Supplemental digital content is available in the text.
Abstract
Background
Human albumin/dextran (HA-D), bovine-gelatin (BG), and packed red blood cells plus plasma have been used in European and North-American clinical trials of normothermic ex situ liver perfusion (NEsLP). We compared the effects of these perfusates in a porcine model during NEsLP and after transplantation.
Methods
Porcine livers were retrieved 30 minutes after circulatory death. After 5 hours of NEsLP, grafts were transplanted. Three groups (n = 6) were assessed (HA-D vs BG vs whole blood [WB]). One group of static cold storage (SCS) was evaluated for comparison with the perfusion groups. Hemodynamic variables, liver and endothelial injury, and function were assessed during NEsLP and posttransplantation.
Results
Hepatic artery flow was higher since the beginning of NEsLP in the HA-D group (HA-D, 238 ± 90 mL/min vs BG, 97 ± 33 mL/min vs WB, 148 ± 49 mL/min; P = 0.01). Hyaluronic acid was lower in the HA-D at the end of perfusion (HA-D, 16.28 ± 7.59 ng/μL vs BG, 76.05 ± 15.30 ng/μL vs WB, 114 ± 46 ng/μL; P < 0.001). After transplant, aspartate aminotransferase was decreased in the HA-D group when compared with the rest of the groups (HA-D, 444 ± 226 IU/L vs BG, 1033 ± 694 IU/L vs WB, 616 ± 444 IU/L vs SCS, 2235 ± 1878 IU/L). At 5 hours after transplant, lactate was lower in the HA-D group (HA-D, 3.88 ± 1.49 mmol/L vs BG, 7.79 ± 2.68 mmol/L vs WB, 8.16 ± 3.86 mmol/L vs SCS, 9.06 ± 3.54 mmol/L; P = 0.04). International Normalized Ratio was improved in HA-D group compared to the rest of the groups (HA-D, 1.23 ± 0.30 vs BG, 1.63 ± 0.20 vs WB, 1.50 ± 0.31 vs SCS, 1.97 ± 1.55; P = 0.03) after transplantation. In contrast, BG displayed lower aspartate aminotransferase levels during NEsLP (HA-D, 183 ± 53 IU/L vs BG, 142 ± 52 IU/L vs WB, 285 ± 74 IU/L; P = 0.01) and less cleaved-caspase-3 staining (HA-D, 2.05 ± 0.73% vs BG, 0.95 ± 1.14% vs WB, 1.74 ± 0.54% vs SCS, 7.95 ± 2.38%) compared with the other groups. On the other hand, the bile from the WB showed higher pH (HA-D, 7.54 ± 0.11 vs BG, 7.34 ± 0.37 vs WB, 7.59 ± 0.18) and lower glucose levels (HA-D, 0.38 ± 0.75 mmol/L vs BG, 1.42 ± 1.75 mmol/L vs WB, 0 ± 0 mmol/L) by the end of perfusion.
Conclusions
Overall HA-D displayed more physiologic conditions during NEsLP that were reflected in less graft injury and improved liver function and survival after transplantation. Optimization of the perfusates based on the beneficial effects found with these different solutions would potentially improve further the outcomes through the use of NEsLP in marginal grafts.
Normothermic ex situ liver perfusion (NEsLP) has emerged as a novel strategy to assess marginal grafts and increase the organ pool. It has now moved from the bench to bedside and several European and North American human clinical trials have assessed the safety and feasibility of this technology.1–6
Over the past decade, grafts from donation after circulatory death (DCD) have become an alternative to neurologic declared death donation to increase the donor pool and reduce the mortality on the waiting list. However, utilization of DCD grafts remains limited due to higher incidence of complications, such as primary nonfunction (PNF) and biliary complications. Studies have shown that DCD grafts are prone to endothelial, hepatocyte, and biliary injury due to the increase of warm ischemia (WI).7,8 Additionally, prior research has shown that DCD livers exposed to prolonged static cold storage (SCS) times increase the risk of hepatic injury and early allograft dysfunction.9,10 Normothermic ex situ liver perfusion offers the opportunity to improve outcomes of liver transplantation with DCD grafts by minimizing the SCS times. Current practices by our institution (clinical trial: NCT02478151, REB ID: 14-8132), and other centers are now focused on NEsLP in DCD grafts with prolonged WI time to extend the donor pool with livers that are currently declined for transplantation.11–13 Hence, the optimal perfusate solution characteristics for the NEsLP of the DCD grafts need to be determined.
Gelofusine, Steen, and packed red blood cells (PRBCs) plus plasma are the solutions that have been used in the published NEsLP trials. Gelofusine is a solution made of bovine-derived gelatin (non–FDA-approved), which contains succinylated gelatin as the source of oncotic pressure. Gelofusine is used in Europe as a plasma expander in trauma and shock situations. Gelofusine provides physiologic concentration of Na+ and Cl−, with low concentrations of K+ and Ca++. It has an important effect on increasing intravascular volume and oxygen delivery but also at decreasing clot formation.14-17 On the other side, Steen solution has physiologic levels of Na+, Cl−, and K+. It also contains human albumin and dextran (D)-40, both characterized for expanding the intravascular volume.15 In addition, these agents decrease the oxidative response by scavenger effect on hydroxyl, superoxide, hydrogen peroxide, and peroxynitrite.18–20 The solution has also shown a decrease in the excessive platelet/leucocyte interaction with the endothelium.21 Steen as perfusate had previously been tested in pig liver transplant studies and has been extensively used in normothermic ex vivo lung perfusion.22,23 Steen is extensively used in clinical trials for ex vivo lung preservation, demonstrating promising results,24–26 as well as in the first North American human trial of NEsLP.3 Gelofusine in contrast has been used for the majority of NEsLP in European clinical studies.5 In addition, PRBCs plus fresh frozen plasma (FFP) have been used as perfusate in a single-center clinical trial with a homemade NEsLP machine.6
In the first clinical trials in Europe and North America, the 3 perfusate solutions have been used with good outcomes. However, the results of the initial clinical studies have to be taken with caution. These clinical trials were designed for safety and feasibility, and it is possible that different effects of the perfusion solutions might become apparent only in higher-risk grafts. In addition, a direct comparison of the 3 solutions in a transplant model is required to determine their differences in regard to outcomes during NEsLP and after transplantation. This question is best addressed in an animal model when all other factors (eg, donor age, degree of injury, etc) can be kept equal, minimizing background noise.
The objective of this study is to compare Gelofusine, Steen, and whole blood (WB) during NEsLP and their impact on hepatic injury and function during NEsLP and after transplantation. This study is focused on comparing perfusion solutions for NEsLP that have been used in published clinical trials.
Other transplantation groups are currently using albumin-, Gelofusine-, and PRBCs/FFP-based perfusates in preclinical and clinical studies with Liver Assist, OCS, and homemade devices.6,11,13,27–29 Therefore, the data from this study could also contribute with some evidence on which solution and what adjustments will be necessary to make the solution more suitable for their perfusion systems.
MATERIALS AND METHODS
Study Design
A bovine-derived gelatin-based solution (n = 6) (Gelofusine; B. Braun, Melsungen, Germany) plus pig washed red blood cells was compared with a human albumin (HA)/D-based solution (n = 6) (Steen; XVIVO, Denver, CO) plus pig washed red blood cells and WB (n = 6) as the perfusate for NEsLP machine. Porcine livers were retrieved after 30 minutes of WI (30min-WI) time. The 3 groups of livers were exposed to 2 hours of SCS while back table surgery, placing of cannulas, and blood preparation for perfusion were performed. After SCS, livers were perfused for 5 hours in a normothermic (37°C) setup of ex situ machine perfusion. Perfused livers were transplanted into recipient pigs with a follow-up time of 3 days. Perfusion setup in the 2 groups was similar to the recent human clinical trials.2–4 The only modification between the 3 groups was the use of a different perfusate solution (Steen, Gelofusine, or WB). An additional group of SCS (n = 6) was included in the study for comparison with the NEsLP groups. The total preservation time for the 4 groups was 7 hours.
Animals
Yorkshire pigs weighing 30 to 34 kg were used for this study. The Animal Care Committee of Toronto General Hospital approved the experiments. Animals received humane care in observance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care of Laboratory Animals” published by the National Institute of Health.
Colloid Solutions for Machine Perfusion
Two colloid perfusion solutions were compared, Gelofusine was used as the bovine-gelatin solution, and Steen was used as the HA-D solution. Several characteristics differ between solutions. Gelofusine is a 4% solution of succinylated-gelatin with a molecular weight of 30 000 Da. Its described theoretical osmolarity and pH are 274 mOsm/L and 7.1 to 7.7 mOsm/L, respectively. Gelofusine is described with a colloid-osmotic pressure of 33 mm Hg. The solution contains 154 and 120 mmol/L of sodium and chloride, respectively. There is no potassium, calcium, or magnesium content in this solution.30 On the other hand, Steen is a solution based in human albumin (64-70 g/L) and D-40 (5 g/L) with a molecular weight of 69 000 and 50 000, respectively. Its described theoretical osmolarity and pH are 275 to 315 mOsm/L and 7.1-7.9 mOsm/L, respectively. Described colloid-osmotic pressure is 25 mm Hg. The solution additionally contains sodium chloride (86 mmol/L), potassium chloride (4.6 mmol/L), calcium chloride dehydrate (1.5 mmol/L), sodium dihydrogen phosphate dehydrate (1.2 mmol/L), sodium bicarbonate (15 mmol/L), magnesium dichloride hexahydrate (1.2 mmol/L), and D(+) glucose monohydrate (11 mmol/L). Components are dissolved in water, and pH is adjusted to physiologic values with sodium hydroxide.31
Donor Surgery, Normothermic Ex Situ Liver Perfusion and Recipient Surgery
The animals were first randomized into the treatment groups. Subsequently, a DCD model with 30minutes-WI was used to simulate the current clinical practice in most of the centers. The NEsLP setup was established to resemble the system used in the human clinical trials.2,3 Liver weight was determined at 2 timepoints, after the retrieval cold flush and following the cold flush after NEsLP. The change in liver weight was determined from the differences between these 2 timepoints. Livers were then transplanted into recipient pigs with the use of a venovenous portojugular bypass shunt during the anhepatic phase as previously described.32 Liver biopsies were performed 2 hours after the PV was reperfused and at the time of euthanasia. The donor surgery, machine setup, circuit priming, posttransplant care, and biopsy processing were performed as described in Supplementary methods (http://links.lww.com/TXD/A191).
Colloid Solutions and Perfusate Characteristics
For the purpose of this study, we referred to the bottled solution as “colloid solution.” We reserved the word “perfusate” to the fluid that flows through the liver after complete circuit priming (WB or PRBCs, colloid solution, bicarbonate, etc). Electrolytes, pH, glucose, albumin, blood urea nitrogen (BUN), colloid-osmotic pressure, osmolarity, viscosity, and density were determined from both colloid solutions directly from the fluid bottle. Baseline perfusate biochemistry was determined 5 minutes after the liver was placed on pump. After baseline values were determined, corrections were made in the 3 groups to reach same acid-base levels as currently performed in the clinical practice. Detailed description of methods can be found in the Supplementary methods (http://links.lww.com/TXD/A191).
Liver Assessment and Bile Composition Evaluation
Liver enzymes were determined by using Piccolo Xpress machine. Serum aspartate aminotransferase (AST) levels were measured as marker of hepatic injury at hours 0, 2, and 4 during perfusion. Additionally, during the posttransplant follow-up AST, total bilirubin (TBil) and alkaline phosphatase (ALP) were assessed 3 and 5 hours after reperfusion and every 24 hours starting on the first postoperative day (POD). Bile characteristics (pH, volume, Na+, and HCO3− content) and bile/perfusate Na+, K+, Ca++ ratio were evaluated at BL, 1, 2, 3, 4, and 5 hours after the liver was placed on pump. Lactate clearance was assessed hourly during NEsLP and after transplantation. International Normalized Ratio (INR) was measured after transplantation. Detailed description can be found in the Supplementary methods (http://links.lww.com/TXD/A191).
Hyaluronic Acid Levels
Hyaluronic acid levels were determined by enzyme-linked immunosorbent assay during NEsLP and at 2 hours after transplantation (quantitative sandwich enzyme immunoassay technique; R&D Systems, Minneapolis, MN) as an indirect marker of endothelial cell (EC) function. Lower levels indicating a better EC function.33
Statistical Analysis
Data were first evaluated for normal distribution. Parametric tests were used to analyze the data with normal distribution. One-way analysis of variance (ANOVA) was used for ordinal data between the 4 different groups. Student t test was used when ordinal variable was analyzed on an individual timepoint between the 2 groups. Data are presented as mean ± SD. Statistical tests were performed by using SPSS 22 statistical package (IBM, Chicago, IL).
RESULTS
Normothermic Ex Situ Liver Perfusion
(a) Colloid Solution Composition
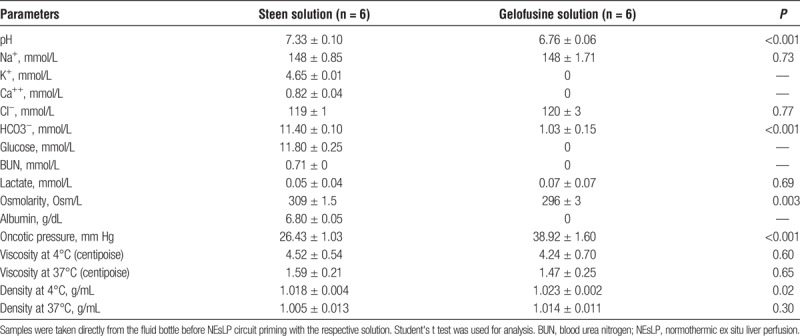
The analysis of solutions composition showed a significantly more acidotic pH in Gelofusine group (Steen, 7.33 ± 0.10 vs Gelofusine, 6.76 ± 0.06; P < 0.001). No potassium, calcium, glucose, or BUN was detected in the Gelofusine solution. In addition, significantly lower levels of bicarbonate were found in the Gelofusine group (Steen, 11.4 ± 0.10 mmol/L vs Gelofusine, 1.03 ± 0.15 mmol/L; P < 0.001). Although both solutions showed hypertonic osmolarity, Steen solution osmolarity was significantly higher when compared with Gelofusine (Steen, 309 ± 1.5 vs Gelofusine, 296 ± 3; P = 0.003). In contrast, oncotic pressure was significantly higher in Gelofusine group (Steen, 26.43 ± 1.03 mm Hg vs Gelofusine, 38.92 ± 1.60 mm Hg; P < 0.001). Of interest, viscosity showed similar values in both groups at 4°C (Steen, 4.52 ± 0.53 cP vs Gelofusine, 4.24 ± 0.07 cP; P = 0.60) and 37°C (Steen, 1.59 ± 0.21 cP vs Gelofusine, 1.47 ± 0.25 cP; P = 0.70). Density determinations showed lower values in the Steen group at 4°C (Steen, 1.018 ± 0.004 g/cm3 vs Gelofusine, 1.0238 ± 0.002 g/cm3; P = 0.02) and 37°C (Steen, 1.005 ± 0.013 g/cm3 vs Gelofusine, 1.014 ± 0.011 g/cm3; P = 0.30) (Table 1).
TABLE 1.
Blood gas analysis, chemistry analysis, osmolarity, oncotic pressure, viscosity, and density from colloid solutions
(b) Perfusate Characteristics at the Beginning of the NEsLP
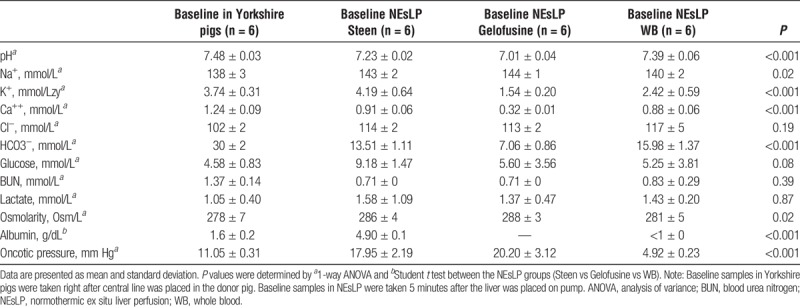
The characteristics of the 3 perfusates (Steen, Gelofusine, and WB) were determined at the start of the NEsLP. The pH of the perfusate from Steen and Gelofusine groups was significantly more acidotic than the WB group (Steen, 7.23 ± 0.02 vs Gelofusine, 7.01 ± 0.04 vs WB, 7.39 ± 0.06; P < 0.001). Although sodium levels in the perfusate were physiologic for all the groups, potassium was within normal range only for the Steen group and significantly higher compared with the Gelofusine and WB groups (Table 2). Corrections for pH and potassium levels were made by adding potassium bicarbonate to allow similar levels for all the groups in pH (Steen, 7.50 ± 0.06 vs Gelofusine, 7.43 ± 0.06 vs WB, 7.47 ± 0.03) and potassium levels (Steen, 3.27 ± 1.36 mmol/L vs Gelofusine, 3.36 ± 0.24 mmol/L vs WB, 3.78 ± 0.93 mmol/L) by the end of perfusion (Supplementary Table 1, http://links.lww.com/TXD/A192). Oncotic pressure analysis showed comparable values in Steen group when compared to Gelofusine (Steen, 17.95 ± 2.19 mm Hg vs Gelofusine, 20.20 ± 3.12 mm Hg; P = 0.24). In contrast, Steen and Gelofusine groups demonstrated significantly higher oncotic pressure when compared with WB group (Table 2).
TABLE 2.
Blood gas analysis, chemistry analysis, osmolarity, and oncotic pressure measured at baseline in serum of Yorkshire pigs and at the start of NEsLP from the circuit perfusate
(c) Hemodynamic Parameters During NEsLP
Overall HA flow was higher throughout the perfusion in the livers that were perfused with Steen. The difference was significantly larger at the start of perfusion (Steen, 238 ± 90 mL/min vs Gelofusine, 97 ± 32 mL/min vs WB, 148 ± 49 mL/min; P = 0.01) and was maintained to the end of perfusion (Steen, 270 ± 110 mL/min vs Gelofusine, 183 ± 33 mL/min vs WB, 188 ± 42; P = 0.12) (Figure 1). Intraarterial resistance was significantly higher in the Gelofusine group when compared with the other groups at the beginning of perfusion (Steen, 0.23 ± 0.05 mm Hg·mL−1·min−1 vs Gelofusine, 0.52 ± 0.18 mm Hg/mL−1·min−1 vs WB, 0.32 ± 0.10; P = 0.01) but not at later timepoints (Figure 1). The PV flow was at physiological levels in the 3 groups since the start of perfusion (Figure 1).
FIGURE 1.
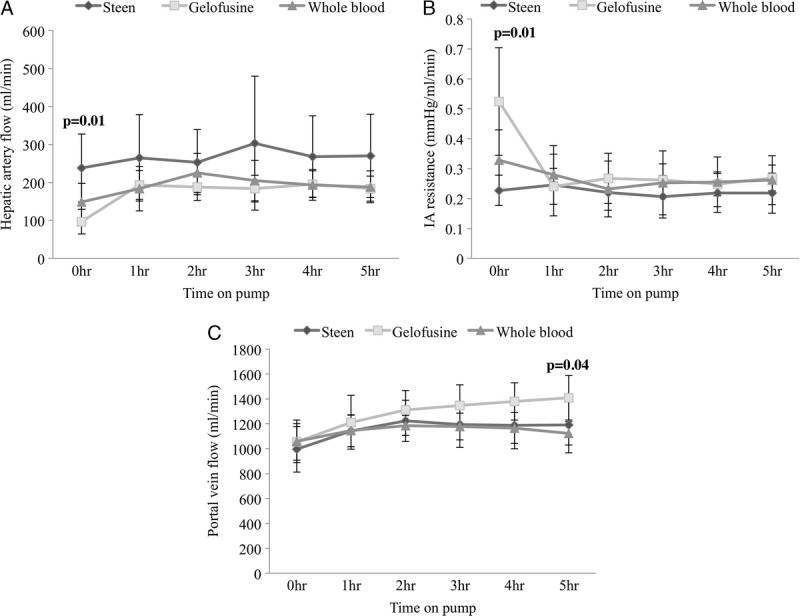
Hemodynamic parameters during perfusion. HA flows were higher during the entire perfusion with significant difference found at the start of perfusion in the Steen vs Gelofusine vs WB group (238 ± 90 mL/min vs 97 ± 33 mL/min vs 148 ± 49 mL/min; P = 0.01) (A). Intraarterial resistance significantly increased at the beginning of the perfusion in the Gelofusine group compared with the rest of the groups (Steen, 0.23 ± 0.05 mm Hg·mL−1·min−1 vs Gelofusine, 0.52 ± 0.18 mm Hg·mL−1·min−1 vs WB, 0.32 ± 0.10 mm Hg·mL−1·min−1; P = 0.01) (B). PV flow was at physiological levels in the 3 groups since the start of the NEsLP (C). Results are expressed as mean ± SD. Six experiments were performed per group (n = 6). One-way ANOVA was used for analysis (A, B, C). ANOVA, analysis of variance; HA, human albumin; NEsLP, normothermic ex situ liver perfusion; PV, portal vein; SD, standard deviation; WB, whole blood.
(d) Hepatocyte and EC Injury and Function During NEsLP
Perfusate levels of AST were significantly higher in the WB group when compared with Steen and Gelofusine groups (Figure 2). Endothelial cell function was assessed by hyaluronic acid clearance with lower values indicating a better EC function. Hyaluronic acid levels were significantly lower in the Steen livers when compared with Gelofusine and WB livers at 1 hour (Steen, 3.09 ± 1.48 ng/μL vs Gelofusine, 32.63 ± 3.55 ng/μL vs WB, 18.89 ± 13.48 ng/μL; P < 0.001) and 5 hours of perfusion (Steen, 16.28 ± 7.59 ng/μL vs Gelofusine, 76.05 ± 15.30 ng/μL vs WB, 113.62 ± 46.43 ng/μL; P < 0.001) (Figure 2), indicating better EC function in the Steen group compared with Gelofusine and WB groups.
FIGURE 2.
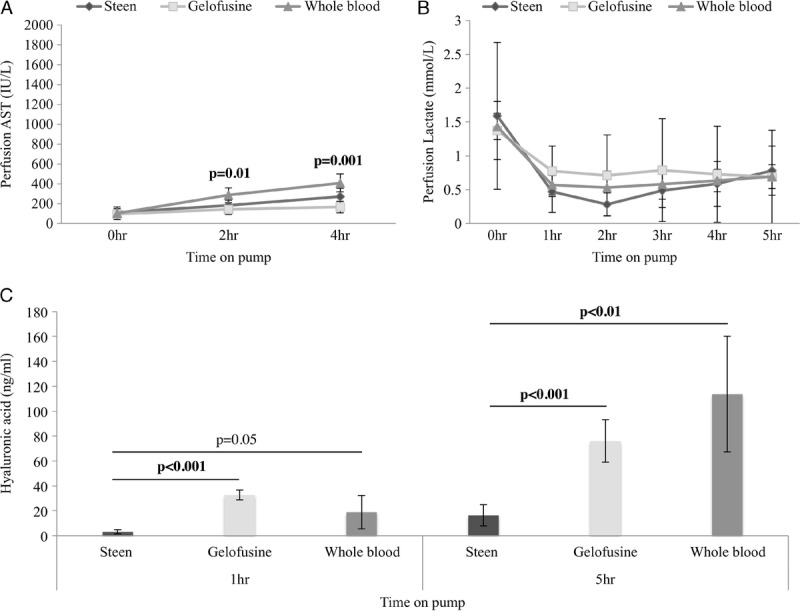
Hepatocytes and ECs injury and function during NEsLP. AST showed significantly lower levels in the Gelofusine group compared to the rest of the groups (Steen, 183 ± 53 vs Gelofusine, 142 ± 52 vs WB, 285 ± 74; P = 0.01) (A). Lactate clearance was similar for the 3 groups at all timepoints (B). Hyaluronic acid was significantly lower in Steen vs Gelofusine vs WB group at 1 hour (3.09 ± 1.48 ng/μL vs 32.63 ± 3.55 ng/μL vs 18.89 ± 13.48 ng/μL; P < 0.001) and 5 hours (16.28 ± 7.59 ng/μL vs 76.05 ± 15.30 ng/μL vs 113.62 ± 46.43 ng/μL; P < 0.001) of perfusion. Results are expressed as mean ± SD. Six experiments were performed per group (n = 6). One-way ANOVA (A, B) and Student t test (C) were used for analysis.ANOVA, analysis of variance; AST, aspartate aminotransferase; EC, endothelial cell; NEsLP, normothermic ex situ liver perfusion; SD, standard deviation; WB, whole blood.
(e) Lactate Clearance During NEsLP
Lactate clearance, as a parameter of liver function, was similar for all the groups, reaching normal levels by the end of the perfusion in all the groups (Steen, 0.78 ± 0.36 mmol/L vs Gelofusine, 0.69 ± 0.69 mmol/L vs WB, 0.69 ± 0.17 mmol/L; P = 0.95) (Figure 2).
(f) Oxygen Consumption During NEsLP
Oxygen consumption (OC) was evaluated as a parameter of aerobic liver metabolism, and no difference was observed between the 3 groups. Oxygen consumption was stable since the 1 hour of perfusion with similar rate in all the groups (Steen, 25 ± 4 mL·min−1·kg−1 vs Gelofusine, 24 ± 4 mL·min−1·kg−1 vs WB, 20 ± 11 mL·min−1·kg−1; P = 0.20) (Figure 3).
FIGURE 3.
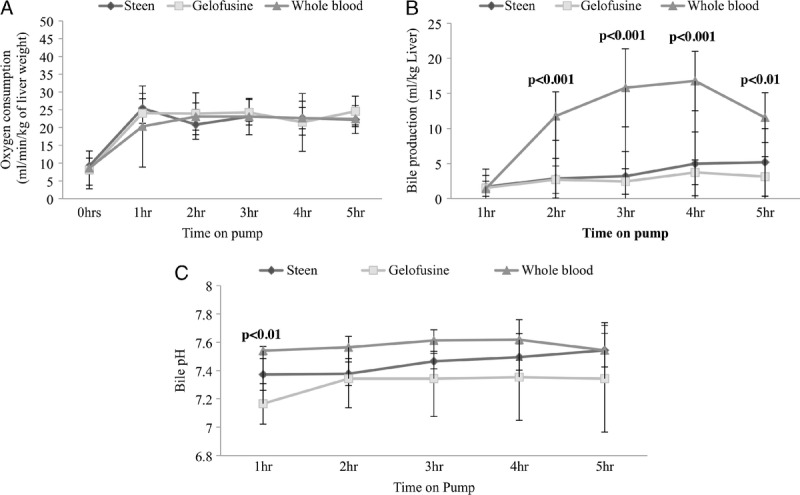
OC and bile production during NEsLP. OC was stable since the 1 hour of perfusion without differences between the groups (A). Bile production was significantly higher in the WB group since 2 hours of NEsLP (B). Significantly higher bile pH was found in the WB at 1 hour of perfusion compared to the rest of the groups (Steen, 7.37 ± 0.13 vs Gelofusine, 7.16 ± 0.12 vs WB, 7.49 ± 0.05; P < 0.01) (C). Results are expressed as mean ± SD. Six experiments were performed per group (n = 6). One-way ANOVA was used for all the analyses (A, B, C). ANOVA, analysis of variance; NEsLP, normothermic ex situ liver perfusion; OC, oxygen consumption; SD, standard deviation; WB, whole blood.
(g) Bile Production and Composition During NEsLP
Bile production was significantly higher in the WB group when compared with Steen and Gelofusine groups since the 2-hour NEsLP (Figure 3). Acidotic bile during NEsLP has been suggested as a potential predictor marker of biliary injury after DCD liver transplantation.13 Bile pH was significantly higher in the WB group when compared with the other groups at 1 hour of perfusion (Steen, 7.37 ± 0.13 vs Gelofusine, 7.16 ± 0.12 vs WB, 7.49 ± 0.05; P < 0.01) (Figure 3). This finding correlated with higher levels of HCO3− in the WB group compared with Steen and Gelofusine groups at 1 hour (Steen, 10.04 ± 1.21 mmol/L vs Gelofusine, 7.15 ± 2.22 mmol/L vs WB, 12.50 ± 2.57 mmol/L; P = 0.02), 2 hours (Steen, 10.70 ± 4.30 mmol/L vs Gelofusine, 9.20 ± 4.36 mmol/L vs WB, 13.88 ± 2.03 mmol/L; P = 0.04), 3 hours (Steen, 9.84 ± 1.51 mmol/L vs Gelofusine, 10.18 ± 5.88 mmol/L vs WB, 17.10 ± 3.96 mmol/L; P < 0.01), 4 hours (Steen, 13.08 ± 4.60 mmol/L vs Gelofusine, 10.08 ± 5.44 mmol/L vs WB, 19.93 ± 6.77 mmol/L; P = 0.02), and 5 hours (Steen, 14.43 ± 4.05 mmol/L vs Gelofusine, 10.32 ± 6.06 mmol/L vs WB, 17.15 ± 7.13 mmol/L; P = 0.16) of perfusion (Figure 4). Of note, all the animals in the NEsLP groups had preserved bile duct structure and epithelium integrity at the end of the follow-up.
FIGURE 4.
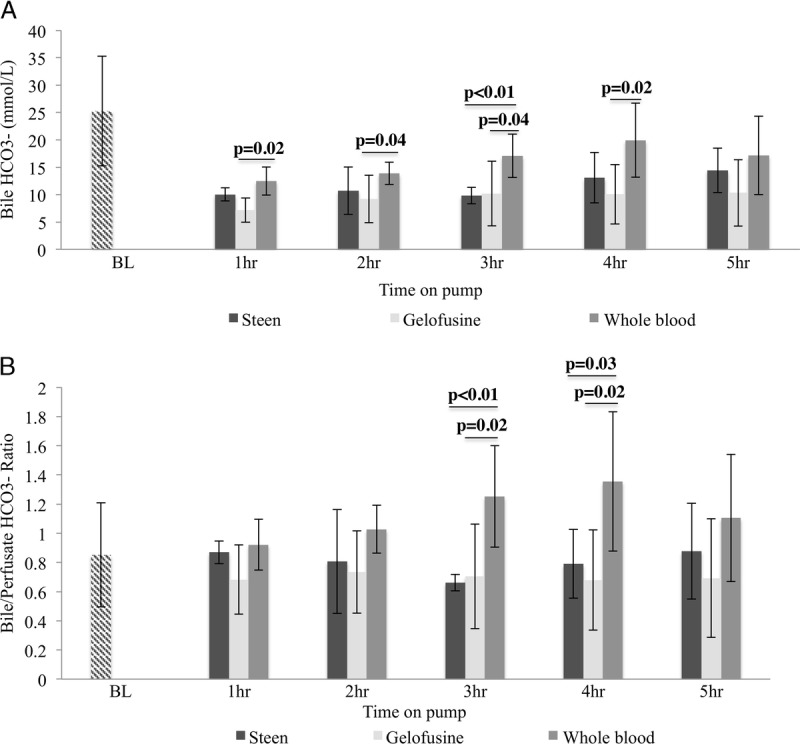
Bile bicarbonate concentration and bile/serum ratio. Bile HCO3- concentration showed significantly higher values in the WB group for the majority of the timepoints (A). WB group showed also higher bile/perfusate HCO3- ratio when compared to Steen and Gelofusine groups (B). Results are expressed as mean ± SD. Six experiments were performed per group (n = 6). Student t test was used for analysis (A, B). Note: BL bile and serum samples represent samples taken directly from the bile duct and central line of donor pigs before any intervention, respectively.SD, standard deviation; WB, whole blood.
Bile sodium content was determined during NEsLP. Analysis showed that Na+ concentration was higher in the bile of livers perfused with Steen in contrast to those perfused with Gelofusine and WB at 2 hours (Steen, 179 ± 16 mmol/L vs Gelofusine, 161 ± 4 mmol/L vs WB, 156 ± 4 mmol/L; P = 0.03), 3 hours (Steen, 177 ± 14 mmol/L vs Gelofusine, 164 ± 5 mmol/L vs WB, 153 ± 4 mmol/L; P < 0.01), 4 hours (Steen, 174 ± 15 mmol/L vs Gelofusine, 167 ± 6 mmol/L vs WB, 150 ± 3 mmol/L; P = 0.001), and 5 hours (Steen, 175 ± 17 mmol/L vs Gelofusine, 162 ± 6 mmol/L vs WB, 149 ± 2 mmol/L; P = 0.001) (Figure 5). The bile/perfusate Na+-ratio also showed higher values in the Steen group during perfusion when compared with the other groups at 2 hours (Steen, 1.22 ± 0.16 vs Gelofusine, 1.12 ± 0.03 vs WB, 1.10 ± 0.02; P = 0.33), 3 hours (Steen, 1.21 ± 0.08 vs Gelofusine, 1.14 ± 0.04 vs WB, 1.08 ± 0.01; P = 0.03), 4 hours (Steen, 1.19 ± 0.08 vs Gelofusine, 1.15 ± 0.04 vs WB, 1.06 ± 01; P < 0.01), and 5 hours (Steen, 1.18 ± 0.21 vs Gelofusine, 1.13 ± 0.04 vs WB, 1.06 ± 0.01; P < 0.01) (Figure 5). Similarly, bile/perfusate Ca++ ratio was also higher in the Steen group, although the difference did not reach significance (Figure 5). Overall bile electrolyte concentration in the Steen group was more similar to the samples obtained from untreated (BL) animals than Gelofusine and WB groups. Of interest, the Steen group showed a trend to significantly lower LDH levels (Steen, 562 ± 490 IU/L vs Gelofusine, 905 ± 450 IU/L vs WB, 694 ± 435 IU/L; P = 0.30) and gamma-glutamyl transferase (Steen, 292 ± 111 IU/L vs Gelofusine, 576 ± 294 IU/L vs WB, 934 ± 552 IU/L; P = 0.07) at 1 hour after the liver was placed on pump. In addition, all the groups showed a trend to a progressive decrease in the levels of bile glucose during NEsLP. Although not significant, the WB showed lower bile glucose concentration at all the timepoints (Figure 6).
FIGURE 5.
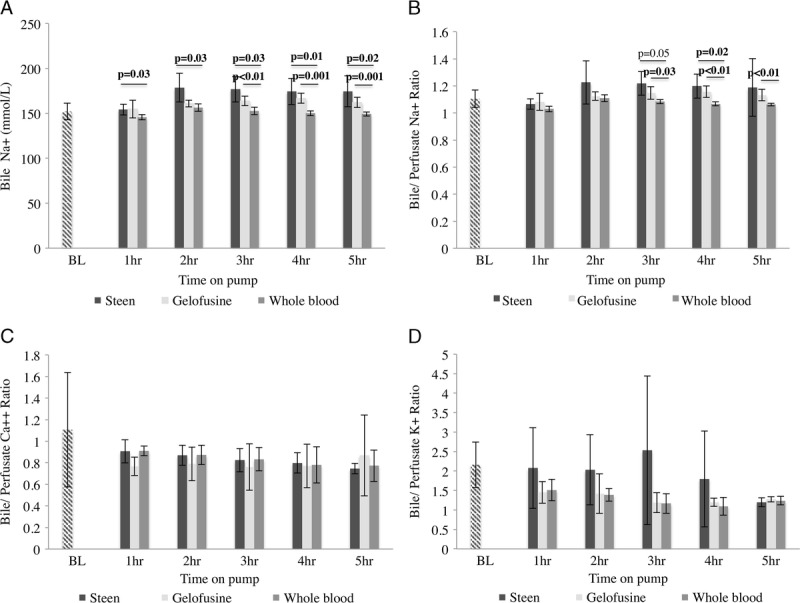
Bile electrolytes. Bile analysis showed higher Na+ concentration in Steen group since the 2 hours of perfusion compared with Gelofusine and WB group (A). The bile/perfusate Na+, Ca++ and K+ ratios also showed higher values in the Steen group compared with Gelofusine and WB groups at the majority of the timepoints (B, C, D). Results are expressed as mean ± SD. Six experiments were performed per group (n = 6). Student t test was used to perform all the analyses. Note: BL bile and serum samples represent samples taken directly from the bile duct and central line of donor pigs before any intervention, respectively; SD, standard deviation; WB, whole blood.
FIGURE 6.
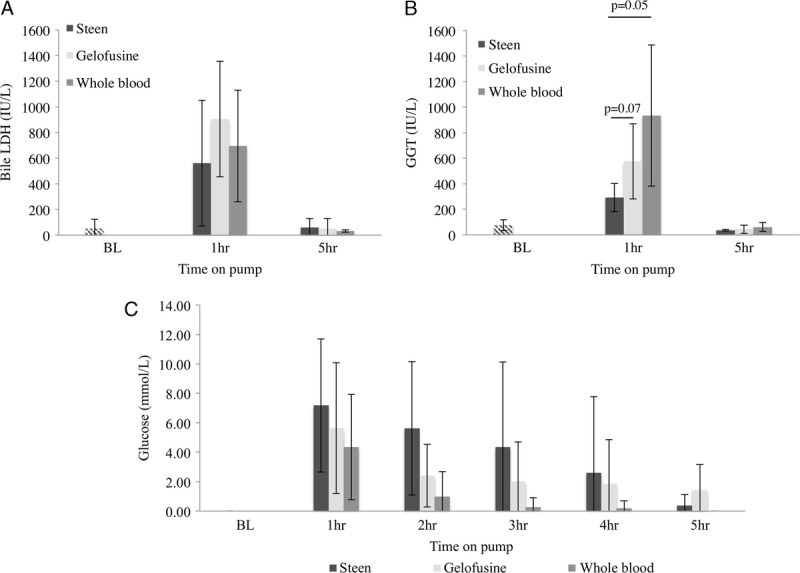
Bile markers of cellular injury. Analysis of LDH and gamma-glutamyl transferase showed a decrease in the levels of these markers by the end of the perfusion in all the groups. Steen vs Gelofusine vs WB group showed a trend to significantly lower levels of LDH (562 ± 490 IU/L vs 905 ± 450 IU/L vs 694 ± 435 IU/L; P = 0.30) and gamma-glutamyl transferase (292 ± 111 IU/L vs 576 ± 294 IU/L vs 934 ± 552 IU/L; P = 0.07) at 1 hour after the liver was placed on pump (A, B). The bile glucose levels were lower in the WB group compared to Steen and Gelofusine groups at all timepoints without reaching significant difference (C). Results are expressed as mean ± SD. Six experiments were performed per group (n = 6). Student t test was used for analysis. Note: BL bile samples represent pig serum samples taken directly from the bile duct of donor pigs before any intervention; SD, standard deviation; WB, whole blood.
(h) Change in Liver Weight
Weight of the livers after the retrieval cold flush was similar in all the groups (Steen, 818 ± 73 g vs Gelofusine, 867 ± 63 g vs WB, 827 ± 83 g; P = 0.47). Interestingly, there was a lower percentage of weight gain in the Steen and WB groups compared with the Gelofusine group after NEsLP (Steen, 4.62 ± 5.75%, Gelofusine, 14.73 ± 5.95%, WB, 5.73 ± 7.58%; P = 0.01) (Figure 7).
FIGURE 7.
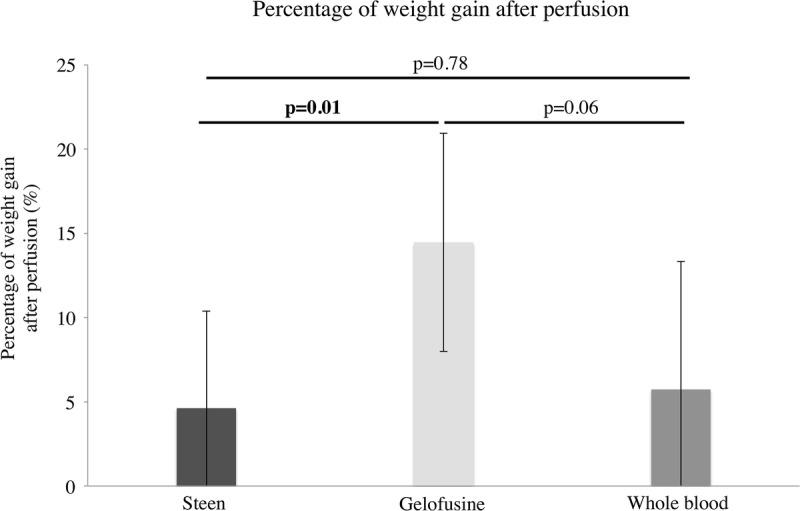
Percentage of gain in liver weight at the end of perfusion. Analysis of liver weight before and after NEsLP demonstrated a significantly lower percentage of weight gain in the grafts perfused with Steen and WB groups compared with the Gelofusine group (Steen, 4.62 ± 5.75%, Gelofusine, 14.73 ± 5.95%, WB, 5.73 ± 7.58%; P = 0.01). Results are expressed as mean ± SD. Six experiments were performed per group (n = 6). Student t test was used for analysis.NEsLP, normothermic ex situ liver perfusion; SD, standard deviation; WB, whole blood.
Outcome After Liver Transplantation
(a) Hepatic and Bile Duct Injury After Liver Transplantation
After transplantation, the Steen group showed persistently lower AST levels compared with the other groups at day 1 (Steen, 1250 ± 742 IU/L vs Gelofusine, 1957 ± 1188 IU/L vs WB, 1425 ± 9571 IU/L vs SCS, 2097 ± 1121 IU/L; P = 0.48), day 2 (Steen, 444 ± 226 IU/L vs Gelofusine, 1033 ± 694 IU/L vs WB, 616 ± 444 IU/L vs SCS, 2235 ± 1878 IU/L; P = 0.05), and day 3 (Steen, 180 ± 89 IU/L vs Gelofusine, 608 ± 554 IU/L vs WB, 219 ± 145 IU/L vs SCS, 652 ± 549 IU/L; P = 0.15) (Figure 8). Serum ALP and TBil levels after transplantation were also decreased in the Steen group compared with Gelofusine, WB, and SCS groups (Figure 8). Significantly lower percentage of cleaved-caspase-3 positive staining at 2 hours after reperfusion was found in the Steen, Gelofusine, and WB groups compared with SCS (Steen, 2.05 ± 0.73% vs Gelofusine, 0.95 ± 1.14% vs WB, 1.74 ± 0.54% vs SCS, 7.95 ± 2.38%; P < 0.001) (Figure 8). Of note, hematoxylin and eosin analysis demonstrated a significantly lower percentage of necrosis at POD3 in the Steen group compared with Gelofusine (Steen, 12.5 ± 6% vs Gelofusine, 20 ± 4.5%; P = 0.03) and SCS group (Steen, 12.5 ± 6% vs SCS, 25 ± 5%; P < 0.01) (Figure 9). Bile duct structure and epithelium integrity was completely preserved in all the animals in the Steen, Gelofusine, and WB group at the time of sacrifice. In contrast, 2 of 6 animals from the SCS showed alternated areas with loss of bile duct epithelium and structure (Figure 9).
FIGURE 8.
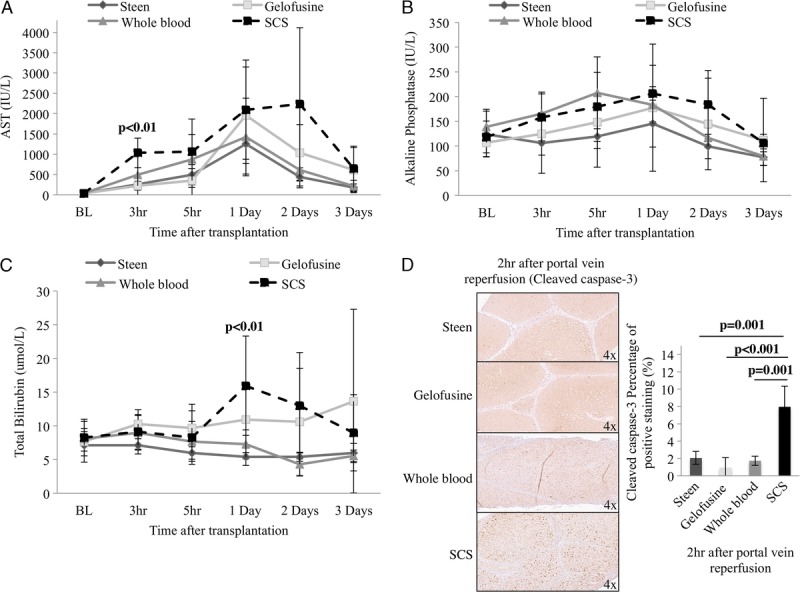
Hepatic injury and apoptosis after transplantation. After transplantation Steen group showed lower AST levels compared with Gelofusine, WB, and SCS groups (day 1, 1250 ± 742 IU/L vs 1957 ± 1188 IU/L vs 1425 ± 9571 IU/L vs 2097 ± 1121 IU/L; P = 0.48), (day 2, 444 ± 226 IU/L vs 1033 ± 694 IU/L vs 616 ± 444 IU/L vs 2235 ± 1878 IU/L; P = 0.05) (day 3, 180 ± 89 IU/L vs 608 ± 554 IU/L vs 219 ± 145 IU/L vs 652 ± 549 IU/L; P = 0.15) (A). Lower levels of ALP and TBil were also found in Steen group compared with Gelofusine, WB, and SCS groups (B, C). Cleaved caspase-3 analysis showed significantly lower levels of positive staining in Gelofusine, Steen, and WB groups compared with SCS group (P < 0.001) (D). Results are expressed as mean ± SD. Six experiments were performed per group (n = 6). One way ANOVA (A, B, C) and Student t test were used for statistical analysis (D). ALP, alkaline phosphatase; ANOVA, analysis of variance; AST, aspartate aminotransferase; SCS, static cold storage; SD, standard deviation; TBil, total bilirubin; WB, whole blood.
FIGURE 9.
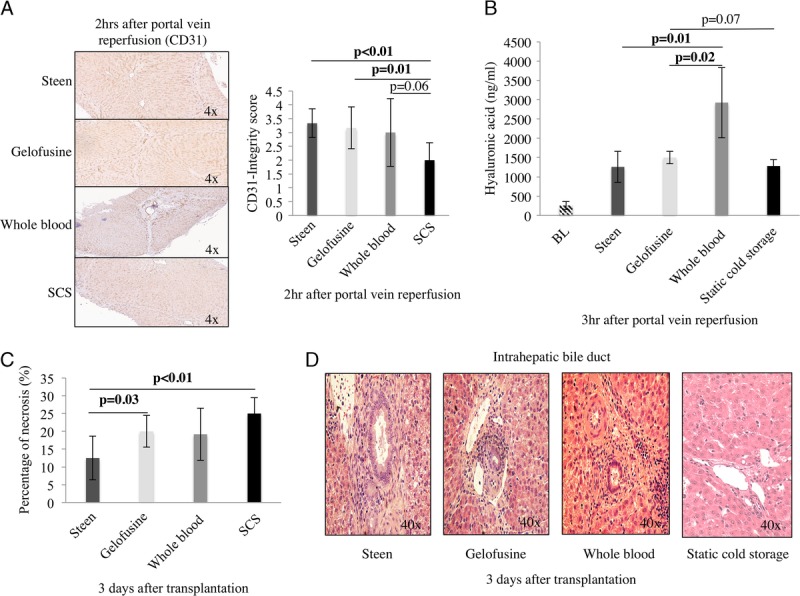
Hepatic necrosis and EC integrity after transplantation. CD31 integrity score showed higher score in the Steen vs Gelofusine vs WB groups compared with SCS group (3.33 ± 0.51 vs 3.16 ± 0.75 vs 3.16 ± 1.16 vs 2 ± 0.63; P < 0.01) (A). Hyaluronic acid levels were significantly lower in the Steen, Gelofusine, and SCS groups when compared with the WB group (1257 ± 358 ng/μL vs 1501 ± 143 ng/μL vs 1280 ± 151 ng/μL vs 2925 ± 912 ng/μL; P = 0.01) (B). Of note, hematoxylin and eosin analysis demonstrated a significantly lower percentage of necrosis at POD3 in Steen group compared with Gelofusine (12.5 ± 6% vs 20 ± 4.5%; P = 0.03) and SCS group (12.5 ± 6% vs 25 ± 5%; P < 0.01) (C). All the animals in the perfusion groups showed preserved bile duct structure and epithelium (Steen vs Gelofusine vs WB). In contrast, 2 of 6 animals showed alternated areas of epithelium loss and distorted structure in the bile duct (D). Six experiments were performed per group (n = 6). Student t test was used for all the analyses. EC, endothelial cell; SCS, static cold storage; WB, whole blood.
(b) Endothelial Integrity and Function After Liver Transplantation
Surface marker-CD31 was used to evaluate the integrity of ECs 2 hours postreperfusion. CD31 integrity score was improved in the Steen, Gelofusine, and WB groups compared with the SCS group (Steen, 3.33 ± 0.51 vs Gelofusine, 3.16 ± 0.75 vs WB, 3.16 ± 1.16 vs SCS, 2 ± 0.63; P < 0.01) (Figure 9). Hyaluronic acid clearance, as a marker of EC function, was determined at the same timepoint. Hyaluronic acid levels were significantly lower in the Steen, Gelofusine, and SCS groups when compared with the WB group (Steen, 1257 ± 358 ng/μL vs Gelofusine, 1501 ± 143 ng/μL vs WB, 2925 ± 912 ng/μL vs SCS, 1280 ± 151 ng/μL; P = 0.01) (Figure 9).
(c) Liver Function After Transplantation
International Normalized Ratio posttransplantation was determined as a marker of hepatocyte function. The INR values were significantly higher in the WB group when compared with the other groups at 3 hours after portal vein (PV) reperfusion (Steen, 1.60 ± 0.26 vs Gelofusine, 1.65 ± 0.16 vs WB, 1.98 ± 0.47 vs SCS, 1.49 ± 0.26; P = 0.07). Of interest, Steen group persistently showed lower INR after transplantation, which correlated with the 100% survival in this group (Figure 10).
FIGURE 10.
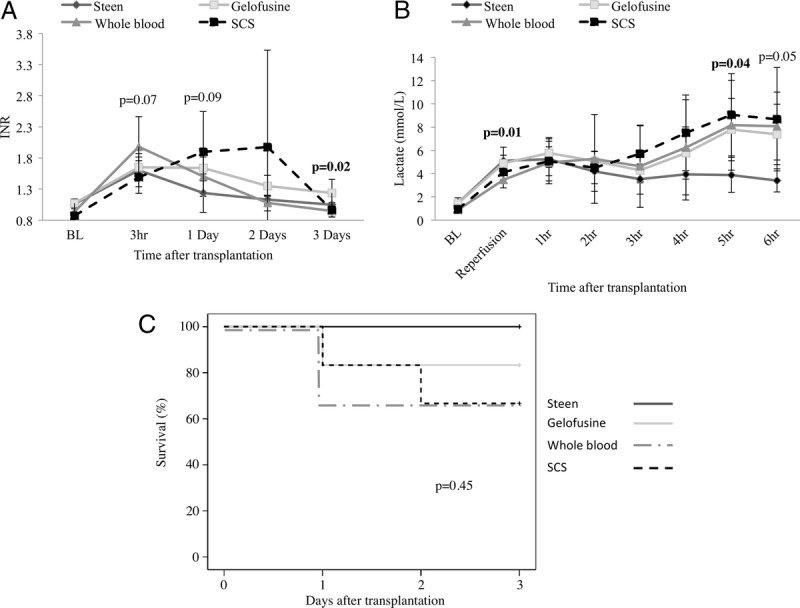
Liver function and animal survival after transplantation. The INR values were lower in the Steen, Gelofusine, and SCS groups when compared with the WB group at 3 hours after PV reperfusion (Steen, 1.60 ± 0.26 vs Gelofusine, 1.65 ± 0.16 vs WB, 1.98 ± 0.47 vs SCS, 1.49 ± 0.26; P = 0.07) after transplant (A). Lactate clearance was significantly improved in the Steen group when compared to the Gelofusine, WB and SCS groups at 5 hours after transplantation (Steen, 3.88 ± 1.49 mmol/L vs Gelofusine, 7.79 ± 2.68 mmol/L vs WB, 8.16 ± 3.86 mmol/L vs SCS, 9.06 ± 3.54 mmol/L; P = 0.04) (B). All animals in the Steen group survived, whereas 1, 2, and 2 pigs from the Gelofusine, WB, and SCS groups, respectively were euthanized due to PNF (C). Results are expressed as mean ± SD. Six experiments were performed per group (n = 6). Student t test (A, B) and Kaplan Meier method with log-rank test (C) were used for analysis. INR, International Normalized Ratio; PNF, primary nonfunction; PV, portal vein; SCS, static cold storage; SD, standard deviation; WB, whole blood.
Lactate clearance was significantly improved in the Steen group when compared with the rest of the groups at 5 hours after transplantation (Steen, 3.88 ± 1.49 mmol/L vs Gelofusine, 7.79 ± 2.68 mmol/L vs WB, 8.16 ± 3.86 mmol/L vs SCS, 9.06 ± 3.54 mmol/L; P = 0.04) (Figure 10).
Animal Survival
All the animals in the Steen group survived until the POD3 without complications and good recovery. In contrast, 1, 2, and 2 animals were euthanized due to PNF and bad clinical appearance (respiratory distress, signs of hypoperfusion, and puncture sites bleeding) in the Gelofusine, WB, and SCS groups, respectively (Figure 10).
DISCUSSION
The focus of previous animal studies has been on the protective effects of the warm perfusion on preservation injury after liver transplantation. Little is known about the impact of the perfusion fluid on NEsLP. Most published porcine studies have used WB, PRBCs, or plasma for warm machine perfusion.34-41 In contrast to the experimental data, the first clinical trial used Gelofusine with PRBCs for NEsLP.2 In SCS preservation, it has been the switch from Eurocollins to UW as preservation solution that provided the breakthrough of organ preservation in the 1990s,42 and it is possible that the type of perfusate fluid plays also an important role in warm perfusion preservation. Of interest, Gelofusine/albumin in combination with acellular hemoglobin-based oxygen carriers have been recently used for NEsLP with results suggesting these combinations to be a safe approach.28,43 This indicates the importance of defining the differences between the perfusate solutions before we move forward to a different setup.
Although the first European perfusion trial for liver transplantation was performed with Gelofusine, the first North American clinical trial used Steen for warm perfusion. In addition, single-center experience has shown the feasibility of using PRBCs plus FFP as perfusate.6 The decision on using different perfusate solutions has been made primarily based on local logistic or preference reasons more than prior experimental evidence.
In our study, the 3 perfusates (Steen, Gelofusine, and WB) showed important differences during NEsLP and after transplantation in a DCD model (30 min-WI), indicating that specific characteristics from each solution might contribute to improve the warm preservation. Of interest, even though the WI injury was moderate and the perfusion was relatively short, we were able to identify important differences between the 3 groups. The lower pH at the beginning of Gelofusine perfusion correlated with the finding of a more acidotic pH (6.76 ± 0.06) in the Gelofusine bags than the one described from the manufacturer (7.1-7.7). In the beginning, the NEsLP perfusions with Steen and WB were associated with a higher HA flow and less hepatic artery resistance. The reduction of hepatic artery flow during Gelofusine perfusion may possibly be related to the initial low potassium content of Gelofusine. It has been demonstrated in prior animal studies that low potassium levels can result in a reduction of nitric oxide production with subsequent increased vasoconstriction.44–46 In addition, the higher density of the gelatin-based perfusate at low temperatures could have also contributed to the lower HA flow and higher intraarterial resistance at the beginning of the perfusion. An additional finding was that although Gelofusine solution does not contain potassium, the perfusate from the Gelofusine group showed an increase of the potassium level of 1.5 mmol/L within 5 minutes after the liver was placed on pump. This increase in potassium can be a result of increased cell injury, or alternatively secondary to the initial acidotic environment of Gelofusine perfusate that favors the exchange of hydrogen ions (intracellular displacement) with potassium (extracellular displacement). In contrast, Gelofusine group showed lower levels of AST during NEsLP and lower cleaved caspase-3 staining when compared with Steen and WB groups. Of note, the WB group displayed a more alkalotic bile pH, a more physiologic bile HCO3- concentration, and reduced bile glucose in comparison with Steen and Gelofusine groups. These findings are important because bile pH and bile glucose have been suggested as potential markers of biliary viability by Watson et al and Matton et al in recent publications.13,47,48 However, these markers should be validated in future clinical studies before establishing them as predictors of ischemic cholangiopathy. Interestingly, the bile pH and bicarbonate content tended to decrease by the end of the perfusion in the WB groups, which might be secondary to the higher hepatocellular and endothelial injury found during NEsLP in this group. On the other hand, the Steen group showed significantly lower levels of hyaluronic acid during NEsLP in contrast to the levels in the Gelofusine and WB groups' results that correlate with the prior reports on the protective effect of dextran on ECs.49,50
An additional finding of this study was the significant increase in liver weight in the Gelofusine group when compared with the Steen and WB groups. This outcome could reflect parenchymal edema, which might have also contributed to the lower HA flow in this group. This factor can become an important element to take into account for prolonged perfusions.
Posttransplantation Steen perfused grafts had significant better early graft function as determined by improved lactate clearance and lower INR. There was additionally a trend toward decreased AST, ALP, and TBil in the Steen group when compared with the rest of the groups. All animals in the Steen group survived with 1 and 2 of 6 animals dying in the Gelofusine and WB groups, respectively. The lower survival in the WB group correlated with significantly higher levels of hyaluronic acid during NEsLP and after transplantation in this group that might be secondary to endothelial activation and injury during perfusion and after transplantation due to the exposure to platelets and remnant leukocytes content in the WB perfusate. As described in prior studies, leukocytes and platelets play an important role during ischemia-reperfusion injury.51–53
Although 30 minutes-WI is the upper limit of acceptable WI time for DCD retrieval with SCS by most centers, it probably represents a mild injury if the liver graft is stored with warm perfused preservation. Therefore, differences in perfusion quality might not result in clinical relevant outcome differences because of the overall good graft quality. It is possible that by further increasing warm ischemic injury or adding additional risk factors for preservation injury that optimize perfusion conditions could become critical. Before establishing what perfusate is ideal for each type of graft (eg, DCD, fatty liver, older donors), it is essential to first optimize the current perfusate solutions.
Based on the results found in this study, we suggest that the future investigations should emphasize identifying factors in the WB perfusate that contributed to more physiologic bile pH and bile glucose content. The identification and supplementation with elements from the WB (eg, secretin, vasoactive intestinal polypeptide, corticosteroids, gastrin, bombesin, etc54) to the colloid-based perfusate (Steen or Gelofusine) might bring an additional benefit in the outcomes. In addition, based on the results of this study, we suggest perfusate hyaluronic acid levels as a potential marker to predict liver viability after transplantation.
Moreover, apart from optimizing the perfusate solution for NEsLP, upcoming studies should explore the possibility of combination with other innovative techniques, such as normothermic regional perfusion, hypothermic perfusion, and controlled oxygenated rewarming, where beneficial effects have been found at decreasing hepatocellular injury in uncontrolled DCD organs,55 restoration of ATP levels in fatty livers,56 and improvement in the peak of transaminases after transplantation with rescue allocation organs,57 respectively.
Our study has several limitations. First, the porcine model of 30 minutes-WI might not be sufficient to detect differences in perfusion quality. Second, mechanisms of preservation injury were not assessed in the model. Third, the correction of perfusate pH was made 5 minutes after the liver was placed on pump. Fourth, the prostacyclin infusion was not dynamically adjusted. Fifth, the short duration of NEsLP may have prevented some of the observed trends from reaching significance. We have chosen 30 minutes-WI because it reflects a clinically relevant withdrawal time for DCD retrievals used by the majority of transplant centers today. Prolonged animal survival is difficult in a porcine model because pigs will develop severe rejection starting day 5 despite immunosuppression. Biochemical and histologic features of severe rejection make the interpretation of later results more difficult.
Overall, this study demonstrates that Steen perfusate improves initial HA flow, markers of bile duct, and hepatocyte and endothelial injury during NEsLP and after transplantation. On the other side, Gelofusine reduces AST levels during NEsLP and apoptosis after transplantation. In contrast, WB displays a more physiologic bile pH and bile glucose levels during NEsLP. Future studies should focus on optimizing warm perfusion solutions for the NEsLP of extreme marginal grafts and possibly combine the advantages of the different perfusion solutions.
Supplementary Material
ACKNOWLEDGMENTS
I.L. expresses his gratitude to the Mexican National Council of Science and Technology (CONACyT, Mexico City, Mexico) for its support for the graduate program at University of Toronto. The authors thank Uwe Mummenhoff and the Birmingham family for their generous support. The study was supported by the Canadian National Transplant Research Program (CNTRP).
Footnotes
Published online 4 March, 2019.
The authors declare no conflicts of interest.
I.L., M.S., and N.S. participated in research design and writing the paper. I.L., D.K., T.G., J.E., J.M.K., M.H., P.U., L.M., R.R., C.B., F.O., and S.G. participated in performance of the research. I.L., O.A., P.Y., M.S., and N.S. participated in data analysis.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Banan B, Xiao Z, Watson R, et al. Novel strategy to decrease reperfusion injuries and improve function of cold-preserved livers using normothermic ex vivo liver perfusion machine. Liver Transpl. 2016;22:333–343. [DOI] [PubMed] [Google Scholar]
- 2.Ravikumar R, Jassem W, Mergental H, et al. Liver transplantation after ex vivo normothermic machine preservation: a phase 1 (first-in-man) clinical trial. Am J Transplant. 2016;16:1779–1787. [DOI] [PubMed] [Google Scholar]
- 3.Selzner M, Goldaracena N, Echeverri J, et al. Normothermic ex vivo liver perfusion using Steen solution as perfusate for human liver transplantation: first North American results. Liver Transpl. 2016;22:1501–1508. [DOI] [PubMed] [Google Scholar]
- 4.Bral M, Gala-Lopez B, Bigam D, et al. Preliminary single-center Canadian experience of human normothermic ex vivo liver perfusion: results of a clinical trial. Am J Transplant. 2017;17:1071–1080. [DOI] [PubMed] [Google Scholar]
- 5.Nasralla D, Coussios CC, Mergental H, et al. A randomized trial of normothermic preservation in liver transplantation. Nature. 2018;557:50–56. [DOI] [PubMed] [Google Scholar]
- 6.Liu Q, Soliman B, Iuppa G, et al. First report of human liver transplantation after normothermic machine perfusion (NMP) in the United States [abstract]. Am J Transplant. 2017;17(suppl 3). [Google Scholar]
- 7.Lee DD, Singh A, Burns JM, et al. Early allograft dysfunction in liver transplantation with donation after cardiac death donors results in inferior survival. Liver Transpl. 2014;20:1447–1453. [DOI] [PubMed] [Google Scholar]
- 8.O'Neill S, Roebuck A, Khoo E, et al. A meta-analysis and meta-regression of outcomes including biliary complications in donation after cardiac death liver transplantation. Transpl Int. 2014;27:1159–1174. [DOI] [PubMed] [Google Scholar]
- 9.Monbaliu D, Liu Q, Vekemans K, et al. Potentiation of adverse effects of cold by warm ischemia in circulatory death donors for porcine liver transplantation. Transplant Proc. 2012;44:2874–2879. [DOI] [PubMed] [Google Scholar]
- 10.Sher L, Quintini C, Fayek SA, et al. Attitudes and barriers to the use of donation after cardiac death livers: comparison of a United States transplant center survey to the united network for organ sharing data. Liver Transpl. 2017;23:1372–1383. [DOI] [PubMed] [Google Scholar]
- 11.Mergental H, Perera MT, Laing RW, et al. Transplantation of declined liver allografts following normothermic ex-situ evaluation. Am J Transplant. 2016;16:3235–3245. [DOI] [PubMed] [Google Scholar]
- 12.Perera T, Mergental H, Stephenson B, et al. First human liver transplantation using a marginal allograft resuscitated by normothermic machine perfusion. Liver Transpl. 2016;22:120–124. [DOI] [PubMed] [Google Scholar]
- 13.Watson CJE, Kosmoliaptsis V, Randle LV, et al. Normothermic perfusion in the assessment and preservation of declined livers before transplantation: hyperoxia and vasoplegia—important lessons from the first 12 cases. Transplantation. 2017;101:1084–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tait AR, Larson LO. Resuscitation fluids for the treatment of hemorrhagic shock in dogs: effects on myocardial blood flow and oxygen transport. Crit Care Med. 1991;19:1561–1565. [DOI] [PubMed] [Google Scholar]
- 15.Van der Linden P, Ickx BE. The effects of colloid solutions on hemostasis. Can J Anaesth. 2006;53(Suppl 6):S30–S39. [DOI] [PubMed] [Google Scholar]
- 16.Barron ME, Wilkes MM, Navickis RJ. A systematic review of the comparative safety of colloids. Arch Surg. 2004;139:552–563. [DOI] [PubMed] [Google Scholar]
- 17.Evans PA, Heptinstall S, Crowhurst EC, et al. Prospective double-blind randomized study of the effects of four intravenous fluids on platelet function and hemostasis in elective hip surgery. J Thromb Haemost. 2003;1:2140–2148. [DOI] [PubMed] [Google Scholar]
- 18.Oettl K, Stauber RE. Physiological and pathological changes in the redox state of human serum albumin critically influence its binding properties. Br J Pharmacol. 2007;151:580–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shibata A, Ishima Y, Ikeda M, et al. Human serum albumin hydropersulfide is a potent reactive oxygen species scavenger in oxidative stress conditions such as chronic kidney disease. Biochem Biophys Res Commun. 2016;479:578–583. [DOI] [PubMed] [Google Scholar]
- 20.Carnevale R, Biondi-Zoccai G, Peruzzi M, et al. New insights into the Steen solution properties: breakthrough in antioxidant effects via NOX2 downregulation. Oxid Med Cell Longev. 2014;2014:242180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frost-Arner L, Bergqvist D. Effect of isovolemic hemodilution with dextran and albumin on thrombus formation in artificial vessel grafts inserted into the abdominal aorta of the rabbit. Microsurgery. 1995;16:357–361. [DOI] [PubMed] [Google Scholar]
- 22.Liu Q, Nassar A, Farias K, et al. Comparing normothermic machine perfusion preservation with different perfusates on porcine livers from donors after circulatory death. Am J Transplant. 2016;16:794–807. [DOI] [PubMed] [Google Scholar]
- 23.Goldaracena N, Echeverri J, Spetzler VN, et al. Anti-inflammatory signaling during ex vivo liver perfusion improves the preservation of pig liver grafts before transplantation. Liver Transpl. 2016;22:1573–1583. [DOI] [PubMed] [Google Scholar]
- 24.Pagano F, Nocella C, Sciarretta S, et al. Cytoprotective and antioxidant effects of Steen solution on human lung spheroids and human endothelial cells. Am J Transplant. 2017;17:1885–1894. [DOI] [PubMed] [Google Scholar]
- 25.Stone JP, Critchley WR, Major T, et al. Altered immunogenicity of donor lungs via removal of passenger leukocytes using ex vivo lung perfusion. Am J Transplant. 2016;16:33–43. [DOI] [PubMed] [Google Scholar]
- 26.Cypel M, Rubacha M, Yeung J, et al. Normothermic ex vivo perfusion prevents lung injury compared to extended cold preservation for transplantation. Am J Transplant. 2009;9:2262–2269. [DOI] [PubMed] [Google Scholar]
- 27.op den Dries S, Karimian N, Sutton ME, et al. Ex vivo normothermic machine perfusion and viability testing of discarded human donor livers. Am J Transplant. 2013;13:1327–1335. [DOI] [PubMed] [Google Scholar]
- 28.Matton APM, Burlage LC, van Rijn R, et al. Normothermic machine perfusion of donor livers without the need for human blood products. Liver Transpl. 2018;24:528–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hassanein W, Khayal TI. TransMedics (OCS) Liver Trial: Preserving and Assessing Donor Livers for Transplantation (Liver PROTECT). US National Library of Medicine. 2018. https://clinicaltrials.gov/ct2/show/NCT02522871. Accessed May 2018.
- 30.Lobo DN, Stanga Z, Aloysius MM, et al. Effect of volume loading with 1 liter intravenous infusions of 0.9% saline, 4% succinylated gelatine (Gelofusine) and 6% hydroxyethyl starch (Voluven) on blood volume and endocrine responses: a randomized, three-way crossover study in healthy volunteers. Crit Care Med. 2010;38:464–470. [DOI] [PubMed] [Google Scholar]
- 31.Steen S. Evaluation and preservation solution. United States Patent and Trademark Office. 2010. https://assignment.uspto.gov/patent/index.html - /patent/search/resultAssignment?searchInput=20040029096&id=25155-612. Accessed May 2018.
- 32.Spetzler VN, Goldaracena N, Knaak JM, et al. Technique of porcine liver procurement and orthotopic transplantation using an active porto-caval shunt. J Vis Exp. 2015;99:e52055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deaciuc IV, Bagby GJ, Lang CH, et al. Hyaluronic acid uptake by the isolated, perfused rat liver: an index of hepatic sinusoidal endothelial cell function. Hepatology. 1993;17:266–272. [PubMed] [Google Scholar]
- 34.Liu Q, Nassar A, Farias K, et al. Sanguineous normothermic machine perfusion improves hemodynamics and biliary epithelial regeneration in donation after cardiac death porcine livers. Liver Transpl. 2014;20:987–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nassar A, Liu Q, Farias K, et al. Ex vivo normothermic machine perfusion is safe, simple, and reliable: results from a large animal model. Surg Innov. 2015;22:61–69. [DOI] [PubMed] [Google Scholar]
- 36.Vogel T, Brockmann JG, Pigott D, et al. Successful transplantation of porcine liver grafts following 48-hour normothermic preservation. PLoS One. 2017;12:e0188494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jamieson RW, Zilvetti M, Roy D, et al. Hepatic steatosis and normothermic perfusion-preliminary experiments in a porcine model. Transplantation. 2011;92:289–295. [DOI] [PubMed] [Google Scholar]
- 38.Reddy S, Greenwood J, Maniakin N, et al. Non-heart-beating donor porcine livers: the adverse effect of cooling. Liver Transpl. 2005;11:35–38. [DOI] [PubMed] [Google Scholar]
- 39.Reddy SP, Bhattacharjya S, Maniakin N, et al. Preservation of porcine non-heart-beating donor livers by sequential cold storage and warm perfusion. Transplantation. 2004;77:1328–1332. [DOI] [PubMed] [Google Scholar]
- 40.Imber CJ, St Peter SD, de Cenarruzabeitia IL, et al. Optimisation of bile production during normothermic preservation of porcine livers. Am J Transplant. 2002;2:593–599. [DOI] [PubMed] [Google Scholar]
- 41.Butler AJ, Rees MA, Wight DG, et al. Successful extracorporeal porcine liver perfusion for 72 hr. Transplantation. 2002;73:1212–1218. [DOI] [PubMed] [Google Scholar]
- 42.Cofer JB, Klintmalm GB, Howard TK, et al. A comparison of UW with Eurocollins preservation solution in liver transplantation. Transplantation. 1990;49:1088–1093. [DOI] [PubMed] [Google Scholar]
- 43.Laing RW, Bhogal RH, Wallace L, et al. The use of an acellular oxygen carrier in a human liver model of normothermic machine perfusion. Transplantation. 2017;101:2746–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen X, Han W, Zhang Y, et al. The molecular pathway of ATP-sensitive potassium channel in endothelial cells for mediating arteriole relaxation. Life Sci. 2015;137:164–169. [DOI] [PubMed] [Google Scholar]
- 45.Villalba N, Sonkusare SK, Longden TA, et al. Traumatic brain injury disrupts cerebrovascular tone through endothelial inducible nitric oxide synthase expression and nitric oxide gain of function. J Am Heart Assoc. 2014;3:e001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Souza Md, Bouskela E. Arteriolar diameter and spontaneous vasomotion: importance of potassium channels and nitric oxide. Microvasc Res. 2013;90:121–127. [DOI] [PubMed] [Google Scholar]
- 47.Matton APM, de Vries Y, Burlage LC, et al. Biliary bicarbonate, pH and glucose are suitable biomarkers of biliary viability during ex situ normothermic machine perfusion of human donor livers. Transplantation. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watson CJE, Kosmoliaptsis V, Pley C, et al. Observations on the ex situ perfusion of livers for transplantation. Am J Transplant. 2018;18:2005–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laumonier T, Walpen AJ, Maurus CF, et al. Dextran sulfate acts as an endothelial cell protectant and inhibits human complement and natural killer cell-mediated cytotoxicity against porcine cells. Transplantation. 2003;76:838–843. [DOI] [PubMed] [Google Scholar]
- 50.Eto N, Kojima I, Uesugi N, et al. Protection of endothelial cells by dextran sulfate in rats with thrombotic microangiopathy. J Am Soc Nephrol. 2005;16:2997–3005. [DOI] [PubMed] [Google Scholar]
- 51.Khandoga A, Biberthaler P, Messmer K, et al. Platelet-endothelial cell interactions during hepatic ischemia-reperfusion in vivo: a systematic analysis. Microvasc Res. 2003;65:71–77. [DOI] [PubMed] [Google Scholar]
- 52.Tamura T, Kondo T, Ogawa K, et al. Protective effect of heme oxygenase-1 on hepatic ischemia-reperfusion injury through inhibition of platelet adhesion to the sinusoids. J Gastroenterol Hepatol. 2013;28:700–706. [DOI] [PubMed] [Google Scholar]
- 53.Esch JS, Jurk K, Knoefel WT, et al. Platelet activation and increased tissue factor expression on monocytes in reperfusion injury following orthotopic liver transplantation. Platelets. 2010;21:348–359. [DOI] [PubMed] [Google Scholar]
- 54.Tabibian JH, Masyuk AI, Masyuk TV, et al. Physiology of cholangiocytes. Compr Physiol. 2013;3:541–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Carlis R, Di Sandro S, Lauterio A, et al. Liver grafts from donors after circulatory death on regional perfusion with extended warm ischemia compared with donors after brain death. Liver Transpl. 2018;24:1523–1535. [DOI] [PubMed] [Google Scholar]
- 56.Kron P, Schlegel A, Mancina L, et al. Hypothermic oxygenated perfusion (HOPE) for fatty liver grafts in rats and humans. J Hepatol. 2018;68:82–91. [DOI] [PubMed] [Google Scholar]
- 57.Hoyer DP, Mathé Z, Gallinat A, et al. Controlled oxygenated rewarming of cold stored livers prior to transplantation: first clinical application of a new concept. Transplantation. 2016;100:147–152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


