Summary:
The physical microenvironment of pancreatic tumors is highly abnormal, and this causes significant challenges for drug delivery through multiple mechanisms. Measurements of tissue elasticity could be used as a physical biomarker to assess aberrant drug delivery, and potentially guide stroma-targeting treatment strategies and patient stratification.
In this issue of Clinical Cancer Research, Wang and colleagues demonstrate that shear modulus, a measure of tissue stiffness measured using elastography, correlates with drug delivery in two orthotopic mouse models of pancreatic tumors (1). While increased matrix density can cause increased stiffness and hinder drug delivery, there are other aspects of the physical tumor microenvironment (PhysTME) that also affect drug delivery in desmoplastic tumors. To give the reader a more accurate view of the complex interplay between the PhysTME and drug delivery, we will start with a brief overview of the key elements of abnormal PhysTME in desmoplastic tumors, describe their etiology, and discuss the mechanisms underlying aberrant drug delivery in tumors. We will also discuss the translational and clinical relevance of elastography, one of the very few non-invasive modalities for characterizing the PhysTME, and discuss the potential use of elastography as a surrogate for abnormal physical properties in tumors.
The three main physical abnormalities in most desmoplastic tumors – including pancreatic adenocarcinomas – are (2): (i) increased stiffness (also known as rigidity and elasticity), (ii) elevated solid stresses, and (iii) elevated interstitial fluid pressure (IFP). Stiffness or rigidity, defined as the resistance to deformation, is the first tumor mechanical abnormality to be recognized, and is the basis for well-established diagnostic and prognostic methods for a variety of cancer types. The biological processes that contribute to increased stiffness are deposition of extracellular matrix (ECM) and increased cross-linking of ECM constituents. Relevant to this discussion, matrix deposition and crosslinking tend to reduce the amount of space in the tissue available for movement of drugs and other materials (i.e., “pore size”) (3), thus restricting drug access into the tumor. It is well known that dense tissues are stiffer and have lower drug diffusion rates (3) (Fig. 1); Wang and colleagues have, for the first time, confirmed this relationship by mapping drug delivery and tissue elasticity.
Figure 1. Drug delivery in tumors are affected by physical microenvironment.
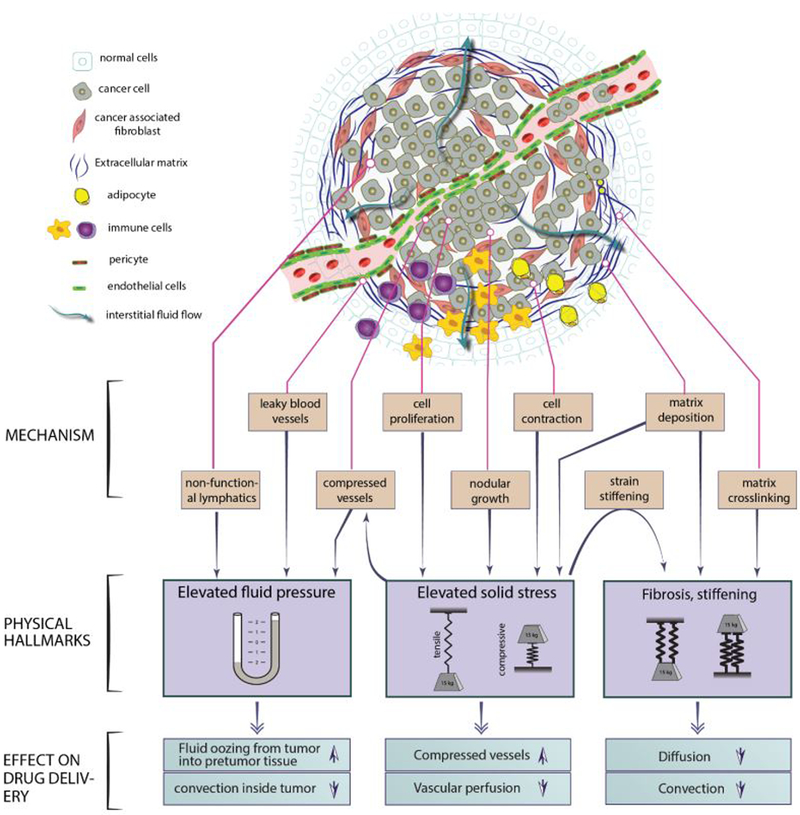
Elevated fluid pressure, solid stress and stiffness, the three main features of physical microenvironment in most tumors with distinct origins and consequences affect drug delivery through different mechanisms.
Tissues can be stiff or soft, but they can also have regions that are under tension or compression. Tension and compression are mechanical solid stresses, the result of forces contained and transmitted by solid components of the tissue including the ECM and cellular elements. Solid stresses are elevated in many fibrotic tumors, but are nearly zero in the corresponding normal tissues (2, 4). Solid stresses are caused by cell proliferation or infiltration, matrix deposition, gel swelling, and resistance to the volume expansion from within and outside the tumor through the ECM and cell adhesion. Solid stresses impede drug delivery by compressing blood and lymphatic vessels (2) (Fig. 1). When solid stress is reduced via anti-fibrotic treatments, drug delivery increases through decompression of blood vessels (5). This results in an improvement in tumor response and overall survival when anti-fibrotic strategies are combined with anti-cancer treatments (2, 5) [JE Murphy et al. A phase II study of neoadjuvant FOLFIRINOX in combination with losartan followed by chemoradiotherapy in locally advanced pancreatic cancer: R0 resection rate and clinical outcomes (submitted)].
Elevated stiffness and pent-up solid stresses are structural/mechanical properties of the tumor; in addition, there are abnormalities in the fluid environment of tumors that interfere with drug delivery. Normally, fluid travels easily through tissue between the blood and lymphatic systems, maintaining a fluid pressure close to zero in the interstitium. However, there are no functional lymphatic vessels in tumors, and the blood vessels are abnormally leaky. Thus, interstitial fluid pressure (IFP) is elevated throughout the tumor, only decreasing at the tumor periphery where functional lymphatics exist. The elevated IFP is approximately uniform across the tumor volume, with an abrupt drop at the tumor-normal tissue interface. The high uniform IFP across the tumor impedes the transport of drug and nutrients from the blood vessels within tumors. The sharp drop in IFP at the tumor edge also causes fluid to “ooze” into the peritumor normal tissue that promotes “washing” of the drug along with growth factors and cytokines from the tumor into the normal surrounding tissue (2).
Because of the well-established links between drug delivery and the PhysTME (Fig. 1), efforts to characterize tumor mechanopathologies have intensified. While there are several approaches to quantify the three major components of the PhysTME, the only one that can currently be measured non-invasively is stiffness. Wang and colleagues proposed using shear wave elastography to map the shear modulus of pancreatic tumors and examine how well the shear modulus correlates with drug delivery in pancreatic tumors. This work is one of the first studies to provide a non-invasive measurement for a physical biomarker to correlate with drug delivery. Wang and colleagues created orthotopic human xenograft models of pancreatic cancer by implanting human cells in immune deficient mice. Although a syngeneic model would have recapitulated the highly desmoplastic human pancreatic tumors more closely, the authors verified the presence of the key ECM components: collagen (via trichome staining) and hyaluronic acid (HA). The delivery of verteporfin – an FDA-approved fluorescent drug – was mapped in the tumor after intravenous perfusion. An important aspect of the study is the correlation of the spatial maps of drug delivery and ECM content with those of shear modulus. They perform these correlations within each tumor as well as globally, i.e., across mice. A key finding is that shear modulus inversely correlates with drug uptake, when compared globally. As expected, shear modulus was shown to correlate strongly with collagen both globally and locally. Interestingly, the correlation between shear modulus and HA has been weak when compared globally, while they seem to correlate reasonably well when compared locally.
The main question that Wang and coworkers attempted to answer was whether stiffness is an appropriate biomarker to predict drug delivery. Another question indirectly addressed in this study was whether stiffness measurements are related to solid stress levels. Although increased matrix deposition is a common source of stiffness and solid stress, solid stress is also accumulated through other mechanisms and is dramatically affected by growth pattern. For example, two tumors generated from the same pancreatic cancer cells might have similar stiffness values, but the solid stress levels can be significantly different (4).
In light of the discussion above, drug delivery is affected by three distinct abnormalities of the PhysTME, all based on different biological and/or physical mechanisms, including transport within blood vessels, transport from vessels into tissue, and transport through the interstitium. Matrix densification, which is reflected in increased stiffness, affects the interstitial transport outside the blood vessels. Although important, this does not reveal the whole story. To rigorously predict drug delivery, we would also need to know whether solid stresses are restricting local fluid flow by compressing blood or lymphatic vessels. In addition, we would need to know whether the drug can easily move across the vessel walls and whether IFP is elevated. Therefore, while tumor tissue elastography indicates how dense the matrix is, it cannot assess other properties that affect drug delivery in tumors. For a more accurate estimation of drug delivery, measurement of both solid stress and IFP would be needed.
Fortunately, elastography may be further extended to help fill this unmet need. Conventional elastography, based on linear elasticity, estimates stiffness, which does not necessarily predict solid stress in tumors. However, by utilizing the strain-stiffening mechanism, a non-linear effect that describes how stiff a tissue becomes under tension or compression, it may be possible to provide a measure of solid stress using modifications of elastography. Ongoing research into the strain-stiffening phenomenon may result in new elastography modalities to decouple stiffness from solid stress and provide a non-invasive measure of solid stress in tumors.
Another challenge is to provide elasticity maps of pancreatic tumors in situ. Wang et al. performed the measurement ex vivo with tumors embedded in a gel for a higher quality of imaging, as extending the methodology to measuring the shear modulus deep in patient tissues is not straightforward. Tumors such as breast and brain are relatively accessible, and thus, shear wave elastography can be performed in vivo. However, pancreatic tumors are deeper in the abdomen, and the surrounding organs confound the use of shear wave elastography. Currently, elastography in pancreatic tumors is limited to endoscopic ultrasound elastography (EUS), which provides a relative map of stiffness within a tumor, and therefore cannot be used longitudinally or to compare results among patients. Non-invasive, large-scale quantification of tumor stiffness, which can be used to compare stiffness of tumors among patients, is an unmet need with high clinical potential for improved detection, diagnosis, and design of anti-fibrotic treatment strategies for pancreatic and other desmoplastic cancers.
Acknowledgments
Funding: This work was supported by the National Cancer Institute (NCI; P01-CA080124, P50-CA165962, R01-CA129371, R01-CA208205, U01-CA 224348), NCI Outstanding Investigator Award (R35-CA197743), the Lustgarten Foundation, the Ludwig Center at Harvard, the National Foundation for Cancer Research, and the Gates Foundation (all to RKJ), R01-HL128168 (LLM), and F32-CA216944–01 (HTN).
Footnotes
Conflict of interest: RKJ received honorarium from AMGEN, consultant fees from Pfizer, Ophthotech, Merck, SPARC, SynDevRx and XTuit. RKJ owns equity in Enlight, Ophthotech and SynDevRx, and serves on the Board of Directors of XTuit and the Boards of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, the Tekla Healthcare Opportunities Fund and the Tekla World Healthcare Fund. LLM receives equity from Bayer Pharmaceuticals. No funding or reagents from these organizations were used in this work.
References
- 1.Wang H, Mislati R, Ahmed R, [...], Doyley M. Elastography can map the local inverse relationship between shear modulus and drug delivery within the pancreatic ductal adenocarcinoma microenvironment. Clinical Cancer Research. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain RK, Martin JD, Stylianopoulos T. The role of mechanical forces in tumor growth and therapy. Annual Review of Biomedical Engineering. 2014;16:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer research. 2000;60(9):2497–503. [PubMed] [Google Scholar]
- 4.Nia HT, Liu H, Seano G, Datta M, Jones D, Rahbari N, et al. Solid stress and elastic energy as measures of tumour mechanopathology. Nature Biomedical Engineering. 2016;1:0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chauhan VP, Martin JD, Liu H, Lacorre DA, Jain SR, Kozin SV, et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nature Communications. 2013;4. [DOI] [PMC free article] [PubMed] [Google Scholar]


