Supplemental digital content is available in the text.
Abstract
Background
Nephrology trials assessing the impact of interventions on “standard” outcomes, such as doubling of creatinine, end-stage renal disease (ESRD), and/or death, are difficult to conduct given the time required for endpoints to accrue. The objective of this study was to determine if using lesser declines in kidney function would alter the interpretation of a previous randomized controlled trial.
Methods
This study was a secondary analysis of a kidney transplant trial comparing the use of a 40% or greater, 30% or greater, or 20% or greater decline in estimated glomerular filtration rate (eGFR) as a substitute for doubling of serum creatinine. Declines in eGFR were determined relative to baseline. This trial enrolled 212 kidney transplant patients with proteinuria and assessed the clinical impact of ramipril versus placebo on a primary outcome of doubling of serum creatinine, ESRD, or death. In this analysis, the declines in eGFR replaced doubling of creatinine in the composite endpoint.
Results
Mean trial follow-up was 41 months. A time-to-event composite of death, ESRD, or a 40% or greater, 30% or greater, or 20% or greater eGFR decline occurred in 45 (26 placebo vs 19 ramipril), 68 (35 vs 33), and 99 (50 vs 49) patients, respectively. Substituting these eGFR declines for doubling of serum creatinine resulted in an increase of 12, 35, and 66 endpoints compared with the original trial. In all 3 eGFR declines, ramipril treatment was not associated with any statistically significant differences despite the increase in events.
Conclusions
Substituting doubling of serum creatinine for lesser eGFR percentage decline thresholds did not alter trial interpretation but did increase the number of events.
Clinical trials in nephrology and kidney transplantation are challenged by low event rates of hard outcomes, such as death and end-stage renal disease (ESRD).1-5 To provide clinical insight, kidney transplant trials assessing the impact of an intervention on ‘hard’ outcomes like death or ESRD require a substantial sample size and/or extended follow-up—both major threats to feasibility.1-5 To increase the number of early events and improve trial feasibility, an endpoint of doubling of baseline serum creatinine is often incorporated into a time-to-event composite outcome that also includes death and ESRD.1-5 Doubling of serum creatinine is a marker of kidney function decline and has been shown to be an effective surrogate for both ESRD and death.1,2,6
However, utilizing doubling of serum creatinine within a composite endpoint has not solved the problem, as issues of low short-term event rates and consequently efficiency continue to persist.7 To increase event rates and enable clinical trials of shorter duration, the use of a percentage reduction in estimated glomerular filtration rate (eGFR) as an alternative surrogate for hard outcomes was recently explored.1 In a large, multinational database of chronic kidney disease (CKD) patients, an endpoint of 30% or greater decline in eGFR over 2 years was considerably more frequent and also predictive of ESRD and death in this population.1 The validity of using percentage eGFR declines as surrogate endpoints in clinical trials were further evaluated in a kidney transplant population.2 The investigators of this study recommended that percentage decline in eGFR should be considered for use as a surrogate endpoint in kidney transplant trials.2 To investigate the impact of such recommendations, we conducted a secondary analysis of a randomized trial in kidney transplantation.8 In this study, we incorporated alternative percentage declines in eGFR into a composite outcome to determine if the “new endpoints” altered the original trial results, interpretation, or conclusions.
MATERIALS AND METHODS
Study Design and Treatment
We conducted a secondary analysis of a double-blind, placebo-controlled, randomized trial involving kidney transplant patients at 14 centers in Canada and New Zealand.8 Eligible patients entered a 2-week open-label trial of ramipril 5 mg daily. If tolerated, patients were randomized (1:1) to receive either ramipril or placebo. Details of the trial protocol9 and primary study results8 have been published previously. The study was approved by the local research ethics board at every participating institution and all trial participants provided written informed consent. The study adhered to the Declaration of Helsinki and Declaration of Istanbul. The trial registered with International Standard Randomized Controlled Trial Number 78129473.
Trial Primary Outcome
The primary outcome was a time-to-event composite consisting of doubling of serum creatinine, ESRD, or death. ESRD was defined as the date of repeat kidney transplantation or initiation of dialysis. Doubling of serum creatinine was defined as a 2-fold increase in serum creatinine relative to baseline and was confirmed by two consecutive tests at least 4 weeks apart by a central laboratory. All outcomes in the composite were measured as time to first occurrence of any component of the composite. However, overall total events of each composite component were recorded and reported as individual components.
Secondary Analysis
In place of doubling of serum creatinine, we incorporated eGFR percentage declines of 40% or greater, 30% or greater, or 20% or greater into the original composite outcome that also included ESRD and death. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation10 was used to calculate all eGFR values. Events of 40% or greater, 30% or greater, or 20% or greater eGFR decline were determined relative to baseline. All outcomes (death, ESRD, or eGFR percentage decline) were measured as time-to-event and only the first event was used in the composite endpoint. Overall total events of each composite component were recorded and reported as individual components. In a sensitivity analysis, the 4-variable Modification of Diet in Renal Disease equation11 was used to calculate eGFR values (Tables S1 and S2, SDC, http://links.lww.com/TXD/A194).
Study Assessments
Study visits occurred at randomization, 1 month, 6 months, and then every 6 months thereafter until trial end. At each study visit, hemoglobin, serum creatinine, and serum potassium concentrations were measured by a central laboratory and any event of doubling of serum creatinine, ESRD, or death was determined. Baseline data for each trial participant included age, gender, ethnic origin (white, black, Asian, or other), proteinuria (mg/day), type of donor (living or deceased), measured GFR (mL/min per 1.73 m2), serum creatinine (μmol/L), diabetes status (present or absent), and time posttransplantation (days).
Statistical Analyses
All analyses were conducted in accordance with the intention-to-treat principle. Kaplan-Meier plots assessing each time-to-event composite over the trial period were constructed while nonparametric log-rank tests were performed as significance tests. Cox proportional hazard ratios along with corresponding 95% confidence intervals were created to compare outcomes between study groups. All analyses were conducted in R statistical software version 3.3.2 (R core team; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
A total of 212 patients were analyzed and both groups were well balanced at baseline (Table 1). Mean follow-up was 41 months (range 1–48). A composite consisting of death, ESRD, and a 40% or greater, 30% or greater, or 20% or greater eGFR decline occurred in a total of 45 (26 placebo vs 19 ramipril), 68 (35 vs 33), and 99 (50 vs 49) patients, respectively. There were no statistically significant differences between Ramipril treatment and placebo on these endpoints (Table 2). The time to occurrence of each composite did not differ significantly between groups (Figure 1).
TABLE 1.
Baseline characteristics from the Canadian ACE inhibitor transplant trial
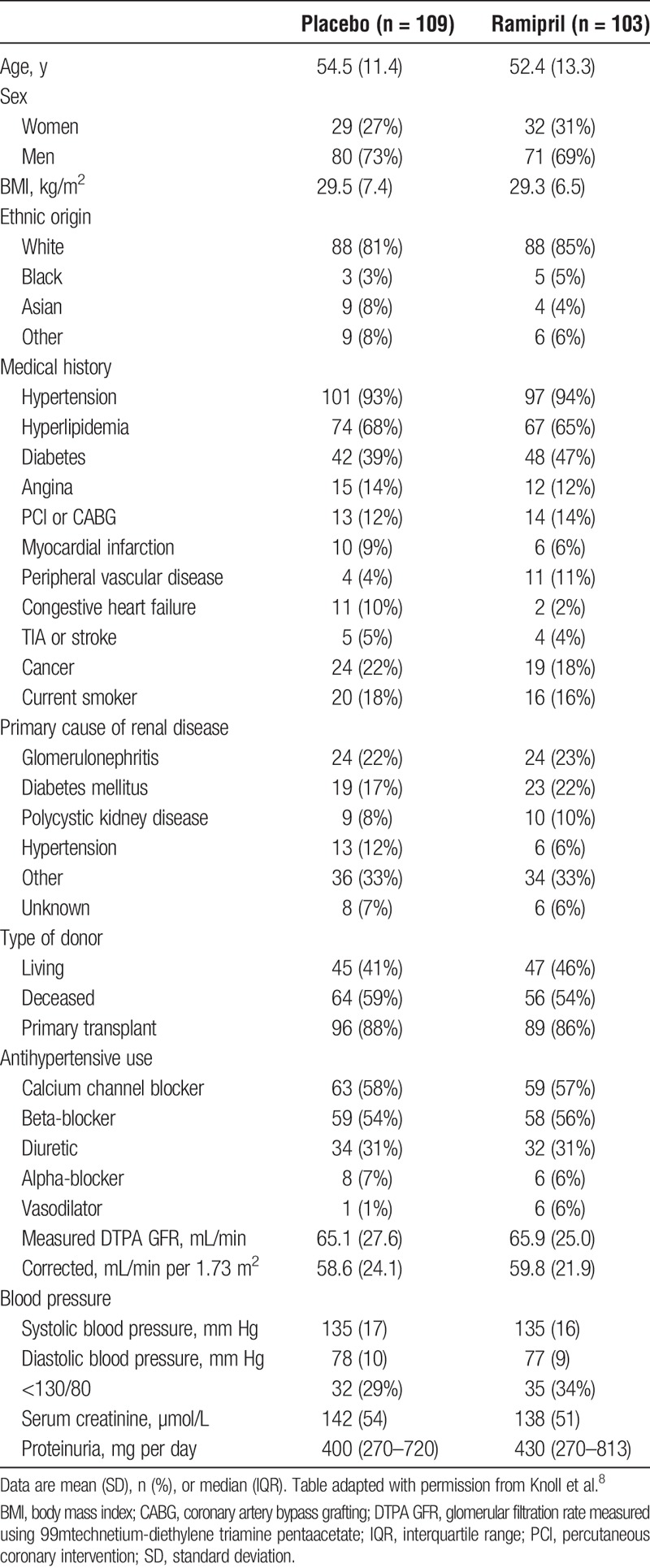
TABLE 2.
Primary outcome, including events of eGFR decline, ESRD, or death
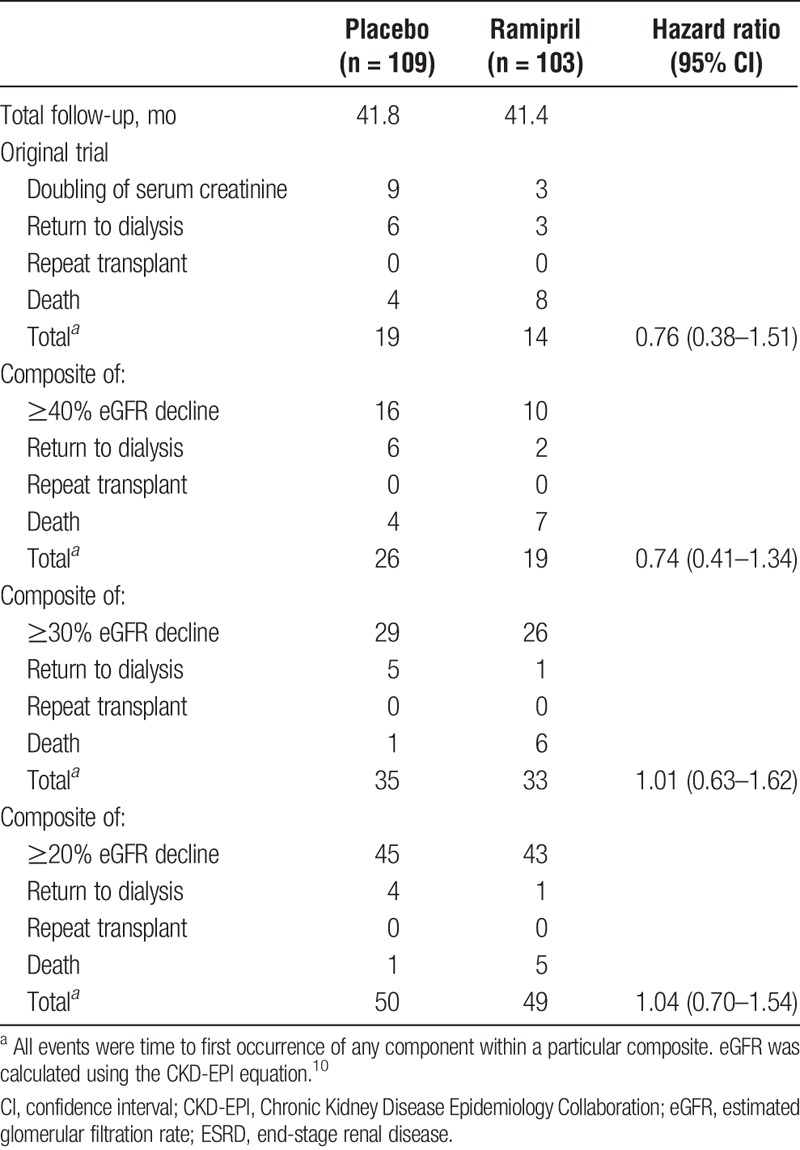
FIGURE 1.
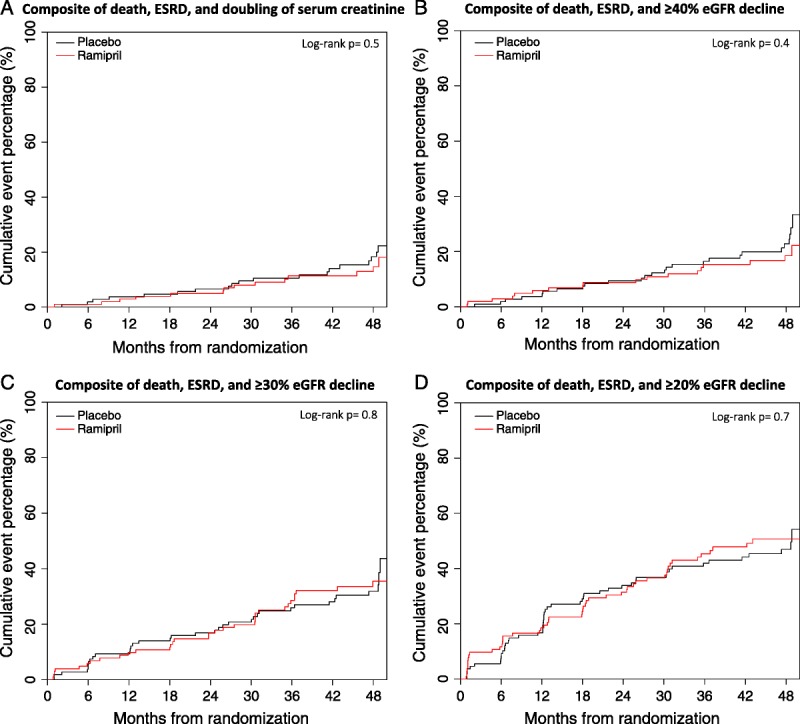
Cumulative hazard plots of trial composite outcomes (mean follow-up, 41 mo). eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease.
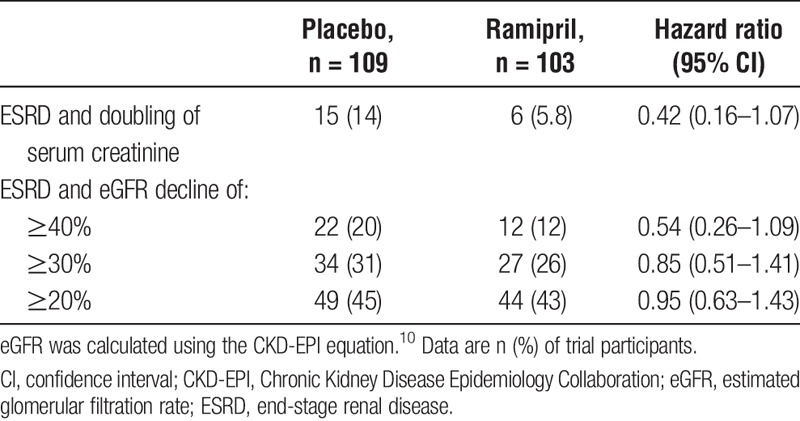
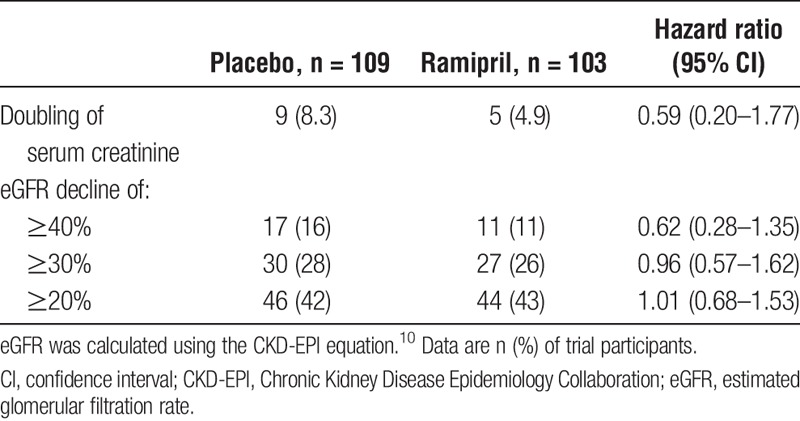
When kidney outcomes were analyzed separately (ie, death was excluded from the composite endpoint), with ESRD or doubling of serum creatinine, 40% or greater, 30% or greater, or 20% or greater eGFR decline occurred in a total of 21 (15 placebo vs 6 ramipril), 34 (22 vs 12), 61 (34 vs 27), and 93 (49 vs 44) patients, respectively. Ramipril treatment versus placebo did not result in any statistically significant difference in these kidney-specific endpoints (Table 3).
TABLE 3.
Kidney outcomes, excluding death
The eGFR declines were analyzed on their own and shown in Table 4. An eGFR decline of 40% or greater, 30% or greater, or 20% or greater occurred in a total of 28 (17 placebo vs 11 ramipril), 57 (30 vs 27), and 90 (46 vs 44) patients, respectively (Table 4). On endpoints of eGFR decline, treatment with Ramipril was not associated with any statistically significant differences compared to placebo (Table 4). In addition, all events of eGFR decline were preceded by consistent overall declines in renal function prior to the event occurring (Figures S1-S3, SDC http://links.lww.com/TXD/A195).
TABLE 4.
eGFR decline outcomes only
DISCUSSION
We found that substituting eGFR declines of 40% or greater, 30% or greater, or 20% or greater for doubling of serum creatinine within a time-to-event composite of death and ESRD did increase the overall number of trial outcomes. However, the increased number of events did not alter the original trial conclusions; there was no statistically significant difference between ramipril and placebo in any of the re-analyzed composite endpoints.
The effectiveness of using eGFR decline as a surrogate endpoint for ESRD has been shown in the CKD population.1 A National Kidney Foundation and US Food and Drug Administration workshop concluded that a 40% or greater eGFR decline over 2 to 3 years was broadly acceptable as a substitute for doubling of serum creatinine in CKD progression trials, whereas a 30% or greater eGFR decline may be acceptable—but only in cases where there lacks an acute effect on eGFR.4 Stemming from this workshop, Badve et al4 conducted a review and concluded that the decision to use a 30% or greater or 40% or greater eGFR decline as a surrogate endpoint in CKD progression trials should be determined on a trial-by-trial basis, depending on the intervention being assessed and its hypothesized impact on eGFR percentage decline. Clayton et al2 recently investigated the relationship between eGFR decline and hard outcomes in 7949 kidney transplants from the Australia and New Zealand Dialysis and Transplant Registry. They determined that both 30% or greater and 40% or greater eGFR decline not only occurred more frequently than doubling of serum creatinine but were also strongly associated with ESRD and death.2 Although the use of these eGFR decline cut-offs will increase event rates in kidney transplant trials, evidence of their impact on statistical power and trial feasibility within the kidney transplant setting is lacking.2 A post hoc analysis of two recent trials in ESRD patients found that the use of lower eGFR decline endpoints do increase the number of events but they also attenuate treatment effect—which would potentially eliminate any increases in statistical efficiency and trial power.7
Our results demonstrate that the number of composite events, and the rate at which they occur, increased as the percentage threshold was lowered. Using a 40% eGFR or greater decline, instead of doubling of serum creatinine in the composite the outcome resulted in an additional 12 events or a 36% relative increase in events. When 30% or greater eGFR decline was used, additional 35 events were noted for a relative increase of 106%. Lastly, the composite using a 20% or greater eGFR decline resulted in an additional 66 total events, a relative increase of 200%.
The increased number of events did lead to more events and, consequently, to narrower 95% confidence intervals. Increasing the frequency of events does increase statistical power, but the practical value of this increase depends critically on whether or not the intervention in question exhibits a similar treatment effect on the alternative endpoints. If the event rate increases and the treatment effect is similar, trial power increases and the same conclusions can be drawn using fewer patients or a shorter duration of follow-up. However, if the treatment effect on eGFR decline was different from the effect on doubling of serum creatinine, the fundamental meaning of the observed effect could change. In other words, power increases, but this may lead to different conclusions. In this trial, the results suggest that a lack of treatment effect was consistently observed regardless of whether a 40% or greater, 30% or greater, or 20% or greater eGFR decline was substituted for doubling of serum creatinine in the composite outcome. This lack of treatment effect made it impossible to assess the impact of these endpoints on treatment attenuation and trial statistical power. Another aspect that requires emphasis is that the choice of renal decline surrogate should be informed by the underlying treatment being assessed. Our study drug ramipril (an ACE-inhibitor) is associated with acute declines in renal function (acute effects) but can, in certain clinical situations, prevent further kidney function decline over time (chronic effects).7 In the analysis by Lambers Heerspink and colleagues,7 they found that the acute effects of angiotensin receptor blockers may be responsible for the attenuation of treatment effect seen when using eGFR decline endpoints of lesser magnitude. For a similar treatment such as ramipril, it would be necessary to assess the appropriateness of using a lower threshold of renal decline as it may lead to an over-emphasis of the expected acute transient fluctuations in renal function, thereby negating any long-term treatment effect. In our study, it did appear that there was higher rate of 20% or greater eGFR events in the first 3 months in the ramipril group versus placebo. However, this higher rate was not seen in renal function endpoints of higher magnitude (≥40%, ≥30% eGFR decline, and doubling of creatinine). If surrogate renal function endpoints are to be used, the clinical context should be evaluated to determine the appropriateness of the surrogate endpoints.
The interpretation of the trial was not changed by substituting alternative endpoints of graft function (≥40%, ≥30%, and ≥20% eGFR declines) for doubling of serum creatinine in a time-to-event composite of death and ESRD. Treatment with an ACE-inhibitor was not associated with any statistically significant differences in any of the endpoints. However, the use of lesser eGFR declines did increase the number of events and lead to narrower confidence intervals, but we stress that without any observed treatment effect, an assessment of whether these endpoints attenuate treatment effect was not possible. The potential implications of using these alternative endpoints should be investigated further; but for now, their use should be determined with respect to the intervention in question and its hypothesized treatment effect.
Supplementary Material
Footnotes
Published online 19 March, 2019.
The trial analyzed in this secondary analysis is registered with International Standard Randomised Controlled Trial Number (ISRCTN) 78129473.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The original randomized trial secondarily analyzed in this article received funding from the Canadian Institutes of Health Research (grant MCT-78844).
The authors declare no conflicts of interest.
N.A.F., T.R., M.C., S.W.E., and G.A.K. were responsible for conceiving and designing the project. N.A.F. performed all statistical analyses with assistance and support from T.R. and M.C. N.A.F. drafted the article, whereas N.A.F., T.R., M.C., S.W.E., and G.A.K. contributed to and critically revised the final report.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Coresh J, Turin TC, Matsushita K, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311:2518–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clayton PA, Lim WH, Wong G, et al. Relationship between eGFR decline and hard outcomes after kidney transplants. J Am Soc Nephrol. 2016;27:3440–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inker LA, Lambers Heerspink HJ, Mondal H, et al. GFR decline as an alternative end point to kidney failure in clinical trials: a meta-analysis of treatment effects from 37 randomized trials. Am J Kidney Dis. 2014;64:848–859. [DOI] [PubMed] [Google Scholar]
- 4.Badve SV, Palmer SC, Hawley CM, et al. Glomerular filtration rate decline as a surrogate end point in kidney disease progression trials. Nephrol Dial Transplant. 2016;31:1425–1436. [DOI] [PubMed] [Google Scholar]
- 5.Connell PJ, Kuypers DR, Mannon RB, et al. Clinical trials for immunosuppression in transplantation: the case for reform and change in direction. Transplantation. 2017;101:1527–1534. [DOI] [PubMed] [Google Scholar]
- 6.Jun M, Turin TC, Woodward M, et al. Assessing the validity of surrogate outcomes for ESRD: a meta-analysis. J Am Soc Nephrol. 2015;26:2289–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambers Heerspink HJ, Weldegiorgis M, Inker LA, et al. Estimated GFR decline as a surrogate end point for kidney failure: a post hoc analysis from the reduction of end points in non-insulin-dependent diabetes with the Angiotensin II Antagonist Losartan (RENAAL) study and Irbesartan Diabetic Nephropathy Trial. Am J Kidney Dis. 2014;63:244–250. [DOI] [PubMed] [Google Scholar]
- 8.Knoll GA, Fergusson D, Chasse M, et al. Ramipril versus placebo in kidney transplant patients with proteinuria: a multicentre, double-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4:318–326. [DOI] [PubMed] [Google Scholar]
- 9.Knoll GA, Cantarovitch M, Cole E, et al. The Canadian ACE-inhibitor trial to improve renal outcomes and patient survival in kidney transplantation—study design. Nephrol Dial Transplant. 2008;23:354–358. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS, Coresh J, Greene T, et al. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


