Abstract
The U.S. Food and Drug Administration (FDA) approved 5-ALA (Gleolan®; photonamic GmbH & Co. KG) for use as an intraoperative optical imaging agent in patients with suspected high-grade gliomas (HGGs) in 2017. This was the first ever optical imaging agent approved as an adjunct for the visualization of malignant tissue during surgery for brain tumors. The approval occurred a decade after European approval and a multicenter, phase III randomized trial which confirmed that surgeons using 5-ALA FGS as a surgical adjunct could achieve more complete resections of tumors in HGG patients and better patient outcomes than with conventional microsurgery. Much of the delay in the US FDA approval of 5-ALA stemmed from its conceptualization as a therapeutic and not as an intraoperative imaging tool. We chronicle the challenges encountered during the US FDA approval process to highlight a new standard for approval of intraoperative optical imaging agents in brain tumors.
Introduction
In June of 2017, the U.S. Food and Drug Administration (FDA) approved oral Gleolan® (5-aminolevulinic acid hydrochloride (5-ALA); photonamic GmbH & Co. KG for use as an optical imaging agent in patients with gliomas (suspected World Health Organization Grades III or IV high-grade gliomas (HGG) on preoperative imaging) as an adjunct for the visualization of malignant tissue during surgery [1]. This heralded the U.S. approval of the first-ever optical imaging agent for use with brain tumors during fluorescence-guided surgery (FGS).
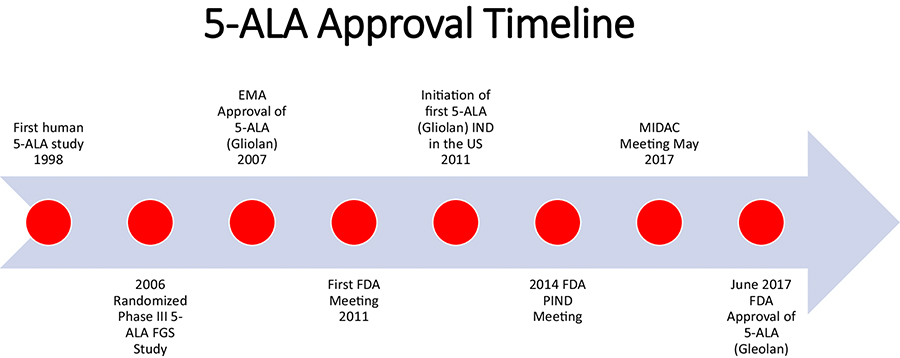
The FDA approval of 5-ALA for HGGs occurred 10 years after the approval by the European Medicine’s Agency (EMA) in 2007 and close to 2 decades after Dr. Walter Stummer’s initial description of 5-ALA FGS in human patients (Fig. 1) [2, 3]. Here we describe the FDA process for 5-ALA approval in the US and some of the challenges faced during this journey that took years for fruition. We hope this may help other future intraoperative optical imaging agents move forward with FDA approval for use in tumor visualization in patients.
Figure 1.
5-ALA approval timeline.
FDA Approved Drugs/Devices in Brain Tumors
Currently there are 5 drugs and 1 device approved by the FDA for brain tumors in the U.S including 5-ALA. The first drugs to be FDA approved for HGGs were oral lomustine (CeeNU®; Bristol-Myers Squibb, Princeton, NJ), which received approval in 1976 [4], and intravenous (i.v.) carmustine (BiCNU®; Bristol-Myers Squibb) which received approval in 1977 [5]. These nitrosoureas were approved as single agents or in combination with other chemotherapeutic agents in patients with HGG tumors who have already undergone surgery and/or radiation therapy. In both drugs, FDA approval was based on the tumor response rate of patients treated. Intraoperative carmustine chemotherapy wafer implants (Gliadel® wafers; Arbor Pharmaceuticals Inc.) were approved by the FDA in 1996 for recurrent HGGs [6]. In 2003, these chemotherapy implants were approved for new HGGs [7, 8]. The approval of carmustine wafers was based on a significant increase in overall survival (OS) of patients in comparison to placebo control groups. Temozolomide (Temodar®; Merck and Co. Inc.) chemotherapy was granted approval for recurrent anaplastic astrocytomas in 1999 and newly diagnosed glioblastoma (GBM) in 2005 [9, 10]. The approval of temozolomide for newly diagnosed GBM was based on the largest OS increase to date in HGG patients undergoing adjuvant and concurrent treatment after fractionated external beam radiotherapy [11].
Bevacizumab (Avastin®; Genentech Corp.) chemotherapy was approved for recurrent GBM in 2009 [12]. The clinical trial that was completed for FDA approval was a randomized, non-comparative (non-controlled), multicenter trial to evaluate the efficacy and safety of bevacizumab alone or in combination with irinotecan. The approval of bevacizumab was based on the progression-free survival rate at 6 months (PFS6) and the durable objective response rate of patients (independent radiologic review and stable or decreasing corticosteroid use) [12–14].
The Optune device (Novocure Inc.), which utilizes alternating electric fields (tumor treatment fields (TTF)) rather than chemotherapy, was approved for recurrent GBM in 2011 and newly diagnosed GBM in 2015 [15–18]. Both of these approvals were based on randomized, controlled studies. In the recurrent GBM setting, there was no improvement in OS, however, efficacy and activity with the device was comparable to chemotherapy regimens commonly used for recurrent GBM. In newly diagnosed GBM patients, there was a significant OS and PFS patient benefit.
5-ALA History
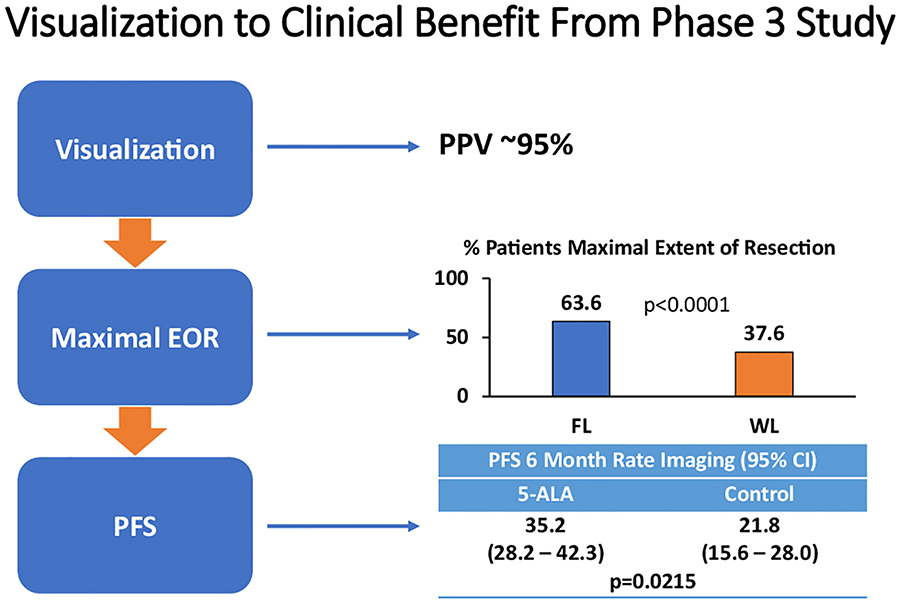
In 2006, the landmark 5-ALA multicenter, randomized phase III study, led by Dr. Walter Stummer, confirmed that surgeons using 5-ALA FGS as a surgical adjunct could achieve more complete resections of tumors in HGG patients and better patient outcomes than with conventional microsurgery [19]. Complete resection of the enhancing portion of newly diagnosed HGGs occurred in 65% of patients assigned to the 5-ALA group versus 36% in those assigned to the conventional surgery white light group (difference between groups 29% [95% CI 17–40], p<0·0001). In addition to greater complete resections, overall progression-free survival at 6 months (PFS-6) was also significantly greater after 5-ALA FGS (41.0% [32·8–49·2] vs. 21.1% [14·0–28·2]; p=0.0003). Safety was also established by the phase III study with use of 5-ALA FGS. No deaths were attributed to the actual use of 5-ALA and there were no significant differences in the NIH stroke scale (NIH-SS) scores for patients in both arms of the study at 6 weeks post-surgery [20]. There was no detectable difference in overall survival (OS) of patients in the study, however, as the phase III was not powered to be an OS study. Furthermore, during the course of the study, the Stupp regimen (concurrent and adjuvant temozolomide after fractionated external beam radiotherapy), which is widely considered a standard of care for HGG patients, was not routinely administered at that time. The results of this study led to approval of 5-ALA (Gliolan®, photonamic GmbH Co & Medac GmbH.) by the European Medicine’s Agency (EMA) in 2007. A decade would go by before FDA approval of 5-ALA in the US.
Patient Clinical Benefit
The FDA was initially approached by a group of medical experts (4 neurosurgeons and a radiation-oncologist) and industry (NX Development Corp. (NXDC) and photonamic GmbH Co.) in 2011 to discuss the approval process of 5-ALA in the US. The European clinical experience, including the randomized, phase III study results, was provided for consideration by the FDA. Two years prior in 2009, the FDA approval of bevacizumab for recurrent GBM was based on a non-comparative study and the “objective response” of patients treated with bevacizumab [12]. The Oncologic Drugs Advisory Committee (ODAC) held a Public Workshop on Clinical Trial End Points in Primary Brain Tumors in 2006 to define an “objective response” to treatment [21]. ODAC stated that an “objective response could be an adequate surrogate for clinical benefit under the proper parameters”. Workshop discussants agreed that a response rate of sufficient magnitude (e.g. >30%) was likely to be associated with clinical benefit. The convincing phase III data showing safety, a close to doubling of the extent of resection (EOR) of HGGs, and a significant PFS-6 with 5-ALA FGS was discussed.
The FDA advisory group, which included members of the Division of Medical Imaging Products and ODAC, felt that further patient clinical benefit in addition to PFS-6 was needed for consideration of 5-ALA approval since the phase III study did not provide a treatment specific OS benefit to patients (even though it was not powered to do so). The FDA requested evidence that 5-ALA FGS provided additional clinical benefit to patients who otherwise would undergo conventional surgical resection. What did this mean? The prospect of a randomized controlled study utilizing 5-ALA FGS in HGG patients for assessing OS as a primary endpoint was daunting. ODAC, at the time, was considering 5-ALA as a therapeutic agent, rather than an adjunct visualization agent and, given this assumption, it was only logical that the FDA should require an outcomes trial. However, the obvious misconception in this assumption was that 5-ALA was envisioned as an imaging tool used by surgeons to improve resection. Surgical resection was the driver of outcome, with resection being driven by much more than simply the knowledge of tumor location, leading to an inflation of patient numbers. HGG disease-related variables including genomics (IDH mutational and MGMT methylation status), frequency of treatment interventions, the lack of ability to control salvage therapy, and the use of surgery at the time of recurrence impacted upon power calculations and the number of patients that would be required to properly design an OS trial [22]. As a result, it became apparent that a properly designed trial would be beyond the scope of the next decade in this orphan disease.
The large patient numbers, lengthy time needed for patient enrollment, and high costs required to conduct such a study were prohibitive even with the prospect of teaming up with well-known cooperative groups that have experience completing such large randomized studies in HGG patients (e.g. Radiation Therapy Oncology Group (RTOG)). Furthermore, randomized OS clinical studies with HGG patients are challenged by clinical equipoise and the high likelihood of patients leaving the study at recurrence to enroll into other trials that are actually therapeutic.
Utilization of quality of life (QOL) as a primary endpoint for clinical benefit in a randomized 5-ALA FGS study was one strategy pursued with guidance by the RTOG [23–27]. At the time, completion of the large, randomized studies for assessing the benefit of dose-dense temozolomide (RTOG 0525) [28] and bevacizumab (RTOG 0825) [29] in newly diagnosed GBM patients served as a data source to assess whether QOL could associated with greater tumor extent of resection (EOR). In both studies, QOL metrics were measured in each arm in addition to tumor EOR. Unfortunately, QOL was not definitively associated with EOR in those studies and EOR analyses in both studies were not performed with rigorous volumetric methods.
Surgical Trial Bias
Attempts were made to initiate a randomized, placebo-controlled multicenter trial of 5-ALA and FGS in combination with conventional chemoradiation and adjuvant temozolomide in the US. The primary endpoints proposed were PFS-6 and volumetric measurement of residual MRI contrast enhancement. This trial was proposed through the RTOG and the National Cancer Institute (NCI) Cancer Therapy Evaluation Program (CTEP) but there were major concerns with surgical trial bias and the use of PFS-6 instead of OS as a primary endpoint. The completion of any randomized, phase III study requires absolute blinding of investigators and patients to each arm of the study to be able to assess the primary outcome (e.g., PFS and/or OS) without bias. How could this be possible with a 5-ALA FGS study? With the flick of a switch on the fluorescence-equipped microscope, a surgeon could determine immediately whether the patient received 5-ALA or not by the presence or absence of tumor fluorescence. This could influence the surgeon’s decision to properly resect the tumor in an unbiased manner. A surgeon might not perform a maximal tumor resection with conventional white light since they would know that the use of blue light and visualization of fluorescent tumor tissue would provide additional information for further surgical resection.
EOR and OS
Almost two decades of volumetric studies have shown that maximal resection of HGG tumors is associated with a survival advantage in patients [30–33]. One small, randomized study has shown that surgical resection leads to greater OS of HGG patients when compared to stereotactic biopsy [34]. Greater EOR of HGG tumors is also associated with better efficacy of adjuvant therapies [35]. Most recently, the largest systematic review and quantitative meta-analysis of 41,117 patient examined EOR and OS in newly diagnosed GBM patients and found that complete resection of the contrast enhancing portion of HGGs, or a gross total resection (GTR), was associated with a significant increase in OS and PFS in comparison to subtotal resection (STR) [36]. Over 50% of HGG patients, however, do not undergo a GTR of their tumor after conventional surgery based on multiple studies [37, 38]. If 5-ALA can permit more complete resections of HGG tumors, based on randomized, phase III data, then the natural assumption should be a survival benefit for those patients that undergo 5-ALA FGS. Does this constitute patient benefit?
Initiation of First 5-ALA (Gliolan®) IND in the US
In 2011, the first patient underwent 5-ALA FGS in the US with 5-ALA (Gliolan®) under the FDA investigational new drug (IND) application (#112246) held by Dr. Constantinos G. Hadjipanayis at Emory University Hospital Midtown in Atlanta, Georgia. The study, entitled “A Phase 2 Study of 5-Aminolevulinic Acid (5-ALA) to Enhance Visualization and Resection of Newly Diagnosed or Recurrent Malignant Gliomas” was performed from 2011–2015 enrolling over 70 patients [39–41]. A current multicenter 5-ALA (Gliolan®) study is underway at 18 centers in the US entitled “A Multicenter Study of 5-Aminolevulinic Acid (5-ALA) to Enhance Visualization of Malignant Tumor in Patients with Newly Diagnosed or Recurrent Malignant Gliomas: A Safety, Histopathology, and Correlative Biomarker Study” (ClinicalTrials.gov ID: NCT02632370).
5-ALA as a Therapeutic or Intraoperative Imaging Agent: A New Standard?
Much of the delay in the US FDA approval of 5-ALA stemmed from its conceptualization as a therapeutic and not as an intraoperative imaging tool. Oncologic therapies are held to a different standard with the FDA approval process that require OS as a primary endpoint and randomized, placebo, controlled studies showing safety and a significant increase in OS with the therapeutic being studied. While safety was established in the phase III study [19] and with 5-ALA administration to thousands of HGG patients globally, the data to confirm a significant OS increase with use of 5-ALA FGS was lacking.
A new standard for FDA approval was needed for 5-ALA as an intraoperative optical imaging agent that provides real-time feedback to the operating surgeon. There was a lack of precedence for FDA approval of an intraoperative optical imaging agent, which was considered a diagnostic/therapeutic crossover, governed by two regulatory paradigms. Cancer drug approval mandates showing clinical patient benefit while imaging drug approval focuses on structural identification. Structural identification relies on how sensitive and specific the agent is at detecting and not detecting cancer and how beneficial this detection is for the patient. The 5-ALA ester derivative hexaminolevulinate (HAL) (Cysview®, Photocure ASA) was FDA approved for topical use in bladder cancer detection (non-muscle invasive papillary cancer) in 2010 (http://wayback.archiveit.org/7993/20170113081150/http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm215515.htm). The approval of HAL was based upon a clinical study that showed a single use of the imaging agent with the Photodynamic Diagnostic D-Light C (PDD) System resulted in the detection of a greater number of Ta and/or T1 bladder lesions, compared to standard (white light) cystoscopy [42].
5-ALA, which is selectively taken up by glioma cells in tumors and metabolized to its fluorescent form, protoporphyrin IX, was shown to have unprecedented intraoperative diagnostic accuracy [43]. Multiple biopsy-driven studies confirmed what surgeons around the world knew, that 5-ALA was highly sensitive at detecting malignant tumor tissue [3, 44–49]. Furthermore, the probability that fluorescent tissue represented malignant tissue, or the positive-predictive value (PPV) of 5-ALA, was close to 100% in almost all studies published [19, 45, 46, 48, 50, 51].
The specificity and negative-predictive value (NPV), however, were not found to be as high in most biopsy-driven studies [45–47, 51–53]. Due to the infiltrative biology of HGGs, tumor cells are known to infiltrate centimeters (cm) away from the tumor bulk, which represents the contrast-enhancing portion of tumor [54]. Walter Dandy showed us in 1928 that even after a hemispherectomy in patients with a HGG, tumors can still recur [55]. When biopsies are obtained in HGGs at their infiltrative margin, fluorescence detection is much lower due to the reduced presence of tumor cells with normal cells in the brain [50].
FDA Orphan Drug Approval and New drug application (NDA)
A successful FDA Orphan Drug Application was submitted for 5-ALA (Gliolan®) in 2012 that resulted in orphan designation by the FDA in 2013. The orphan designation of 5-ALA was for the visualization of malignant tissue during surgery for malignant gliomas (WHO grades III and IV). This designation brought new life into the movement for 5-ALA FDA approval in the US based on its strengths as an intraoperative imaging agent.
In 2014, a group of neurosurgeons (Drs. Constantinos Hadjipanayis, Georg Widhalm, and Walter Stummer) and industry experts NX Development Corp. (NXDC) and photonamic GmbH Co.) approached the FDA (Medical Imaging Division) to discuss how to move forward with a New Drug Application (NDA) and FDA approval based on 5-ALA use as an intraoperative diagnostic imaging agent. A Pre-Investigational New Drug Application (PIND) for 5-ALA was provided to the FDA for the meeting. The intraoperative diagnostic accuracy and safety data of 5-ALA were presented to the Medical Imaging group at the FDA. No pursuit of claims related to the adequacy of surgical resection or survival with 5-ALA FGS was made during this crucial meeting. A new drug application (NDA) submission was proposed based on 5-ALA as a real-time imaging agent based upon strong PPV data and overwhelming safety in pre-marketing and 26,000 patient post marketing exposure.
After the PIND meeting and extensive communications with the FDA, a new drug application (NDA) would be submitted in December 2016 that consisted of the phase III study results (greater tumor resection and PFS-6 with 5-ALA FGS) and biopsy-driven diagnostic studies (Studies 3, 28, 30) completed (high PPV) (Table 1) to support 5-ALA use as an intraoperative imaging agent [19, 48, 50] (Figure 2). Progression-free survival (PFS) as a surrogate for OS in GBM patients had been shown recently in a meta-analysis from 91 trials [56]. Industry and FDA support for the NDA application included painstaking review of all the biopsy-driven data from studies with a focus on the PPV, NPV, sensitivity, specificity, and safety of 5-ALA. The NDA submission and ultimate FDA approval could not have been possible without the support of NXDC and photonamic GmbH Co.
Table 1.
Pivotal Studies: Biopsy-Based Diagnostic Measures
| Measure | Study 3 | Study28 | Study 30 |
|---|---|---|---|
| (Primary) | (Primary) | (Recurrent) | |
| PPV | 97.8 | 96.2 | 96.6 |
| NPV | 18.8 | 24.1 | 18.8 |
| Sensitivity | 70.6 | 67.7 | 96.3 |
| Specificity | 81.1 | 79.4 | 20.0 |
Study 3: Stummer W. et al. (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7: 392–401
Study 28: Stummer W. et al. (2014) 5-Aminolevulinic acid-derived tumor fluorescence: the diagnostic accuracy of visible fluorescence qualities as corroborated by spectrometry and histology and postoperative imaging. Neurosurgery 74: 310–319; discussion 319–320
Study 30: Nabavi A, Thurm H, Zountsas B, Pietsch T, Lanfermann H, Pichlmeier U, Mehdorn M, Group ALARGS (2009) Five-aminolevulinic acid for fluorescence-guided resection of recurrent malignant gliomas: a phase ii study. Neurosurgery 65: 1070–1076; discussion 1076–1077
Figure 2.
Summary of clinical benefit for patients undergoing 5-ALA FGS including tumor visualization with high diagnostic accuracy (high PPV), maximal EOR, and better PFS-6.
5-ALA Medical Advisory Committee Meeting
After receipt of the NDA and release of key discussion questions by the FDA (https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/MedicalImagingDrugsAdvisoryCommittee/UCM559370.pdf) for the potential approval of 5-ALA, a Medical Imaging Drugs Advisory Committee (MIDAC) Meeting was set for May 10, 2017 (https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/MedicalImagingDrugsAdvisoryCommittee/UCM559368.pdf). Eleven members of the committee were appointed by the FDA Medical Imaging Division that included two neurosurgeons and one neuro-oncologist (https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/MedicalImagingDrugsAdvisoryCommittee/UCM557133.pdf). Presentations by Drs. Ezrin (NXDC), Hadjipanayis, and Stummer were made to address the unmet medical need, efficacy, safety, and risk-benefit of 5-ALA for detection of malignant tissue during surgery after administration. An open comment session was held to permit a surviving glioma patient (Jennifer Giliberto) and her son (Tucker Giliberto), brain tumor patient advocacy leaders (Mr. Al Musella from the Musella Foundation and Mrs. Geri Shaffer from the Southeastern Brain Tumor Foundation (SBTF)), and other expert neurosurgeons (both academic (Drs. Steven Kalkanis (Henry Ford Health System) and Georg Widhalm (University of Vienna, Austria) and community-based (Dr. Lloyd Zucker, Delray Hospital) to discuss the potential benefits of 5-ALA use for HGGs. The MIDAC committee voted unanimously (11 votes in favor and 0 votes against) for the approval of 5-ALA (Gleolan®). The official FDA approval of 5-ALA shortly followed on June 6 2017 (http://www.raredr.com/news/fda-approves-gleolan).
A new standard for regulatory approval of optical imaging agents and devices
The 5-ALA regulatory path to approval created a new understanding of the importance of intraoperative detection and delineation of tumor tissue for real-time decision-making for surgeons. The appropriate clinical trial end-points that meet FDA requirements are still ill defined for optical imaging technologies. A one-day workshop on May 4, 2016, which included representatives from the NCI, the FDA, members of the American Society of Image Guided Surgery (ASIGS), and members of the World Molecular Imaging Society was held to discuss how to better define optical imaging drug/device approval [57]. The workshop provided clarity to the research community on data collection and trial design for approval of novel optical drugs and devices.
Conclusions and Moving Forward
5-ALA is a reliable diagnostic intraoperative imaging agent that provides real-time guidance to the neurosurgeon that can permit more complete resection of HGGs [43]. The road to 5-ALA (Gleolan®) approval in the US uncovered the difficulties associated with regulatory requirements for an intraoperative imaging agent. We believe the 5-ALA regulatory path to approval may permit other intraoperative optical imaging agents to move forward in the future.
Acknowledgements:
We acknowledge the great effort provided by a large number of individuals in academia, industry, patients, families, and the community who provided their strong support for 5-ALA approval in the US. We wish to especially acknowledge Dr. Alan Ezrin (CEO and President of NXDC) and his team (Dr. Joe Wyse, Linda Kearns, and Jeff Cooper) in addition to Drs. Ulrich Kosciessa, Anne Moor, and Markus Stocker (Photonamic GmbH Co.) for their strong commitment and dedication to moving 5-ALA forward to FDA approval in the US. We also wish to acknowledge the Scilucent (Beth Silverstein, Cindy Fink, Alura Johnston, and Dipali MacAllister) and Pharmapprove (Len Baum, Lisa Peluso, and Lisa Starke) teams for their herculean efforts in preparing the core medical team for the MIDAC meeting.
Funding: This study did not receive any funding
Footnotes
Conflict of Interest:
Constantinos Hadjipanayis is a consultant for NXDC and Synaptive Medical Inc. He will receive royalties from NXDC. He has also received speaker fees by Carl Zeiss and Leica.
Walter Stummer has received speaker and consultant fees by Carl Zeiss, Leica, Photonamic, Medac, and NXDC
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Lakomkin N, Hadjipanayis CG (2018) Fluorescence-guided surgery for high-grade gliomas. J Surg Oncol 118: 356–361 doi: 10.1002/jso.25154 [DOI] [PubMed] [Google Scholar]
- 2.Stummer W, Stocker S, Wagner S, Stepp H, Fritsch C, Goetz C, Goetz AE, Kiefmann R, Reulen HJ (1998) Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery 42: 518–525; [DOI] [PubMed] [Google Scholar]
- 3.Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ (2000) Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg 93: 1003–1013 doi: 10.3171/jns.2000.93.6.1003 [DOI] [PubMed] [Google Scholar]
- 4.Parney IF, Chang SM (2003) Current chemotherapy for glioblastoma. Cancer J 9: 149–156 [DOI] [PubMed] [Google Scholar]
- 5.Walker MD, Alexander E Jr., Hunt WE, MacCarty CS, Mahaley MS Jr., Mealey J Jr., Norrell HA, Owens G, Ransohoff J, Wilson CB, Gehan EA, Strike TA (1978) Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg 49: 333–343 doi: 10.3171/jns.1978.49.3.0333 [DOI] [PubMed] [Google Scholar]
- 6.Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, Black K, Sisti M, Brem S, Mohr G, et al. (1995) Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet 345: 1008–1012 [DOI] [PubMed] [Google Scholar]
- 7.Valtonen S, Timonen U, Toivanen P, Kalimo H, Kivipelto L, Heiskanen O, Unsgaard G, Kuurne T (1997) Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: a randomized double-blind study. Neurosurgery 41: 44–48; [DOI] [PubMed] [Google Scholar]
- 8.Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, Whittle IR, Jaaskelainen J, Ram Z (2003) A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol 5: 79–88 doi: 10.1093/neuonc/5.2.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newlands ES, Stevens MF, Wedge SR, Wheelhouse RT, Brock C (1997) Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat Rev 23: 35–61 [DOI] [PubMed] [Google Scholar]
- 10.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for R, Treatment of Cancer Brain T, Radiotherapy G, National Cancer Institute of Canada Clinical Trials G (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987–996 doi: 10.1056/NEJMoa043330 [DOI] [PubMed] [Google Scholar]
- 11.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352: 997–1003 doi: 10.1056/NEJMoa043331 [DOI] [PubMed] [Google Scholar]
- 12.Cohen MH, Shen YL, Keegan P, Pazdur R (2009) FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist 14: 1131–1138 doi: 10.1634/theoncologist.2009-0121 [DOI] [PubMed] [Google Scholar]
- 13.Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Marcello J, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Sampson J, Wagner M, Bailey L, Bigner DD, Friedman AH, Friedman HS (2007) Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 25: 4722–4729 doi: 10.1200/JCO.2007.12.2440 [DOI] [PubMed] [Google Scholar]
- 14.Bokstein F, Shpigel S, Blumenthal DT (2008) Treatment with bevacizumab and irinotecan for recurrent high-grade glial tumors. Cancer 112: 2267–2273 doi: 10.1002/cncr.23401 [DOI] [PubMed] [Google Scholar]
- 15.Stupp R, Wong ET, Kanner AA, Steinberg D, Engelhard H, Heidecke V, Kirson ED, Taillibert S, Liebermann F, Dbaly V, Ram Z, Villano JL, Rainov N, Weinberg U, Schiff D, Kunschner L, Raizer J, Honnorat J, Sloan A, Malkin M, Landolfi JC, Payer F, Mehdorn M, Weil RJ, Pannullo SC, Westphal M, Smrcka M, Chin L, Kostron H, Hofer S, Bruce J, Cosgrove R, Paleologous N, Palti Y, Gutin PH (2012) NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer 48: 2192–2202 doi: 10.1016/j.ejca.2012.04.011 [DOI] [PubMed] [Google Scholar]
- 16.Stupp R, Taillibert S, Kanner AA, Kesari S, Steinberg DM, Toms SA, Taylor LP, Lieberman F, Silvani A, Fink KL, Barnett GH, Zhu JJ, Henson JW, Engelhard HH, Chen TC, Tran DD, Sroubek J, Tran ND, Hottinger AF, Landolfi J, Desai R, Caroli M, Kew Y, Honnorat J, Idbaih A, Kirson ED, Weinberg U, Palti Y, Hegi ME, Ram Z (2015) Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA 314: 2535–2543 doi: 10.1001/jama.2015.16669 [DOI] [PubMed] [Google Scholar]
- 17.Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, Toms S, Idbaih A, Ahluwalia MS, Fink K, Di Meco F, Lieberman F, Zhu JJ, Stragliotto G, Tran D, Brem S, Hottinger A, Kirson ED, Lavy-Shahaf G, Weinberg U, Kim CY, Paek SH, Nicholas G, Bruna J, Hirte H, Weller M, Palti Y, Hegi ME, Ram Z (2017) Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 318: 2306–2316 doi: 10.1001/jama.2017.18718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taphoorn MJB, Dirven L, Kanner AA, Lavy-Shahaf G, Weinberg U, Taillibert S, Toms SA, Honnorat J, Chen TC, Sroubek J, David C, Idbaih A, Easaw JC, Kim CY, Bruna J, Hottinger AF, Kew Y, Roth P, Desai R, Villano JL, Kirson ED, Ram Z, Stupp R (2018) Influence of Treatment With Tumor-Treating Fields on Health-Related Quality of Life of Patients With Newly Diagnosed Glioblastoma: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol 4: 495–504 doi: 10.1001/jamaoncol.2017.5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, Group AL-GS (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7: 392–401 doi: 10.1016/S1470-2045(06)70665-9 [DOI] [PubMed] [Google Scholar]
- 20.Stummer W, Tonn JC, Mehdorn HM, Nestler U, Franz K, Goetz C, Bink A, Pichlmeier U, Group AL-GS (2011) Counterbalancing risks and gains from extended resections in malignant glioma surgery: a supplemental analysis from the randomized 5-aminolevulinic acid glioma resection study. Clinical article. J Neurosurg 114: 613–623 doi: 10.3171/2010.3.JNS097 [DOI] [PubMed] [Google Scholar]
- 21.Administration USFaD (January 16, 2006) FDA Workshop Brain Cancer End-Points
- 22.Alexander BM, Cloughesy TF (2017) Adult Glioblastoma. J Clin Oncol 35: 2402–2409 doi: 10.1200/JCO.2017.73.0119 [DOI] [PubMed] [Google Scholar]
- 23.Chiu L, Chiu N, Zeng L, Zhang L, Popovic M, Chow R, Lam H, Poon M, Chow E (2012) Quality of life in patients with primary and metastatic brain cancer as reported in the literature using the EORTC QLQ-BN20 and QLQ-C30. Expert Rev Pharmacoecon Outcomes Res 12: 831–837 doi: 10.1586/erp.12.70 [DOI] [PubMed] [Google Scholar]
- 24.Archibald YM, Lunn D, Ruttan LA, Macdonald DR, Del Maestro RF, Barr HW, Pexman JH, Fisher BJ, Gaspar LE, Cairncross JG (1994) Cognitive functioning in long-term survivors of high-grade glioma. J Neurosurg 80: 247–253 doi: 10.3171/jns.1994.80.2.0247 [DOI] [PubMed] [Google Scholar]
- 25.Meyers CA, Rock EP, Fine HA (2012) Refining endpoints in brain tumor clinical trials. J Neurooncol 108: 227–230 doi: 10.1007/s11060-012-0813-8 [DOI] [PubMed] [Google Scholar]
- 26.Armstrong TS, Wefel JS, Wang M, Gilbert MR, Won M, Bottomley A, Mendoza TR, Coens C, Werner-Wasik M, Brachman DG, Choucair AK, Mehta M (2013) Net clinical benefit analysis of radiation therapy oncology group 0525: a phase III trial comparing conventional adjuvant temozolomide with dose-intensive temozolomide in patients with newly diagnosed glioblastoma. J Clin Oncol 31: 4076–4084 doi: 10.1200/JCO.2013.49.6067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armstrong TS (2013) Measuring clinical benefit: use of patient-reported outcomes (PRO) in primary brain tumor clinical trials. Curr Oncol Rep 15: 27–32 doi: 10.1007/s11912-012-0276-2 [DOI] [PubMed] [Google Scholar]
- 28.Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA, Armstrong TS, Wefel JS, Won M, Blumenthal DT, Mahajan A, Schultz CJ, Erridge S, Baumert B, Hopkins KI, Tzuk-Shina T, Brown PD, Chakravarti A, Curran WJ Jr., Mehta MP (2013) Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol 31: 4085–4091 doi: 10.1200/JCO.2013.49.6968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M, Jeraj R, Brown PD, Jaeckle KA, Schiff D, Stieber VW, Brachman DG, Werner-Wasik M, Tremont-Lukats IW, Sulman EP, Aldape KD, Curran WJ Jr., Mehta MP (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370: 699–708 doi: 10.1056/NEJMoa1308573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, Hess K, Michael C, Miller D, Sawaya R (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95: 190–198 doi: 10.3171/jns.2001.95.2.0190 [DOI] [PubMed] [Google Scholar]
- 31.Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS (2011) An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 115: 3–8 doi: 10.3171/2011.2.JNS10998 10.3171/2011.7.JNS10238 [DOI] [PubMed] [Google Scholar]
- 32.Li YM, Suki D, Hess K, Sawaya R (2016) The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross-total resection? J Neurosurg 124: 977–988 doi: 10.3171/2015.5.JNS142087 [DOI] [PubMed] [Google Scholar]
- 33.Kuhnt D, Becker A, Ganslandt O, Bauer M, Buchfelder M, Nimsky C (2011) Correlation of the extent of tumor volume resection and patient survival in surgery of glioblastoma multiforme with high-field intraoperative MRI guidance. Neuro Oncol 13: 1339–1348 doi: 10.1093/neuonc/nor133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vuorinen V, Hinkka S, Farkkila M, Jaaskelainen J (2003) Debulking or biopsy of malignant glioma in elderly people - a randomised study. Acta Neurochir (Wien) 145: 5–10 doi: 10.1007/s00701-002-1030-6 [DOI] [PubMed] [Google Scholar]
- 35.Stummer W, Meinel T, Ewelt C, Martus P, Jakobs O, Felsberg J, Reifenberger G (2012) Prospective cohort study of radiotherapy with concomitant and adjuvant temozolomide chemotherapy for glioblastoma patients with no or minimal residual enhancing tumor load after surgery. J Neurooncol 108: 89–97 doi: 10.1007/s11060-012-0798-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown TJ, Brennan MC, Li M, Church EW, Brandmeir NJ, Rakszawski KL, Patel AS, Rizk EB, Suki D, Sawaya R, Glantz M (2016) Association of the Extent of Resection With Survival in Glioblastoma: A Systematic Review and Meta-analysis. JAMA Oncol 2: 1460–1469 doi: 10.1001/jamaoncol.2016.1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orringer D, Lau D, Khatri S, Zamora-Berridi GJ, Zhang K, Wu C, Chaudhary N, Sagher O (2012) Extent of resection in patients with glioblastoma: limiting factors, perception of resectability, and effect on survival. J Neurosurg 117: 851–859 doi: 10.3171/2012.8.JNS12234 [DOI] [PubMed] [Google Scholar]
- 38.McGirt MJ, Chaichana KL, Gathinji M, Attenello FJ, Than K, Olivi A, Weingart JD, Brem H, Quinones-Hinojosa AR (2009) Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg 110: 156–162 doi: 10.3171/2008.4.17536 [DOI] [PubMed] [Google Scholar]
- 39.Cordova JS, Schreibmann E, Hadjipanayis CG, Guo Y, Shu HK, Shim H, Holder CA (2014) Quantitative tumor segmentation for evaluation of extent of glioblastoma resection to facilitate multisite clinical trials. Transl Oncol 7: 40–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cordova JS, Gurbani SS, Holder CA, Olson JJ, Schreibmann E, Shi R, Guo Y, Shu HK, Shim H, Hadjipanayis CG (2016) Semi-Automated Volumetric and Morphological Assessment of Glioblastoma Resection with Fluorescence-Guided Surgery. Mol Imaging Biol 18: 454–462 doi: 10.1007/s11307-015-0900-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cordova JS, Shu HK, Liang Z, Gurbani SS, Cooper LA, Holder CA, Olson JJ, Kairdolf B, Schreibmann E, Neill SG, Hadjipanayis CG, Shim H (2016) Whole-brain spectroscopic MRI biomarkers identify infiltrating margins in glioblastoma patients. Neuro Oncol 18: 1180–1189 doi: 10.1093/neuonc/now036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daniltchenko DI, Riedl CR, Sachs MD, Koenig F, Daha KL, Pflueger H, Loening SA, Schnorr D (2005) Long-term benefit of 5-aminolevulinic acid fluorescence assisted transurethral resection of superficial bladder cancer: 5-year results of a prospective randomized study. J Urol 174: 2129–2133, discussion 2133 doi: 10.1097/01.ju.0000181814.73466.14 [DOI] [PubMed] [Google Scholar]
- 43.Hadjipanayis CG, Widhalm G, Stummer W (2015) What is the Surgical Benefit of Utilizing 5-Aminolevulinic Acid for Fluorescence-Guided Surgery of Malignant Gliomas? Neurosurgery 77: 663–673 doi: 10.1227/NEU.0000000000000929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao S, Wu J, Wang C, Liu H, Dong X, Shi C, Shi C, Liu Y, Teng L, Han D, Chen X, Yang G, Wang L, Shen C, Li H (2013) Intraoperative fluorescence-guided resection of high-grade malignant gliomas using 5-aminolevulinic acid-induced porphyrins: a systematic review and meta-analysis of prospective studies. PLoS One 8: e63682 doi: 10.1371/journal.pone.0063682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roberts DW, Valdes PA, Harris BT, Fontaine KM, Hartov A, Fan X, Ji S, Lollis SS, Pogue BW, Leblond F, Tosteson TD, Wilson BC, Paulsen KD (2011) Coregistered fluorescence-enhanced tumor resection of malignant glioma: relationships between delta-aminolevulinic acid-induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. Clinical article. J Neurosurg 114: 595–603 doi: 10.3171/2010.2.JNS091322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coburger J, Engelke J, Scheuerle A, Thal DR, Hlavac M, Wirtz CR, Konig R (2014) Tumor detection with 5-aminolevulinic acid fluorescence and Gd-DTPA-enhanced intraoperative MRI at the border of contrast-enhancing lesions: a prospective study based on histopathological assessment. Neurosurg Focus 36: E3 doi: 10.3171/2013.11.FOCUS13463 [DOI] [PubMed] [Google Scholar]
- 47.Diez Valle R, Tejada Solis S, Idoate Gastearena MA, Garcia de Eulate R, Dominguez Echavarri P, Aristu Mendiroz J (2011) Surgery guided by 5-aminolevulinic fluorescence in glioblastoma: volumetric analysis of extent of resection in single-center experience. J Neurooncol 102: 105–113 doi: 10.1007/s11060-010-0296-4 [DOI] [PubMed] [Google Scholar]
- 48.Nabavi A, Thurm H, Zountsas B, Pietsch T, Lanfermann H, Pichlmeier U, Mehdorn M, Group ALARGS (2009) Five-aminolevulinic acid for fluorescence-guided resection of recurrent malignant gliomas: a phase ii study. Neurosurgery 65: 1070–1076; discussion 1076–1077 doi: 10.1227/01.NEU.0000360128.03597.C7 [DOI] [PubMed] [Google Scholar]
- 49.Hefti M, von Campe G, Moschopulos M, Siegner A, Looser H, Landolt H (2008) 5-aminolevulinic acid induced protoporphyrin IX fluorescence in high-grade glioma surgery: a one-year experience at a single institutuion. Swiss Med Wkly 138: 180–185 doi:2008/11/smw-12077 [DOI] [PubMed] [Google Scholar]
- 50.Stummer W, Tonn JC, Goetz C, Ullrich W, Stepp H, Bink A, Pietsch T, Pichlmeier U (2014) 5-Aminolevulinic acid-derived tumor fluorescence: the diagnostic accuracy of visible fluorescence qualities as corroborated by spectrometry and histology and postoperative imaging. Neurosurgery 74: 310–319; discussion 319–320 doi: 10.1227/NEU.0000000000000267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lau D, Hervey-Jumper SL, Chang S, Molinaro AM, McDermott MW, Phillips JJ, Berger MS (2016) A prospective Phase II clinical trial of 5-aminolevulinic acid to assess the correlation of intraoperative fluorescence intensity and degree of histologic cellularity during resection of high-grade gliomas. J Neurosurg 124: 1300–1309 doi: 10.3171/2015.5.JNS1577 [DOI] [PubMed] [Google Scholar]
- 52.Idoate MA, Diez Valle R, Echeveste J, Tejada S (2011) Pathological characterization of the glioblastoma border as shown during surgery using 5-aminolevulinic acid-induced fluorescence. Neuropathology 31: 575–582 doi: 10.1111/j.1440-1789.2011.01202.x [DOI] [PubMed] [Google Scholar]
- 53.Valdes PA, Fan X, Ji S, Harris BT, Paulsen KD, Roberts DW (2010) Estimation of brain deformation for volumetric image updating in protoporphyrin IX fluorescence-guided resection. Stereotact Funct Neurosurg 88: 1–10 doi: 10.1159/000258143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kelly PJ, Daumas-Duport C, Kispert DB, Kall BA, Scheithauer BW, Illig JJ (1987) Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg 66: 865–874 doi: 10.3171/jns.1987.66.6.0865 [DOI] [PubMed] [Google Scholar]
- 55.Dandy WE (1928) Removal of right cerebral hemisphere for certain tumors with hemiplegia. JAMA 90: 823–825 [Google Scholar]
- 56.Han K, Ren M, Wick W, Abrey L, Das A, Jin J, Reardon DA (2014) Progression-free survival as a surrogate endpoint for overall survival in glioblastoma: a literature-based meta-analysis from 91 trials. Neuro Oncol 16: 696–706 doi: 10.1093/neuonc/not236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tummers WS, Warram JM, Tipirneni KE, Fengler J, Jacobs P, Shankar L, Henderson L, Ballard B, Pogue BW, Weichert JP, Bouvet M, Sorger J, Contag CH, Frangioni JV, Tweedle MF, Basilion JP, Gambhir SS, Rosenthal EL (2017) Regulatory Aspects of Optical Methods and Exogenous Targets for Cancer Detection. Cancer Res 77: 2197–2206 doi: 10.1158/0008-5472.CAN-16-3217 [DOI] [PMC free article] [PubMed] [Google Scholar]