 4 should be considered for enrollment into clinical trials with adjuvant therapies given shortly after LT to reduce post-LT HCC recurrence risk, rather than waiting to treat patients after they have developed recurrent HCC.
Obviously, a priority for research in this field is to further improve candidate selection to limit HCC recurrence after LT. Some progress has been made by incorporating AFP23–25 and response to LRT 26–29 in the selection of LT candidates with HCC within and beyond Milan criteria (downstaging). A fundamental problem facing the transplant community is inaccurate tumor staging by imaging before LT. There is inconsistent correlation between radiographic and pathologic tumor assessment with frequent understaging by imaging even after the implementation of the Liver Imaging Reporting and Data System.30 This is clearly an area that needs substantial improvements. The presence of microvascular invasion has been widely accepted as one of the most important factors associated with HCC recurrence after LT31 and is 1 of the 3 variables used in the RETREAT score. While microvascular invasion cannot be reliably ascertained before LT,31 there have been efforts to identify surrogates for microvascular invasion before LT, including the TRAIN (time–radiological response–AFP–inflammation) score32 and radiogenomic biomarkers.33,34 Nevertheless, the problem with HCC recurrence will not easily go away until we see a major breakthrough in the development of reliable biomarkers to identify candidates at high risk for HCC recurrence after LT who should be excluded from LT. Developing better post-LT strategies for preventing and treating HCC, guided by an accurate and validated prognostic scoring system, remains an important focus of future research.
There are several limitations of the present study. We excluded nearly 20% of the UNOS cohort due to missing information on AFP at the time of LT. Some potential risk factors for HCC recurrence, including neutrophil:lymphocyte ratio35 and response to LRT, were not available in the UNOS database. However, RETREAT in part accounts for the effects of preoperative LRT as those with no viable tumor(s) as a result of complete response to LRT receive no added RETREAT points, reflecting a low risk for HCC recurrence.36,37 In addition, hepatitis C treatment history was not available in the UNOS database, and thus we could not assess the potential effects of treatment with direct-acting antivirals on posttransplantation HCC recurrence.38,39 Because the study period predates the widespread use of Interferon-free therapy, we do not believe that there is a significant impact of these treatments on posttransplantation HCC recurrence. Although the UNOS HCC recurrence data have been validated to exclude systematic underreporting and overreporting by centers,40 no mandate requires centers to report HCC recurrence, and thus some cases of recurrence may have been misclassified. The median post-LT follow-up of 1.9 years in this study was also relatively short. These factors could have resulted in an underestimation of the overall cumulative incidence of HCC recurrence (4.4% in this UNOS validation cohort vs 11.6% in the original development cohort11). Despite this limitation, our analysis has demonstrated that a higher RETREAT score predicts not only greater risk for HCC recurrence but also worse post-LT survival. Finally, there were notable differences in this UNOS cohort compared with our original development cohort,11 including a lower percentage with hepatitis B (5.5% vs 16%), shorter wait time to LT (5 vs 8 months), and a lower percentage beyond Milan on explant (14% vs 22%) in the UNOS cohort. Despite these variations, the RETREAT score still performs well in this national cohort and center-specific differences in clinical practice do not seem to affect the prognostic power of the RETREAT score.
In conclusion, RETREAT is a simple and novel risk score for predicting individual risk for HCC recurrence after LT. This study validated RETREAT as a prognostic scoring system when applied on a broader scale using the UNOS database. RETREAT also outperformed the Milan criteria in predicting HCC recurrence. This prognostic score may help standardize post-LT HCC surveillance strategies, provide a framework for tumor staging and risk stratification, and select appropriate candidates for adjuvant therapies.
ACKNOWLEDGMENTS
This work was supported by the Clinical and Translational Core of the UCSF Liver Center (P30 DK026473).
Funding information
National Institutes of Health, Grant/Award Number: P30 DK026473
Abbreviations:
- AFP
α-fetoprotein
- CI
confidence interval
- HCC
hepatocellular carcinoma
- IQR
interquartile range
- LRT
locoregional therapy
- LT
liver transplant, transplantation
- MELD
Model for End-stage Liver Disease
- OPTN
Organ Procurement and Transplantation Network
- RETREAT
Risk Estimation of Tumor Recurrence After Transplant
- TRAIN
time–radiological response–AFP–inflammation
- UNOS
United Network for Organ Sharing
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
REFERENCES
- 1.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693–699. [DOI] [PubMed] [Google Scholar]
- 2.Pomfret EA, Washburn K, Wald C, et al. Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transpl 2010;16:262–278. [DOI] [PubMed] [Google Scholar]
- 3.Levi DM, Tzakis AG, Martin P, et al. Liver transplantation for hepatocellular carcinoma in the Model for End-stage Liver Disease era. J Am Coll Surg 2010;210:727–734. [DOI] [PubMed] [Google Scholar]
- 4.Clavien PA, Lesurtel M, Bossuyt PM, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol 2012;13:e11–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zimmerman MA, Ghobrial RM, Tong MJ, et al. Recurrence of hepatocellular carcinoma following liver transplantation: a review of preoperative and postoperative prognostic indicators. Arch Surg 2008;143:182–188. [DOI] [PubMed] [Google Scholar]
- 6.Ioannou GN, Perkins JD, Carithers RL Jr. Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology 2008;134:1342–1351. [DOI] [PubMed] [Google Scholar]
- 7.Freeman RB Jr, Steffick DE, Guidinger MK, Farmer DG, Berg CL, Merion RM. Liver and intestine transplantation in the United States, 1997–2006. Am J Transplant 2008;8:958–976. [DOI] [PubMed] [Google Scholar]
- 8.Massie AB, Caffo B, Gentry SE, et al. MELD exceptions and rates of waiting list outcomes. Am J Transplant 2011;11:2362–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sapisochin G, Goldaracena N, Astete S, et al. Benefit of treating hepatocellular carcinoma recurrence after liver transplantation and analysis of prognostic factors for survival in a large Euro-American series. Ann Surg Oncol 2015;22:2286–2294. [DOI] [PubMed] [Google Scholar]
- 10.Bodzin AS, Lunsford KE, Markovic D, Harlander-Locke MP, Busuttil RW, Agopian VG. Predicting mortality in patients developing recurrent hepatocellular carcinoma after liver transplantation: impact of treatment modality and recurrence characteristics. Ann Surg 2017;266:118–125. [DOI] [PubMed] [Google Scholar]
- 11.Mehta N, Heimbach J, Harnois DM, et al. Validation of a risk estimation of tumor recurrence after transplant (RETREAT) score for hepatocellular carcinoma recurrence after liver transplant. JAMA Oncol 2017;3:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer 1954;7:462–503. [DOI] [PubMed] [Google Scholar]
- 13.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med 2004;23:2109–2123. [DOI] [PubMed] [Google Scholar]
- 14.Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–172. [DOI] [PubMed] [Google Scholar]
- 15.Matter MS, Decaens T, Andersen JB, Thorgeirsson SS. Targeting the mTOR pathway in hepatocellular carcinoma: current state and future trends. J Hepatol 2014;60:855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geissler EK, Schnitzbauer AA, Zulke C, et al. Sirolimus use in liver transplant recipients with hepatocellular carcinoma: a randomized, multicenter, open-label phase 3 trial. Transplantation 2016;100:116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanimine N, Ishiyama K, Ohira M, Shimizu S, Yano T, Ohdan H. Adoptive immunotherapy with liver allograft-derived NK cells improves recurrence-free survival after living-donor liver transplantation in patients with hepatocellular carcinoma. Am J Transplant 2015. http://atcmeetingabstracts.com/abstract/adoptive-immunotherapy-with-liver-allograft-derived-nk-cells-improves-recurrence-free-survival-after-living-donor-liver-transplantation-in-patients-with-hepatocellular-carcinoma/ [Google Scholar]
- 18.Tanimine N, Ohdan H. Impact of multiplicity of functional KIR-HLA compound genotypes on hepatocellular carcinoma. Oncoimmunology 2015;4:e983765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J, Shen ZY, Chen XG, et al. A randomized controlled trial of Licartin for preventing hepatoma recurrence after liver transplantation. Hepatology 2007;45:269–276. [DOI] [PubMed] [Google Scholar]
- 20.Kudo M. Immune checkpoint inhibition in hepatocellular carcinoma: basics and ongoing clinical trials. Oncology 2017;92(suppl 1):50–62. [DOI] [PubMed] [Google Scholar]
- 21.Jung HI, Jeong D, Ji S, et al. Overexpression of PD-L1 and PD-L2 Is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Res Treat 2017;49:246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gnoni A, Santini D, Scartozzi M, et al. Hepatocellular carcinoma treatment over sorafenib: epigenetics, microRNAs and microenvironment. Is there a light at the end of the tunnel? Expert Opin Ther Targets 2015;19:1623–1635. [DOI] [PubMed] [Google Scholar]
- 23.Duvoux C, Roudot-Thoraval F, Decaens T, et al. Liver transplantation for hepatocellular carcinoma: a model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143:986–894. [DOI] [PubMed] [Google Scholar]
- 24.Hakeem AR, Young RS, Marangoni G, Lodge JP, Prasad KR. Systematic review: the prognostic role of alpha-fetoprotein following liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther 2012;35:987–999. [DOI] [PubMed] [Google Scholar]
- 25.Hameed B, Mehta N, Sapisochin G, Roberts JP, Yao FY. Alphafetoprotein level > 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl 2014;20:945–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai Q, Avolio AW, Graziadei I, et al. Alpha-fetoprotein and modified response evaluation criteria in solid tumors progression after locoregional therapy as predictors of hepatocellular cancer recurrence and death after transplantation. Liver Transpl 2013;19:1108–1118. [DOI] [PubMed] [Google Scholar]
- 27.Kim DJ, Clark PJ, Heimbach J, et al. Recurrence of hepatocellular carcinoma: importance of mRECIST response to chemoembolization and tumor size. Am J Transplant 2014;14:1383–1390. [DOI] [PubMed] [Google Scholar]
- 28.Yao FY, Mehta N, Flemming J, et al. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology 2015;61:1968–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehta N, Yao FY. Moving past “one size (and number) fits all” in the selection of candidates with hepatocellular carcinoma for liver transplantation. Liver Transpl 2013;19:1055–1058. [DOI] [PubMed] [Google Scholar]
- 30.Harper AM, Edwards E, Washburn WK, Heimbach J. An early look at the Organ Procurement and Transplantation Network explant pathology form data. Liver Transpl 2016;22:757–764. [DOI] [PubMed] [Google Scholar]
- 31.Gouw AS, Balabaud C, Kusano H, Todo S, Ichida T, Kojiro M. Markers for microvascular invasion in hepatocellular carcinoma: where do we stand? Liver Transpl 2011;17(Suppl 2):S72–S80. [DOI] [PubMed] [Google Scholar]
- 32.Lai Q, Nicolini D, Inostroza Nunez M, et al. A novel prognostic index in patients with hepatocellular cancer waiting for liver transplantation: time-radiological-response-alpha-fetoprotein-inflammation (TRAIN) score. Ann Surg 2016;264:787–796. [DOI] [PubMed] [Google Scholar]
- 33.Banerjee S, Wang DS, Kim HJ, et al. A computed tomography radiogenomic biomarker predicts microvascular invasion and clinical outcomes in hepatocellular carcinoma. Hepatology 2015;62:792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Segal E, Sirlin CB, Ooi C, et al. Decoding global gene expression programs in liver cancer by noninvasive imaging. Nat Biotechnol 2007;25:675–680. [DOI] [PubMed] [Google Scholar]
- 35.Halazun KJ, Najjar M, Abdelmessih RM, et al. Recurrence after liver transplantation for hepatocellular carcinoma: a new MORAL to the story. Ann Surg 2017;265:557–564. [DOI] [PubMed] [Google Scholar]
- 36.Agopian VG, Morshedi MM, McWilliams J, et al. Complete pathologic response to pretransplant locoregional therapy for hepatocellular carcinoma defines cancer cure after liver transplantation: analysis of 501 consecutively treated patients. Ann Surg 2015;262:536–545. [DOI] [PubMed] [Google Scholar]
- 37.Montalti R, Mimmo A, Rompianesi G, et al. Absence of viable HCC in the native liver is an independent protective factor of tumor recurrence after liver transplantation. Transplantation 2014;97:220–226. [DOI] [PubMed] [Google Scholar]
- 38.Yang JD, Aqel BA, Pungpapong S, Gores GJ, Roberts LR, Leise MD. Direct acting antiviral therapy and tumor recurrence after liver transplantation for hepatitis C-associated hepatocellular carcinoma. J Hepatol 2016;65:859–860. [DOI] [PubMed] [Google Scholar]
- 39.ANRS collaborative study group on hepatocellular carcinoma. Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: data from three ANRS cohorts. J Hepatol 2016;65:734–740. [DOI] [PubMed] [Google Scholar]
- 40.Samoylova ML, Dodge JL, Vittinghoff E, Yao FY, Roberts JP. Validating posttransplant hepatocellular carcinoma recurrence data in the United Network for Organ Sharing database. Liver Transpl 2013;19:1318–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]


