Supplemental digital content is available in the text.
Abstract
Background
A multicenter, randomized, open-label, parallel group, pilot, 52-week study in Asian countries that assessed the renal function, efficacy, and safety of reduced-exposure versus standard-exposure prolonged-release tacrolimus (PR-T) in adult kidney transplant recipients (KTRs).
Methods
Posttransplantation, KTRs received PR-T from weeks 0 to 4 (initial dose, 0.2–0.3 mg/kg; target trough level, 6–10 ng/mL). At week 4, KTRs were randomized (1:1) to receive reduced-exposure PR-T (target 4–6 ng/mL, weeks 4–12; 3–5 ng/mL, weeks 12–52) or standard-exposure PR-T (target: 6–10 ng/mL, weeks 4–52). Primary end point: estimated glomerular filtration rate (eGFR) over 52 weeks. Secondary end points (week 52) included creatinine clearance, serum creatinine, graft/patient survival, biopsy-confirmed acute rejection (AR), composite of graft loss/patient death/biopsy-confirmed AR, and steroid-resistant AR. Treatment-emergent adverse events were recorded.
Results
Sixty-six KTRs received PR-T (reduced-exposure, n = 32; standard-exposure, n = 34) and were analyzed. After per-protocol dose adjustment, mean ± standard deviation tacrolimus trough level was lower with reduced- versus standard-exposure PR-T (week 52, 4.5 ± 1.1 ng/mL vs 8.0 ± 2.2 ng/mL). In the reduced- versus standard-exposure group, eGFR was similar at weeks 8 to 52 (overall least-square mean difference, –2.82; 95% confidence interval, −7.91 to 2.27; P = 0.272). At week 52, there was no significant difference in creatinine clearance (P = 0.375) or serum creatinine (P = 0.547) between groups. All grafts/patients survived, no steroid-resistant AR was reported, and 4 and 3 patients had AR in reduced- and standard-exposure groups, respectively. Drug-related treatment-emergent adverse events were reported in 34.4% and 38.2% of patients, respectively.
Conclusions
Reducing exposure to PR-T resulted in a clinically acceptable short-term safety profile and was generally as effective as standard tacrolimus exposure for Asian patients.
Kidney transplant recipients are generally required to adhere to a lifelong immunosuppressive regimen in order to preserve long-term graft function and to prevent graft rejection. Immunosuppressive regimens after kidney transplantation typically involve a calcineurin inhibitor (CNI).1 Nevertheless, there remains concern that long-term use of CNIs may be associated with renal function deterioration. The CNI withdrawal after kidney transplantation would prevent development of CNI-related renal dysfunction and has been attempted. However, such strategies have been shown to reduce long-term graft and patient survival2,3 due to inadequate rejection prophylaxis provided by other immunosuppressive regimens. Indeed, alloimmunity may be a major mechanism leading to late kidney allograft failure.4 Therefore, dose minimization, whereby the dose of the CNI is adjusted to target lower exposure within the therapeutic window, may be the preferred option for preserving long-term renal function compared with CNI avoidance or withdrawal.5
Tacrolimus is now the CNI of choice in over 90% of kidney transplant recipients,1 and a once-daily, prolonged-release formulation of tacrolimus was licensed for use in adult kidney or liver transplant recipients in Taiwan and South Korea in 2009. Compared with twice-daily, immediate-release tacrolimus, the prolonged-release formulation has been shown to improve adherence with immunosuppressive therapy6,7 and reduce intrapatient variability in tacrolimus exposure,8,9 which has the potential to improve long-term transplant outcomes.4,10,11 However, studies of dose-minimization strategies with prolonged-release tacrolimus-based immunosuppression in de novo kidney transplant recipients are lacking, and few well-controlled tacrolimus studies have been conducted in Asian patients. The ADHERE study, which was conducted in 18 European and Asia–Pacific countries, demonstrated comparable renal function at week 52 in patients receiving prolonged-release tacrolimus plus mycophenolate mofetil (MMF), or prolonged-release tacrolimus with ≥25% dose reduction on day 42, plus sirolimus.12 However, more adverse events (AEs) led to study discontinuation with reduced-dose prolonged-release tacrolimus versus standard-dose tacrolimus, possibly due to tolerability issues associated with sirolimus.13 This study, therefore, assessed renal function, efficacy, and safety of targeting a reduced versus a standard tacrolimus trough level, when administered as prolonged-release tacrolimus in combination with MMF and corticosteroids, in an Asian population of adult kidney transplant recipients for up to 52 weeks posttransplantation.
MATERIALS AND METHODS
Study Design and Patients
This was a multicenter, randomized, open-label, parallel-group, pilot, exploratory study, conducted at 4 sites in South Korea and 2 sites in Taiwan (protocol number 506-MA-1001). Approvals for the study were obtained from the relevant independent ethics committee or institutional review board (approval numbers: in Korea, Asan Medical Center, 2014-0200; Samsung Medical Center, SMC 2014-01-124; Severance Hospital, 4-2013-0865; Dongsan Medical Center, DSMC 2014-02-001; in Taiwan, Chang Gung Memorial Hospital, 103-0766A; Tri-Service General Hospital, 2-104-05-075). The study was conducted in accordance with the Declaration of Helsinki and International Conference of Harmonisation guidelines. All patients provided written informed consent and could withdraw from the study at any time.
Patients were included at screening if they were aged 20–65 years, had end-stage kidney disease, were undergoing primary kidney transplantation or retransplantation, and received their transplant from a donor (deceased or living) with a compatible ABO blood type.
The full list of exclusion criteria is presented in SDC, Materials and Methods (http://links.lww.com/TXD/A193). Key exclusion criteria at screening were receipt of an organ transplant other than a kidney, receipt of an organ from an HLA-identical donor, cold ischemia time >24 hours, and receipt of a graft from a non–heart-beating donor (other than of Maastricht category 3). Patients were also excluded if they had high immunologic risk (panel-reactive antibody level >50% in the previous 52 weeks, donor-specific antibody [DSA] before transplantation, T cell and/or B cell crossmatch-positive before transplantation, or previous graft loss within 52 weeks of the current transplantation for immunologic reasons). Patients were additionally excluded at week 4 posttransplantation if they did not receive basiliximab induction therapy or if prolonged-release tacrolimus dose adjustment after week 4 was contraindicated due to a rejection episode. Patients were also excluded at week 4 if they had delayed graft function (required more than 1 dialysis treatment in the first week posttransplantation) or if their pretransplant DSA test was found after screening to be positive (as most sites analyzed DSA once weekly, it could take up to 7 days to receive the results).
Immediately after transplantation, kidney transplant patients received prolonged-release tacrolimus (Advagraf, Astellas Pharma Europe BV, Netherlands) from week 0 until week 4 at an initial daily dose of 0.2–0.3 mg/kg. Doses were adjusted based on clinical evidence of efficacy, occurrence of AEs, and to observe target tacrolimus trough levels.
During the initial 4-week period, the recommended target tacrolimus trough level was 6–10 ng/mL (Figure 1). At week 4, patients with a tacrolimus trough level of 6–10 ng/mL, and not meeting any of the week 4 exclusion criteria, were randomized (1:1) to receive either reduced-exposure or standard-exposure to prolonged-release tacrolimus (Figure 1). The randomization sequence for allocation of patients to treatment groups at week 4 was coordinated centrally and stratified by the donor type and site. The data for each patient were typed on the WEB/randomization screen by the site, and a third-party vendor subsequently confirmed that the patient was eligible and randomized them to a treatment group.
FIGURE 1.
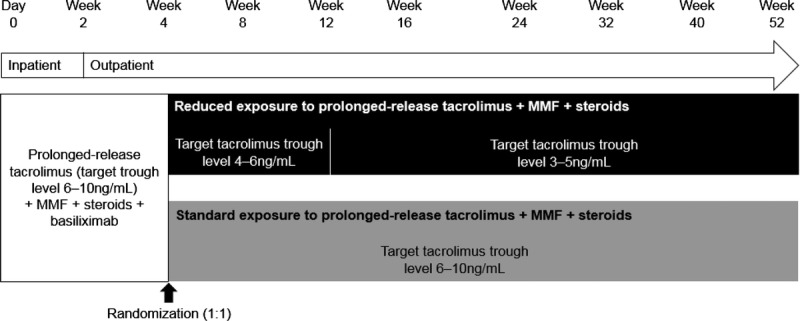
Study design. MMF, mycophenolate mofetil.
Patients randomized to the reduced-exposure group had their prolonged-release tacrolimus dose adjusted to achieve target tacrolimus trough levels of 4–6 ng/mL between weeks 4 and 12 and 3–5 ng/mL between weeks 12 and 52. Patients randomized to the standard-exposure group received prolonged-release tacrolimus, adjusted to retain the target tacrolimus trough level at 6–10 ng/mL between weeks 4 and 52. All patients received 1 dose of basiliximab on day 0 (20 mg within 2 hours before the start of surgery) and a further dose on day 4 (20 mg). Steroids and MMF were administered as per routine clinical practice at individual study sites.
Tacrolimus whole-blood trough levels were monitored according to local practice using microparticle enzyme immunoassay, enzyme-multiplied immunoassay technique, high-performance liquid chromatography–tandem mass spectrometry, or antibody-conjugated magnetic immunoassay analysis. Immunoassays were commercially available assays, and the high-performance liquid chromatography-tandem mass spectrometry method was center specific. The same assay was used consistently throughout the study. For each assessment, up to 2 mL of blood per sample was taken in the morning before administration of tacrolimus. The whole-blood trough levels were assessed 2–3 times per week during hospitalization, at each outpatient visit, and whenever clinically indicated.
Data were collected during 12 visits to the clinic: at baseline (visit 1) and at weeks 1, 2, 3, 4, 8, 12, 16, 24, 32, 40, and 52 (visits 2–12, respectively). There was a window of ±1 day for visit 2, ±3 days for visits 3–5, ±7 days for visits 6–7, ±14 days for visit 8, ±21 days for visits 9–11, and ±28 days for visit 12. Tacrolimus trough levels were assessed at all visits.
End Points
The primary efficacy variable was the estimated glomerular filtration rate (eGFR; Modified Diet in Renal Disease [MDRD] method) over 52 weeks with reduced versus standard exposure to prolonged-release tacrolimus. The MDRD formulae used were as follows:
Korean patients14: 175 × serum creatinine−1.154 × age−0.203 (× 0.742 if female)
Taiwanese patients15: 175 × serum creatinine−1.234 × age−0.179 (× 0.79 if female)
Secondary efficacy assessments at week 52 were calculated creatinine clearance (using the Cockcroft–Gault formula), serum creatinine, graft survival, patient survival, biopsy-confirmed acute rejection (BCAR), and a composite of graft loss, patient death, and BCAR with reduced versus standard exposure to prolonged-release tacrolimus. Graft loss was defined as retransplantation, transplant nephrectomy, death, or dialysis ongoing at study end or at time of discontinuation of the patient from the study. The incidence of acute rejection, steroid-resistant acute rejection, and time between transplantation and the first rejection event were also recorded, as were tacrolimus dose, and trough levels, and DSA development. Treatment-emergent AEs (TEAEs), laboratory assessments, and vital signs/weight were assessed at every visit.
Statistical Analyses
Ninety patients would provide 69% power to detect a mean difference in eGFR between groups of 10 mL/min per 1.73 m2 (standard deviation [SD] 19 mL/min per 1.73 m2) at a 0.05 level of significance. Given that up to 5% of patients were expected to drop out between weeks 0 and 4,16 the aim was to enroll 100 patients in the study. The full-analysis set (FAS) included all randomized patients who received at least 1 dose of study drug postrandomization, had an eGFR value at week 4, and had at least 1 eGFR value after randomization. The safety-analysis set (SAF) comprised all randomized patients who received at least 1 dose of study drug postrandomization. Efficacy data were assessed using the FAS, with the SAF used for summaries of demographic and baseline characteristics, and all safety- and tolerability-related variables.
Comparison of eGFR between study groups was made by repeated-measures analysis of covariance mixed model, with treatment group, donor type (deceased or living), and scheduled visit as factors and week 4 eGFR as a covariate. The same model was repeated to include a treatment-by-visit interaction, in order to assess whether there were interactions between the treatment groups and study time points in relation to eGFR. The differences in time-to-event data were analyzed using the log-rank test, with day 0 as the starting time. Kaplan–Meier survival rates at week 52 are provided with 95% confidence interval (CI; based on Greenwood's formula for standard error). A Cox regression model with treatment as a factor was applied. Incidence rates for acute rejection and steroid-resistant acute rejection at week 52 were compared between treatment groups using the Fisher exact test.
For the primary analysis of the primary end point and for secondary end points, no imputation was performed. P < 0.05 was considered statistically significant, and all analyses were performed using SAS version 9.2 or higher. No adjustments for multiplicity were made.
RESULTS
Patient Characteristics
The date of first enrollment was June 26, 2014, and the date of last evaluation was December 22, 2016. Patient enrollment was terminated before recruitment of 100 patients due to delay in enrollment, the expected additional timeline to recruit 100 patients, and because this was a pilot and exploratory study. Baseline characteristics were, however, comparable between the reduced-exposure and standard-exposure groups when two-thirds of the target population had been enrolled. Early termination of patient enrollment impacted the power of the study to detect a mean difference in eGFR between groups of 10 mL/min per 1.73 m2.
Overall, 79 kidney transplant recipients were enrolled, and 74 received at least 1 prerandomization dose of prolonged-release tacrolimus. Of these 74 patients, 66 formed the FAS and the SAF (reduced-exposure group, n = 32; standard-exposure group, n = 34) (Figure 2).
FIGURE 2.
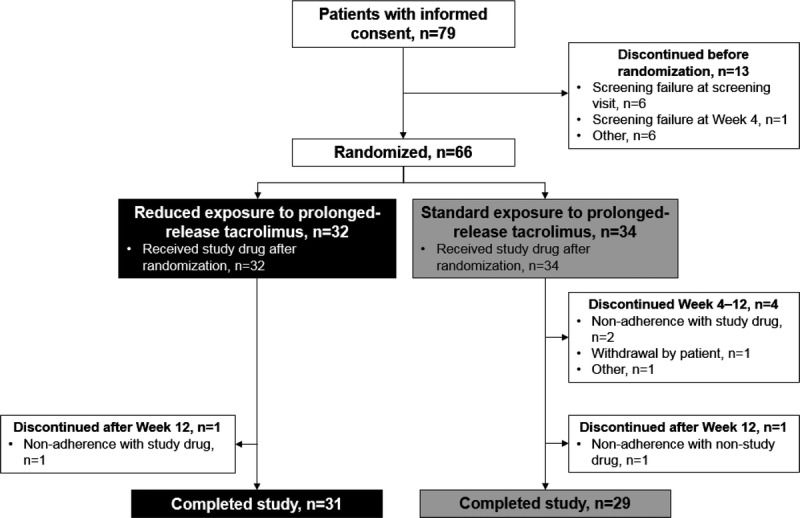
Flow of patients through the study.
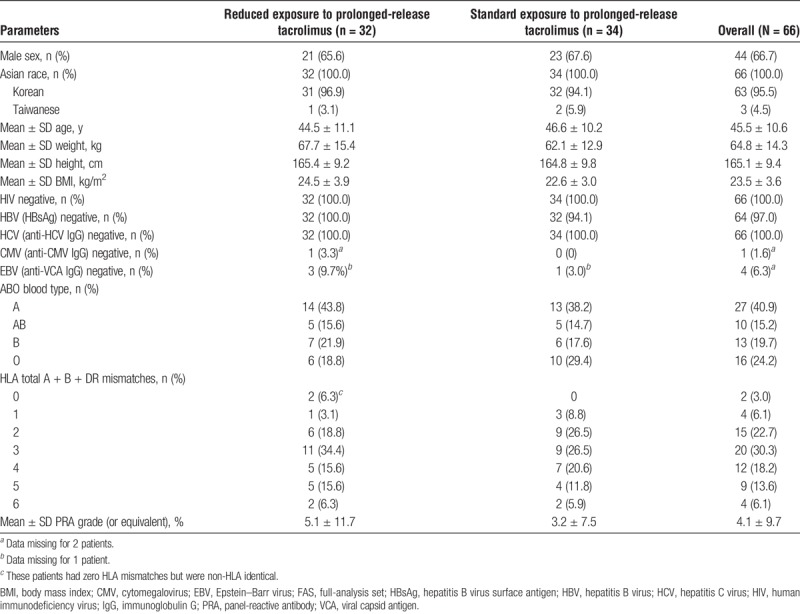
All patients were Asian, and most (95.5%) were Korean. Overall, the mean ± SD age of patients was 45.5 ± 10.6 years, and 66.7% of patients were male. All patients were negative for human immunodeficiency virus and hepatitis C virus, 2 patients (standard-exposure group) were positive for hepatitis B virus, and most patients (93.8%) with available data were positive for Epstein–Barr virus (Table 1). The numbers of HLA total A + B + DR mismatches were 1 (6.1%) for 4 patients, 2 (22.7%) for 15 patients, 3 (30.3%) for 20 patients, 4 (18.2%) for 12 patients, 5 (13.6%) for 9 patients, and 6 (6.1%) for 4 patients; 2 (3.0%) patients had zero HLA mismatches but were non-HLA identical (Table 1). The ABO blood type was A for 40.9% of patients, AB for 15.2%, B for 19.7%, and O for 24.2%, and the mean ± SD panel-reactive antibody grade (or equivalent) was 4.1 ± 9.7%. Patient baseline characteristics were generally similar between treatment groups (Table 1). The most commonly reported previous medications were drugs for constipation (30.3%) and acid-related disorders (25.8%).
TABLE 1.
Patient demographics and baseline characteristics (FAS)
Overall, 60 patients completed the study, whereas 6 patients discontinued (reduced-exposure group, n = 1; standard-exposure group, n = 5). The most common reason for study discontinuation was nonadherence with study drug (n = 3). Other reasons for discontinuation were withdrawal by the patient (n = 1), nonadherence with nonstudy drug (n = 1), and other (n = 1).
Tacrolimus Dose and Trough Levels
The mean ± SD duration of treatment with prolonged-release tacrolimus was 336.2 ± 80.2 days (reduced-exposure group 352.8 ± 46.7 days; standard-exposure group 320.6 ± 100.5 days). The mean daily dose of prolonged-release tacrolimus was similar between the reduced- and standard-exposure groups over the first 4 weeks of treatment (Figure 3A). After the first and second dose adjustments at weeks 4 and 12, the mean dose of prolonged-release tacrolimus was lower in the reduced-exposure than the standard-exposure group (Figure 3A). In the reduced-exposure group, the mean ± SD daily dose of prolonged-release tacrolimus was 0.16 ± 0.07 mg/kg at week 4, 0.11 ± 0.06 mg/kg at week 12, and 0.07 ± 0.03 mg/kg at week 52. In the standard-exposure group, the mean ± SD daily dose of prolonged-release tacrolimus was 0.16 ± 0.09 mg/kg, 0.15 ± 0.09 mg/kg, and 0.11 ± 0.07 mg/kg, respectively.
FIGURE 3.
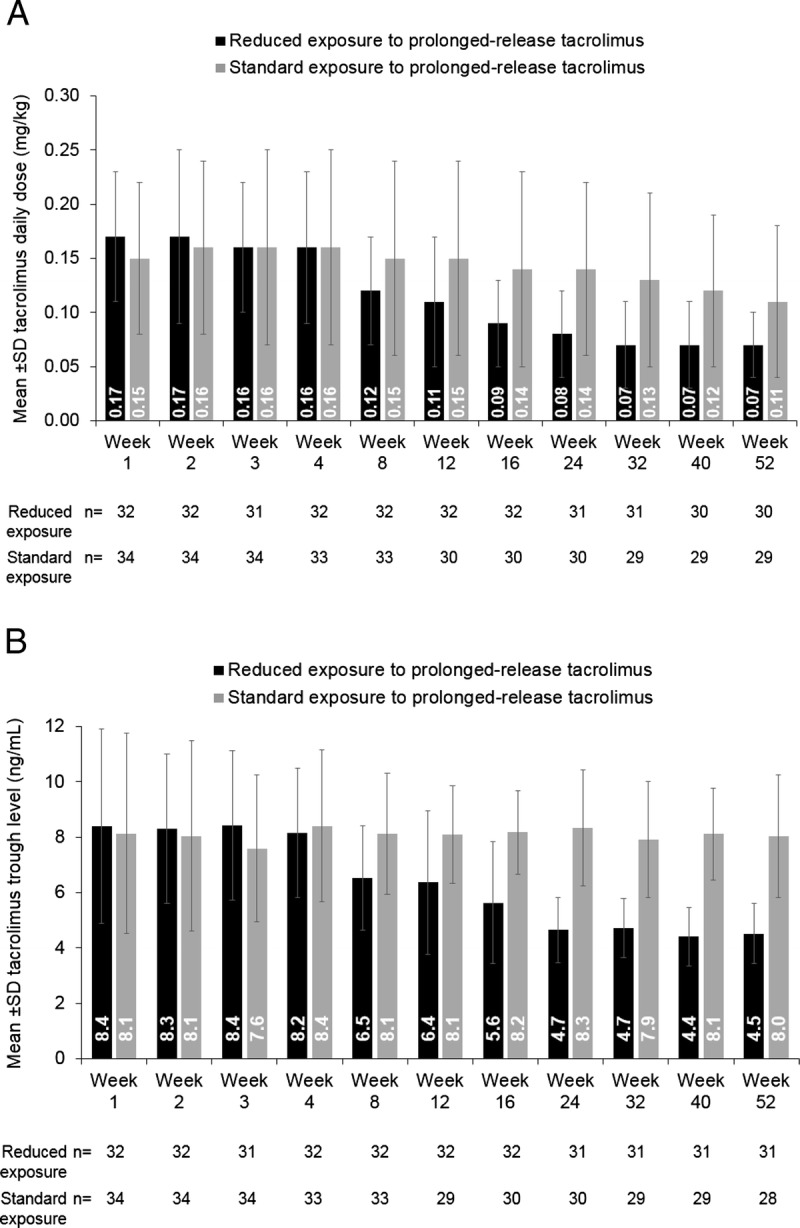
Mean ± SD (A) daily tacrolimus dose and (B) tacrolimus trough levels stratified by reduced versus standard exposure to prolonged-release tacrolimus-based treatment (full-analysis set).
As per protocol, the mean tacrolimus trough level was comparable between the reduced-exposure and standard-exposure groups over the first 4 weeks of treatment. After dose adjustments, the mean tacrolimus trough level was lower in the reduced-exposure group versus the standard-exposure group (Figure 3B). In the reduced-exposure group, the mean ± SD tacrolimus trough level was 8.2 ± 2.3 ng/mL at week 4, 6.4 ± 2.6 ng/mL at week 12, and 4.5 ± 1.1 ng/mL at week 52. In the standard-exposure group, the mean ± SD tacrolimus trough level was 8.4 ± 2.8 ng/mL, 8.1 ± 1.8 ng/mL, and 8.0 ± 2.2 ng/mL, respectively. After a decrease in tacrolimus dosing at weeks 4 and 12, the mean tacrolimus trough level was within target from week 16 through week 52 in the reduced-exposure group. The mean tacrolimus trough levels were within target at all time points in the standard-exposure group (Figure 3B).
Renal Function
Figure 4A presents the mean ± SD eGFR up to week 52. Mean ± SD eGFR numerically decreased between weeks 4 and 8 (from 69.2 ± 20.2 to 64.6 ± 18.4 mL/min per 1.73 m2, respectively) after the first reduction in tacrolimus trough level in the reduced-exposure group and, generally, was numerically lower in the reduced-exposure group during follow-up compared with the standard-exposure group. However, there was no significant difference in eGFR between treatment groups from week 8 to week 52 (overall difference least-squares [LS] mean: −2.82 mL/min per 1.73 m2, 95% CI −7.91 to 2.27; P = 0.272). When treatment-by-visit interaction was included in the model, there were no significant interactions between treatment groups and time points (P = 0.812).
FIGURE 4.
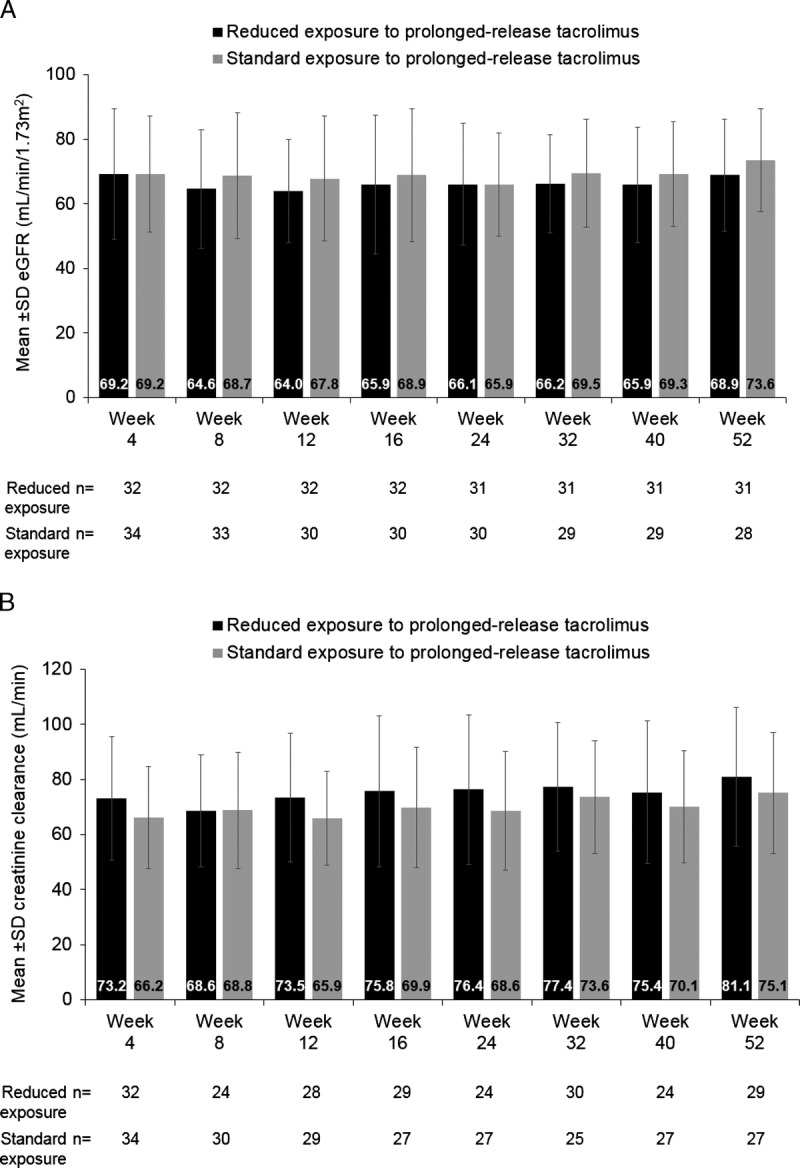
Mean ± SD (A) eGFR and (B) creatinine clearance over 52 weeks, stratified by reduced versus standard exposure to prolonged-release tacrolimus-based treatment (full-analysis set). eGFR, estimated glomerular filtration rate.
Of note, the mean ± SD calculated creatinine clearance was higher in the reduced- versus the standard-exposure group at week 4 (73.2 ± 22.5 versus 66.2 ± 18.6 mL/min, respectively) but was similar between groups at week 8 (Figure 4B). The mean ± SD calculated creatinine clearance was higher at week 52 compared with week 8 in both treatment groups. The values in the reduced-exposure group were 68.6 ± 20.4 mL/min at week 8 versus 81.1 ± 25.2 mL/min at week 52 and in the standard-exposure group were 68.8 ± 21.2 mL/min versus 75.1 ± 22.0 mL/min, respectively (Figure 4B). There was no overall significant difference in LS mean between treatment groups (P = 0.375).
The mean ± SD serum creatinine was 1.1 ± 0.3 mg/dL in both the reduced- and standard-exposure groups at week 4. The mean ± SD serum creatinine was similar between weeks 8 and 52 in the reduced-exposure group (1.2 ± 0.3 versus 1.1 ± 0.3 mg/dL) and the standard-exposure group (1.1 ± 0.3 versus 1.0 ± 0.2 mg/dL). There was no overall significant difference in LS mean between treatment groups (P = 0.547).
Survival and Rejection Rates
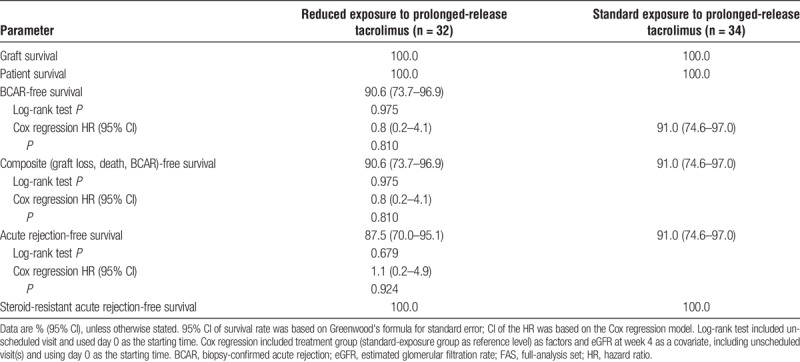
There were no graft losses, patient deaths, or episodes of steroid-resistant acute rejection in either treatment group during the study (Table 2). There was no significant difference between treatment groups for the rate of BCAR-free, composite event-free, or acute rejection-free survival at week 52 (Table 2). The week 52 Kaplan–Meier BCAR-free survival rates in the reduced-exposure and standard-exposure groups were 90.6% and 91.0%, respectively (P = 0.810; data identical for the composite end point), and the week 52 Kaplan–Meier acute rejection-free survival rate was 87.5% and 91.0%, respectively (P = 0.679).
TABLE 2.
Kaplan–Meier estimated graft survival, patient survival, BCAR-free survival, composite (graft loss, patient death, and BCAR)-free survival, and acute rejection-free survival at week 52, stratified by reduced vs standard exposure to prolonged-release tacrolimus-based treatment (FAS)
Seven of the randomized patients experienced acute rejection episodes (4 and 3 patients in the reduced- and standard-exposure groups, respectively); 3 patients in the reduced-exposure group and 2 patients in the standard-exposure group had their rejection confirmed by biopsy. The first acute rejection episode was reported 28 days after transplantation (Figure 5). Acute antibody-mediated grade I rejection and borderline changes were reported for 1 patient in the initial phase (at day 13; the last tacrolimus trough level before onset of acute rejection was 4.2 ng/mL [day 7]). This patient was then randomized to the standard-exposure group and reported borderline changes in the postrandomization period (at day 29; the last tacrolimus trough level before onset of acute rejection was 3.4 ng/mL [day 22]). In both instances, rejection and borderline changes resolved without prolonged-release tacrolimus dose adjustment. The patient was subsequently discontinued from the study on day 64 due to difficulties maintaining their tacrolimus trough levels. In the postrandomization period, borderline changes were reported for 1 patient in the reduced-exposure group (day 57; the tacrolimus trough level measurement closest to the event was 6.4 ng/mL [day 50]); acute antibody-mediated rejection II was reported for 1 patient in the reduced-exposure group (day 36; 2.5 ng/mL [day 28]); acute T-cell-mediated rejection IA was reported for 2 patients in the reduced-exposure group (days 55 and 63, respectively; 8.9 ng/mL [day 54] and 8.8 ng/mL [day 34], respectively) and 1 patient in the standard-exposure group (day 43; 8.9 ng/mL [day 29]); and acute T-cell-mediated rejection IIA was reported for 1 patient in the standard-exposure group (day 55; 9.3 ng/mL [day 26]). All events resolved with steroid treatment.
FIGURE 5.
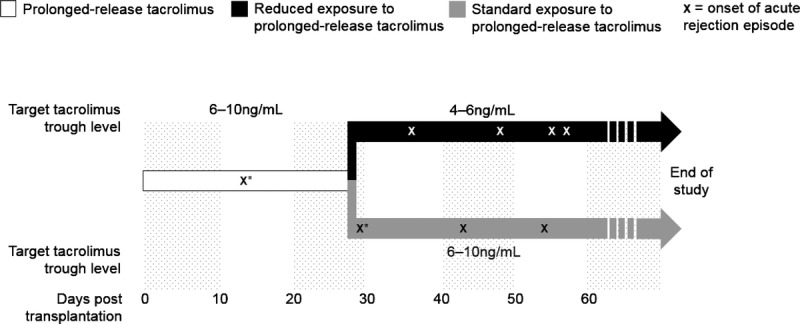
Time of acute rejection after transplantation in the prerandomization and postrandomization phases (FAS). *Both acute rejection episodes occurred in the same patient.
Safety
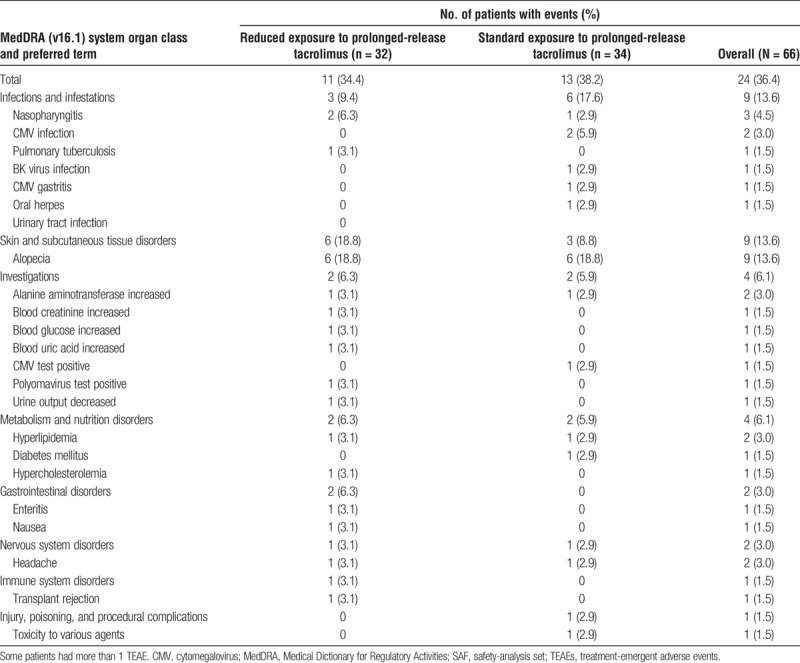
Overall, the safety profile was similar between the reduced- and standard-exposure groups. All patients in both treatment groups experienced TEAEs. Serious TEAEs were reported for 34.4% (11/32; 14 events) of patients in the reduced-exposure group and 38.2% (13/34; 19 events) of patients in the standard-exposure group.
Drug-related TEAEs were reported for 34.4% (11/32; 21 events) and 38.2% (13/34; 16 events) of patients in the reduced- and standard-exposure groups, respectively. The most common drug-related TEAEs in both treatment groups were infections and infestations (9.4% and 17.5% of patients in the reduced- or standard-exposure groups, respectively) and skin and subcutaneous disorders (18.8% and 8.8%) (Table 3). Drug-related serious TEAEs were reported for 6.3% (2/32; 3 events, enteritis, transplant rejection, and pulmonary tuberculosis) and 8.8% (3/34; 4 events, cytomegalovirus [CMV] gastritis, CMV infection, urinary tract infection, CMV test positive) of patients, respectively.
TABLE 3.
Drug-related TEAEs by system organ class and stratified by reduced vs standard exposure to prolonged-release tacrolimus-based treatment (SAF)
No TEAEs led to permanent discontinuation of study drug in either treatment group.
Laboratory Parameters and Vital Signs
Laboratory test results at baseline were below the reference range for erythrocytes (reduced-exposure group 81.3% of patients; standard-exposure group 91.2%), hematocrit (81.3%, 85.3%), and hemoglobin (78.1%, 94.1%). Mean values improved over time in both treatment groups up to week 52. At baseline and week 52, the mean ± SD erythrocyte count was 3.5 ± 0.5 × 1012 versus 4.7 ± 0.6 × 1012, respectively, in the reduced-exposure group and 3.4 ± 0.5 × 1012 versus 4.7 ± 0.8 × 1012 in the standard-exposure group. The mean ± SD hematocrit was 0.32 ± 0.04 versus 0.43 ± 0.06 at baseline and week 52, respectively, in the reduced-exposure group and 0.31 ± 0.05 versus 0.43 ± 0.07 in the standard-exposure group. At baseline and week 52, the mean ± SD hemoglobin level was 108.3 ± 14.5 versus 139.6 ± 19.6 g/L, respectively, in the reduced-exposure group and 104.9 ± 15.2 versus 139.1 ± 22.9 g/L in the standard-exposure group.
At baseline, the leukocyte count was above the reference range for 25.0% (8/32) of patients in the reduced-exposure group and 11.8% (4/34) of patients in the standard-exposure group but improved over time in the reduced-exposure group up to week 52. At baseline and week 52, the mean ± SD leukocyte count was 7.6 ± 3.5 × 109 versus 7.5 ± 2.3 × 109, respectively, in the reduced-exposure group and 6.8 ± 2.9 × 109 versus 7.4 ± 2.3 × 109 in the standard-exposure group.
There were no notable differences between dosing groups in other hematology, biochemistry, or urinalysis parameters or vital signs/weight at week 52 (data not shown). Additionally, there were no clinically meaningful postbaseline changes in systolic blood pressure, diastolic blood pressure, and pulse rate.
At the end of the study, 3 (9.4%) of 32 patients and 2 (5.9%) of 34 patients in the reduced- and standard-exposure groups, respectively, had DSA. None of these patients had DSA before kidney transplantation.
DISCUSSION
The study demonstrated that reduced systemic exposure to tacrolimus when administered as prolonged-release tacrolimus-based immunosuppression was not associated with any beneficial effect on renal function at week 52 posttransplantation in this Asian population of adult de novo kidney transplant recipients. Furthermore, there was no graft loss or patient death reported during the study, and the Kaplan–Meier BCAR-free and acute rejection-free survival rates were comparable between treatment groups. Seven acute rejection episodes were reported, none of which were steroid-resistant. Although all patients in both treatment groups experienced TEAEs, there were few serious drug-related events, and no new safety signals were reported for prolonged-release tacrolimus.
In this study, eGFR decreased after the first tacrolimus trough level reduction in the reduced-exposure group. As there were no protocol biopsies during the study, it is unclear whether this numeric change related to subclinical rejection due to the dose reduction. However, there was no further decline in eGFR after the second tacrolimus trough level reduction at week 12 in the reduced-exposure group, and most rejection episodes occurred within 8 weeks after transplantation. This suggests that it might be preferable to reduce tacrolimus trough levels after the second or third month posttransplantation, rather than after the first month.
During follow-up, eGFR was generally numerically lower in the reduced- versus standard-exposure group, and this difference could be considered clinically meaningful at week 52 in this short-term study. However, there was no statistically significant difference in renal function between the 2 dosing groups from week 8 to week 52, based on eGFR and creatinine clearance. Failure to reject the hypothesis that reduced exposure to prolonged-release tacrolimus would result in better renal function than with standard exposure may be due to the low power (69%) used to estimate a sufficient sample size. Additionally, the assumed 10-mL/min per 1.73 m2 difference in eGFR between the treatment groups used in the sample size calculation may have been unrealistically high. Alternatively, our data may suggest that tacrolimus dose minimization (and thereby drug exposure minimization) strategies may not be associated with better renal function than standard tacrolimus dosing regimens within the first year after transplantation (the interval assessed in this short-term study). This study does, however, confirm previous reports that prolonged-release tacrolimus-based immunosuppression supports long-term renal function in de novo kidney transplant recipients.17 Similar findings have also been reported in stable kidney recipients converted from immediate- to prolonged-release tacrolimus.17-20
As expected, the mean daily dose of prolonged-release tacrolimus was lower in the reduced- versus standard-exposure group after week 4, to achieve lower tacrolimus trough levels. The mean tacrolimus trough level was within the target range throughout the study in the standard-exposure group but above the target range or at the top end of the target range after the first (weeks 4–12) and second (weeks 12–52) dose adjustments between weeks 4 and 52 in the reduced-exposure group. However, tacrolimus trough levels were generally better controlled during our study than in the randomized SYMPHONY trial, which compared the efficacy and safety of low-dose immediate-release tacrolimus, standard- or low-dose cyclosporine, and low-dose sirolimus-based immunosuppressive regimens in kidney transplant recipients.16 In the SYMPHONY study, although the tacrolimus target trough range was 3–7 ng/mL, the mean trough level was greater than 7 ng/mL during the first 8 weeks of the study, and was in the upper end of the target range for the remainder of the study.16 This may have been due to a reluctance to target reduced tacrolimus trough levels because of the risks associated with underimmunosuppression, as low exposure to tacrolimus has been associated with increased risk of graft dysfunction and graft loss.21,22 In our study, although we attempted to adjust the tacrolimus trough level to within the target range, there was a tendency for the levels to be at the top end of the target range, while avoiding underimmunosuppression. It should be noted that failure to achieve reduced tacrolimus trough levels within the target range for the intended duration could have decreased the ability to detect a significant difference in eGFR between treatment groups in our study.
At week 52, there was no significant difference between dosing groups in graft and patient survival, BCAR, composite end point (graft loss, patient death, and BCAR), acute rejection, and steroid-resistant acute rejection. There was 1 additional rejection episode in the reduced-exposure compared with the standard-exposure group. Whether this was related to underimmunosuppression in the reduced-exposure group is unclear but might suggest that the dose of tacrolimus should be decreased after the second or third month posttransplantation, rather than after the first month. The data suggest that targeting the lower end of the tacrolimus therapeutic range may not impact short-term kidney transplant outcomes; potential benefits for long-term renal function beyond 1 year posttransplantation should be assessed.
Despite the difference in tacrolimus exposure between treatment groups, the tolerability profile was comparable in patients in the reduced- versus standard-exposure groups. This may be due to the small patient numbers. However, our data support the hypothesis posed by the ADHERE study that the higher number of AEs that led to study discontinuation with reduced-dose prolonged-release tacrolimus versus standard-dose tacrolimus may have been due to tolerability issues associated with sirolimus.13 Importantly, in our study, prolonged-release tacrolimus had a clinically acceptable safety profile in both the reduced- and standard-exposure groups, no new safety signals were detected, and there were no notable differences in laboratory parameters between groups.
This study was associated with limitations, including its open-label and exploratory design. The methods for measuring tacrolimus trough levels varied between centers, although the tacrolimus trough levels were well maintained in this study, reflecting real clinical practice in Asia. Due to the small number of patients (and therefore a decreased statistical power), data should be interpreted with caution. Indeed, a larger number of patients might have increased the power of the study to detect differences between the treatment groups. Furthermore, the rigorous inclusion criteria, which defined a patient group with low immunologic risk, may limit the applicability of these results to a broader patient population. Further studies are required to assess reduced tacrolimus exposure regimens in patients with high immunologic risk. The study design did not include a protocol biopsy as, due to their clinical practice, not every study site could conduct a protocol biopsy. This may have limited the detection of acute rejection, real-time pathologic changes, and borderline changes associated with reduced- or standard-exposure to prolonged-release tacrolimus after transplantation. Inclusion of protocol biopsies would have provided useful additional information regarding the safety of reducing tacrolimus exposure. Furthermore, this study reports data up to 52 weeks. Longer-term follow-up studies that compare reduced versus standard target tacrolimus trough levels may be required to fully elucidate any potential renal-sparing effects with tacrolimus reduced-dose strategies and their utility in clinical practice.
Despite its limitations, this study adds to the limited available data on dose-minimization strategies with prolonged-release tacrolimus-based immunosuppression in de novo kidney transplant recipients. Additionally, the study was conducted in Asian patients—a population in which few tacrolimus studies have been conducted to date.
CONCLUSIONS
This study suggested that during 1 year, reducing exposure to tacrolimus was generally as effective as standard exposure to tacrolimus for Asian patients. Importantly, prolonged-release tacrolimus had a clinically acceptable safety profile in both the reduced- and standard-exposure groups, and no new safety signals were detected with prolonged-release tacrolimus. Renal function was comparable between the reduced- and standard-exposure groups between weeks 4 and 52. Longer-term follow-up studies will be required to further assess any effect on renal function of targeting low tacrolimus trough levels.
Data Statement
Access to anonymized individual participant level data collected during the trial, in addition to supporting clinical documentation, is planned for trials conducted with approved product indications and formulations, as well as compounds terminated during development. Conditions and exceptions are described under the Sponsor Specific Details for Astellas on www.clinicalstudydatarequest.com. Study-related supporting documentation is redacted and provided if available, such as the protocol and amendments, statistical analysis plan, and clinical study report. Access to participant level data is offered to researchers after publication of the primary article (if applicable) and is available as long as Astellas has legal authority to provide the data. Researchers must submit a proposal to conduct a scientifically relevant analysis of the study data. The research proposal is reviewed by an Independent Research Panel. If the proposal is approved, access to the study data is provided in a secure data sharing environment after receipt of a signed Data Sharing Agreement.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank the following investigators: Joo Hee Jung, Seung Yeup Han, Jae Berm Park, Kyo Won Lee, Kyu Ha Huh, Yu Seun Kim, and Shou-Hung Tang. The authors would also like to thank Yutaka Komukai, Richard Croy, Shino Hagikura, Ryoji Hirabayashi, Osamu Yamamoto, Adrian Yit Reen Ooi, Sungmin Lee, Joanna You, Yoshihiko Watarai, and Hideki Ishida for supporting the study. This study was sponsored by Astellas Pharma, Inc. Daniella T Draper, PhD, CMPP, and Anna Thompson, PhD, from Cello Health MedErgy assisted in drafting the initial version of the article under the direction of the authors and provided editorial support throughout its development. Editorial support was funded by Astellas Pharma, Inc.
Footnotes
Published online 25 March, 2019.
Y.H.K. and Y.-J.C. contributed equally.
This study was sponsored by Astellas Pharma, Inc. Medical writing support in the development of this article was provided by Astellas Pharma, Inc.
All authors report nonfinancial support from Astellas Pharma, Inc. in the development of this article. Y.H.K., Y.-J.C., S.-J.K., M.S.K., S.B.P., S.-T.W., and D.-J.H. have no other conflicts to disclose. K.H., Y.N., and H.J. are employees of Astellas Pharma, Inc.
All authors critically reviewed the article and revised it for important intellectual content. Y.-J.C., H.J., and D.-J.H. participated in the design of the research. Y.H.K., Y.-J.C., S.-J.K., M.S.K., S.B.P., S.-T.W., and D.-J.H. performed the research. K.H. and Y.N. participated in the data analysis. Y.H.K., Y.-J.C., K.H., Y.N., H.J., and D.-J.H. participated in writing the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2015 annual data report: kidney. Am J Transplant. 2017;17(Suppl 1):21–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Opelz G, Döhler B. Effect on kidney graft survival of reducing or discontinuing maintenance immunosuppression after the first year posttransplant. Transplantation. 2008;86:371–376. [DOI] [PubMed] [Google Scholar]
- 3.Isakova T, Xie H, Messinger S, et al. Inhibitors of mTOR and risks of allograft failure and mortality in kidney transplantation. Am J Transplant. 2013;13:100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiebe C, Gibson IW, Blydt-Hansen TD, et al. Rates and determinants of progression to graft failure in kidney allograft recipients with de novo donor-specific antibody. Am J Transplant. 2015;15:2921–2930. [DOI] [PubMed] [Google Scholar]
- 5.Sawinski D, Trofe-Clark J, Leas B, et al. Calcineurin inhibitor minimization, conversion, withdrawal, and avoidance strategies in renal transplantation: a systematic review and meta-analysis. Am J Transplant. 2016;16:2117–2138. [DOI] [PubMed] [Google Scholar]
- 6.Kuypers DR, Peeters PC, Sennesael JJ, et al. Improved adherence to tacrolimus once-daily formulation in renal recipients: a randomized controlled trial using electronic monitoring. Transplantation. 2013;95:333–340. [DOI] [PubMed] [Google Scholar]
- 7.Cassuto E, Pageaux GP, Cantarovich D, et al. Adherence to and acceptance of once-daily tacrolimus after kidney and liver transplant. Transplantation. 2016;100:2099–2106. [DOI] [PubMed] [Google Scholar]
- 8.Wu M-J, Cheng C-HC-Y, Chen C-H, et al. Lower variability of tacrolimus trough concentration after conversion from Prograf to Advagraf in stable kidney transplant recipients. Transplantation. 2011;92:648–652. [DOI] [PubMed] [Google Scholar]
- 9.Stifft F, Stolk LM, Undre N, et al. Lower variability in 24-hour exposure during once-daily compared to twice-daily tacrolimus formulation in kidney transplantation. Transplantation. 2014;97:775–780. [DOI] [PubMed] [Google Scholar]
- 10.Gaynor JJ, Ciancio G, Guerra G, et al. Graft failure due to noncompliance among 628 kidney transplant recipients with long-term follow-up: a single-center observational study. Transplantation. 2014;97:925–933. [DOI] [PubMed] [Google Scholar]
- 11.Sapir-Pichhadze R, Wang Y, Famure O, et al. Time-dependent variability in tacrolimus trough blood levels is a risk factor for late kidney transplant failure. Kidney Int. 2014;85:1404–1411. [DOI] [PubMed] [Google Scholar]
- 12.Rummo OO, Carmellini M, Rostaing L, et al. ADHERE: randomized controlled trial comparing renal function in de novo kidney transplant recipients receiving prolonged-release tacrolimus plus mycophenolate mofetil or sirolimus. Transpl Int. 2017;30:83–95. [DOI] [PubMed] [Google Scholar]
- 13.Vitko S, Wlodarczyk Z, Kyllönen L, et al. Tacrolimus combined with two different dosages of sirolimus in kidney transplantation: results of a multicenter study. Am J Transplant. 2006;6:531–538. [DOI] [PubMed] [Google Scholar]
- 14.Lee CS, Cha R, Lim Y-H, et al. Ethnic coefficients for glomerular filtration rate estimation by the Modification of Diet in Renal Disease study equations in the Korean population. J Korean Med Sci. 2010;25:1616–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Y-C, Zuo L, Chen J-H, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2937–2944. [DOI] [PubMed] [Google Scholar]
- 16.Ekberg H, Tedesco-Silva H, Demirbas A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357:2562–2575. [DOI] [PubMed] [Google Scholar]
- 17.van Hooff JP, Alloway RR, Trunečka P, et al. Four-year experience with tacrolimus once-daily prolonged release in patients from phase II conversion and de novo kidney, liver, and heart studies. Clin Transpl. 2011;25:E1–E12. [DOI] [PubMed] [Google Scholar]
- 18.Spagnoletti G, Gargiulo A, Salerno MP, et al. Conversion from Prograf to Advagraf in stable kidney transplant recipients: better renal function after 3-year follow-up. Transplant Proc. 2014;46:2224–2227. [DOI] [PubMed] [Google Scholar]
- 19.Guirado L, Burgos D, Cantarell C, et al. Medium-term renal function in a large cohort of stable kidney transplant recipients converted from twice-daily to once-daily tacrolimus. Transplant Direct. 2015;1:e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barreto P, Malheiro J, Vieira P, et al. Conversion from twice-daily to once-daily tacrolimus in stable kidney graft recipients. Transplant Proc. 2016;48:2276–2279. [DOI] [PubMed] [Google Scholar]
- 21.Kuypers DR, Claes K, Evenepoel P, et al. Clinical efficacy and toxicity profile of tacrolimus and mycophenolic acid in relation to combined long-term pharmacokinetics in de novo renal allograft recipients. Clin Pharmacol Ther. 2004;75:434–447. [DOI] [PubMed] [Google Scholar]
- 22.Rodrigo E, Segundo DS, Fernández-Fresnedo G, et al. Within-patient variability in tacrolimus blood levels predicts kidney graft loss and donor-specific antibody development. Transplantation. 2016;100:2479–2485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


