Abstract
Objective:
Depression in Parkinson disease (PD) is a common problem that worsens quality of life and causes disability. However, little is known about the longitudinal impact of depression on disability in PD. This study examined the association between disability and DSM-IV-TR depression status across six years.
Methods:
Longitudinal cohort study with assessments at study entry, year two, four, and six conducted in the Morris K. Udall Parkinson Disease Research Center. Recruitment totaled 137 adult men and women with idiopathic PD in which up to six years of data on demographic, motor, and non-motor variables was collected. Movement disorder specialists used the structured interview for DSM-IV-TR depressive disorders and the Northwestern Disability Scale to assess depression and disability. A generalized linear mixed model was fitted with Northwestern Disability Scale score as the dependent variable to determine the effect of baseline depression status on disability.
Results:
43 participants were depressed at baseline compared to 94 without depression. Depressed participants were more likely to be female, were less educated, were less likely to take dopamine agonists, and more likely to have motor fluctuations. Controlling for these variables, symptomatic depression predicted greater disability compared to both never depressed (p=0.0133) and remitted depression (p=0.0009). Disability associated with symptomatic depression at baseline was greater over the entire six-year period compared to participants with remitted depressive episodes or who were never depressed.
Conclusions:
Persisting depression is associated with a long-term adverse impact on daily functioning in PD. Adequate treatment or spontaneous remission of depression improves ADL function.
Keywords: Parkinson disease, depression, disability, longitudinal, nonmotor
INTRODUCTION
Parkinson disease (PD) is diagnosed according to its motor features: bradykinesia, tremor, rigidity, and postural instability. The progression of motor symptoms parallels worsening daily function over the course of the disease. However, non-motor symptoms, especially psychiatric disorders such as depression, are also recognized as disease features that have an adverse impact on health-related quality of life in PD, and cause additional impairment in activities of daily living (ADLs).(Barone et al., 2009; Carod-Artal et al., 2008; Quelhas et al., 2009; Rahman et al., 2008) Studies on the impact of depression on ADLs in PD have been mostly cross-sectional. They suggest that depression is associated with disability beyond what is expected from motor symptoms alone and that disability is reduced when depression is treated.(Cole et al., 1996; Holroyd et al., 2005; Pankratz et al., 2008; Weintraub et al., 2004; Starkstein et al., 1992; Ravina et al., 2007, Menza et al., 2009) Longitudinal studies within the general population demonstrate that disability level changes with the severity of depression and that daily functioning returns to normal levels when the depression improves.(Ormel et al., 1993) Thus, depression produces disability in all patients and is a significant burden especially for individuals already compromised by the other symptoms of PD.
Longitudinal data on depression and disability in PD are limited; it is unclear whether impaired ADL function associated with depression is stable over long periods and whether ADL function improves at any point in its course if depression remits. This study examined the impact of depression on disability using the first 6 years of data from an ongoing longitudinal study of PD.
METHODS
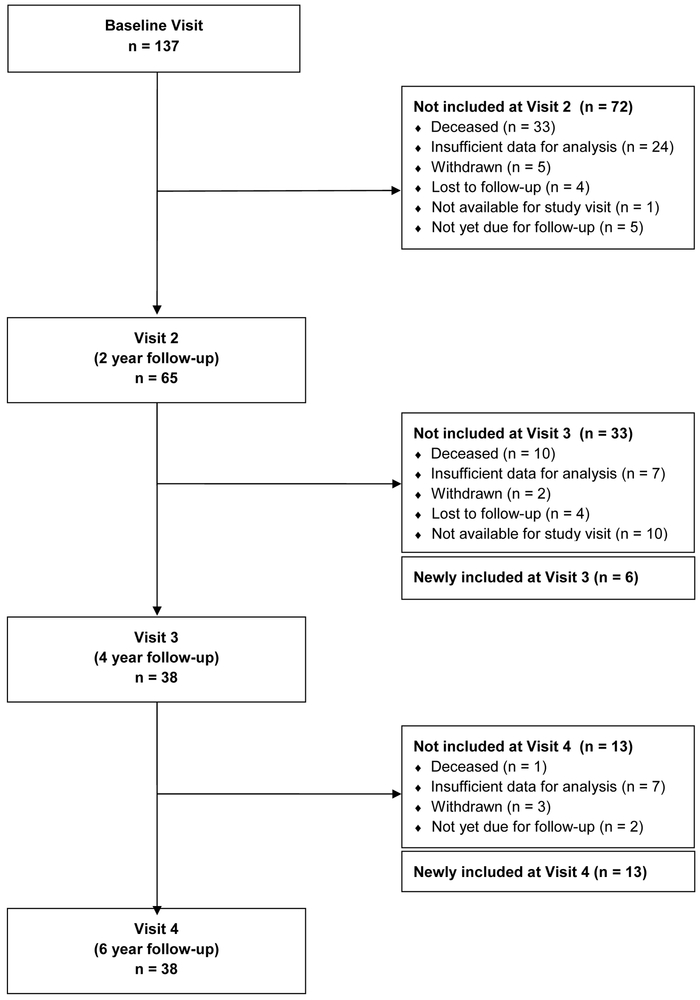
Participants with idiopathic PD (Hughes et al., 1992) were enrolled in a prospective brain donation program with a longitudinal research component that assessed the cognitive, motor, psychiatric, and clinical-pathological features of PD. The cohort was recruited from tertiary care and community practices and included older individuals with advanced disease along with younger less affected participants who could be followed for a longer duration. Assessments were conducted in-person every two years until autopsy or loss to follow up. The analyses for this study draw on data for up to six years of follow-up from 137 participants. Participants were assessed at baseline and then at 2-year intervals (baseline-visit 1, visit 2-year 2, visit 3-year 4, and visit 4-year 6) for a total of 268 visits included in the study. The average number of visits per participant was 2.21 with 137 participants evaluated at visit 1, 65 at visit 2, 38 at visit 3, and 38 at visit 4. Attrition is accounted for in the consort diagram in Figure 1. The Johns Hopkins University Institutional Review Board approved the study protocol. Participants or their legally authorized representative gave written informed consent to participate.
Figure 1.
Consort diagram for Parkinson disease longitudinal study
Depressive syndromes were assessed by a geriatric psychiatrist and research nurse using a combination of a semi-structured clinical interview [Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient edition (SCID)] (First et al., 2002), informant interviews, and medical records review. Using this information and the mental status exam, the psychiatrist assigned final psychiatric diagnoses using DSM-IV-TR criteria for Major Depressive Disorder and Depressive Disorder Not Otherwise Specified (NOS). The Northwestern Disability Scale (NWDS) scores five types of ADLs: walking, speech, hygiene, eating and feeding, and dressing, each on a 0 to 10 point scale; lower scores indicate greater disability.(Canter et al., 1961) Standardized data forms were used to collect demographic information and, based on the clinical interview, to indicate whether participants diagnosed with a depressive disorder were symptomatic or asymptomatic at each visit and whether they were receiving medication for the treatment of depression. This allowed for categorization into three depressive states; 1) symptomatic depression (SD), 2) remitted depression (RD), or 3) never depressed (ND). Depressive episodes that were determined by psychiatric history at interview, but resolved before enrollment, were coded as “remitted depression” at baseline. Depression status of all participants (whether symptomatic, remitted, or never depressed at baseline) was re-evaluated at each follow-up visit using the SCID for DSM-IV-TR diagnostic criteria and an informant interview, participants were reclassified if any change in depression status occurred.
PD motor features were assessed using the Unified Parkinson’s Disease Rating Scale (UPDRS) part III motor subscale and part IV motor complications of therapy (Fahn et al., 1987) and Hoehn Yahr (HY) stage.(Hoehn and Yahr, 1967) Levodopa equivalent daily dose was calculated using conversion factors detailed in Tomlinson et al.(Tomlinson et al., 2010)
Analyses were conducted using Stata Statistical Software 2011: Release 12, College Station, TX: StataCorp LP. In table 2, group means were assessed using ANOVA and percentages were compared using χ2. A generalized linear mixed model was fitted to quantify the observations; nwdsij ~ Poisson(λij) log (λij) = α0i + α1 * visit + α2 * depression + α3 * edu + α4 * sex + α5 * agonist + α6 * motorfluc +εij. The dependent variable, NWDS score, had integer values, which were fitted with a Poisson generalized linear model. The symptomatically depressed status was used as the reference. The model considered only baseline depression status. Sex and dopamine agonist status were binary variables. All other variables were continuous. Random intercepts were used, instead of a fixed one, to capture subject effects (α0i). The model controlled for visit effect, education, and dopamine agonist usage as these variables differed significantly between depressed and non-depressed subjects at baseline and were correlated with NWDS scores. Items from the UPDRS part III score overlapped with items on the NWDS and were therefore not included in the model to avoid confounding despite being significantly different between symptomatically depressed and non-depressed groups.
Table 2:
Sociodemographic Features
| Depressed at Baseline (n = 43) |
Never depressed at Baseline (n = 72) |
Remitted Depression at Baseline (n = 22) |
Significance | |
|---|---|---|---|---|
| Age (yrs), mean (sd) | 67.9 (9.8) | 68.6 (10.4) | 68.1 (10.5) | p = 0.924 |
| Gender | 19 M, 24 F | 51 M, 21 F | 10 M, 12 F | p = 0.008 |
| Education (yrs), mean (sd) | 15.2 (2.3) | 16.9 (3.4) | 15.9 (2.8) | p = 0.017 |
| MMSE, mean (sd) (range 0-30)* | 25.4 (5.5) | 26.8 (3.1) | 27.2 (2.7) | p = 0.130 |
| Parkinson Features | ||||
| Age symptom onset, mean (sd) | 56.9 (12.0) | 58.6 (11.2) | 59.0 (12.4) | p = 0.692 |
| Symptom duration (yrs), mean (sd) | 11.0 (7.8) | 10.0 (6.3) | 9.1 (5.2) | p = 0.529 |
| Presence of motor fluctuations (% n) ** | 70.7% | 54.3% | 50.0% | p = 0.158 |
| Presence of early morning dystonia (% n)** | 34.1% | 27.1% | 36.4% | p = 0.615 |
| Presence of other psychiatric disorders (% n) | 53.5% | 52.8% | 68.2% | p = 0.423 |
| Use of antidepressant medication *** | 62.8% | 23.9% | 54.5% | p = 0.000 |
| Total L-dopa equivalents, mean (sd) | 787.6 (566.7) | 718.8 (438.3) | 622.2 (419.2) | p = 0.418 |
| Dopamine agonist use (% n) | 32.6% | 50.0% | 50.0% | p = 0.163 |
| Disability Severity | ||||
| Northwestern Disability Scale score, mean (sd)**** | 33.6 (11.3) | 38.5 (8.6) | 39.8 (5.9) | p = 0.010 |
| UPDRS part III, motor sub-score, mean (sd)*** | 38.9 (20.3) | 29.7 (15.9) | 22.2 (10.7) | p = 0.001 |
| Hoehn and Yahr Stage* | I 1, II 20, III 7, IV 6, V 6 | I 4, II 52, III 11, IV 2, V 3 | I 2, II 14, III 5, IV 1, V 0 | p =0.044 |
n=134
n=133
n=136
n=135
RESULTS
At baseline 72 participants were assessed as never depressed, 43 were symptomatically depressed, and 22 had depressive disorders that were currently in remission. Table 1 summarizes the sociodemographic, Parkinson features, and disability severity for the total sample of the 137 participants at their baseline visit (Visit 1). The baseline measures for each of the three groups, symptomatically depressed, never depressed, and remitted depression, were also compared across the same categories of sociodemographic, Parkinson features, and disability variables (Table 2).
Table1:
Sociodemographic Features
| n = 137 | |
|---|---|
| Age (yrs), mean (sd) | 68.3 (10.2) |
| Gender | 80 M, 57 F |
| Education (yrs), mean (sd) | 16.2 (3.2) |
| MMSE, mean (sd) (range 0-30)* | 26.4 (4.0) |
| Parkinson Features | |
| Age symptom onset, mean (sd) | 58.1 (11.6) |
| Symptom duration (yrs), mean (sd) | 10.2 (6.7) |
| Presence of motor fluctuations (% n)** | 58.6% |
| Presence of early morning dystonia (% n)** | 30.8% |
| Presence of other psychiatric disorders (% n) | 55.5% |
| Antidepressant medication status, % taking | 35.6% |
| Total L-dopa equivalents, mean (sd) | 724.9 (479.1) |
| Dopamine agonist use (% n) | 44.5% |
| Disability Severity | |
| Northwestern Disability Scale score, mean (sd)**** | 37.2 (9.4) |
| UPDRS part III, motor sub-score, mean (sd)*** | 31.3 (17.5) |
| Hoehn and Yahr Stage* | I 7, II 86, III 23, IV 9, V 9 |
n= 134
n=133
n=136
n=135
A generalized linear mixed model was used to determine the impact of depression status at baseline (never depressed vs. remitted depression vs. symptomatically depressed) on disability as measured by the NWDS. An uncontrolled model found that symptomatic depression predicted worse disability as measured by the NWDS compared to both never depressed (p=0.002) and remitted depression (p=0.0003). After controlling for potential confounders in the relationship between NWDS and depression status (education, dopamine agonist use), the model’s findings remained significant with symptomatic depression predicting worse disability as measured by the NWDS compared to both never depressed (p=0.0233) and remitted depression (p=0.0012) (Table 3). The model, uncontrolled and controlled, found no difference in ADL disability between the never depressed and remitted depression groups (controlled/uncontrolled, estimate 0.0333/0.0237, std. error 0.0392/0.0399, z-value 0.851/0.59, and p-values=0.3949/0.5534).
Table 3:
Model Fit – for symptomatic depression and Northwestern Disability Scale
| Fixed Effects | Controlled model | |||
|---|---|---|---|---|
| Variable | Estimate | Std Err | Z value | p-value |
| Intercept | 3.21219 | 0.11626 | 27.6 | p≤0.001 |
| Visit | −0.05645 | 0.01096 | −5.2 | p≤0.001 |
| Remitted depression | 0.13633 | 0.04198 | 3.2 | p=0.001 |
| Never depressed | 0.09640 | 0.04248 | 2.3 | p=0.023 |
| Education | 0.01872 | 0.00698 | 2.7 | p=0.007 |
| Dopamine agonist | 0.11728 | 0.04334 | 2.7 | p=0.007 |
| Random Effects | ||||
| Groups | Name | Variance | Std Dev | |
| id | Intercept | 0.041789 | 0.20442 | |
| Uncontrolled model | ||||
| Variable | Estimate | Std Err | Z value | p-value |
| Intercept | 3.5480 | 0.03922 | 90.47 | p≤0.001 |
| Visit | −0.05588 | 0.01102 | −5.07 | p≤0.001 |
| Remitted depression | 0.15294 | 0.04245 | 3.60 | p≤0.001 |
| Never depressed | 0.12928 | 0.04273 | 3.03 | p=0.002 |
Normal tests are performed; Number of observations: 268, groups: id, 135
Figure 2 shows that the slope of NWDS differs by baseline depression status. The rate of decline in the NWDS score was 1.06 times greater in the symptomatic depressed group than the never depressed group (slope difference, estimate 0.0588, std. error 0.0272, z-value −0.826, and p-value=0.0399). However, comparing SD and RD the slopes of NWDS decline were not significantly different (slope difference, estimate 0.0363, std. error 0.0328, z-value 1.109, and p-value=0.2676), indicating that both groups decline at the same rate despite RD having an overall lower level of disability (higher NWDS) at anytime point. Finally, NWDS scores for RD appear to decline faster than ND, but this did not reach statistical significance (slope difference, estimate −0.0225, std. error 0.0272, z-value −0.826, and p-value=0.409).
Figure 2. Northwestern disability score by study visit and depression status.
ND=never depressed, RD=remitted depression, SD=symptomatically depressed
*Note to editor, please make black and white in print (we do not wish to pay for color in print) allowing the different shapes to distinguish the lines. However, free online color would be good.
DISCUSSION
This longitudinal study of PD patients of varied duration and disease severity shows that symptomatically depressed individuals have greater ADL disability at any point over a six-year span. Remitted depression, whether because of antidepressant treatment or spontaneous remission, appears to return patients to a non-depressed functional baseline, as there was no difference in ADL function between remitted depression and never depressed patients. Depressed individuals’ ADL function declined faster than individuals who had never been depressed. However, there was no difference in the rate of decline in ADL function in individuals with remitted depression compared to those who remain depressed, despite a return to higher ADL function overall.
The finding that ADL functioning in patients with remitted depression was comparable to those who were never depressed suggests that depressive disorders do not cause persistent functional impairment, even after several years, unless the patient’s mood disorder remains symptomatic. Evidence that depression is associated with a faster rate of decline in ADL function underscores the importance of early recognition and concerted treatment of depressive disorders, in addition to treatment of motor symptoms to preserve ADL function.( Cole et al., 1996; Holroyd et al., 2005; Pankratz et al., 2008; Weintraub et al., 2004; Starkstein et al., 1992; Ravina et al., 2007, Menza et al., 2009) These data also indicate that although ADL function improves with depression remission, the rate of decline in ADL function with remitted depression may be more aggressive (compared to never depressed) and similar in rate to when actively depressed. If true, this suggests that remission of depressive symptoms is similar to the effects that levodopa and other symptomatic antiparkinsonian therapies have in PD; they improve ADL function by reducing symptom severity, but do not modify the overall rate of disease progression.
The association between depression and ADL disability is consistent across studies, regardless of whether depression is assessed categorically (by diagnosis present/absent) or dimensionally (by scale ascertained severity or symptom load). In a study of PD patients assessed at baseline and 12-months later, Starkstein et al. found that major depression diagnosed using a psychiatric interview was associated with a greater decline in NWDS-assessed ADL function as compared to individuals with no depression. (Starkstein et al., 1992) In addition, they found that the decline in ADL function in major depression was greater than occurred in minor depression, suggesting that depression severity is an important determinant of functional impact. Several other studies support the idea that depression severity is proportionate to the degree of ADL disability. The largest study, a cross-sectional examination of 840 non-demented PD participants, found a strong positive association between depressive symptoms measured by the Geriatric Depression Scale (GDS) and disability measured by several disability instruments, Schwab and England (SE), Blessed Functional Activity Scale, and the UPDRS II. (Pankratz et al., 2008) Similarly, Weintraub et al. reported that increasing depression severity, measured by the Hamilton Depression Rating Scale (HAMD), was associated with greater disability on SE and UPDRS II. (Weintraub et al., 2004) This association between depression and disability is also evident in early stages of PD, untreated with dopaminergic medications. Ravina et al. analyzed data from two phase II clinical trials in early untreated PD and found that a GDS score greater than or equal to 5 was associated with more impaired ADLs, as measured by the UPDRS part II. (Ravina et al., 2007)
Motor impairment is a significant part of disability in PD and was, along with the HY, reported in several previous studies of depression and disability in PD. UPDRS part III motor severity score and HY stage are sometimes treated as clinical proxies for disability severity in PD, while others conceptualize disability more formally as an amalgam of motor impairment and other variables. In our study, individuals with symptomatic depression had, on average, an almost 10-point higher UPDRS part III motor severity score in the on-state and a higher proportion of depressed individuals with more severe HY stages compared to non-depressed. In the study of 840 non-demented PD subjects mentioned above, there was also a positive association between depressive symptoms on the GDS and motor impairment on the UPDRS part III.(Pankratz et al. 2008) However, four other studies, using the GDS, GDS-15 short form, HAMD, and the Zung self-rating depression scale respectively, did not find an association between depression and greater motor impairment on UPDRS part III.(Holroyd et al., 2005; Ravina et al., 2007; Kostic et al., 1987; Iwasaki et al., 2006) In two depression treatment trials by Menza et al., UPDRS part III motor severity did not change significantly in treatment responders, even when disability improved, suggesting that factors in addition to motor impairment are important determinants of disability in PD.(Menza et al., 2009; Menza et al., 2004) Finally, we also found that compared to the non-depressed subjects, symptomatically depressed individuals were more likely to have motor fluctuations on the UPDRS part IV. On-off fluctuations were associated with depression and anxiety in previous studies, including a controlled trial of levodopa infusion in which off-state levodopa administration was associated with both motor improvement and mood elevation.(Maricle et al., 1995; Witjas et al., 2002)
Depression is typically an episodic illness and our findings suggest that the disability associated with depression can be improved regardless of whether remission was spontaneous or due to treatment with antidepressants. We found a higher rate of antidepressant medication use in depressed compared to non-depressed subjects. Short-term improvement of disability associated with the treatment of depression has been demonstrated in two previous clinical trials by Menza et al mentioned above. The other studies of depression and disability discussed above either did not account for antidepressant use, (Cole et al., 1996; Starkstein et al., 1992) excluded individuals on antidepressants, (Pankratz et al., 2008) or did not control for the potential effect on disability (Holroyd et al., 2005; Weintraub et al., 2004; Ravina et al., 2007). In our study, there was no difference between depressed and non-depressed subjects in regard to use of agonists. However, several studies suggest that dopamine agonists may have antidepressant properties.(Leentjens et al., 2011) The most persuasive of these studies are two controlled trials by Barone et al., which found that pramipexole was both superior to placebo and equal in efficacy to sertraline for the treatment of depression in PD.(Barone et al., 2010; Barone et al., 2006) While more than half of the Weintraub et al. sample was taking dopamine agonists, the authors found no correlation between agonists and disability, and similar to our findings also found no difference in agonist use between depressed and non-depressed subjects.(Weintraub et al., 2004) Agonists have been shown to improve motor function, fluctuations, and depression in PD and therefore could influence the relationship between depression and disability in several ways; therefore agonist use was controlled for in the current study.
The study has several important limitations. The high attrition rate is in large part due to the study’s primary goal of brain donation to provide tissue for basic science research on disease modifying treatments for PD. Therefore, enrollment was often prioritized based on the likelihood of tissue acquisition, which may have introduced a bias for more severely affected individuals with greater medical comorbidity. The study enrolled both early and typical onset PD subjects, which may have produced a cohort effect given that early onset cases typically have a different rate of symptom progression and differences in ability to perform ADLs. However, studies have not found a difference between early-onset and typical onset PD with regard to depression classification. (Pankratz et al., 2008) Another limitation is the difficulty in determining whether antidepressants were at therapeutic doses for the treatment of depression. We used an inclusive approach and considered anyone taking an antidepressant to be “treated”. However, as active or remitted depression status was determined independently using a structured diagnostic interview, we doubt that this impacted the finding of depression status on disability. The model included only baseline depression status and did not account for depression status at each visit; however the percentage of status change was low and comparable across the groups, likely obviating the need to include visit-specific status. Finally, we combined all severities of depression into “case or non-case” as the study was insufficiently powered to determine whether depression severity, i.e. DSM-IV-TR designations-mild, moderate, or severe, has a differential impact on disability.
CONCLUSIONS
This study is the first to demonstrate the longitudinal impact of full criteria DSM-diagnosed depression on physical ADL disability in PD and that spontaneous or treated remission of depressive episodes results in improved ADL functioning over the course of our six year study. However, just as symptomatic motor treatments do not affect overall rate of disease progression in PD, improvement in depression status (remission) does not appear to change the rate of decline in ADL function. Therefore prevention of depression might be of even greater importance; otherwise depressed patients—whether symptomatically depressed or remitted—may experience a more aggressive rate of decline than those who were never depressed. Future studies should determine whether categorical diagnosis of depression or a continuous measure of depression symptom severity is the most appropriate clinical indicator of depression in PD and whether the primary treatment should be traditional antidepressants, dopamine agonists, psychotherapy, or a novel agent. These findings reinforce the importance of early recognition and treatment of depression in PD and provide encouraging evidence that even longstanding ADL disability associated with depression can be improved.
Acknowledgements:
We thank Vanessa Johnson for subject recruitment and proctoring, data collection, and database management. Data from a portion of this sample was presented in preliminary form in 2001 at the Movement Disorders and Mental Dysfunction Conference, Montreal, Canada.
Sources of support: Supported by the Morris K. Udall Parkinson’s Disease Research Center of Excellence at Johns Hopkins (NIH-P50-NS-58377).
Footnotes
Author disclosures: Dr. Williams is a Biogen Idec employee and stockholder. All other authors have no disclosures relevant to this study or manuscript.
References
- Barone P, Antonini A, Colosimo C, et al. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord 2009. Mov Disord 2009;24:1641–9. [DOI] [PubMed] [Google Scholar]
- Barone P, Poewe W, Albrecht S, et al. Pramipexole for the treatment of depressive symptoms in patients with Parkinson’s disease: A randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010;9:573–580. [DOI] [PubMed] [Google Scholar]
- Barone P, Scarzella L, Marconi R, et al. Pramipexole versus sertraline in the treatment of depression in Parkinson’s disease: A national multicenter parallel-group randomized study. J Neurol 2006;253:601–607. [DOI] [PubMed] [Google Scholar]
- Carod-Artal FJ, Ziomkowski S, Mourao Mesquita H, Martinez-Martin P. Anxiety and depression: Main determinants of health-related quality of life in Brazilian patients with Parkinson’s disease. Parkinsonism Relat Disord 2008;14:102–108. [DOI] [PubMed] [Google Scholar]
- Canter GJ, De La Torre R, Mier M. A method for evaluating disability in patients with Parkinson’s disease. J Nerv Ment Dis 1961;133:143–147. [DOI] [PubMed] [Google Scholar]
- Cole SA, Woodard JL, Juncos JL, Kogos JL, Youngstrom EA, Watts RL. Depression and disability in Parkinson’s disease. J Neuropsychiatry Clin Neurosci 1996;8:20–25. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL, members of the UPDRS Development Committee. Unified Parkinson’s Disease Rating Scale In: Fahn S, Marsden CD, Goldstein M, eds. Recent developments in Parkinson’s disease II. New York: Macmillan, 1987;153–63. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, et al. Structured clinical interview for DSM-IV-TR axis I disorders, research version, non-patient edition. (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute, November 2002. [Google Scholar]
- Holroyd S, Currie LJ, Wooten GF. Depression is associated with impairment of ADL, not motor function in Parkinson disease. Neurology 2005;64:2134–2135. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: Onset, progression, and mortality. 1967. Neurology 2001;57:S11–26. [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki Y, Ikeda K, Igarashi O, et al. Depression is associated with impairment of ADL, not motor function in Parkinson disease. Neurology;2006;66:956. [DOI] [PubMed] [Google Scholar]
- Kostic VS, Djuricic BM, Covickovic-Sternic N, Bumbasirevic L, Nikolic M, Mrsulja BB. Depression and Parkinson’s disease: possible role of serotonergic mechanisms. J Neurology 1987;234:94–96. [DOI] [PubMed] [Google Scholar]
- Leentjens AF. The role of dopamine agonists in the treatment of depression in patients with Parkinson’s disease: A systematic review. Drugs 2011;71:273–286. [DOI] [PubMed] [Google Scholar]
- Maricle RA, Nutt JG, Valentine RJ, Carter JH. Dose-response relationship of levodopa with mood and anxiety in fluctuating Parkinson’s disease: A double-blind, placebo-controlled study. Neurology 1995;45:1757–1760. [DOI] [PubMed] [Google Scholar]
- Menza M, Dobkin RD, Marin H, et al. A controlled trial of antidepressants in patients with Parkinson disease and depression. Neurology 2009;72:886–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menza M, Marin H, Kaufman K, Mark M, Lauritano M. Citalopram treatment of depression in Parkinson’s disease: The impact on anxiety, disability, and cognition. J Neuropsychiatry Clin Neurosci 2004;16:315–319. [DOI] [PubMed] [Google Scholar]
- Ormel J, Von Korff M, Van den Brink W, Katon W, Brilman E, Oldehinkel T. Depression, anxiety, and social disability show synchrony of change in primary care patients. Am J Public Health 1993;83:385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankratz N, Marder KS, Halter CA, et al. Clinical correlates of depressive symptoms in familial Parkinson’s disease. Mov Disord 2008;23:2216–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quelhas R, Costa M. Anxiety, depression, and quality of life in Parkinson’s disease. J Neuropsychiatry Clin Neurosci 2009;21:413–419. [DOI] [PubMed] [Google Scholar]
- Rahman S, Griffin HJ, Quinn NP, Jahanshahi M. Quality of life in Parkinson’s disease: The relative importance of the symptoms. Mov Disord 2008;23:1428–1434. [DOI] [PubMed] [Google Scholar]
- Ravina B, Camicioli R, Como PG, Marsh L, Jankovic J, Weintraub D, Elm J. The impact of depressive symptoms in early parkinson disease. Neurology 2007;69:342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkstein SE, Mayberg HS, Leiguarda R, Preziosi TJ, Robinson RG. A prospective longitudinal study of depression, cognitive decline, and physical impairments in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 1992;55:377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in parkinson’s disease. Mov Disord 2010;25:2649–2653. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Moberg PJ, Duda JE, Katz IR, Stern MB. Effect of psychiatric and other nonmotor symptoms on disability in Parkinson’s disease. J Am Geriatr Soc 2004;52:784–788. [DOI] [PubMed] [Google Scholar]
- Witjas T, Kaphan E, Azulay JP, et al. Nonmotor fluctuations in Parkinson’s disease: Frequent and disabling. Neurology 2002;59:408–413. [DOI] [PubMed] [Google Scholar]