Abstract
Given the increasing incidence of hepatocellular carcinoma (HCC) and regional variation in liver transplantation (LT) rates for HCC, we investigated temporal and geographic disparities in LT and wait-list dropout. LT candidates receiving Model for End-Stage Liver Disease (MELD) exception from 2005 to 2014 were identified from the United Network for Organ Sharing database (n = 14,320). Temporal differences were compared across 2 eras (2005–2009 and 2010–2014). Regional groups were defined based on median wait time as long-wait region (LWR; regions 1, 5, and 9), mid-wait region (MWR; regions 2, 4, 6, 7, and 8), and short-wait region (SWR; regions 3, 10, and 11). Fine and Gray competing risk regression estimated risk of wait-list dropout as hazard ratios (HRs). The cumulative probability of LT within 3 years was 70% in the LWR versus 81% in the MWR and 91% in the SWR (P < 0.001). From 2005–2009 to 2010–2014, median time to LT increased by 6.0 months (5.6 to 11.6 months) in the LWR compared with 3.8 months (2.6 to 6.4 months) in the MWR and 1.3 months (1.0 to 2.3 months) in the SWR. The cumulative probability of dropout within 3 years was 24% in the LWR versus 16% in the MWR and 8% in the SWR (P < 0.001). From 2005–2009 to 2010–2014, the LWR also had the greatest increase in probability of dropout. Risk of dropout was increased in the LWR (HR, 3.5; P < 0.001) and the MWR (HR, 2.2; P < 0.001) compared with the SWR, and year of MELD exception 2010–2014 (HR, 1.9; P < 0.001) compared with 2005–2009. From 2005–2009 to 2010–2014, intention-to-treat 3-year survival decreased from 69% to 63% in the LWR (P < 0.001), 72% to 69% in the MWR (P = 0.008), and remained at 74% in the SWR (P = 0.48). In conclusion, we observed a significant increase in wait-list dropout in HCC patients in recent years that disproportionately impacted LWR patients. Widening geographical disparities call for changes in allocation policy as well as enhanced efforts at increasing organ donation and utilization.
The landscape of liver transplantation (LT) for hepatocellular carcinoma (HCC) has changed dramatically since the introduction of the Model for End-Stage Liver Disease (MELD) priority exception system for HCC in 2002. This policy was initially implemented to establish equitable dropout rates for HCC and non-HCC patients,(1) but even with adjustments in 2003 and 2005, HCC patients continued to receive excess priority.(2–5) A confounding factor has been the regional variation in this discrepancy.(5,6) Patients in regions with short wait-list time have greater access to LT and improved wait-list outcomes compared with patients in regions with longer wait-list time.(7) For example, in 2010, 90% of HCC patients in regions with short wait-list time underwent LT within 3 months of listing compared with 20%−30% in regions with long wait-list time.(8) After a 10-year span (2005–2015) during which the same allocation system applies for all HCC patients, the United Network for Organ Sharing (UNOS)/Organ Procurement and Transplantation Network made 2 policy changes in October 2015: a 6-month delay in awarding exception points and a MELD exception cap at 34 points.(9) These changes were brought about in large part by simulation data(10) showing that a 6-month delay would allow for improved equity between HCC and non-HCC patients. The HCC MELD cap at 34 was introduced to limit regional sharing under the “Share 35” policy to medically urgent patients with a likely effect of this change being to benefit non-HCC patients because there would be less competition for these organs.
Although these policy changes were introduced with important objectives to address the inequity between HCC and non-HCC patients in access to LT, the incidence of HCC has continued to increase due to several factors, including the aging cohort with chronic hepatitis C virus (HCV) as well as increasing rates of the metabolic syndrome and nonalcoholic fatty liver disease.(11,12) The burden of HCC from HCV is not expected to peak until approximately 2030,(13) and nonalcoholic fatty liver disease is currently the fastest growing indication for LT in patients with HCC.(14) Additionally, HCC screening in at-risk populations is recommended by all liver societies and increases the proportion diagnosed at an early stage to be within conventional LT criteria.(15,16) HCC now accounts for nearly 25% of all LTs performed in the United States compared with only 15% from 2002 to 2005.(4,17,18) The impact of the growing demands of LT for patients with HCC in recent years on wait-list outcome overall and by region has not been clearly elucidated.
Given the increasing incidence of wait-list registrants with HCC and ongoing regional differences in LT rates for HCC, we investigated temporal and geographical trends in LT and wait-list dropout in the UNOS database to test the hypothesis that HCC patients in regions with the longest wait time are further disadvantaged over time.
Patients and Methods
STUDY DESIGN AND PATIENT POPULATION
This study included consecutive patients with HCC in the UNOS database aged 18 years and older who were always within Milan criteria and listed for LT with MELD exception for HCC from 2005 to 2014. This study period was chosen because it reflected a continuous period where initial MELD exception was uniformly granted at 22 points for type 2 HCC (1 lesion 2–5 cm or 2–3 lesions none >3 cm) with upgrades every 3 months.(19) The end date was chosen to allow for adequate follow-up on the waiting list and to exclude patients affected by UNOS policy changes enacted in 2015. Patients listed for a multiorgan transplant (n = 432) or those receiving a living donor (n = 148) were excluded from this analysis.
Study variables collected from the UNOS database at the time of listing with MELD exception included age, sex, race/ethnicity, etiology of liver disease, body mass index, laboratory MELD and Child-Pugh score, size and number of HCCs, alpha-fetoprotein (AFP), and local regional therapy (LRT) received. Year of MELD exception approval and UNOS region where each patient was listed were captured to assess temporal and regional changes. Year of MELD exception was dichotomized into early (2005–2009) and late (2010–2014) eras in 5-year increments. The 11 UNOS regions were subdivided based on median time from listing to LT into long-wait region (LWR; regions 1, 5, and 9), mid-wait region (MWR; regions 2, 4, 6, 7, and 8), and short-wait region (SWR; regions 3, 10, and 11) (Table 1). Per UNOS listing policy, patients underwent contrast-enhanced computed tomography or magnetic resonance imaging at a minimum of once every 3 months after LT listing.
TABLE 1.
Wait Time From Initial HCC MELD Exception to LT and Wait-List Dropout by Individual UNOS Region During the 2005–2014 Study Period (n = 14,320)
| UNOS Region | Time to LT (months) |
Time to Dropout (months) |
|---|---|---|
| LWR | 7.7 (4.0–13.3) | 7.2 (3.3–12.9) |
| 1 | 8.2 (3.9–13.1) | 7.4 (4.5–12.5) |
| 5 | 7.7 (4.2–13.6) | 6.3 (3.0–12.0) |
| 9 | 7.2 (3.6–12.7) | 8.3 (4.2–14.3) |
| MWR | 4.2 (1.7–7.7) | 5.2 (2.5–9.3) |
| 7 | 5.7 (3.1–9.8) | 6.3 (2.9–10.5) |
| 2 | 4.3 (2.0–8.0) | 4.9 (2.3–9.8) |
| 4 | 4.2 (1.7–7.6) | 5.1 (2.7–9.0) |
| 8 | 3.5 (1.2–6.3) | 5.1 (2.8–8.6) |
| 6 | 2.4 (0.8–5.7) | 4.2 (2.4–7.7) |
| SWR | 1.6 (0.6–3.2) | 3.0 (1.3–5.5) |
| 11 | 1.7 (0.7–3.4) | 3.0 (1.3–6.1) |
| 10 | 1.5 (0.5–3.1) | 3.1 (1.3–5.6) |
| 3 | 1.5 (0.6–3.2) | 3.0 (1.2–5.2) |
| P value (LWR versus MWR versus SWR) | <0.001 | <0.001 |
NOTE: Data are given as median (IQR).
OUTCOMES AND STATISTICAL ANALYSIS
The primary outcome was dropout from the LT waiting list for any of the following reasons: death without LT, HCC tumor progression beyond Milan criteria, or being too sick to undergo LT. Secondary outcomes included LT, intention-to-treat survival, and post-LT survival. Outcomes were assessed for the overall cohort and stratified by both wait-time region and MELD exception year to investigate geographic and temporal disparities, respectively.
Patient characteristics were summarized using medians and interquartile ranges (IQRs) for continuous variables and proportions for categorical variables. Characteristics were stratified by UNOS wait-time regions (LWR, MWR, and SWR) and compared with Kruskal-Wallis and Pearson’s chi-square tests, as appropriate. The cumulative incidence of wait-list dropout and LT were estimated while accounting for competing risks.(20) Patient follow-up time was measured from the date of first MELD exception to wait-list outcome (dropout or LT) or last date on the waiting list. For dropout, follow-up was terminated on the date of dropout with LT considered a competing event. For LT, patient follow-up ended at the date of LT with dropout considered a competing event. Patients removed from the waiting list for other reasons or remaining on the waiting list were censored at the last date on the waiting list. Intention-to-treat and post-LT survival were estimated using the Kaplan-Meier method and compared by year of MELD exception and UNOS wait-time region using the log-rank test. For intention-to-treat survival, patient follow-up time was measured from the date of first MELD exception to the date of wait-list or post-LT death. Patients were censored on the date of last follow-up (for those without LT), date of retransplant, or date of last post-LT follow-up. Follow-up for post-LT survival was measured from the date of LT to death with patients remaining alive censored at the date of retransplant or last follow-up. Univariate and multivariate hazard ratios (HRs) and 95% confidence intervals (CIs) for risk of wait-list dropout were estimated using Fine and Gray competing risk regression. Characteristics with a univariate P value of <0.1 were included in the multivariate analysis with the final model selected by backward elimination (P for removal >0.05). Statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC) and Stata/IC 14.2 (StataCorp, College Station, TX). This study was approved by the UCSF Committee for Human Research.
Results
PATIENT CHARACTERISTICS
Baseline demographic and clinical characteristics of the 14,320 patients comprising the study population are summarized in Table 2. There were 33.3% of the cohort listed in the LWR, 42.8% listed in the MWR, and 23.9% listed in the SWR. A significantly higher percentage of non-Caucasians were listed in the LWR. HCV was the most common etiology of liver disease. Hepatitis B virus (HBV) was more common in the LWR, whereas nonalcoholic fatty liver disease was more common in the SWR. At the time of LT listing, the overall median laboratory MELD score was 11. The LWR had the highest proportion of Child-Pugh A patients, and the SWR had the highest proportion of Child-Pugh C patients. At listing, 70.9% had a single lesion, 21.4% had 2 lesions, and 7.8% had 3 lesions. Patients in the LWR and the MWR more commonly received at least 1 LRT (71.5% and 69.5%, respectively) than those in the SWR (55.4%).
TABLE 2.
Baseline Characteristics of the Study Population by Wait-Time Region (n = 14,320)
| Overall (n = 14,320) | LWR (n = 4764) | MWR (n = 6134) | SWR (n = 3422) | P Value | |
|---|---|---|---|---|---|
| Age, years | 58 (54–63) | 59 (54–63) | 58 (54–62) | 58 (54–63) | <0.001 |
| Sex, male | 11,010 (76.9) | 3660 (76.8) | 4737 (77.2) | 2613 (76.4) | 0.62 |
| Ethnicity | <0.001 | ||||
| Caucasian | 9437 (65.9) | 2632 (55.2) | 4165 (67.9) | 2640 (77.1) | |
| Hispanic | 2133 (14.9) | 1072 (22.5) | 787 (12.8) | 274 (8.0) | |
| Black | 1355 (9.5) | 308 (6.5) | 681 (11.1) | 366 (10.7) | |
| Asian | 1215 (8.5) | 690 (14.5) | 419 (6.8) | 106 (3.1) | |
| Other | 180 (1.3) | 62 (1.3) | 82 (1.3) | 36 (1.1) | |
| Etiology | <0.001 | ||||
| HCV | 8438 (58.9) | 2797 (58.7) | 3645 (59.4) | 1996 (58.3) | |
| EtOH | 1205 (8.4) | 431 (9.0) | 515 (8.4) | 259 (7.6) | |
| NAFLD | 824 (5.8) | 231 (4.8) | 321 (5.2) | 272 (7.9) | |
| HBV | 779 (5.4) | 365 (7.7) | 299 (4.9) | 115 (3.4) | |
| Autoimmune* | 361 (2.5) | 101 (2.1) | 168 (2.7) | 92 (2.7) | |
| Other | 2713 (18.9) | 839 (17.6) | 1186 (19.3) | 688 (20.1) | |
| BMI, kg/m2 | 28.2 (25.1–31.8) | 27.7 (24.7–31.3) | 28.3 (25.1–31.9) | 28.4 (25.4–32.3) | <0.001 |
| Listing laboratory MELD | 11 (8–14) | 10 (8–14) | 11 (8–14) | 11 (9–14) | <0.001 |
| MELD ≥ 15 | 3142 (22.2) | 982 (21.1) | 1385 (22.8) | 775 (22.7) | 0.008 |
| MELD ≥ 20 | 683 (4.8) | 250 (5.4) | 309 (5.1) | 124 (3.6) | 0.001 |
| Child-Pugh class | <0.001 | ||||
| A | 5181 (36.2) | 2039 (42.8) | 2079 (33.9) | 1063 (31.1) | |
| B | 6761 (47.2) | 2034 (42.7) | 3028 (49.4) | 1699 (49.6) | |
| C | 2378 (16.6) | 691 (14.5) | 1027 (16.7) | 660 (19.3) | |
| AFP, ng/mL | 13 (5–51) | 12 (5–53) | 13 (5–49) | 13 (5–53) | 0.86 |
| Initial tumor burden | 0.01 | ||||
| 1 lesion of 2–3 cm | 6645 (46.4) | 2189 (46.0) | 2920 (47.6) | 1536 (44.9) | |
| 1 lesion of 3.1–5.0 cm | 3502 (24.5) | 1168 (24.5) | 1505 (24.5) | 829 (24.2) | |
| 2 lesions | 3060 (21.4) | 1001 (21.0) | 1279 (20.9) | 780 (22.8) | |
| 3 lesions | 1113 (7.8) | 406 (8.5) | 430 (7.0) | 277 (8.1) | |
| Received LRT | 9566 (66.8) | 3404 (71.5) | 4265 (69.5) | 1897 (55.4) | <0.001 |
| MELD exception listing year | <0.001 | ||||
| 2005–2009 | 6194 (43.3) | 2128 (44.7) | 2577 (42.0) | 1489 (43.5) | |
| 2010–2014 | 8126 (56.7) | 2636 (55.3) | 3557 (58.0) | 1933 (56.5) |
NOTE: Data are given as n (%) and median (IQR). LWR is regions 1, 5, and 9; MWR is regions 2, 4, 6, 7, and 8; and SWR is regions 3, 10, and 11.
Includes autoimmune hepatitis, primary sclerosing cholangitis, and primary biliary cholangitis.
TEMPORAL LISTING TRENDS
Nearly 2000 more HCC patients received MELD exception approval from 2010 to 2014 (n = 8126) than from 2005 to 2009 (n = 6194). Registrations from 2010 to 2014 in the LWR, MWR, and SWR comprised 55.3%, 58.0%, and 56.5%, respectively, of the regional cohorts (Table 2). Nearly twice as many HCC patients received MELD exception in 2013 (n = 1713) than in 2005 (n = 901).
LT AND WAIT TIMES
Wait-list outcomes for the study cohort are summarized in Table 3. Of the 14,320 patients in the cohort, 10,399 (72.6%) underwent LT after a median of 3.8 months from listing with MELD exception for HCC. Median wait-list time from MELD exception to LT was 7.7 months in the LWR, 4.2 months in the MWR, and 1.6 months in the SWR (P < 0.001). Median MELD exception at LT was 28 in the LWR, 25 in the MWR, and 22 in the SWR. Only 60.7% of patients underwent LT in the LWR compared with 72.8% in the MWR and 88.9% in the SWR (P < 0.001). The LWR had the highest proportion of donors >60 years (19.8% versus 18.6% in the MWR [P = 0.04 versus LWR] and 13.8% in the SWR [P < 0.001 versus LWR]) and the longest cold ischemia time (6.6 versus 6.2 hours in the MWR [P = 0.01 versus LWR] and 5.9 hours in the SWR [P < 0.001 versus LWR]). Donation after circulatory death was lowest in the SWR (4.6% versus 6.7% in the LWR [P < 0.001 versus the SWR] and 6.8% in the MWR [P < 0.001 versus the SWR; P = not significant versus LWR]). The median wait-list time to LT in the LWR increased by 6.0 months (5.6 to 11.6 months) from 2005–2009 to 2010–2014, compared with 3.8 months (2.6 to 6.4 months) in the MWR and 1.3 months (1.0 to 2.3 months) in the SWR (all P < 0.001). From 2005–2009 to 2010–2014, the median MELD exception points at LT increased from 25 to 31 in the LWR and from 22 to 28 in the MWR, but it remained the same at 22 in the SWR (Table 3).
TABLE 3.
Outcomes on the LT Waiting List for HCC Patients by Region and MELD Exception Listing Year
| Overall (n = 14,320) | LWR (n = 4764) | MWR (n = 6134) | SWR (n = 3422) | P Value | |
|---|---|---|---|---|---|
| Dropout due to death/delisting | 2163 (15.1) | 1028 (21.6) | 882 (14.4) | 253 (7.4) | <0.001 |
| Median time to dropout (months) | <0.001 | ||||
| Overall | 5.6 (2.7–10.7) | 7.2 (3.3–12.9) | 5.2 (2.5–9.3) | 3.0 (1.3–5.5) | |
| Listed 2005–2009 | 5.0 (2.3–10.1) | 6.4 (3.1–13.3) | 4.0 (1.9–8.5) | 2.7 (1.0–5.1) | |
| Listed 2010–2014 | 6.0 (3.0–11.0) | 7.4 (3.5–12.9) | 5.6 (3.1–9.9) | 3.1 (1.3–5.7) | |
| Cumulative probability of dropout (95% CI) | <0.001 | ||||
| Within 12 months, % | |||||
| Overall | 12.7 (12.1–13.3) | 16.3 (15.2–17.4) | 13.1 (12.3–14.0) | 6.9 (6.1–7.8) | |
| Listed 2005–2009 | 9.8 (9.0–10.6) | 13.1 (11.6–14.6) | 9.7 (8.6–11.0) | 5.3 (4.2–6.5) | |
| Listed 2010–2014 | 14.9 (14.1–15.7) | 18.9 (17.4–20.5) | 15.5 (14.3–16.8) | 8.2 (7.0–9.5) | |
| Within 24 months, % | |||||
| Overall | 16.1 (15.4–16.7) | 22.5 (21.3–23.8) | 15.7 (14.8–16.7) | 7.7 (6.8–8.7) | |
| Listed 2005–2009 | 11.6 (10.8–12.5) | 16.5 (14.9–18.3) | 10.9 (9.6–12.2) | 5.8 (4.6–7.1) | |
| Listed 2010–2014 | 19.5 (18.6–20.4) | 27.3 (25.5–29.2) | 19.2 (17.8–20.6) | 9.3 (8.0–10.7) | |
| LT | 10,399 (72.6) | 2891 (60.7) | 4465 (72.8) | 3043 (88.9) | <0.001 |
| Laboratory MELD at LT | 0.22 | ||||
| MELD | 12 (9–15) | 12 (9–16) | 12 (9–16) | 12 (9–15) | 0.003 |
| Laboratory MELD at LT ≥ 15 | 3144 (30.3) | 927 (32.1) | 1363 (30.6) | 854 (28.1) | <0.001 |
| Laboratory MELD at LT ≥ 20 | 1185 (11.4) | 408 (14.1) | 538 (12.1) | 239 (7.9) | |
| DRI | 1.39 (1.13–1.69) | 1.45 (1.17–1.78) | 1.38 (1.12–1.67) | 1.34 (1.11–1.63) | <0.001 |
| DRI > 1.5 | 4156 (40.0) | 1344 (46.5) | 1742 (29.0) | 1070 (35.2) | <0.001 |
| DRI > 1.8 | 1858 (17.9) | 669 (23.1) | 739 (16.6) | 450 (14.8) | <0.001 |
| Donor age | <0.001 | ||||
| <40 years | 4404 (42.4) | 1196 (41.4) | 1835 (41.1) | 1373 (45.1) | |
| 40–49 years | 2013 (19.4) | 550 (19.0) | 874 (19.6) | 589 (19.4) | |
| 50–59 years | 2161 (20.8) | 574 (19.8) | 927 (20.8) | 660 (21.7) | |
| 60–69 years | 1315 (12.6) | 381 (13.2) | 592 (13.3) | 342 (11.2) | |
| ≥70 years | 506 (4.9) | 190 (6.6) | 237 (5.3) | 79 (2.6) | |
| Donation after circulatory death | 637 (6.1) | 193 (6.7) | 305 (6.8) | 139 (4.6) | <0.001 |
| Cold ischemia time, hours | 6.2 (5.0–8.0) | 6.6 (5.1–8.5) | 6.2 (5.0–8.0) | 5.9 (4.7–7.2) | <0.001 |
| Time to transplant, months | <0.001 | ||||
| Overall | 3.8 (1.4–7.8) | 7.7 (4.0–13.3) | 4.2 (1.7–7.7) | 1.6 (0.6–3.2) | |
| Listed 2005–2009 | 2.5 (0.9–5.4) | 5.6 (2.9–9.2) | 2.6 (0.9–4.9) | 1.0 (0.4–2.0) | |
| Listed 2010–2014 | 5.4 (2.4–10.4) | 11.6 (6.3–15.9) | 6.4 (3.4–10.2) | 2.3 (1.0–4.0) | |
| HCC MELD exception at LT | <0.001 | ||||
| Overall | 25 (22–28) | 28 (25–31) | 25 (22–28) | 22 (22–25) | |
| Listed 2005–2009 | 22 (22–25) | 25 (25–29) | 22 (22–25) | 22 (22–22) | |
| Listed 2010–2014 | 25 (22–29) | 31 (28–33) | 28 (25–29) | 22 (22–25) | |
| Cumulative probability of transplant (95% CI) | <0.001 | ||||
| Within 12 months, % | |||||
| Overall | 66.4 (65.6–67.2) | 44.9 (43.5–46.4) | 70.0 (68.8–71.2) | 90.3 (89.2–91.3) | |
| Listed 2005–2009 | 78.7 (77.7–79.8) | 63.2 (61.0–65.3) | 83.7 (82.1–85.1) | 92.6 (91.2–93.9) | |
| Listed 2010–2014 | 57.2 (56.1–58.3) | 30.4 (28.6–32.3) | 60.3 (58.6–61.9) | 88.5 (86.9–89.9) | |
| Within 24 months, % | |||||
| Overall | 78.8 (78.0–79.5) | 68.3 (66.8–69.7) | 80.1 (79.0–81.2) | 90.9 (89.9–91.9) | |
| Listed 2005–2009 | 83.9 (82.9–84.8) | 74.9 (72.9–76.8) | 86.0 (84.5–87.4) | 92.9 (91.5–94.2) | |
| Listed 2010–2014 | 75.1 (74.0–76.1) | 63.3 (61.2–65.3) | 76.0 (74.4–77.5) | 89.4 (87.8–90.7) | |
| 3-year post-LT survival | 0.02 | ||||
| Overall | 80.0 (79.2–80.8) | 81.1 (79.5–82.7) | 80.1 (78.7–81.3) | 78.9 (77.3–80.5) | Not significant* |
| Listed 2005–2009 | 78.8 (77.6–79.9) | 80.0 (77.8–82.0) | 78.7 (76.8–80.4) | 77.6 (75.2–79.8) | |
| Listed 2010–2014 | 81.3 (80.0–82.5) | 82.3 (79.6–84.7) | 81.6 (79.6–83.3) | 80.2 (77.8–82.3) |
NOTE: Data are given as n (%) or median (IQR). LWR is regions 1, 5, and 9; MWR is regions 2, 4, 6, 7, and 8; and SWR is regions 3, 10, and 11.
All P values >0.05 for post-LT survival comparisons between listing eras both overall and when stratified by wait region.
The cumulative probability of LT within 3 years of MELD exception was 69.8% (95% CI, 68.3–71.2) for the LWR compared with 80.5% (95% CI, 79.3–81.5) in the MWR and 90.9% (95% CI, 89.9–91.9) in the SWR (P < 0.001). When stratified by year of MELD exception, the overall 3-year cumulative probability of LT decreased from 84.2% in 2005–2009 to 76.0% in 2010–2014 (P < 0.001). This decrease was most pronounced in the LWR (75.7% to 65.4%) and the MWR (86.1% to 76.6%), whereas the SWR was the least affected (92.9% to 89.4%; all P < 0.001).
DROPOUT WHILE ON THE WAITING LIST
Overall, 2163 (15.1%) patients experienced dropout from the waiting list because of death without LT, tumor progression, or being too sick to undergo LT. The median time from MELD exception to dropout was 5.6 months (IQR, 2.7–10.7 months). Median time to dropout was 7.2 months in the LWR, 5.2 months in the MWR, and 3.0 months in the SWR (P < 0.001). Dropout occurred in 21.6% of patients in the LWR compared with 14.4% in the MWR and 7.4% in the SWR (P < 0.001). Nearly half of all dropouts (1028/2163, 47.5%) were in the LWR. Cumulative probabilities of dropout were 9.4% within 6 months, 16.3% within 12 months, and 22.5% within 24 months of listing in the LWR, versus 8.5%, 13.1%, and 15.7% in the MWR and 5.8%, 6.9%, and 7.7% in the SWR, respectively (P < 0.001; Table 3). Center-specific median 2-year cumulative incidence of dropout was 22.1% (IQR, 16.0%−28.2%; range, 7.7%−34.7%) for the LWR (n = 28 centers), 15.4% (IQR, 10.0%−22.2%; range, 6.8%−43.8%) for the MWR (n = 50), and 8.6% (IQR, 4.7%−10.7%; range, 1.4%−19.1%) for the SWR (n = 31).
From 2005–2009 to 2010–2014, the median time to dropout increased from 5.0 to 6.0 months. When stratified by MELD exception era, the overall cumulative probability of dropout within 3 years of MELD exception by competing risks increased from 12.4% in 2005–2009 to 20.5% in 2010–2014 (P < 0.001). The probability of dropout increased from 18.0% to 29.4% in the LWR, from 11.4% to 20.0% in the MWR, and from 6.4% to 9.4% in the SWR (all P < 0.001; Fig. 1; Supporting Table 1).
FIG. 1.
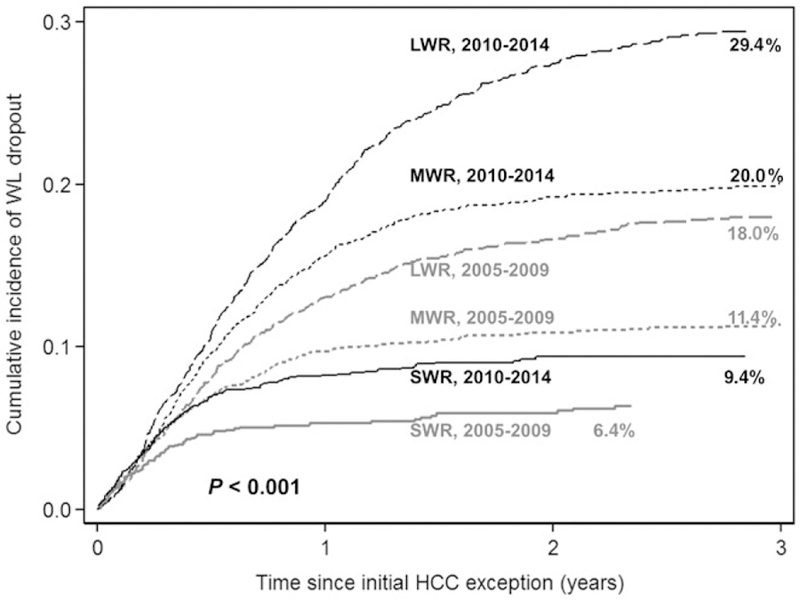
Cumulative probability of wait-list dropout stratified by UNOS wait region (LWR, regions 1, 5, and 9; MWR, regions 2, 4, 6, 7, and 8; and SWR, regions 3, 10, and 11) and MELD exception era (2005–2009 versus 2010–2014).
Given these trends in wait-list dropout by MELD exception era and wait-time region, we performed a post hoc analysis comparing wait-list dropout between HCC and non-HCC patients (n = 60,075) by both wait-time region and listing period (Supporting Table 2). The cumulative incidence of dropout at 2 years in the LWR was not significantly different between HCC and non-HCC patients during the period 2010–2014 (27.7% versus 27.3%, respectively; P = 0.08). In contrast, the wait-list dropout rates at 2 years in the period 2010–2014 were significantly higher for non-HCC patients compared with HCC patients in both the MWR and SWR.
FACTORS ASSOCIATED WITH WAIT-LIST DROPOUT
The univariate analysis of factors associated with wait-list dropout due to death or delisting are summarized in Table 4. Compared with the reference region 5 with the greatest number of listed HCC patients during the study period, regions 1 and 9 had no statistically significant difference in wait-list dropout, whereas risk of dropout was decreased in the other 8 regions. When the regions were combined, the LWR (regions 1, 5, and 9) had a HR of 3.06 and the MWR (regions 2, 4, 6, 7, and 8) had a HR of 2.04 in the risk of wait-list dropout compared with the SWR (regions 3, 10, and 11). In terms of temporal trends, each listing year starting with 2010 was associated with an increased rate of dropout compared with the reference listing year of 2005. When individual listing years were combined, first approved MELD exception in 2010–2014 was associated with increased risk of wait-list dropout with a HR of 1.59 compared with years 2005–2009.
TABLE 4.
Univariate Analysis of Predictors of Wait-list Dropout Because of Death or Delisting by Competing Risks
| Predictor | Univariate HR (95% CI) | P Value |
|---|---|---|
| Wait-time region | ||
| Region 1 (versus 5) | 0.89 (0.75–1.06) | 0.18 |
| Region 2 (versus 5) | 0.68 (0.59–0.78) | <0.001 |
| Region 3 (versus 5) | 0.29 (0.24–0.36) | <0.001 |
| Region 4 (versus 5) | 0.71 (0.61–0.83) | <0.001 |
| Region 6 (versus 5) | 0.52 (0.38–0.71) | <0.001 |
| Region 7 (versus 5) | 0.78 (0.66–0.92) | 0.003 |
| Region 8 (versus 5) | 0.55 (0.45–0.68) | <0.001 |
| Region 9 (versus 5) | 1.13 (0.99–1.29) | 0.08 |
| Region 10 (versus 5) | 0.40 (0.32–0.52) | <0.001 |
| Region 11 (versus 5) | 0.33 (0.26–0.42) | <0.001 |
| LWR (versus SWR) | 3.06 (2.67–3.52) | <0.001 |
| MWR (versus SWR) | 2.04 (1.77–2.35) | <0.001 |
| MELD exception listing year | ||
| 2006 (versus 2005) | 0.90 (0.68–1.19) | 0.46 |
| 2007 (versus 2005) | 1.02 (0.78–1.33) | 0.88 |
| 2008 (versus 2005) | 1.17 (0.91–1.50) | 0.22 |
| 2009 (versus 2005) | 1.19 (0.93–1.53) | 0.16 |
| 2010 (versus 2005) | 1.73 (1.37–2.18) | <0.001 |
| 2011 (versus 2005) | 1.64 (1.30–2.07) | <0.001 |
| 2012 (versus 2005) | 1.76 (1.40–2.22) | <0.001 |
| 2013 (versus 2005) | 1.70 (1.35–2.14) | <0.001 |
| 2014 (versus 2005) | 1.72 (1.36–2.18) | <0.001 |
| 2010–2014 (versus 2005–2009) | 1.59 (1.46–1.75) | <0.001 |
| Age (per year) | 1.02 (1.01–1.02) | <0.001 |
| Sex, female | 1.09 (0.99–1.20) | 0.09 |
| Ethnicity | ||
| Hispanic (versus Caucasian) | 1.26 (1.13–1.41) | <0.001 |
| Black (versus Caucasian) | 1.03 (0.89–1.19) | 0.72 |
| Asian (versus Caucasian) | 0.88 (0.75–1.04) | 0.13 |
| Other (versus Caucasian) | 1.04 (0.71–1.52) | 0.84 |
| Disease etiology | ||
| E†OH (versus HCV) | 1.19 (1.03–1.37) | 0.02 |
| NASH (versus HCV) | 0.92 (0.76–1.11) | 0.36 |
| HBV (versus HCV) | 0.63 (0.50–0.79) | <0.001 |
| Autoimmune* (versus HCV) | 1.10 (0.84–1.43) | 0.48 |
| Other (versus HCV) | 1.02 (0.91–1.14) | 0.72 |
| Laboratory MELD (per point) | 1.09 (1.08–1.10) | <0.001 |
| Laboratory MELD ≥15 (versus <15) | 1.98 (1.82–2.18) | <0.001 |
| Laboratory MELD ≥13 (versus <13) | 1.89 (1.74–2.06) | <0.001 |
| Child-Pugh class | ||
| B (versus A) | 1.59 (1.43–1.76) | <0.001 |
| C (versus A) | 2.65 (2.36–2.99) | <0.001 |
| AFP | ||
| 21–100 ng/mL (versus ≤20) | 1.13 (1.01–1.26) | 0.02 |
| 101–500 ng/mL (versus ≤20) | 1.42 (1.25–1.61) | <0.001 |
| 501–1000 ng/mL (versus ≤20) | 1.76 (1.39–2.22) | <0.001 |
| >1000 ng/mL (versus ≤20) | 2.60 (2.17–3.12) | <0.001 |
| Initial tumor burden | ||
| 1 lesion 3.1–5 cm (versus 1 lesion 2–3 cm) | 1.30 (1.17–1.44) | <0.001 |
| 2 lesions (versus 1 lesion 2–3 cm) | 1.16 (1.04–1.29) | 0.009 |
| 3 lesions (versus 1 lesion 2–3 cm) | 1.31 (1.12–1.53) | 0.001 |
| Received LRT Prior to LT | 0.81 (0.74–0.88) | <0.001 |
NOTE: LWR is regions 1, 5, and 9; MWR is regions 2, 4, 6, 7, and 8; and SWR is regions 3, 10, and 11.
Includes autoimmune hepatitis, primary sclerosing cholangitis, and primary biliary cholangitis.
Additional factors associated with dropout in univariate analysis included increasing age, AFP, laboratory MELD, and Child-Pugh scores as well as Hispanic race/ethnicity versus Caucasians. The risk of dropout was increased among candidates with alcoholic liver disease and decreased among candidates with HBV (both compared with HCV). A single 3.1–5.0 cm lesion or multiple lesions (compared with a single 2–3 cm lesion) at listing was associated with an increased risk of waitlist dropout, whereas receiving at least 1 LRT prior to LT was associated with a decreased risk of dropout.
On multivariate analysis (Table 5), the risk of wait-list dropout remained significantly higher in the LWR (HR, 3.45; P < 0.001) and the MWR (HR, 2.20; P < 0.001) when compared with the SWR, and for the approved MELD exception listing period 2010–2014 (HR, 1.86; P < 0.001) when compared with the period 2005–2009. In addition, dropout was associated with increasing age, AFP > 20 ng/mL, laboratory MELD ≥ 15, and a single 3.1–5.0 cm tumor or multiple tumors (versus a single 2–3 cm tumor). The risk of dropout was lower among patients receiving LRT prior to LT and those with HBV.
TABLE 5.
Multivariate Analysis of Predictors of Wait-List Dropout Because of Death or Delisting by Competing Risks
| Predictor | Multivariate HR (95% CI) |
P Value |
|---|---|---|
| Wait-time region | ||
| LWR (versus SWR) | 3.45 (2.99–3.98) | <0.001 |
| MWR (versus SWR) | 2.20 (1.91–2.55) | <0.001 |
| MELD exception listing year | ||
| 2010–2014 (versus 2005–2009) | 1.86 (1.69–2.04) | <0.001 |
| Age (per year) | 1.02 (1.01–1.02) | <0.001 |
| HBV (versus HCV) | 0.64 (0.51–0.81) | <0.001 |
| Laboratory MELD ≥15 (versus <15) | 2.06 (1.88–2.26) | <0.001 |
| AFP | ||
| 21–100 ng/mL (versus ≤20) | 1.25 (1.12–1.40) | <0.001 |
| 101–500 ng/mL (versus ≤20) | 1.55 (1.36–1.76) | <0.001 |
| 501–1000 ng/mL (versus ≤20) | 2.19 (1.74–2.77) | <0.001 |
| >1000 ng/mL (versus ≤20) | 3.01 (2.50–3.62) | <0.001 |
| Initial tumor burden | ||
| 1 lesion 3.1–5.0 cm (versus 1 lesion 2–3 cm) | 1.38 (1.24–1.53) | <0.001 |
| 2 lesions (versus 1 lesion 2–3 cm) | 1.23 (1.10–1.37) | <0.001 |
| 3 lesions (versus 1 lesion 2–3 cm) | 1.37 (1.17–1.61) | <0.001 |
| Received LRT Prior to LT | 0.70 (0.64–0.77) | <0.001 |
NOTE: LWR is regions 1, 5, and 9; MWR is regions 2, 4, 6, 7, and 8; and SWR is regions 3, 10, and 11.
TRANSPLANT AT A DIFFERENT CENTER THAN FIRST LISTED CENTER
At the end of follow-up, 12.3% of patients remained active on the LT waiting list or had been censored at the time of wait-list removal for reasons including being transferred to another LT center, patient decision not to undergo LT, or being lost to follow-up. Of 10,399 LT recipients, 517 (5.0%) received LT at a different center than where they were originally listed. Of these, 212 (41.0%) and 284 (54.9%) patients were initially listed in the LWR and MWR, respectively, whereas only 21 (4.1%) patients were initially listed in the SWR (P < 0.001).
INTENTION-TO-TREAT AND POSTTRANSPLANT SURVIVAL
The intention-to-treat survival from the date of receiving HCC MELD exception was 82.9% at 1 year and 69.8% at 3 years for the entire cohort. The 3-year intention-to-treat survival for MELD exception era 2005–2009 to 2010–2014 decreased from 71.5% to 68.4%, respectively (P < 0.001). Intention-to-treat survival at 3 years was 65.8% in the LWR, 70.3% in the MWR, and 74.1% in the SWR (P < 0.001). The 3-year intention-to-treat survival decreased from 69.2% to 62.8% in the LWR from the period 2005–2009 to 2010–2014. In comparison, the 3-year intention-to-treat survival decreased from 71.9% to 69.3% in the MWR, but it was unchanged at 74% in the SWR over these 2 time periods (Fig. 2; Supporting Table 3).
FIG. 2.
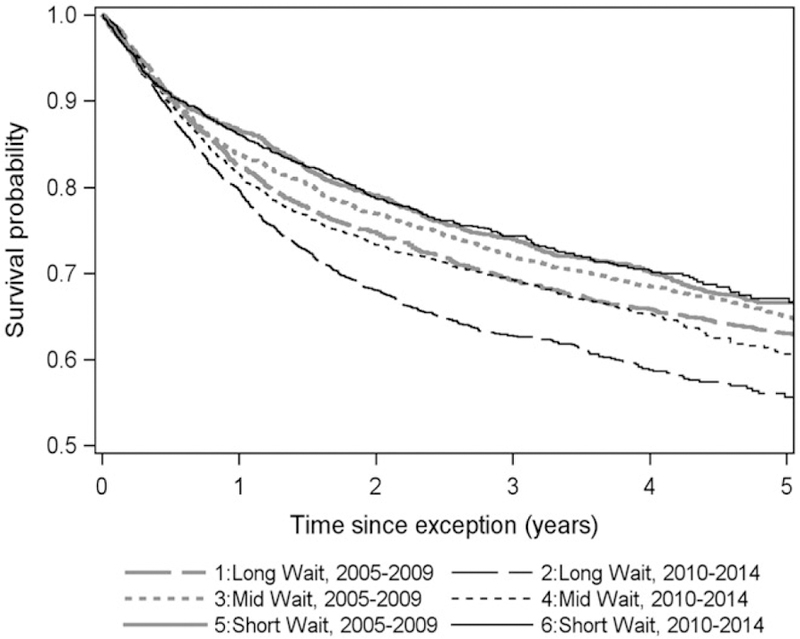
Intention-to-treat survival probability from listing with MELD exception stratified by UNOS wait region (LWR, regions 1, 5, and 9; MWR, regions 2, 4, 6, 7, and 8; and SWR, regions 3, 10, and 11) and MELD exception era (2005–2009 versus 2010–2014).
Median post-LT follow-up time was 3.0 years (IQR, 1.1–5.8 years). The overall post-LT survival was 91.0% (95% CI, 90.4%−91.5%) at 1 year and 80.0% (95% CI, 79.2%−80.8%) at 3 years. The 3-year post-LT survival was 81.1% in the LWR, 80.1% in the MWR, and 78.9% in the SWR (P = 0.02). No significant difference was detected when post-LT survival was stratified by the period receiving MELD exception approval (Table 3).
Discussion
In this analysis of the UNOS database of over 14,000 HCC patients listed with MELD exception from 2005 to 2014, wait-list registration for HCC increased from 43% during the period 2005–2009 to 57% in 2010–2014, and time from listing with MELD exception to LT increased by an average of 3 months between these 2 time periods. What was remarkable, however, was that the LWR bore much of the burden of the increase in wait-list time as well as wait-list dropout. The time from listing to LT increased by 6 months in the LWR compared with 3.8 months and 1.3 months in the MWR and SWR, respectively. The disproportionate increase in wait-list times also translated into further widening of the disparities in wait-list outcomes across regions. The probability of wait-list dropout increased significantly from 18% to 29% in the LWR compared with a very minor increase in wait-list dropout from 6% to 9% in the SWR. Importantly, the intention-to-treat survival at 3 years from LT listing fell from 69% to 63% in the LWR but remained the same at 74% in the SWR. In other words, the likelihood of a given HCC patient being alive at 3 years after LT listing decreased by 11% solely on the basis of where the patient is listed for LT. Furthermore, although we identified other significant predictors of wait-list dropout including increasing age, laboratory MELD score, AFP, and tumor burden, being listed in the LWR carried the highest risk of dropout (HR, 3.45).
These findings raise serious concerns, and yet, they are already familiar to patients and providers in the LWR. This study showed that wait-listed HCC patients in the LWR received grafts from older donors with a higher donor risk index (DRI) and longer cold ischemia time, and they were much more likely to travel to another region to receive a LT than patients from the SWR. Of the 517 such occurrences of receiving LT at a center different from the first center of listing during the study period, only 21 (4%) were in patients initially listed in the SWR. Yet, patients from the LWR had a statistically better 2-year post-LT survival than those from the SWR. There are perceived risks of transplanting patients with HCC too quickly without a minimal period of observation for tumor progression, as illustrated in the “ablate and wait” concept,(21) leading to inclusion of aggressive tumors for LT with a high risk for post-LT recurrence. This in part led to a recent UNOS policy change mandating a 6-month wait before awarding MELD exception.(9) Widening regional dis-parities in the access to LT as well as wait-list outcomes among patients with HCC support the need for additional changes in the organ allocation scheme.
The recently implemented mandatory waiting period of 6 months before MELD exception and a cap of 34 in the MELD exception points for HCC(9) were intended to address inequities in the wait-list outcomes for HCC versus non-HCC patients and probably would not change the widening disparities in wait-list outcomes for HCC patients across regions, as shown in the present study. The 6-month delay should not affect the LWR and MWR where median wait times have generally surpassed 6 months. The effects of the 6-month delay in the SWR are less clear. In a recent multicenter study involving centers in the LWR, MWR, and SWR, dropout rates were low and nearly identical in centers within the MWR and SWR with a median wait-list time of 7.3 and 4.4 months, respectively.(7) Typically, the rate of dropout for HCC patients tends to be low during the first 6 months on the waiting list, but then increases significantly over time.(22) Consequently, assuming that the median time to LT within the SWR does not increase by much beyond 6 months following this recent policy change, wait-list dropout rate in the SWR is unlikely to increase significantly. On the other hand, the MELD exception cap of 34 may further prolong wait-list time and result in an even higher rate of wait-list dropout for HCC patients in the LWR. High rates of wait-list dropout along with the increased risk of post-LT recurrence with long wait-list time >18 months(7) portend poor outcomes for HCC patients in the LWR.
Given the existing geographical disparities in access to LT for HCC patients by region and the likelihood that these disparities may continue to worsen, what should be the next step in bringing about changes in the organ allocation policy to narrow this gap? Reducing priority to LT for HCC patients with a single small, well-treated tumor and well-compensated liver disease who have a very low risk of wait-list dropout(23) would improve access to organs for other HCC patients in more urgent need for LT. This approach, however, would not likely reduce regional disparities. A second option is to assign a fixed HCC MELD exception slightly below the median laboratory MELD score at LT within the donor service area (DSA) rather than using the current “escalator” approach whereby patients receive additional MELD exception every 3 months as they remain on the LT waiting list. After careful consideration, the UNOS board has very recently approved a national liver review board that will assign HCC MELD exception after a 6-month delay based on the median laboratory MELD at LT by DSA minus 3 points.(9) Finally, a concerted effort to improve rates of both organ donation and utilization is imperative. Organ procurement organization (OPO) liver donation rates per 100 eligible deaths range from as low as 44.9 to as high as 87.3, with many of the OPOs in the LWR falling toward the lower end of this spectrum. Similarly, the proportion of livers authorized to be transplanted nationally is 75.7%, but as low as 57.1% in some OPOs located in the LWR.(24) Any improvement in these OPO metrics in the LWR would go a long way to reduce the high wait-list dropout rate.
To our knowledge, this is the first study using the UNOS database to evaluate temporal and geographic trends affecting wait-list outcomes for HCC patients listed for LT. We based our findings on data from a very large cohort using clear distinctions in the stratification of regions as well as listing time periods. Halazun et al.(17) previously designated regions 5 and 9 as LWR and regions 3 and 10 as SWR. In the present study, we included an additional region in each classification using LT wait-time data. Importantly, the rates of wait-list dropout were similar in regions 1, 5, and 9, but they were significantly higher than all other 8 regions, supporting our initial regional wait-time classifications. Furthermore, each listing year from 2010 to 2014 was associated with an increase in the rate of dropout compared with listing years of 2005–2009, allowing for an accurate comparison of recent versus more distant wait-list HCC outcomes to understand temporal trends.
Our study has limitations, most notably the retrospective study design and inherent limitations of the UNOS database. The response to LRT is an important surrogate of tumor biology and a factor predicting wait-list dropout,(23,25,26) but this information is not available in the UNOS database. The pattern of LT listing is also not captured, as only listed patients are included in the UNOS database. Date of initial HCC diagnosis is not accurately recorded in the UNOS database, and this date can vary in relation to date of listing with MELD exception.(7) Finally, the effects of the changes in UNOS policy in October 2015 could not be analyzed because patients listed for LT after 2015 were not included. Given the long wait times to LT in regions 1, 5, and 9, it may take at least another year before the true effects of these policy changes become evident in wait-list outcomes.
In conclusion, in this large national study evaluating temporal and regional trends in wait-list outcomes among patients with HCC, we observed a significant increase in wait-list dropout in recent years that disproportionately impacted patients in the LWR. The widening geographical disparities in wait-list outcomes support both recent UNOS policy approval to assign variable MELD exception points based on the median MELD at transplant by DSA minus 3 points(9) and the need for a change in the geographic boundaries of the current allocation system.
Supplementary Material
Additional supporting information may be found in the online version of this article.
Acknowledgments
This work was supported in part by the Biostatistics Core of the UCSF Liver Center (National Institutes of Diabetes and Digestive and Kidney Diseases P30 DK026473).
Abbreviations:
- AFP
alpha-fetoprotein
- BMI
body mass index
- CI
confidence interval
- DRI
donor risk index
- DSA
donor service area
- EtOH
ethanol
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HR
hazard ratio
- IQR
interquartile range
- LRT
local regional therapy
- LT
liver transplantation
- LWR
long-wait region
- MELD
Model for End-Stage Liver Disease
- MWR
mid-wait region
- NAFLD
nonalcoholic fatty liver disease
- OPO
organ procurement organization
- SWR
short-wait region
- UNOS
United Network for Organ Sharing.
Footnotes
View this article online at wileyonlinelibrary.com.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1).Wiesner RH, Freeman RB, Mulligan DC. Liver transplantation for hepatocellular cancer: the impact of the MELD allocation policy. Gastroenterology 2004;127(suppl 1):S261–S267. [DOI] [PubMed] [Google Scholar]
- 2).Volk ML. Unfair priority for HCC: A problem whose ideal solution remains unsolved. Am J Transplant 2010;10:1507–1508. [DOI] [PubMed] [Google Scholar]
- 3).Goldberg D, French B, Abt P, Feng S, Cameron AM. Increasing disparity in waitlist mortality rates with increased model for endstage liver disease scores for candidates with hepatocellular carcinoma versus candidates without hepatocellular carcinoma. Liver Transpl 2012;18:434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Massie AB, Caffo B, Gentry SE, Hall EC, Axelrod DA, Lentine KL, et al. MELD exceptions and rates of waiting list outcomes. Am J Transplant 2011;11:2362–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Washburn K, Edwards E, Harper A, Freeman R. Hepatocellular carcinoma patients are advantaged in the current liver transplant allocation system. Am J Transplant 2010;10:1643–1648. [DOI] [PubMed] [Google Scholar]
- 6).Kadry Z, Schaefer EW, Uemura T, Shah AR, Schreibman I, Riley TR 3rd. Impact of geographic disparity on liver allocation for hepatocellular cancer in the United States. J Hepatol 2012;56:618–625. [DOI] [PubMed] [Google Scholar]
- 7).Mehta N, Heimbach J, Lee D, Dodge JL, Harnois D, Burns J, et al. Wait time of less than 6 and greater than 18 months predicts hepatocellular carcinoma recurrence after liver transplantation: proposing a wait time “sweet spot”. Transplantation 2017;101:2071–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Washburn K. Model for End-Stage Liver Disease and hepatocellular carcinoma: a moving target. Transplant Rev (Orlando) 2010;24:11–17. [DOI] [PubMed] [Google Scholar]
- 9).Organ Procurement and Transplantation Network. Policies Available from https://optn.transplant.hrsa.gov/. Accessed September 1, 2017.
- 10).Heimbach JK, Hirose R, Stock PG, Schladt DP, Xiong H, Liu J, et al. Delayed hepatocellular carcinoma Model for End-Stage Liver Disease exception score improves disparity in access to liver transplant in the United States. Hepatology 2015;61:1643–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118–1127. [DOI] [PubMed] [Google Scholar]
- 12).Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol 2013;47(suppl):S2–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Rein DB, Wittenborn JS, Weinbaum CM, Sabin M, Smith BD, Lesesne SB. Forecasting the morbidity and mortality associated with prevalent cases of pre-cirrhotic chronic hepatitis C in the United States. Dig Liver Dis 2011;43:66–72. [DOI] [PubMed] [Google Scholar]
- 14).Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014;59:2188–2195. [DOI] [PubMed] [Google Scholar]
- 15).Santi V, Trevisani F, Gramenzi A, Grignaschi A, Mirici-Cappa F, Del Poggio P, et al. for Italian Liver Cancer (ITA.LI.CA) Group. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol 2010;53:291–297. [DOI] [PubMed] [Google Scholar]
- 16).Trevisani F, De Notariis S, Rapaccini G, Farinati F, Benvegnù L, Zoli M, et al. for Italian Liver Cancer Group. Semiannual and annual surveillance of cirrhotic patients for hepatocellular carcinoma: effects on cancer stage and patient survival (Italian experience). Am J Gastroenterol 2002;97:734–744. [DOI] [PubMed] [Google Scholar]
- 17).Halazun KJ, Patzer RE, Rana AA, Verna EC, Griesemer AD, Parsons RF, et al. Standing the test of time: outcomes of a decade of prioritizing patients with hepatocellular carcinoma, results of the UNOS natural geographic experiment. Hepatology 2014;60:1957–1962. [DOI] [PubMed] [Google Scholar]
- 18).Organ Procurement and. Transplantation Network. National Data: Liver Transplants 2017. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/.
- 19).Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693–699. [DOI] [PubMed] [Google Scholar]
- 20).Fine JP, Gray RJ. A proportional hazards model for the sub-distribution of a competing risk. J Am Stat Assoc 1999;94: 496–509. [Google Scholar]
- 21).Roberts JP, Venook A, Kerlan R, Yao F. Hepatocellular carcinoma: ablate and wait versus rapid transplantation. Liver Transpl 2010;16:925–929. [DOI] [PubMed] [Google Scholar]
- 22).Yao FY, Bass NM, Nikolai B, Merriman R, Davern TJ, Kerlan R, et al. A follow-up analysis of the pattern and predictors of dropout from the waiting list for liver transplantation in patients with hepatocellular carcinoma: implications for the current organ allocation policy. Liver Transpl 2003;9:684–692. [DOI] [PubMed] [Google Scholar]
- 23).Mehta N, Dodge JL, Goel A, Roberts JP, Hirose R, Yao FY. Identification of liver transplant candidates with hepatocellular carcinoma and a very low dropout risk: implications for the current organ allocation policy. Liver Transpl 2013;19: 1343–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Scientific Registry of Transplant Recipients. Organ Procurement Organization (OPO) Reports 2017 https://www.srtr.org/reports-tools/opo-specific-reports. Accessed August 22, 2018.
- 25).Mehta N, Yao FY. Moving past “One size (and number) fits all” in the selection of candidates with hepatocellular carcinoma for liver transplantation. Liver Transpl 2013;19:1055–1058. [DOI] [PubMed] [Google Scholar]
- 26).Cucchetti A, Cescon M, Bigonzi E, Piscaglia F, Golfieri R, Ercolani G, et al. Priority of candidates with hepatocellular carcinoma awaiting liver transplantation can be reduced after successful bridge therapy. Liver Transpl 2011;17:1344–1354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article.


