Supplemental digital content is available in the text.
Abstract
Background
Older donors and recipients are increasingly considered for liver transplantation. Both donor and recipient age have a negative impact on outcomes. Large registry analyses show that older donors are frequently matched to older recipients. Whether age-related risks accumulate in a synergic negative effect on outcomes because of donor-recipient age matching is poorly understood.
Methods
We investigated the impact of donor-recipient age interaction on patient and death-censored graft survival in multivariate Cox regressions in 849 transplants (January 2000 to December 2015).
Results
Donors 70 years or older did not affect long-term patient or graft survival. Recipient age independently increased the risk of death (hazard ratio [HR], 1.03; 95% confidence interval [CI], 1.02-1.05, P < 0.0001), but donor-recipient age interaction was noninfluential. The negative impact of recipient age on patient survival was significant as early as 6 months after transplantation (HR, 1.06; 95% CI, 1.03-1.09; P = 0.00008). The adjusted risk of death was significant for patients aged 60 to 69 years (HR, 1.995; 95% CI, 1.40-2.85; P < 0.0001) and 70 years or older (HR, 2.001; 95% CI, 1.10-2.66; P = 0.04). In contrast, the risk of graft loss was not influenced by recipient age (HR, 1.02; 95% CI, 0.996-1.04; P = 0.11) or age interaction.
Conclusions
Older livers can be safely used in older recipients without jeopardizing graft and patient survival if other risk factors are minimized.
Liver transplantation (LT) is increasingly being offered to older candidates as a consequence of a combination of increasing life expectancy1 and improved care of elderly.2,3 At the same time, grafts from older donors are increasingly accepted to satisfy the ever-growing demand for LT.4,5
Because both donor and recipient age are well-known risk factors for transplant failure, the question arises whom these older livers are best offered to. It is generally accepted that recipients with lower likelihood of success, that is, older patients with higher Model for End-stage Liver Disease (MELD) score, should not face the risk of failure associated with donors of suboptimal quality, that is, older donors with higher Donor Risk Index (DRI).6 Conversely, younger recipients are believed to endure the transplantation of high-risk grafts with no or minimal impact on outcomes.6 Surprisingly, recent large registry analyses have shown that older donors are frequently matched with older recipients.3,5,7,8
Aging induces functional changes in both graft and recipient, affecting the pathophysiology of ischemia-reperfusion injury and postoperative course. Aged livers are more frequently fibrotic and have lower regeneration capacities,9,10 resulting in more severe injuries after graft reperfusion.11 Unsurprisingly, donor age is the risk factor with the highest weight in the DRI score.12,13 Nevertheless, several single-center reports showed that careful selection of older donors can mitigate the risks associated with advanced donor age.7,14-17
Senescence impairs the innate immunity in older recipients, increasing the susceptibility to infections, whereas the adaptive response is hyperactivated with exaggerated production of proinflammatory cytokines and aggravation of ischemia-reperfusion injury.18,19 Older liver transplant candidates typically have more comorbidities, lower likelihood of success of surgical procedures,2 and are more carefully selected for LT. Previous studies have associated recipient age with both poor survival3,5,20 and satisfactory results after transplantation.21
It is not clear if matching older donor livers to older transplant candidates affects long-term outcomes because of synergic amplification or age-related risks. We assessed if the interaction of donor and recipient age influences long-term patient and death-censored graft survival after adjustment for other predictors of poor outcomes.
MATERIALS AND METHODS
Study Population and Design
A prospectively collected clinical database was retrospectively reviewed to identify all deceased-donor LTs performed between January 1, 2000, and December 31, 2015, at the University Hospitals of Leuven (Belgium). Pediatric recipients, partial grafts, and combined transplantations were excluded. This study was approved by the local ethical committee (s60518).
Older donors were defined as being 70 years or older (D ≥ 70) based on the most commonly accepted definition in recently published literature.5,7,20,22-24 Recipient age was considered as a continuous variable. In post hoc subgroup analyses, recipients were divided into the following age groups: younger than 60 years, between 60 and 69 years, and equal to or older than 70 years.
Donor and recipient demographics, transplant data, posttransplant complications, and graft and patient survival were collected. The effect of the interaction of donor and recipient age on patient and graft survival was investigated in the entire cohort in multivariate analyses. Survival of recipients was censored at the time of death or at the time of last follow-up (August 31, 2016). Graft loss was defined as retransplantation or failure of the graft leading to recipient's death.
Statistical Analyses
Continuous data are expressed as median (interquartile range), and the difference between groups was estimated with the Mann-Whitney U test. Categorical variables are expressed as numbers (percentage), and the difference in incidence was evaluated with the χ2 test or Fisher exact test. Bonferroni correction for multiple testing was applied when appropriate. The correlation between donor and recipient age was investigated with Spearman's rho.
The existence of age interaction was assessed first visually with interpolation plots. Dot plots were used to visualize the effect of age interaction on outcomes. Mean patient/graft survival (Y-axis) was plotted per recipient age (X-axis) with dots of different colors representing either younger (black) or older donors (gray). Next, interpolation lines were fitted separately for younger and older donors, the slope of which represented the effect of age interaction so that equal slopes (or parallel lines) would indicate absence of relevant interaction.
The effect of age interaction on the risk of recipient's death and graft loss was assessed in univariate analysis with moderated Cox regressions, in which the only variables considered were recipient age, donor 70 years or older, and their interaction product. This effect was further adjusted for other predictors in multivariate Cox regressions. Variables that were significantly associated with the risk of patient's death or graft loss in univariate analysis were considered and retained in the final multivariable models based on backward stepwise selection. The DRI was calculated for every transplant to estimate donor quality, but in multivariate regression only its single components (type, age, race, height, cause of death, cold ischemia, allocation, graft type) were considered to avoid excessive collinearity with donor age. Recipient age, donor 70 years or older, and their interaction product were added to the multivariate models irrespectively from their performance in univariate analyses.
Additionally, an explorative multivariate Cox regression was performed to identify predictors of patient's death in the subgroup of recipients 60 years or older. Finally, given the longevity of the study period, analyses were repeated, adjusting for the effect of transplant era.
Survival curves were plotted based on adjusted hazard ratios (HRs). A 2-sided P value of less than 0.05 was considered statistically significant. Statistical analyses were performed using SPSS (SPSS Inc. Chicago, IL, version 20).
RESULTS
Characteristics of the Study Population
Eight hundred forty-nine deceased-donor LTs meeting the inclusion criteria were performed between January 1, 2000, and December 31, 2015. Donor and recipient demographics are summarized in Table 1; posttransplant outcomes in Table 2. A significant correlation between donor and recipient age was observed in our center, although the strength of this association was weak (Spearman rho = 0.23, P < 0.0001) (SDC, Figure S1, http://links.lww.com/TXD/A196).
TABLE 1.
Overview of donor and recipient demographics of the study population and transplants performed with grafts from donors younger or older than 70 years
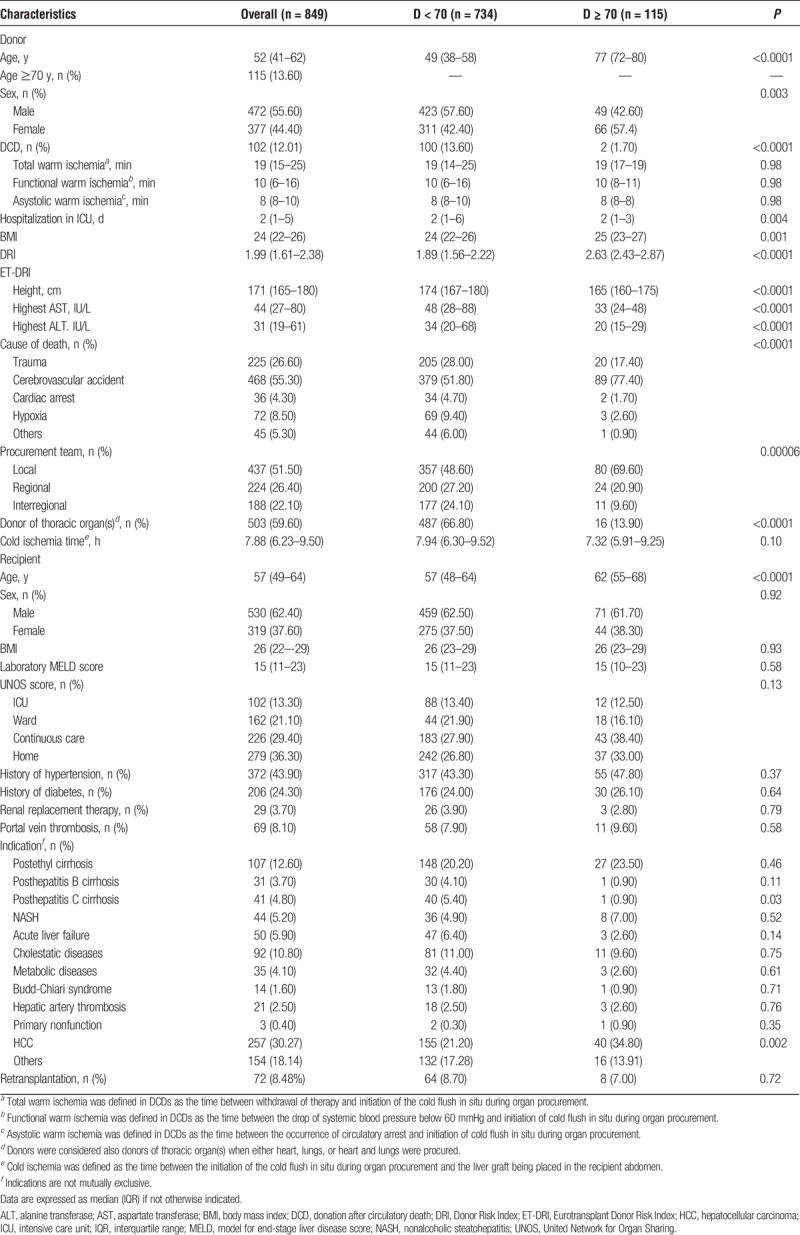
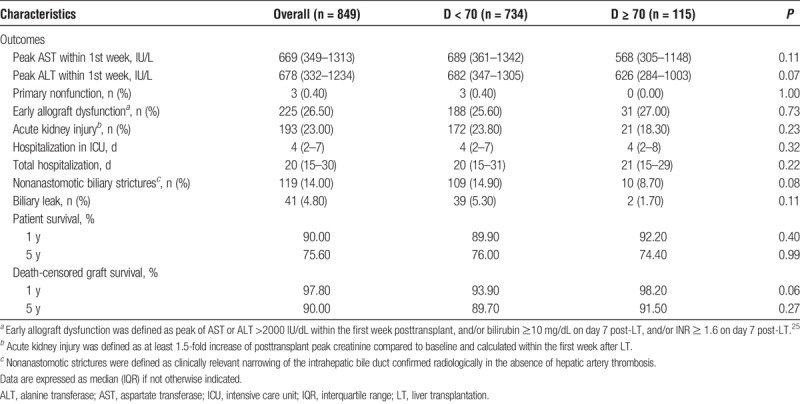
TABLE 2.
Overview of postoperative outcomes after transplantation of the study population and after LT of grafts procured from donors younger or older than 70 years
Median donor age was 52 years (41–62 years). One hundred fifteen donors were 70 years or older (13.60%). Donation after circulatory death (DCD) donors accounted for 12% of LTs. In DCD, total donor warm ischemia time was 19 minutes (15–25 minutes). Cold ischemia time was 7.88 hours (6.23–9.50 hours). No difference in the duration of cold ischemia time was observed between D < 70 and D ≥ 70 (Table 1).
Compared to younger donors, D ≥ 70 were more often female, died more frequently of cerebrovascular events, had shorter hospitalization in intensive care before donation, higher DRI [D ≥ 70: 2.63 (2.43–2.87), D < 70: 1.89 (1.56–2.22); P < 0.0001], and were more frequently procured by our own team. DCDs were less common in D ≥ 70 (1.70%) than in D < 70 (13.60%, P < 0.0001). Predonation peak-transaminases were lower in D ≥ 70 (Table 1).
Recipients were aged 57 years (49–64 years) and were transplanted with a median laboratory MELD at the time of LT of 15 points (11–23). The two most frequent indications for transplantation were hepatocellular carcinoma (HCC; 30.27%) and postethyl cirrhosis (12.60%) (Table 1).
The median follow-up was 5.26 years (2.38–9.35 years). Patient survival was 90% at 1 year and 75.60% at 5 years after transplantation, whereas graft survival was 97.80% and 90% at 1 and 5 years after LT, respectively (Table 2).
Older Recipients Are at Higher Risk of Death Independently of Age Matching
Figure 1A depicts the interpolation plot for patient survival, in which divergent interpolation lines suggest the existence of significant interaction of age. In univariate analysis, recipient age was associated with higher risk of death after transplantation (HR, 1.04 per additional year; 95% confidence interval [CI], 1.02-1.05, P < 0.0001), whereas patient survival was not influenced by D ≥ 70 (HR, 5.96; 95% CI, 0.45-79.09; P = 0.18) or age interaction (HR, 0.97; 95% CI,: 0.93-1.01; P = 0.17).
FIGURE 1.
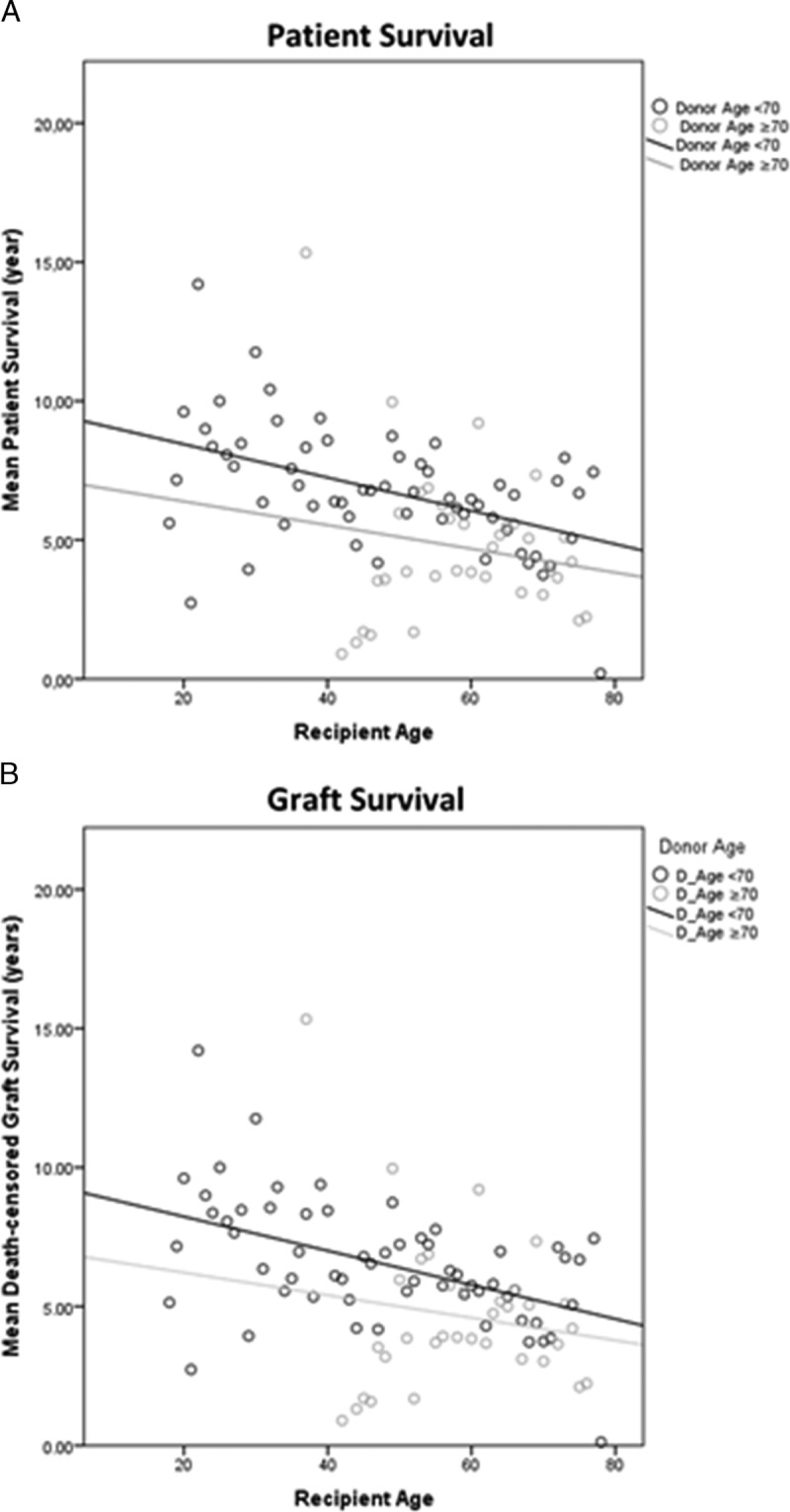
Interpolation plots depicting the interaction between recipient age and donors of younger (<70 years) or older age (≥70 years). The mean patient survival (A) and graft survival (B) are plotted per every age of recipients (x-axis), and dots represent either younger (in black) or older donors (in gray). The slope of the interpolation lines fitted separately for donors of different age corresponds to the effect of age interaction on the outcome measured, where equal slopes (or parallel lines) would represent a nonsignificant interaction.
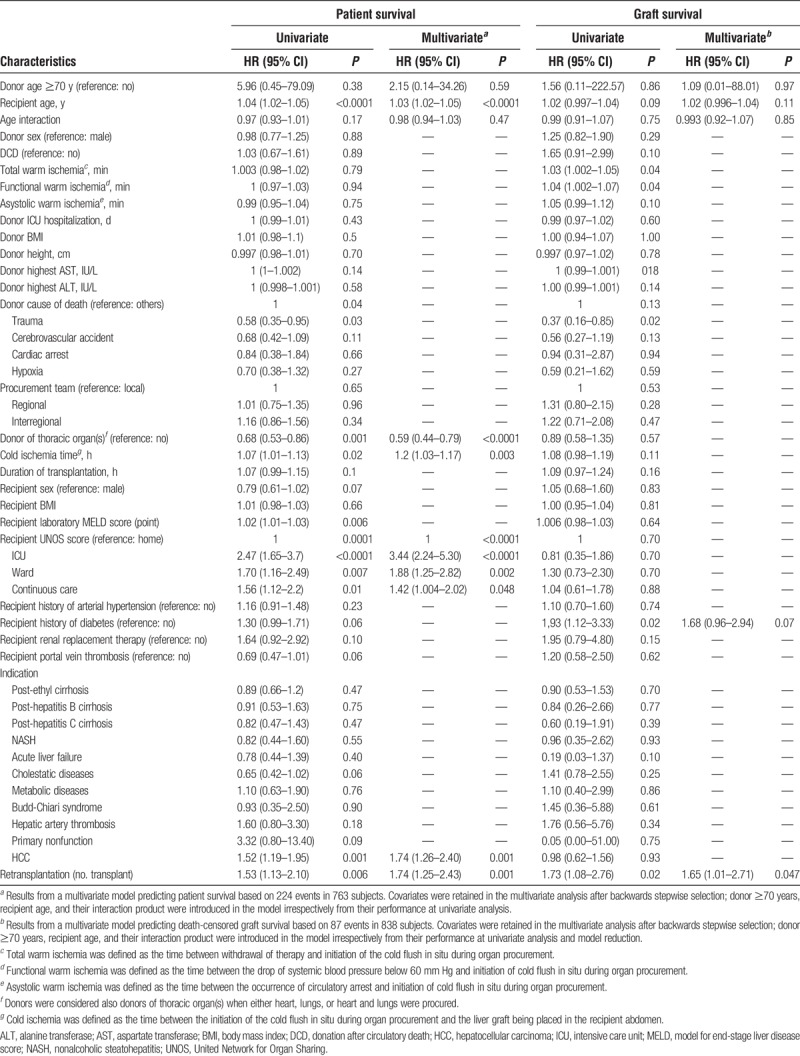
The effect of older donors, recipient age, and age interaction on patient survival was further tested against other predictors in multivariate Cox regression (Table 3). Among the variables significantly associated with worse patient survival in univariate analysis, the United Network for Organ Sharing score, transplantation for HCC, redo-LT, and cold ischemia remained independent predictors for patient's death in multivariate analysis. Donors in which thoracic organs were also donated (either heart, lungs, or heart and lungs) were associated with better survival after transplantation. D ≥ 70 did not affect patient survival (adjusted HR, 2.15; 95% CI, 0.14-34.26; P = 0.59). In contrast, recipient's age independently increased the risk of death after transplantation (adjusted HR, 1.03 per additional year; 95% CI, 1.02-1.05; P < 0.0001), but matching older donors to older recipients did not confer additional risk (adjusted HR of the interaction product, 0.98; 95% CI, 0.94-1.03, P = 0.47) (Table 3).
TABLE 3.
Univariate and multivariate Cox regression for patient and death-censored graft survival
Results remained unchanged when introducing the era of the transplant in the multivariate model, which had no effect on patient survival (adjusted HR, 0.98; 95% CI, 0.93-1.03; P = 0.35).
After stratification per recipient age, the adjusted risk of patient's death was significant for patients aged 60 to 69 years (HR, 1.995; 95% CI, 1.40-2.85; P < 0.0001) and for recipients 70 years or older (HR, 2.001; 95% CI, 1.10-2.66; P = 0.02) (Figure 2; SDC, Table S1, http://links.lww.com/TXD/A197). The adjusted negative effect of recipient age on patient survival was significant already 6 months (HR, 1.06 per additional year; 95% CI, 1.03-1.09; P = 0.00008) and 1 year after transplantation (HR, 1.06 per additional; 95% CI, 1.03-1.09, P = 0.00003) (SDC, Table S2, http://links.lww.com/TXD/A198).
FIGURE 2.
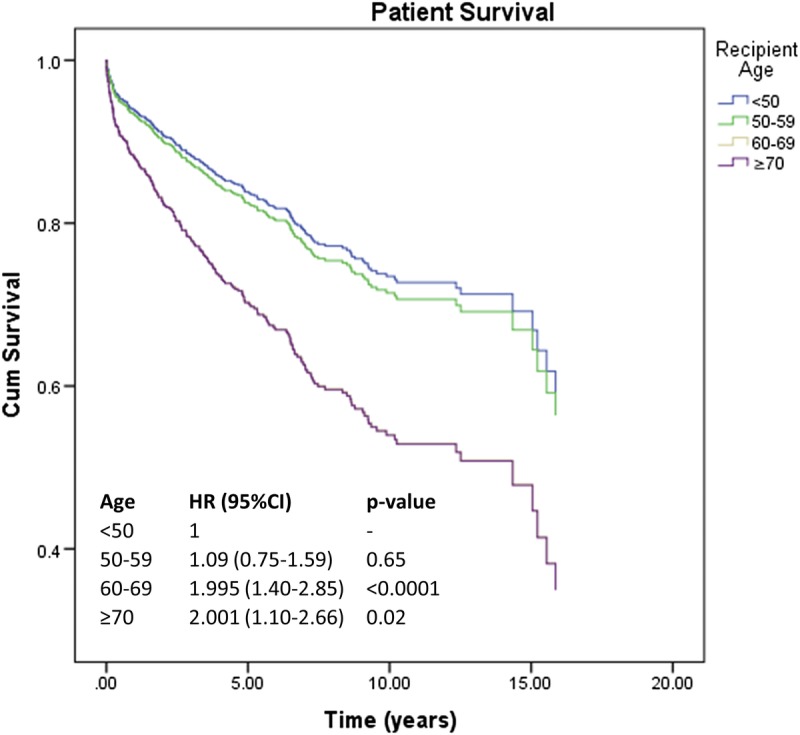
Adjusted patient survival according to different subgroups of recipient age. Survival curves are plotted based on HR from a multivariable Cox regression predicting the risk of patient's death and adjusted for D ≥ 70, donor cause of death, DRI, cold ischemia time, recipient laboratory MELD score, recipient UNOS score, transplantation for HCC, and retransplantation (SDC, Table S1, http://links.lww.com/TXD/A197). CI, confidence interval; DRI, Donor Risk Index; HCC, hepatocellular carcinoma; HR, hazard ratio; MELD, model for end-stage liver disease; UNOS, United Network for Organ Sharing.
Five-year patient survival in recipients 70 years or older transplanted with grafts from D ≥ 70 years was 72.60%. The absolute difference on 5-year patient survival of recipients transplanted with grafts procured from younger or older donors was similar between recipients younger than 60 years (7%) or 70 years or older (8%, P = 0.53), further confirming the absence of significant interaction of donor and recipient age (Figure 3).
FIGURE 3.
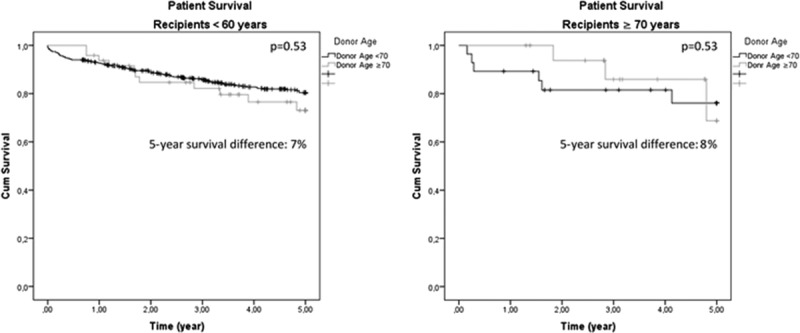
Unadjusted 5-year patient survival of recipients younger than 60 years (A) and ≥70 years (B) stratified per donor's age. The absolute difference in 5-year survival between the 2 groups of recipients was comparable, showing no relevant interaction of donor and recipient age.
Older Recipients Are Not at Higher Risk of Graft Loss
Figure 1B depicts the interpolation plot for graft survival, in which divergent interpolation lines suggested the existence of significant interaction of age. However, in univariate analysis, the risk of graft loss was not associated with recipient age (HR, 1.02 per additional year; 95% CI, 0.997-1.04; P = 0.09), D ≥ 70 (HR, 1.56; 95% CI, 0.11-222.57; P = 0.86), or age interaction (HR, 0.99; 95% CI, 0.91-1.07; P = 0.75).
The effect of older donors, recipient age, and age interaction on graft survival was further tested against other predictors in multivariate Cox regression (Table 3). Among the predictors associated with graft loss in univariate analysis, retransplantation and recipient with a history of diabetes remained significant predictors in multivariate regression, whereas the risk of graft loss was not influenced by D ≥ 70 (adjusted HR, 1.09; 95% CI, 0.01-88.01; P = 0.97), recipient age (adjusted HR, 1.02; 95% CI, 0.996-1.04; P = 0.11), or age interaction (adjusted HR, 0.993; 95% CI, 0.92-1.07; P = 0.85) (Table 3).
Older Recipients Exhibited a Low-risk Profile Pretransplantation
To identify predictors of worse outcomes in older recipients, donor and recipient demographics, transplant characteristics, and postoperative complications were compared post hoc between the age group showing the best outcomes, that is, recipients younger than 60 years (R < 60, n = 514 LT), and the 2 age groups showing a significantly increased risk of death after LT, that is, recipients of 60 to 69 years (R60-69, n = 289 LT) and recipients 70 years or older (R ≥ 70, n = 46 LT).
Both R60-69 and R ≥ 70 were transplanted with grafts procured from older donors. Cold ischemia was significantly shorter in both R60-69 (7.64 hours [6.02–9.34], P = 0.002) and R ≥ 70 (6.55 hours [5.14–8.27], P = 0.002) than R < 60 (8.03 hours [6.45–9.67]). DCD grafts were more frequently transplanted in R60-69 (17.30%) than R < 60 (9.30%, P = 0.004) (Table 4).
TABLE 4.
Comparison of donor and recipient demographics and posttransplant results between recipients that achieved the best patient survival (R < 60 years) and 2 age groups at higher risk of worse outcomes (R60-69 years and R ≥ 70 years)
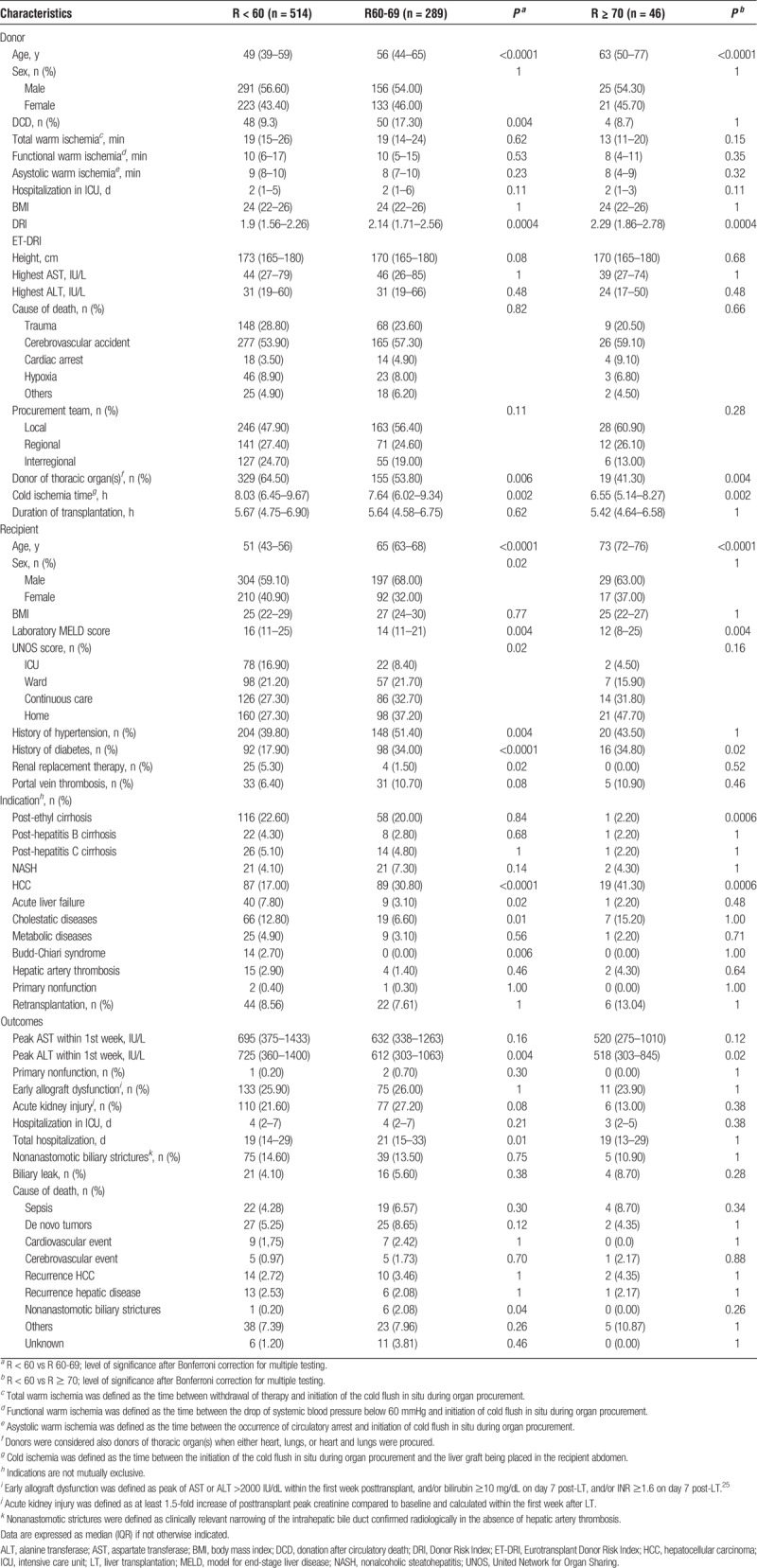
Older recipients had a more stable disease when compared to R < 60. Indeed, both R60-69 and R ≥ 70 were transplanted more frequently for HCC within Milan criteria,26 had lower laboratory MELD score at the time of LT [R < 60: 16 (11–25); versus R60-69: 14 (11–21), P = 0.004; versus R ≥ 70: 12 (8–25), P = 0.004], and were less frequently admitted to the intensive care at the time of organ offer (R < 60: 16.90%; versus R60-69: 8.40%, P = 0.02; versus R ≥ 70: 4.50%, P = 0.06). Additionally, R60-69 needed renal replacement therapy before LT less frequently than R < 60 (Table 4).
There was no difference between groups in the incidence of primary nonfunction, early allograft dysfunction,25 acute kidney injury, and nonanastomotic biliary strictures. R60-69 and R ≥ 70 showed higher frequencies of diabetes than R < 60, and R60-69 showed a higher frequency of arterial hypertension. Nevertheless, there was no difference in death rate caused by either cardiac or cerebrovascular events, nor a more prevalent cause of death in R60-69 or R ≥ 70 could be identified (Table 4).
An explorative analysis of risk factors for patient death in older recipients (R ≥ 60, n = 335 LT) identified hospitalization in the intensive care at the time of organ offer as the only predictor of poor patient survival (adjusted HR, 2.91; 95% CI, 1.53-5.52; P = 0.001), whereas D ≥ 70 did not influence the risk of death after LT (adjusted HR, 0.66; 95% CI, 0.39-1.11; P = 0.12) (SDC, Table S3, http://links.lww.com/TXD/A199).
DISCUSSION
This single-center retrospective analysis of 849 deceased-donor LTs shows that matching older donor livers to older liver transplant recipients does not affect long-term outcomes because there was no synergic amplification of age-related risks when other risk factors are minimized. Advanced recipient age was an independent predictor of patient's death but did not influence the risk of graft loss. Lastly, older donors were not associated with worse outcomes, in contrast with results from large registry studies12,13 but in line with previously published single-center analyses.7,14-17
Previous studies have assessed the risk of death/graft loss of transplants with different combinations of donor and recipient age.27-29 In a large retrospective analysis, Chapman et al27 did not observe any difference in patient and graft survival in LTs matched or mismatched per age, but the actual interaction product of donor and recipient age was not considered. Using a similar approach, Pagano et al28 observed worse graft and patient survival in multivariate analysis in age mismatched LT, but the absence of correction for donor and recipient age, the small sample size, and small number of events do not allow to draw any solid conclusion on the relevance of age interaction. Very recently, Bittermann and Goldberg29 observed in a large-registry analysis that the detrimental effect of increasing donor age was greater in younger (<40 years) than older recipients (≥60 years), and younger recipients transplanted with older grafts (≥60 years) had significantly worse outcomes in multivariate analysis. These results led the authors to conclude that donor and recipient age have a significant synergic interaction influencing outcomes after LT, although it is not clear if recipient age was a risk factor for death and if analyses were adjusted for donor age. In the absence of such adjustment, a higher risk of death/graft loss in transplants in which older grafts are used might reflect the detrimental effect of advanced donor age rather than indicating a significant effect of true interaction of age. Furthermore, in the study by Bitterman and Goldberg, donors younger than 40 years constituted almost 50% of the donor pool, and only 17% were older donors (≥60 years), whereas in our cohort, they accounted for 20% and 30% of LTs, respectively. Such difference in the donor pool resulted in a more stringent selection of older donors in our population (shorter hospitalization, small number of DCDs with shorter warm ischemia time, and more frequent local procurement/allocation), making direct comparison of results difficult. When clustering the transplants according to different age matches similarly to the study by Bittermann and Goldberg, our results did not change: older recipients were at higher risk of death independently from various combinations of donor and recipient age (SDC, Table S4, http://links.lww.com/TXD/A200).
Despite the fact that older patients were at higher risk of death, our adjusted analysis showed that LT in older recipients achieves 5-year patient survival more than 80% (Figure 2), which is in line with the best achievable results identified in a recent benchmark analysis in LT.30 Although the benefit in years-gain after LT should be quantified based on the expected survival on the waiting list rather than on the absolute value of post-LT survival,6 our data justify the conclusion that LT in older candidates can achieve excellent results. Indeed, their remarkable 80% patient survival at 5 years after transplantation is likely to be superior to the expected 5-year survival of age peers suffering from end-stage liver disease still on the waiting list and is substantially greater than the 60% cutoff generally accepted as threshold to overcome the harm of a futile transplant.31 Therefore, older candidates should not be excluded from LT a priori based on “chronological” age; rather, a more comprehensive risk stratification and evaluation of their health status should guide the decision for listing.
Older recipients exhibited a pretransplant profile, suggesting a high likelihood of success (they were transplanted mostly for HCC within Milan criteria,26 with a low laboratory MELD score, and short cold ischemia), yet they had higher risk of death. Although the effect of recipient age remained largely unexplained in our analyses, it did not seem related to graft failure since recipient age did not influence death-censored graft survival. We do acknowledge, however, that our analysis may have been less precise for this outcome due to the small number of graft loss observed in our cohort (n = 87). Lastly, the adjusted effect of recipient age on patient's death was significant as early as 6 months after LT; therefore, the negative effect of recipient age cannot be related solely to the expected inferior longevity of older recipients.
Cardiovascular diseases have been associated with mortality after solid organ transplantation in older patients2 but did not seem to play a major role in our series. The incidence of the most common risk factors for cardiovascular events was similar in older and younger recipients, with the exception of diabetes mellitus. We could not evaluate the incidence of risk factors for metabolic syndrome,32 which is closely related to the risk of both cardiac and cerebrovascular events.33,34 Nevertheless, patients of advanced age in this series underwent full cardiological assessment (including MIBI myocardial perfusion image test or coronary angiography when appropriate) on single-case basis before listing, and we did not observe higher incidence of fatal cardiac or cerebrovascular accidents in older recipients.
Recipient's age may be a surrogate marker of other factor(s) not adequately captured in our analysis. For instance, recipient's global fitness and functional reserves rather than “chronological age” may determine the increased risk of death observed in older recipients. “Physical frailty” is a syndrome of the geriatric population characterized by increased vulnerability to external stressors and predisposes to adverse outcomes and death.35 The loss of muscular mass, the impairment of muscular performance, and the malnutrition almost invariably associated with liver cirrhosis are also hallmarks of the frailty syndrome.36 The recently developed “liver frailty index,” which evaluates the extrahepatic manifestation of cirrhosis (nutritional status, extremity strength, and neuromotor function) with 3 straightforward performance tests (grip strength, chair stands, and balance),37 quantifies patient's functional reserves, predicts waitlist mortality better than MELD,37 and, more importantly, correlates with post-LT mortality in preliminary studies.38,39 We hypothesize that older recipients in our cohort suffered from unknown degrees of physical frailty, which, in turn, might have predisposed them to worse outcomes despite the favorable pretransplant profile. Indeed, an explorative analysis identified hospitalization in intensive care as the only predictors of patient's death in recipients 60 years or older (SDC, Table S3, http://links.lww.com/TXD/A199), which may also reflect the more fragile phenotype of these patients. To date, the pretransplant assessment of (older) candidates suffering from end-stage liver disease focuses mostly on quantifying their probability to survive surgery mainly by stratifying their risk of cardiovascular events.2 The systematic and objective quantification of functional reserves of older transplant candidates might allow us not only to improve risk stratification and selection of older recipients but also to prompt targeted rehabilitation therapies aiming to improve their global fitness and endurance before LT, ultimately improving results in the long-term.
Our study is a single-center retrospective analysis in which donors ≥70 years with a low-risk profile were selected and, as such, donor-related risk factors as donor type and warm ischemia were not associated with worse outcomes. It may be argued that an unequal distribution between younger and older DCD donors in our cohort may confound our findings on the safety of using well-selected older donors. However, DCD grafts of 50 or older or 60 years or older can be safely used without impact on long-term graft survival, provided that other risk factors are minimized.40,41 We repeated our analysis excluding DCD liver transplants, and the results did not change (SDC, Table S5, http://links.lww.com/TXD/A201).
The low median laboratory MELD of the transplants included in our study (15 [11–23]) may also be considered as a limitation to the generalizability of our results. However, de Boer et al analyzing the utilization of older grafts for LTs performed within the Eurotransplant region in the same period considered in our study (2000–2015) reported a similar median laboratory MELD score of 16 points (11–27). Therefore, we believe that the LTs included in our study are a fair representation of the current practice in LTs in the majority of Europe.
Older recipients had more stable disease than younger patients, and the intrinsic effect of recipient's age after adjustment for other predictors in our analysis might have been underestimated. Additionally, the effects of other factors, such as the severity of hepatic steatosis and physical deconditioning and malnutrition, were not considered in this study, and we might have overestimated the effect of recipient's age on long-term outcomes. Therefore, some degree of caution is warranted while interpreting our results.
We acknowledge that the experience of our center with older donors17 may partially influence the good results obtained with this type of graft and that carefully selecting and matching older donors and recipients may limit the generalizability of our study. Nonetheless, this strategy reflects a real-life practice aiming to use older donor grafts without jeopardizing outcomes by minimizing other risk factors well known to influence long-term results. For example, we preferably but not exclusively accept D ≥ 70 with normal liver function tests predonation, a short intensive care stay (≤3 days), procured by our own team allowing us to minimize the duration of cold ischemia. Similar observations have been made in 2 registry analyses. Indeed, Halazun et al observed that adjusting for cold ischemia (>8 hours) and some recipient characteristics (>60 years, hepatitis C, pretransplant hospitalization, retransplantation, previous abdominal surgery) resulted in similar patient survival after transplantation of ≥70 and <70 years donor grafts.20 Similar to our findings, Segev et al identified a subgroup of “low-risk” recipients (nonurgent first transplant for HCC or indications other than hepatitis C, >45 years, cold ischemia <8 hours) in which posttransplant outcomes were not affected by older donor age.5 Such findings were recently confirmed in a Eurotransplant registry analysis.42 However, our results also highlight that older “low-risk” recipients have a considerable increased risk of death independent of age matching, stressing the emergent need to improve the preoperative risk stratification of these patients.
In conclusion, older livers can be safely used in older recipients without jeopardizing graft and patient survival if other risk factors are minimized. However, carefully selected older recipients have higher risk of death after LT, although their 5-year survival can be excellent. The preoperative evaluation of older liver transplant candidates should include the objective assessment of their global fitness and functional reserves because it might improve stratification of risks, allow targeted pretransplant rehabilitation, and ultimately further ameliorate long-term outcomes.
Supplementary Material
ACKNOWLEDGMENTS
An abstract of this manuscript was presented during the 27th international congress of The Transplantation Society (Madrid, June 30, 2018, to July 5, 2018).
Footnotes
Published online 26 March, 2019.
The authors declare no conflicts of interest.
No funding was provided for this study. J.P., D.M., and I.J. hold a CAF chair for abdominal transplant surgery. J.P. holds an IGL chair for abdominal transplant surgery. D.M., C.V., S.v.d.M., and D.C. are senior researchers of The Research Foundation (FWO)-Flanders. The department of abdominal transplant surgery has received unrestricted grants from Astellas and Roche.
N.G. and D.M. designed the study, interpreted data and results, wrote the article, and take responsibility for the integrity of the data and accuracy of data analyses. N.G. acquired data and performed the analyses. I.J., M.S.B., F.N., S.v.d.W., W.L., D.C., L.V., H.v.M., T.R., and J.P. contributed to the interpretation of data and results and critically revised and approved the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Oeppen J, Vaupel JW. Demography. Broken limits to life expectancy. Science. 2002;296:1029–1031. [DOI] [PubMed] [Google Scholar]
- 2.Abecassis M, Bridges ND, Clancy CJ, et al. Solid-organ transplantation in older adults: current status and future research. Am J Transplant. 2012;12:2608–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su F, Yu L, Berry K, et al. Aging of liver transplant registrants and recipients: trends and impact on waitlist outcomes, post-transplantation outcomes, and transplant-related survival benefit. Gastroenterology. 2016;150:441–453.e6. [DOI] [PubMed] [Google Scholar]
- 4.Adam R, Karam V, Delvart V, et al. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J Hepatol. 2012;57:675–688. [DOI] [PubMed] [Google Scholar]
- 5.Segev DL, Maley WR, Simpkins CE, et al. Minimizing risk associated with elderly liver donors by matching to preferred recipients. Hepatology. 2007;46:1907–1918. [DOI] [PubMed] [Google Scholar]
- 6.Schaubel DE, Sima CS, Goodrich NP, et al. The survival benefit of deceased donor liver transplantation as a function of candidate disease severity and donor quality. Am J Transplant. 2008;8:419–425. [DOI] [PubMed] [Google Scholar]
- 7.Paterno F, Wima K, Hoehn RS, et al. Use of elderly allografts in liver transplantation. Transplantation. 2016;100:153–158. [DOI] [PubMed] [Google Scholar]
- 8.Adam R, Karam V, Cailliez V, et al. 2018 annual report of the European Liver Transplant Registry (ELTR)—50-year evolution of liver transplantation. Transpl Int. September 2018. [DOI] [PubMed] [Google Scholar]
- 9.Wakabayashi H, Nishiyama Y, Ushiyama T, et al. Evaluation of the effect of age on functioning hepatocyte mass and liver blood flow using liver scintigraphy in preoperative estimations for surgical patients: comparison with CT volumetry. J Surg Res. 2002;106:246–253. [DOI] [PubMed] [Google Scholar]
- 10.Timchenko NA. Aging and liver regeneration. Trends Endocrinol Metab. 2009;20:171–176. [DOI] [PubMed] [Google Scholar]
- 11.Kireev RA, Cuesta S, Ibarrola C, et al. Age-related differences in hepatic ischemia/reperfusion: gene activation, liver injury, and protective effect of melatonin. J Surg Res. 2012;178:922–934. [DOI] [PubMed] [Google Scholar]
- 12.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–790. [DOI] [PubMed] [Google Scholar]
- 13.Braat AE, Blok JJ, Putter H, et al. The Eurotransplant Donor Risk Index in liver transplantation: ET-DRI. Am J Transplant. 2012;12:2789–2796. [DOI] [PubMed] [Google Scholar]
- 14.Anderson CD, Vachharajani N, Doyle M, et al. Advanced donor age alone does not affect patient or graft survival after liver transplantation. J Am Coll Surg. 2008;207:847–852. [DOI] [PubMed] [Google Scholar]
- 15.Ghinolfi D, Marti J, De Simone P, et al. Use of octogenarian donors for liver transplantation: a survival analysis. Am J Transplant. 2014;14:2062–2071. [DOI] [PubMed] [Google Scholar]
- 16.Barbier L, Cesaretti M, Dondero F, et al. Liver transplantation with older donors: a comparison with younger donors in a context of organ shortage. Transplantation. 2016;100:2410–2415. [DOI] [PubMed] [Google Scholar]
- 17.Darius T, Monbaliu D, Jochmans I, et al. Septuagenarian and octogenarian donors provide excellent liver grafts for transplantation. Transplant Proc. 2012;44:2861–2867. [DOI] [PubMed] [Google Scholar]
- 18.Shaw AC, Joshi S, Greenwood H, et al. Aging of the innate immune system. Curr Opin Immunol. 2010;22:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lo DJ, Weaver TA, Stempora L, et al. Selective targeting of human alloresponsive CD8+ effector memory T cells based on CD2 expression. Am J Transplant. 2011;11:22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halazun KJ, Rana AA, Fortune B, et al. No country for old livers? Examining and optimizing the utilization of elderly liver grafts. Am J Transplant. 2018;18:669–678. [DOI] [PubMed] [Google Scholar]
- 21.Lipshutz GS, Hiatt J, Ghobrial RM, et al. Outcome of liver transplantation in septuagenarians: a single-center experience. Arch Surg. 2007;142:775–781. [DOI] [PubMed] [Google Scholar]
- 22.Dasari BV, Mergental H, Isaac JR, et al. Systematic review and meta-analysis of liver transplantation using grafts from deceased donors aged over 70 years. Clin Transplant. 2017;31:e13139. [DOI] [PubMed] [Google Scholar]
- 23.Ghinolfi D, Lai Q, Pezzati D, et al. Use of elderly donors in liver transplantation: a paired-match analysis at a single center. Ann Surg. 2017;1. [DOI] [PubMed] [Google Scholar]
- 24.Bertuzzo VR, Cescon M, Odaldi F, et al. Actual risk of using very aged donors for unselected liver transplant candidates. Ann Surg. 2017;265:388–396. [DOI] [PubMed] [Google Scholar]
- 25.Olthoff KM, Kulik L, Samstein B, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transplant. 2010;16:943–949. [DOI] [PubMed] [Google Scholar]
- 26.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. [DOI] [PubMed] [Google Scholar]
- 27.Chapman WC, Vachharajani N, Collins KM, et al. Donor age-based analysis of liver transplantation outcomes: short- and long-term outcomes are similar regardless of donor age. J Am Coll Surg. 2015;221:59–69. [DOI] [PubMed] [Google Scholar]
- 28.Pagano D, Grosso G, Vizzini G, et al. Recipient-donor age matching in liver transplantation: a single-center experience. Transplant Proc. 2013;45:2700–2706. [DOI] [PubMed] [Google Scholar]
- 29.Bittermann T, Goldberg DS. Quantifying the effect of transplanting older donor livers into younger recipients. Transplantation. 2018;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller X, Marcon F, Sapisochin G, et al. Defining benchmarks in liver transplantation: a multicenter outcome analysis determining best achievable results. Ann Surg. 2017;1. [DOI] [PubMed] [Google Scholar]
- 31.Volk ML, Vijan S, Marrero JA. A novel model measuring the harm of transplanting hepatocellular carcinoma exceeding Milan criteria. Am J Transplant. 2008. [DOI] [PubMed] [Google Scholar]
- 32.Alberti KG, Zimmet P, Shaw J. IDF epidemiology task force consensus group. The metabolic syndrome—a new worldwide definition. Lancet. 2005;366:1059–1062. [DOI] [PubMed] [Google Scholar]
- 33.Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. [DOI] [PubMed] [Google Scholar]
- 34.Hajhosseiny R, Matthews GK, Lip GY. Metabolic syndrome, atrial fibrillation, and stroke: tackling an emerging epidemic. Heart Rhythm. 2015;12:2332–2343. [DOI] [PubMed] [Google Scholar]
- 35.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 36.Lai JC, Dodge JL, Sen S, et al. Functional decline in patients with cirrhosis awaiting liver transplantation: results from the functional assessment in liver transplantation (FrAILT) study. Hepatology. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lai JC, Covinsky KE, Dodge JL, et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology. 2017;66:564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lai JC, Segev DL, McCulloch CE, et al. Physical frailty after liver transplantation. Am J Transplant. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Englesbe MJ, Patel SP, He K, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schlegel A, Scalera I, Perera MTPR, et al. Impact of donor age in donation after circulatory death liver transplantation: is the cutoff “60”; still of relevance? Liver Transpl. 2018;24:352–362. [DOI] [PubMed] [Google Scholar]
- 41.Croome KP, Mathur AK, Lee DD, et al. Outcomes of donation after circulatory death liver grafts from donors 50 years or older. Transplantation. 2018;102:1108–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Boer JD, Blok JJ, Putter H, et al. Optimizing the use of geriatric livers for transplantation in the Eurotransplant region. How to deal with an ageing donor population? Liver Transplant. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


