Abstract
Parkinson’s disease is a progressive neurodegenerative disorder of aging. The hallmark pathophysiology includes the development of neuronal Lewy bodies in the substantia nigra of the midbrain with subsequent loss of dopaminergic neurons. These neuronal losses lead to the characteristic motor symptoms of bradykinesia, rigidity, and rest tremor. In addition to these cardinal motor symptoms patients with PD experience a wide range of non-motor symptoms, the most important being cognitive impairments that in many circumstances lead to dementia. People with PD experience a wide range of cognitive impairments; in this review we will focus on memory impairment in PD and specifically episodic memory, which are memories of day-to-day events of life. Importantly, these memory impairments severely impact the lives of patients and caregivers alike.
Traditionally episodic memory is considered to be markedly dependent on the hippocampus; therefore, it is important to understand the exact nature of PD episodic memory deficits in relation to hippocampal function and dysfunction. In this review, we discuss an aspect of episodic memory called recognition memory and its subcomponents called recollection and familiarity. Recognition memory is believed to be impaired in PD; thus, we discuss what aspects of the hippocampus are expected to be deficient in function as they relate to these recognition memory impairments. In addition to the hippocampus as a whole, we will discuss the role of hippocampal subfields in recognition memory impairments.
Keywords: Episodic memory, Recognition memory, Hippocampus, Parkinson’s disease, Hippocampal subfields
1. Parkinson’s Disease Cognitive Impairment
Parkinson’s disease is the second most common neurodegenerative disorder of aging. While the diagnosis of PD is based on characteristic motor symptoms, such as bradykinesia (Mata et al, Tsuang et al), rigidity, and rest tremor, it is well established that most patients eventually experience a wide range of cognitive and neuropsychiatric symptoms (for details see (Weintraub & Burn 2011). Cognitive impairments in PD patients can include executive, memory, visuospatial, attentional and language function (Aarsland et al 2010, Cronin-Golomb & Braun 1997, Dujardin et al 1999, Lewis et al 2003, Weintraub & Burn 2011, Weintraub et al 2004). Studies have shown that in early PD patients who are non-demented, the largest effect sizes of impairment are observed in the memory domain, especially immediate and delayed free recall (Muslimovic et al 2005). PD cognitive impairment, and memory impairment in particular, can have a significant impact on both patient and caregiver quality of life as well as increase the risk of morbidity and mortality (Aarsland et al 1999, Aarsland et al 2000). Despite this, there are limited treatments for memory impairment in PD, and none to stop or slow this devastating consequence of disease. In this review we will discuss the types of memory impairment experienced by patients with PD and summarize recent literature on plausible neural correlates.
1.1. Episodic Memory
Memories of our daily lives that pertain to worldly knowledge, facts and everyday events and can be consciously brought to mind and declared (Cohen & Squire 1980) are called Declarative Memories. One subcomponent of declarative memory, called semantic memory, deals with memory of knowledge and facts of the world, by contrast the episodic memory subcomponent refers to daily events of life (Sabbagh et al). Episodic memory entails details of spatial and temporal aspects of an event, i.e., where an event happened and what (Mormino, Mormino et al) was the event. There are four necessary stages in the process of learning and memory (Jack et al): encoding, storage, consolidation and retrieval (Dudai 2004, Melton 1963). Encoding is defined as initial learning of information, storage is maintenance of information over time, consolidation is a process that stabilizes memory after it has been formed and retrieval is the ability to access information when needed. There is another process called reconsolidation wherein previously consolidated memory is recalled and consolidated again (Rodriguez-Ortiz & Bermudez-Rattoni 2007).
A crucial subcomponent of the retrieval stage of episodic memory is recognition memory (Winer et al), which is the ability to recognize previously encountered events, objects or people (Myskiw et al 2008).
Figure 1 shows different subcomponents of Declarative memory.
Figure 1.
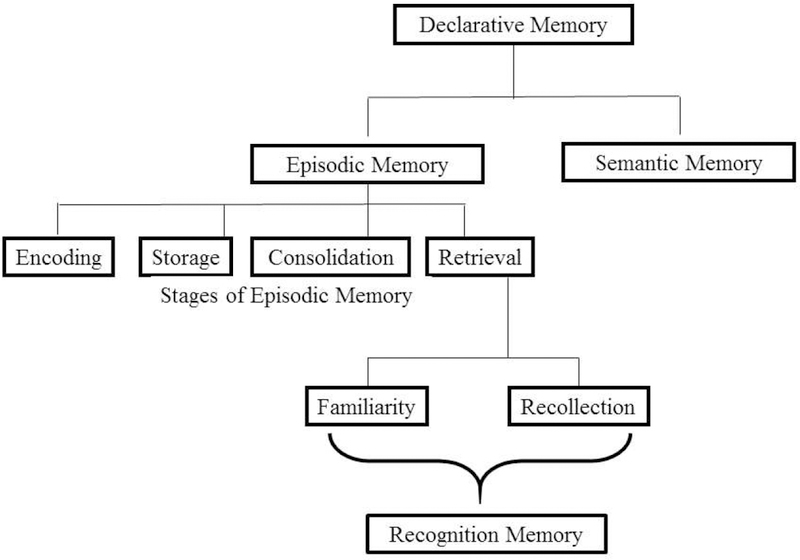
Declarative Memory, Episodic Memory and Subcomponents
Research over the past few decades has demonstrated people with PD can experience deficits in each of these stages of episodic memory (encoding, consolidation, storage and retrieval) as well as other forms of memory like working memory (Labutta et al 1994, Malapani et al 2002, Pillon et al 1993, Warden et al 2016). Here, we will focus on the current literature understanding episodic recognition memory impairment as it happens in PD.
1.2. Episodic Recognition Memory and its Subcomponents
Experimental psychologists had asserted the presence of two major subcomponents of recognition memory, namely familiarity and recollection, which form the foundational cornerstone of recognition memory, sometimes also referred to as knowing and remembering respectively (Jacoby & Dallas 1981, Yonelinas & Jacoby 1994). While familiarity is the feeling that an event has been encountered before, recollection is complete or partial retrieval of the previously encountered event with item and contextual details (Jacoby & Dallas 1981). Since these are thought to be two distinct independent processes involved in recognition, the familiarity-recollection difference in recognition memory is called a dual process theory. According to this theory, familiarity is a signal detection process and recollection is a threshold retrieval process (Yonelinas 1994, Yonelinas & Jacoby 1994). As soon as a previously encountered item seems familiar, it generates a sense of “having-experienced” the previously studied item without retrieval of every single detail and contextual information. Recollection on the other hand is hypothesized to be an all-or-none process where once threshold of recognition is crossed the details of the item are retrieved, either in entirety or partially. The contrasting view of the dual process theory is single process theory, which suggests recollection and familiarity to lie on a continuum and former being a stronger, more vivid representation of latter. The single process theory is supported by an electrode recording study in epileptic patients where spiking activity was analyzed in the hippocampus as patients retrieved items from memory (Rutishauser et al 2006) (For details of the hippocampus, refer to Box. 1). In this study, the authors observed stronger neuronal responses during complete recollection of items with contextual details, weaker responses as items became familiar but not recollected, and weakest responses for forgotten items. They suggested their data was thus compatible with a continuous signal of memory strength; the stronger the neuronal response the better the memory. By contrast, evidence for the dual process theory came from a scalp-recorded event-related brain potential (ERP) study wherein the amnestic drug midazolam selectively impaired putative ERP-correlate of recollection and not putative ERP-correlate of familiarity (Curran & Mintzer 2006). In control saline conditions, accuracy correlated with recollection-related ERP but not familiarity-related ERP. Hence the researchers proposed that recollection was the dominant default process and after midazolam administration, recollection was adversely affected, thereafter the brain resorted to familiarity for recognition.
Box. 1. Hippocampus and its subfields.
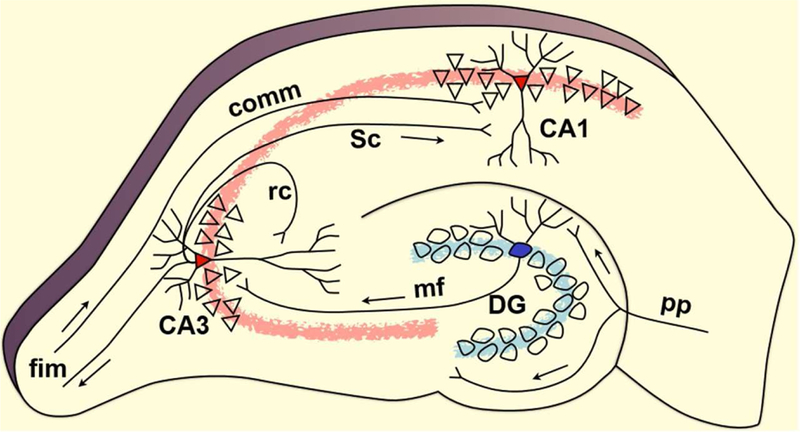
The complex episodic memory processes happen in a small seahorse-shaped structure of brain called the hippocampus, which is located within the medial temporal lobe. The crucial role of the hippocampus and its adjacent regions including the entorhinal cortex in episodic memory was demonstrated when patient H.M. became amnesic after surgical removal of the bilateral hippocampus (Scoville & Milner, 1957). The hippocampus proper consists of subfields called cornu ammonis (CA) fields, namely CA1, CA2, CA3, and CA4, dentate gyrus (DG), and subiculum. Along with the surrounding structures called entorhinal cortex (EC), perirhinal cortex and parahippocampal cortex, hippocampus proper forms the hippocampal complex or hippocampal formation, which carries out episodic memory functions efficiently (Figure 2).
The hippocampal formation is a convergence zone where information from neocortical outputs come together (Tamminga, Stan, & Wagner, 2010). These are information from association areas and sensory areas of neocortex that project onto perirhinal and parahippocampal cortices, which in turn project onto EC. The EC has direct connections with each of the subfields of hippocampus proper. In addition, EC has indirect connections in the form of a trisynaptic pathway; EC projects onto DG through perforant pathway (pp), DG projects onto CA3 via mossy fibers (mf), and CA3 projects onto CA1 via Schaffer collaterals (Sc). CA3’s own pyramidal cells project back onto themselves called recurrent collaterals (rc), and to CA1 through Schaffer collaterals. This trisynaptic pathway is mostly feedforward with very little feedback. The fimbria (fim) is one of the principal output pathways of hippocampus that also brings in commissural (comm) input from contralateral hippocampus.
This systematic, hierarchical organization of information coming from the neocortex converges in the hippocampus and binds together many different associative episodes into a conjunctive representation (Tamminga et al., 2010). Even though different subfields within the hippocampus might have different roles in information processing and mnemonic function; collectively the individual computations and interactions within the whole system flexibly captures co-occurrence of related episodes into a bound representation and in the process, enable episodic memory (Lavenex et al 2007, Leutgeb & Leutgeb 2007, Suzuki & Amaral 2003, Tamminga et al 2010). To this end, studies have indicated the role of CA3 in pattern separation and pattern completion wherein differences between associated events are captured in former; and related events are bound together, completing the picture in latter.
Figure 2 shows a schematic of hippocampus, taken from (Yassa & Stark 2011) with permission. The schematic depicts the trisynaptic pathway, where neurons in Layer II of Entorhinal Cortex project onto dentate gyrus (DG) through perforant pathway (pp), granule cells in DG project onto CA3 field through mossy fibers (mf) pathway, CA3’s own pyramidal cells project back onto themselves called recurrent collaterals (rc), and to CA1 through Schaffer collaterals (Sc). This trisynaptic pathway is mostly feedforward with very little feedback. The fimbria (fim) is one of the principal output pathways of hippocampus that also brings in commissural (comm) input from contralateral hippocampus.
Further evidence for dual process theory comes from behavioral studies of recognition using receiver operating characteristic (ROC) plots. For details on ROC refer to Box. 2 and see (Yonelinas 1994, Yonelinas & Jacoby 1994, Yonelinas et al 2005). Briefly, ROC curves can be used to plot the proportion of correct recognition for items (hit rates) on y-axis and proportion of incorrect recognition (false alarms) on x-axis. The ROC curve is usually asymmetrical and the amount of asymmetry increases with accuracy, in which case the curve is pushed towards top left of plot (Box. 2 and Figure 3). But experimental evidence demonstrates recognition accuracy and degree of ROC curve asymmetry to be functionally independent (Ratcliff et al 1992, Yonelinas & Jacoby 1994), strongly suggesting that recognition memory has at least two separate memory components. The single process theory is insufficient in describing the variations in ROC curve asymmetry, whilst the dual process theory can describe this in the context of familiarity and recollection. As more items are recollected accurately, the hit rate increases and thus the ROC curve shifts more towards the top left of the plot and becomes asymmetrical. Contrastingly, if performance relies exclusively on familiarity, then the ROC curve is symmetrical.
Box. 2. Receiver Operating Characteristics (ROC).
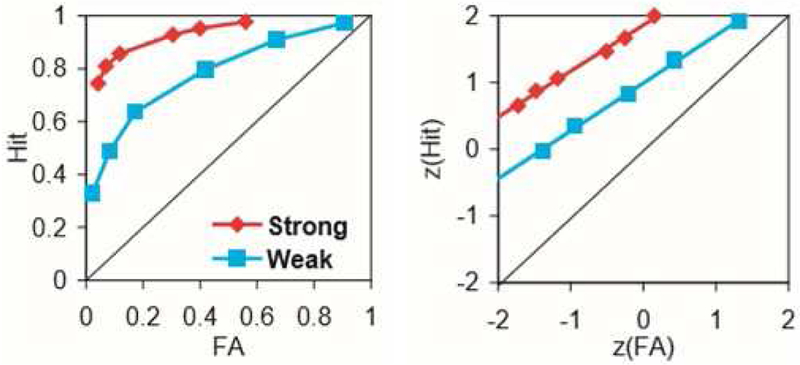
Recognition memory is the memory of objects, events or people that have been encountered before. In a cognitive task where items that have been previously presented, are shown again, and subjects are asked to respond if the item has been encountered in the study before or not. A correct response is called “hit” and incorrect response is “false alarm” (F A). Plotting hits against FA rates gives the Receiver Operating Characteristics (ROC) plot. The ROC curve can be plotted in two different ways: first in probability space where y-axis is proportion of hit rate and x-axis is proportion of FA rate, and this plot is curvilinear (see Figure 3 left). Secondly, ROC can also be plotted by z-transforming hits and FA and plotting them on y and x-axes respectively. In z-space ROC is approximately linear (Figure 3 right).
As the hit rates increase, i.e., as accuracy increases, the ROC curve becomes more asymmetrical because it’s pushed towards top left of the plot corresponding to increased hit rates (Figure 3 left and right boxes, red ROC). On the other hand, as accuracy drops, hit rates decrease and the curve starts to become more symmetrical. Chance performance is represented by the diagonal in each plot. In the plot shown in Figure 3, adapted from (Eichenbaum et al 2007), two types of encoding were performed, strong deep encoding in red (items studied twice) and weak shallow encoding in blue (items studied once). Deep encoding involved meaning while shallow encoding involved knowing only perceptual features of items. In items that were learnt through deep encoding the subjects showed higher hits and thus the ROC curve is pushed higher up in the plot, is more asymmetrical and the bow of the curve more towards left. The bow of the curve depicts sensitivity, higher the bowing of curve, higher is ROC sensitivity in detecting hit rate. In Figure 3 left, red plot has higher bowing and thus more hits. While, for items learnt using shallow encoding, the ROC attains more symmetry and lies lower in the plot (Figure 3 left).
When ROC is plotted in z-space, increased accuracy or hit rate is manifested in higher z-intercept and decrease in slope (Figure 3 right). Z-intercept for red plot is higher than blue and thus red plot shows higher accuracy than blue plot.
When applied to recognition memory, several studies (Yonelinas 2001, Yonelinas & Jacoby 1994, Yonelinas & Levy 2002) showed a dissociation between sensitivity and asymmetry of the recognition memory ROC curve, indicating presence of two independent recognition memory processes. According to dual process theory of recognition memory, these two processes are recollection and familiarity, wherein recollection is retrieval of item memory with its contextual details partially or in entirety and familiarity being a feeling of “previously seen” bu t without retrieval of item or its context. The y-intercept in z-space or probability space plot gives a measure of recollection because y-axis is the hit rate. Additionally, degree of curvilinearity in ROC provides a measure of familiarity, more symmetrical ROC curve represents familiar, and asymmetrical ROC curve represents recollection. For a detailed reading on ROC plots in the context of recognition memory, see (Yonelinas 1994, Yonelinas et al 1996, Yonelinas et al 2005).
Figure 3 (from (Eichenbaum et al 2007) with permission) shows ROC, left plot is a plot of hit rates of correctly recognized items on y-axis and false alarm rates on x-axis; right plot is z-transform plot of hits vs false alarm rates.
Figure. 3.
Receiver Operating Characteristic plot (ROC) in probability space (left) and z-space (right)
1.3. Hippocampus in Recollection
There is broad consensus that the hippocampus has a significant role in declarative memory and has been implicated in the recollection aspect of recognition memory (Bird 2017, Brown & Aggleton 2001, Kim et al 2014), while other studies show crucial role of perirhinal cortex in familiarity (Brown & Banks 2015). It has been traditionally thought that the prefrontal cortex (PFC) has a crucial role in executive functions like selection (Badre et al 2005, Badre & Wagner 2007, Dulas & Duarte 2016), engagement of retrieval mode (Lepage et al 2000), monitoring (Dulas & Duarte 2014, Henson et al 1999) and inhibition (Aron et al 2004). These very same PFC regions have also been reported to contribute towards both formation and retrieval of both long-term declarative and working memory (Aron et al 2004, Blumenfeld & Ranganath 2006, Ranganath et al 2004, Ranganath et al 2003, Rubin et al 2017). For example, replay of hippocampal cell ensembles is believed to contribute to memory consolidation (Karlsson & Frank 2009) and this has been shown to be accompanied by concomitant activity in PFC ensembles (Benchenane et al 2010, Brockmann et al 2011) as well. Hence, the hippocampus and PFC work together as a network, contributing towards memory formation, consolidation and retrieval or recollection. In this review we will focus on the less appreciated hippocampal contributions to episodic memory recollection in PD (as discussed in section 2).
Further, episodic memory recollection has been shown to be associated with the successful execution of two processes called Pattern Completion and Pattern Separation. Hippocampal subfield CA3 has recurrent collaterals (Box. 1), which are capable of forming auto-associative networks, first suggested by David Marr (Marr 1971). In situations of incomplete stimulus or degraded representations, these auto-associative networks can possibly fill in and complete the picture based on previously stored representations. This is pattern completion, which aids in recollection even during insufficient input. Since daily life events are overlapping, there is another mechanism that separates similar events into distinct and non-overlapping ones, called pattern separation (Yassa & Stark 2011). Even though recollection might be possible in both presence and absence of pattern separation, accuracy of recollection is more driven by the presence of pattern separation (Kim & Yassa 2013). Theories of episodic relational memory suggest that hippocampus binds together arbitrarily co-occurring elements of experience into a wholesome representation and aids in recollection (Aggleton & Brown 1999, Eichenbaum et al 2007, Montaldi & Mayes 2010, Rugg & Yonelinas 2003), it has been implicated in forming associations between different memories as well. Therefore, selective hippocampal damage drastically affects recognition memory recollection (Bird 2017, Mumby 2001, Winters et al 2004) and recollection is intricately associated with successful pattern separation and pattern completion. Furthermore, the PFC also contributes towards episodic memory as it builds more inherently meaningful relations, taking the larger context in which, the memory happened (Preston & Eichenbaum 2013, Rubin et al 2017, Schwarb et al 2015, Zeithamova et al 2008). The hippocampus-PFC network has also been proposed to work iteratively, that is as new memories are formed, they are integrated into already existing schemas and modified as needed (Preston & Eichenbaum 2013, van Kesteren et al 2012).
Studies in non-human primates have shown a crucial role of the hippocampus in recollection. Selective damage to the hippocampus or surrounding area has demonstrated declarative memory impairment using two tasks: delayed matching to sample (DMS) and delayed non-matching to sample (DNMS). In these tasks, an item is shown and after some variable delay is presented again with a novel unstudied item and the subject indicates either the previously studied (DMS) or unstudied (DNMS) item (Gaffan 1974, Mishkin 1978). In primates with hippocampus or fornix damage, recognition memory performance was affected only with repeated items, not with items that were never repeated (Charles et al 2004). As discussed earlier, since the hippocampus is known to bind context and items producing recollection, therefore, when items were repeated to primates with selective hippocampal damage, they were unable to bind contextual information of previously seen items and failed to recollect.
Findings in human patients with hippocampal damage support the dual process theory as well. Hippocampal or fornix damage has been shown to be congruent with deficit in recollection but not familiarity (Aggleton et al 2000, Tsivilis et al 2008). These patients had preserved single item memory, most likely because contextual details like background scene were not required to be recollected in these studies. On the other hand, patients with unilateral damage to the perirhinal or entorhinal cortex exhibited deficits in familiarity but not recollection (Brandt et al 2016).These findings indicate a significant role of hippocampus in recollection of an item with all contextual details possible, while perirhinal and parts of entorhinal cortex for familiarity. These patients therefore, exhibited a double dissociation between recollection and familiarity, which strongly points out the two processes to be independent, as proposed by the dual process theory.
Using functional MRI, the hippocampus and the posterior parahippocampal gyrus have been shown to elicit activation for recollected than non-recollected items (Davachi et al 2003, Eichenbaum et al 2007, Uncapher & Rugg 2005, Yonelinas et al 2005), but also see (Merkow et al 2015). By contrast, the anterior parahippocampal gyrus (which includes the perirhinal and entorhinal cortex) exhibits significant activations for familiarity and rarely for recollection (Daselaar et al 2006, Davachi et al 2003, Gold et al 2006, Gonsalves et al 2005, Kensinger & Schacter 2006, Kirwan & Stark 2004, Montaldi et al 2006, Weis et al 2004a, Weis et al 2004b).
It’s important to understand the function of individual subfield of hippocampus in recollection. In one such 7T fMRI study (Suthana et al 2015), an associative memory task was used, where face-name pair had to be learnt and recollected later. Activations were observed in anterior CA2, CA3 during learning (encoding) and posterior CA2, CA1 and posterior subiculum during recollection. In another study, the subiculum elicited strong and consistent activation for items that were consistently recollected at short (10 minutes) or long (one week) delays, but not for familiar or forgotten items (Viskontas et al 2009). Other studies have shown overlapping videos that had to be recollected later and found strong activations in CA3 with successful recollection that was dependent on the size of CA3 as well (Chadwick et al 2014); although studies have also shown activation suppression in CA3-DG as an item is repeated and accuracy of recollection increases (Reagh et al 2014). In a study, when houses were shown in different videos, a multivariate pattern analysis demonstrated activations in CA2–3-DG when same houses were presented in different videos and activations in CA1 when same houses were presented in same videos (Dimsdale-Zucker et al 2018). In support of these findings, rodent electrophysiological studies also show that CA3 could be responsible for both pattern separation and pattern completion (Guzowski et al 2004, Lee et al 2004, Leutgeb et al 2004, Vazdarjanova & Guzowski 2004). These studies demonstrate that in cases of small difference between two environments, there is greater overlap in CA3 pertaining to the representations of the two environments (pattern completion) than in CA1. On the other hand, with larger differences between the two environments, overlap between representations of two environment is lesser in CA3 (pattern separation) than CA1. Therefore, the evidences so far implicate a strong role of hippocampal subfields subiculum, CA2, 3 and DG in recollection, and especially CA3 in deciphering similar overlapping events as distinct, which is a non-trivial process for successful recollection to happen.
The fMRI studies discussed above demonstrate crucial roles of CA1, CA2–3-DG and subiculum during recollection. It has been shown that pre and parasubiculum (which are parts of subicular complex) have access to holistic representations of objects and scenes, as these cortical areas receive integrated information from foveal and peripheral visual inputs (Dalton & Maguire 2017). Hence subiculum and surrounding areas receive rich visual inputs (Dalton & Maguire 2017) and aid in recollection after short and long delays (Viskontas et al 2009). We also discussed the roles of CA3 in pattern separation and pattern completion: CA3 receives afferents from DG via mossy fibers (see Box. 1) and entorhinal cortex, these afferents can modulate pattern separation and pattern completion, in turn modifying the processes of encoding and recollection (Kesner 2013). Moreover, recurrent collaterals within the CA3 have been suggested to aid in pattern completing an incomplete information or scene (Kesner 2013), which might explain why participants showed CA3 activation when same houses were shown in different videos (Dimsdale-Zucker et al 2018), probably they tried to remember the same houses from different videos and attempted to pattern complete. Further, it has been suggested that CA3 holds information for short-term, due to neural activity within its recurrent network. Hence, CA3 acts as a buffer and helps retrieve information from short-term memory, which is usually the case in cognitive tasks used in an experimental set-up, for example the recollection tasks (Kesner et al 2008, Kesner & Rolls 2001, Rolls & Kesner 2006). When the delay is prolonged beyond the short-term range of 5 minutes to 24 hours, CA1 seems to become more dominant (Lee & Kesner 2003). Also, CA1 is known to be critical for recollecting long term autobiographical memories. Hence CA1 has a key role in recollection, particularly re-experiencing a memory. In the fMRI study (Dimsdale-Zucker et al 2018), wherein they observed activations in CA1 when same houses were presented in the same videos, the participants were plausibly re-experiencing the memory of houses in the same set-up. Thus, within the hippocampus, subfields CA2, CA3, DG and subiculum have been specifically implicated to play a role in recollection; whilst the surrounding areas of perirhinal, parts of parahippocampal and entorhinal cortex have been implicated more in familiarity.
2. Episodic Memory and Cognition in Parkinson’s Disease
Parkinson’s disease is characterized by neuronal Lewy bodies in the substantia nigra of the midbrain with subsequent loss of dopaminergic neurons, which lead to typical motor symptoms of bradykinesia (slowness of movement), rigidity, and rest tremors. In addition to these cardinal motor symptoms, patients with PD experience a wide range of non-motor symptoms, most importantly cognitive and memory impairment that in many patients will eventually lead to dementia (Hely et al 2008, Kehagia et al 2010).
In PD patients, cognitive difficulty can be present in multiple domains, but episodic memory impairment is one of the most common cognitive symptoms reported, which can have a substantial impact on quality of life. One study found over 20% of newly diagnosed patients endorsed a memory complaint (Breen & Drutyte 2013) and episodic memory was the most commonly impaired domain at baseline in two large cohorts of newly diagnosed patients (Weintraub et al 2015, Yarnall 2014). Specifically, in the PPMI cohort 17% of de novo PD patients showed impairment on the Hopkins Verbal Learning Test (HVLT), a test for recognition memory. Furthermore, in the ICICLE-PD cohort 15–20% showed impairment on Pattern and Spatial Recognition Memory (Yarnall et al 2014). Episodic memory impairment is also common later in the disease. One meta-analysis of 1,346 patients from 8 different cohorts of PD patients with mild cognitive impairment (PD-MCI) found that over 50% of PD-MCI had memory impairment whereas only 39% had executive dysfunction (Aarsland & Kurz 2010)
PD-MCI is defined by impairments beyond what is expected for age, but not severe enough to affect activities of daily living. As these memory deficits continue to progress most patients will eventually be diagnosed with PD dementia (PDD), which occurs when cognition is impaired enough to affect daily functioning (Emre et al 2007). Older age of onset (Cholerton et al 2013, Hely et al 2005, Hely et al 2008, Levy et al 2002) and longer duration of motor symptoms are the most commonly reported predictors of PD memory decline and dementia (Aarsland et al 2009, Chahine et al 2016). However, recent reports have also found cerebral amyloid (Leaver & Poston 2015), APOEɛ4 (Bekris et al 2015, Mata et al 2014, Tsuang et al 2013) and male gender associated with increased risk of PD memory impairment (Cholerton et al 2018, Watson et al 2013) and PDD (Irwin et al 2012). The Lewy body pathology progresses from brain stem to limbic to neocortical brain regions in later stages of the disease (Braak et al 2003). Generally speaking Lewy body pathology is more severe and extensive in PDD than PD without dementia (Aarsland et al 2003, Apaydin et al 2002, Braak et al 2005, Compta et al 2011, Irwin et al 2013, Irwin et al 2012, Tsuboi et al 2007).
In addition to Lewy body pathology, a significant percentage of PD patients can have comorbid Alzheimer’s disease (AD)-related pathology at autopsy with diffuse Amyloid-β (Aβ) plaques and tau neurofibrillary tangles (NFT) (Kalaitzakis et al 2008). These aggregates have been found to be inversely correlated with cognitive status in PDD (Compta et al 2011, Jellinger 2007, Jellinger & Attems 2008, Jellinger et al 2002, Kovari et al 2003). PDD with concomitant AD is associated with older age of onset (Irwin et al 2013, Irwin et al 2012, Jellinger et al 2002, Sabbagh et al 2009), more malignant disease progression (Compta et al 2011, Halliday et al 2008), shortened survival time (Compta et al 2011, Halliday et al 2008, Jellinger et al 2007, Kotzbauer et al 2012) and shorter disease duration prior to dementia (Irwin et al 2012, Jellinger et al 2002, Jellinger et al 2007, Mikolaenko et al 2005, Sabbagh et al 2009). However, PD with and without AD co-pathology is extremely difficult, if not impossible, to distinguish based on pre-mortem movement, clinical, and neuropsychologic assessments (Sabbagh et al 2009). Recent advances in AD biomarkers could shed light on this challenge. As previously mentioned, abnormal CSF or PET Aβ is common in PD patients (Leaver & Poston 2015), however it is unclear if this is a marker for fulminant AD-type co-pathology or an earlier stage of pathology (such as that seen in otherwise cognitively normal older adults) (Mormino 2014, Mormino et al 2014). Indeed, Aβ plaque formation is thought to be necessary, but not sufficient, to produce clinical dementia from AD (Jack et al 2013). By contrast, abnormal CSF or PET tau is more specific (Jack et al 2018) to the clinical dementia seen in AD. Initial tau PET studies in PD patients without dementia did not identify measurable tau abnormalities associated with PD-MCI (Winer et al 2018), but future studies using tau PET in PDD would be of great benefit.
Collectively, these studies all agree that progression of Lewy bodies from subcortical to limbic to cortical areas seems to be the major driving force for development of dementia in PDD and PDD becomes increasingly common as the disease advances and is responsible for considerable PD-related disability (Hely et al 2005, Hely et al 1999) with loss of employment, caregiver stress and fatigue, increased cost to health systems, patient institutionalization, and decreased survival (Armstrong et al 2014, Svenningsson et al 2012).
Despite the frequency and severity of PD memory impairments, their exact neural underpinnings have been controversial. Early studies suggested that impaired memory in PD was largely due to executive dysfunction resulting in inefficient use of strategies during memory formation or memory retrieval (Massman et al 1990). It was argued that these impairments stemmed mostly from subcortical pathology and subsequent failure of projections to the frontal cortex via the fronto-striatal pathway. More recent studies have challenged this notion that PD memory impairment is entirely a result of dysfunction, an idea that is substantiated by autopsy studies demonstrating hippocampal alpha-synuclein and Lewy-body pathology (Adamowicz et al 2017, Galvin et al 1999). Other autopsy studies have confirmed that PDD can occur in the presence of hippocampal Lewy bodies alone, without concomitant diffuse cortical Lewy bodies (Churchyard & Lees 1997). Thus, it is now generally thought that the mechanisms underlying episodic memory deficits in PD stem not only from fronto-striatal associated executive dysfunction but also from the medial temporal lobe (MTL) (Gratwicke et al 2015). Specifically, episodic memory deficits in PD can be due to dysfunctional executive system in addition to impairment in the hippocampus (as discussed in section 1.3). For the purposes of this review, our focus will be to discuss the contributions of the hippocampus towards recollection sub-component of episodic memory in PD.
2.1. Episodic Recognition Memory in PD
Several investigators have studied recollection and familiarity focused paradigms to better understand episodic recognition memory deficits in PD. We performed a literature search in PubMed for recognition memory impairment in PD and found 67 articles (search words used were: recognition memory, recollection, familiarity, Parkinson’s disease). We then selected the 10 studies dealing with recollection and familiarity using behavior or functional MRI paradigms in PD. Two of these articles studied recognition in terms of cued and free recall (Breen 1993, Higginson et al 2005), and were thus not included in this review, leaving 8 articles discussing recollection and familiarity in PD (Table 1).
Table 1.
Behavior and fMRI studies of recognition memory in PD
| Study | Task used | Finding |
No. of Participants Mean Age |
Mean Disease Duration (years) |
PD and Dementia Diagnostic Criteria |
Major Clinical Assessment Tools |
|
|---|---|---|---|---|---|---|---|
| Recollection | Familiarity | ||||||
| Davidson et al., 2006 | Yes-no recognition memory test and remember-know-new judgement |
No significant impairmentin PD |
Impaired in PD |
19 PD 66.56 (experiment1), 16 PD 67.05 (experiment 2) |
5.79 6.13 |
Criteria for PD diagnosis not specified |
MMSE MHV |
| Edelstyn et al., 2007 | Yes-no recognition memory test and remember-know judgement |
Impaired in PD |
Unimpaired in PD |
17 PD 65.4 |
8.6 | Criteria for PD diagnosis not specified |
MMSE NART WAIS WR |
| Cohn et al.,2010 | Noun-noun pairs recognition memory test, deep and shallow encoded |
Impaired at deep encoding in PD |
Impaired at shallow encoding in PD |
11 PD 67 |
6.4 | Criteria for PD diagnosis not specified |
MMSE |
| Algarabel et al., 2010 | Yes-no recognition memory test |
Impaired in PDD and Advanced PD |
Impaired only in PDD |
20 Early PD 72.55 19 Advanced PD 75.58 10 PD Dementia 80.00 |
3.22 10.95 6.72 |
-PD diagnosis: UK Brain Bank Criteria -Dementia diagnosis based on DSM-IV criteria |
MMSE BNT WMS TMT-A |
| Weiermann et al., 2010 | Yes-no recognition memory for high and low frequency words; remember-know judgement |
Unimpaired for high frequency words in PD |
Poorer sensitivity index d’ for familiarity in PD |
14 PD 63.2 |
9.43 | Criteria for PD diagnosis not specified |
MMSE Verbal Intelligence (NART) |
| Rodriguez et al., 2014 | Word pairs recognition memory test, 2-alternative- forced-choice test |
Impaired in PD |
Unimpaired in PD |
20 PD 71.45 |
5.32 | - PD diagnosis: UK Brain Bank Criteria -Dementia diagnosis based on MDS criteria (Emre et al., 2007) - Dementia was exclusion criteria |
MMSE |
| Cohn et al., 2016† | Associative Reinstatement Memory Task: fMRI study |
Impaired with decreased hippocampa l activation |
Unimpaired in PD |
6 PD-noMCI 9 multidomain PD-MCI 59.1 |
6.20 | -PD diagnosis: UK Brain Bank Criteria -MCI diagnosis based on MDS level 2 criteria (Litvan et al., 2012) |
MoCA Color TMT- A and B WAIS HVLT-R Category Fluency- Animals |
| Pitarque et al., 2017 | Face-scene picture pairs recognition memory test |
Impaired in PD |
Unimpaired in PD |
32 non- demented PD 69.63 |
Not available |
- PD diagnosis: UK Brain Bank Criteria -Criteria for Dementia diagnosis not specified -Dementia was exclusion criteria |
MMSE WAIS Vocabulary |
Abbreviations: PDD: PD Dementia, MMSE: Mini-Mental State Examination, NART: The National Adult Reading Test, MHV: Mill Hill Vocabulary, WAIS: Wechsler Abbreviated Intelligence Scales, WR: Warrington Recognition, BNT: Boston Naming Test, WMS: Wechsler Memory Scale, TMT: Trail Making Test, MoCA: Montreal Cognitive Assessment, HVLT-R: Hopkins Verbal Learning Test-Revised MDS: Movement Disorders
All studies use behavioral methods except Cohn et al., 2016, which is an fMRI study.
With regard to recollection and familiarity, most studies suggest a disrupted recollection as the critical episodic memory deficit in PD, while familiarity is normal with respect to healthy controls (Algarabel et al 2010, Cohn et al 2016, Cohn et al 2010, Edelstyn et al 2007, Rodriguez et al 2014). However, a few studies have showed the opposite effect, i.e., disruption of familiarity but normal recollection in PD as compared to heathy controls (Davidson et al 2006, Weiermann et al 2010). Weiermann et al., used word-frequency mirror effect and remember-know method to test recollection and familiarity. In the former test, words belonging to low and high frequency categories were shown on computer screen. Thereafter, the words were re-presented after 30 minutes, randomly intermixed with new words and participants responded if words were ‘old’ or ‘new’. In case words were respo nded as ‘old’, they were further asked if they recollected the word with confidence or if they could not recollect but believed they had seen in study phase, in a Remember/Know judgement study. The false alarm rates were lower and hit rates were higher for low frequency than high frequency words in both the PD and healthy control cohort. Recollection was lower for high frequency words in both groups. Additionally, based on Remember/Know judgement, patients were significantly impaired in familiarity for low frequency words.
It is possible that the findings by Weiermann et al may be dependent upon how similar the ‘new’ low frequency words were as compared to the studied words. Pitarque et al (Pitarque et al 2017) extended this idea by investigating how recollection and familiarity change as the familiar items become more similar. By using face-scene pairs, as opposed to the Wiermann et al word pairs, Pitarque et al found that PD patients performed erroneously when cues were more similar to already presented stimuli. This study suggests that patients with PD do exhibit deficits in episodic memory recollection, but as the test items become more and more similar to previously studied items, deficits in familiarity domain start to appear.
One possible explanation for the seemingly opposing findings in the above studies (impaired recollection with unimpaired familiarity versus unimpaired recollection with impaired familiarity) is that there could be a double dissociation between recollection and familiarity in PD, depending on the level of encoding. For example, Cohen et al (Cohn et al 2010) presented word (noun-noun) pairs where some pairs were encoded using deep encoding (i.e. generating sentences) and others with shallow encoding (i.e. just reading aloud). Patients with PD demonstrated poor recollection even after deep encoding and poor familiarity after shallow encoding, hence exhibiting a double dissociation.
Cohn and colleagues (Cohn et al 2016) further used an associative reinstatement memory (ARM) task to study whether deficient recollection in PD was associated with lower hippocampal activation. Associative memory, like recognition memory, is a subcomponent of episodic memory and is the ability to remember relationships between unrelated items and eventually forming a relation between them, hence is also called relational memory. The task consisted of word pairs that were learnt using deep encoding (by generating sentences with the two words in the pair). In the test phase, words were presented either as intact, rearranged, half-old (old-new or new-old) or novel pairs or individual words. Participants had to tell whether words were presented in the study phase, however, they were not asked if the word pairs were presented together. This is called a ‘reinstatement’ and it m inimizes impact of executive deficits in PD as it does not require retrieval of two items together in the same order, which is effortful, requires post-retrieval monitoring and verification (Cohn et al 2008). These executive deficits namely, post-retrieval monitoring and verification problems are evident in PD. For example Pitarque and colleagues (Pitarque et al 2017) had used face-scene pairs in study phase and on re-presentation of the pairs as intact, rearranged or completely novel, participants had to tell if the pairs in test phase were exactly same as those in study phase. This retrieval process requires monitoring and verification whether the two items were paired together during study phase, PD patients responded to the rearranged pairs as original (false alarms). Although this could be an impaired recollection in PD as they could not recollect the pairs in same order, it could very well be due to executive deficit in remembering order of the face-scene picture pair. This aspect of remembering order of items has been shown to be dependent on dorsolateral prefrontal cortex (Ragland et al 2012), and was bypassed by Cohn and his colleagues (Cohn et al 2016) in this study by using the reinstatement task, which in turn minimized the involvement of executive function (that are deficient in PD) and thus helped in studying recollection-specific performance. It is crucial to control for executive function because recollection is not only dependent upon memory-related aspects like encoding and storage, but also upon executive functioning (Rubin et al 2017). In a study, patients with PD who were deficient in executive functioning were impaired in recollection as well, even though the patients did not have any correlation between tests of executive function and recollection scores, while familiarity memory was preserved in this study (Edelstyn et al 2007). The gain observed in performance when item was presented in test phase as intact pair versus when item was presented alone or with a novel counterpart determined the associative memory. ARM was calculated as hit rate for intact pairs minus hit rate for rearranged pairs, therefore higher the ARM score, higher is the associative memory. Hippocampus is known to be involved in reinstatement, triggering the process of recollection replete with rich contextual details (Cohn et al 2009). Cohn and colleagues (Cohn et al 2016) demonstrated that patients with PD had lower ARM scores compared to the healthy controls with simultaneous lower hippocampus activation for the ARM condition. This study clearly showed a lower recollection in PD and concomitant lower associative memory. This can have important implications in PD as being unable to form associations between events or items in day to day life can hamper reliable memory formation and thus subsequent memory recollection is also severely impacted. This study also alludes that recollection aspect of episodic memory retrieval is more severely affected in PD than the familiarity as the hit rates for intact pairs were lesser than rearranged pairs (in ARM score).
2.2. Hippocampal Atrophy in Parkinson’s disease
Several studies have reported cortical atrophy in PD patients with MCI, as compared to PD without MCI and healthy controls (Mak et al 2015, Segura et al 2014). In the ICICLE-PD study (Mak et al 2015), PD-MCI patients at 18 months demonstrated more severe atrophy in the temporal and parietal cortices, including the hippocampus. In addition, they also demonstrated frontal lobe cortical thinning. Interestingly PD patients with no-MCI, who later converted to MCI, were shown to have bilateral temporal lobe atrophy at the baseline visit, which became more severe at the 18 months. This study, along with others, suggest that temporal cortical thinning (in addition to other brain regions, such as the parietal and frontal lobes) could be used to predict which PD patients are at greatest risk of developing MCI (Mak et al 2015, Segura et al 2014).
Regions of MTL implicated in recollection (hippocampus and posterior parahippocampal gyrus) and those implicated in familiarity (perirhinal and entorhinal cortices) can both be atrophied in PD (Weintraub et al 2011) to variable extents, depending on the patient disease severity. Therefore, it is not improbable that there are deficits in both these recognition processes associated with hippocampal atrophy. To investigate this possibility, initial studies used a standardized 5-point atrophy severity scale (Scheltens et al 1992) to visually grade medial temporal lobe atrophy on MRI scans. They found that MTL atrophy was less severe in non-demented PD compared to AD (Tam et al 2005) and they found that MTL atrophy could readily discriminate pathologically confirmed AD compared to Dementia with Lewy bodies (Burton et al 2009). Using this grading system, they did not find any relationship between MTL atrophy and severity of cognitive impairment in PD with or without dementia. Thus, these initial studies alluded that in PD the MTL atrophy was not related to disease-state, severity of memory impairment, or presence of PDD.
However, the preponderance of newer evidence supports MTL and hippocampal atrophy in PD, which is related to the degree of cognitive and memory impairments (Table 2). Indeed, there are several related explanations for these early negative findings. First, the degree of hippocampal atrophy in PD is less severe than that in AD, which was the comparison disease in most of these initial studies. Indeed, the consensus of studies now agree that PD patients with dementia have more hippocampal atrophy than healthy age-matched control subjects but less than patients with clinical AD (Bruck et al 2004, Camicioli et al 2003). Therefore, the effect size needed to detect between-group differences is possibly larger in PD than in AD. Second, while there is more consistent evidence supporting whole hippocampal atrophy in PD-MCI and PDD (Camicioli et al 2003, Danti et al 2015, Jokinen et al 2009, Kandiah et al 2014, Melzer et al 2012, Rektorova et al 2014, Summerfield et al 2005) atrophy is not consistently observed in PD with normal cognition (PD-NC) (Melzer et al 2012) (Table 2). This suggests that using whole hippocampus, there is an association between atrophy and development of cognitive impairment in PD (Bouchard et al 2008, Camicioli et al 2003, Danti et al 2015, Jokinen et al 2009, Junque et al 2005, Melzer et al 2012, Rektorova et al 2014, Tam et al 2005, Yang & Yu 2017). Third, the degree of hippocampal atrophy and the degree of memory impairment have been found by some to be correlated in PD and some studies suggest low hippocampal volume is predictive of progression along the spectrum from PD-NC, through PD-MCI, to PDD (Kandiah et al 2014). This correlation of hippocampal atrophy and extent of memory impairment is likely to become more variable as atrophy becomes more evident during this progression, and it is likely dependent on the sensitivity of the hippocampal measures being used. Fourth, there might be other non-motor PD symptoms related to hippocampal atrophy, which need consideration in these analyses. For example, memory impairments often occur in PD patients with depression or visual hallucinations, many of whom have dementia (Ozer et al 2007, Ramirez-Ruiz et al 2006). These depressive symptoms have also been shown to be correlated with recall and recollection, most likely exacerbated by hippocampal disruption, and prefrontal disruption alone might not be responsible for the depressive symptoms (Edelstyn et al 2015). Complementary research in adults with depression has shown that dysfunction in hippocampal memory system is intricately interwoven with depression (Bremner et al 2000, Caetano et al 2004, Malchow et al 2015, Sheline et al 1996, Thoma et al 2009) and in psychotic disorders like schizophrenia, researchers have suggested a relationship between the hippocampus and psychotic symptoms of delusions and hallucinations (Tamminga et al 2010). Fifth, the vast majority of these studies use MRI to study the hippocampus as a whole, but autopsy studies suggest that different hippocampal subfields are more vulnerable to structural, and likely functional changes due to PD pathology. In the next section we discuss volumetric studies that have investigated hippocampal subfields in PD.
Table 2:
Whole hippocampal atrophy studies in PD
| Study | Results | No. of Participants: Mean Age | Mean Disease Duration (years) | Parkinson’s and Dementia Diagnosis | Taking Levodopa, DA agonist or MAO inhibitors | Major Clinical Assessment Tools |
|---|---|---|---|---|---|---|
| (Camicioli et al.,2003) | Progressive decline inHippocampal volume Control>PD>PDD> Alzheimer’s disease | 10PD: 73.3 10 PDD: 74.7 |
5.1 7.3 |
-PD: UK Brain Bank Criteria (Ward & Gibb, 1990) -Dementia: DSM-IV criteria, CDR and patient history |
# | -MMSE -NPI |
| (Bruck, Kurki,Kaasinen,Vahlberg, &Rinne, 2004) | PD: Hippocampal atrophy in PD, atrophy correlated with verbal memory | 20PD: 61.3 | 1.7 | -PD: UK Brain Bank Criteria -Dementia: Patients non-demented and criteria for dementia diagnosis not specified |
-After MRI and cognitive testing, treated with DA medications first time | -MMSE -WMS-R -Word-list Memory and Recognition |
| (Summerfield et al., 2005) | PD: atrophy in right hippocampus PDD: volume decrease in bilateral hippocampus |
13PD: 72.77 16 PDD: 70.06 |
10.61 12.94 |
-PD: UK Brain Bank Criteria -Dementia: DSM-IV, CDR and MMSE |
Yes | -MMSE -HDRS |
| (Junque et al.,2005) | PD: atrophy in hippocampus and amygdala, but not statistically significant. PDD: atrophy in hippocampus and amygdala |
16PD: 72.87 16 PDD: 70.06 |
11.25 13.33 |
-PD: UK Brain Bank Criteria -Dementia: DSM-IV and MMSE |
# | -MMSE -modified RAVLT |
| (Tam, Burton,McKeith, Burn,& O’Brien,2005) | MTL atrophy: AD>PDD>PD-NC. Suggested involvement of other anatomic structures other than hippocampus for dementia. | 33 PD: 75.44 31 PDD: 71.88 |
PD: no information PDD: Mean age of onset 68.29 years, mean duration of cognitive symptoms 3.22 years |
-PD: Diagnosis based on consensus and UK Brain Bank Criteria -Dementia: patient history, MMSE or CAMCOG score<80 DLB and PDD: McKeith criteria (McKeith et al., 1996) |
# | -MMSE -CAMCOG |
| (Bouchard et al.,2008) | Correlation between age and overall hippocampal volume in PD. | 44PD: 71.1 13PDD: 71.9 |
8.4 10.3 |
-PD: UK Brain Bank Criteria -PDD: CDR score >0.5 |
Yes | -MMSE -FAB -CVLT-II -BVMT |
| (Jokinen et al.,2009) | Hippocampus and prefrontal cortex atrophy in PD. Hippocampal atrophy related to memory impairment. | 19PD: 64.4 | Not available | -PD: At least 2 main symptoms of PD- tremor, rigidity and hypokinesia. MRI of patients had no findings incompatible with PD diagnosis -Criteria for dementia diagnosis not specified -MMSE, FAB used for cognitive assessment |
Yes | -MMSE -FAB -WMS-R -WAIS-R -TMT- A and B |
| (Melzer et al.,2012) | PD-NC: not different from HC PD-MCI: atrophy in temporal, parietal and frontal cortex, bilateral caudal hippocampus, amygdala and right putamen PDD: widespread atrophy in hippocampal formation |
57PD-NC: 64.3 23 PD-MCI: 70.8 16 PDD: 73.3 |
3.8 7.2 12.9 |
-PD: UK Brain Bank Criteria -Dementia: MDS criteria (Emre et al., 2007) -Classification of PD as PD- NC, PD-MCI and PDD based on neuropsychological testing |
Yes | -MMSE -MoCA -GDS |
| (Kandiah et al., 2014) | Hippocampal volume was a significant predictor for development of mild cognitive impairment and dementia | 44PD-NC: 62.68 34PD-MCI: 67.10 8 PDD: 70.94 |
5.72 4.70 5.95 |
-NINCDS Criteria for PD diagnosis -Dementia: MDS criteria |
# | -MMSE -MoCA -FAB -Color TMT -ONT |
| (Rektorova etal., 2014) | PDD: atrophied hippocampus and overall temporal lobe, fronto-parietal regions and increases in midbrain-cerebellum | 75PD-NC: 64.2 29PD-MCI: 67.0 22PDD: 70.7 |
5 9 5 |
-PD: UK Brain Bank Criteria -Dementia: MDS criteria |
Yes | -MMSE -ACE-R (Mathuranath, Nestor, Berrios, Rakowicz, & Hodges, 2000) |
| (Danti et al.,2015) | PD-MCI as compared to PD-NC: atrophy in right frontal, middle temporal, left insula, bilateral thalamus, left hippocampus | 18PD-NC: 60.6 18PD-MCI: 66.5 |
Mean age of onset in months: PD-NC: 18 PD-MCI: 20 |
-PD: UK Brain Bank Criteria (Ward & Gibb, 1990) -123I FP-CIT SPECT: supportive criterion to confirm nigrostriatal degeneration -PD-MCI established using MDS criteria |
# | -MMSE -MoCA -FAB -TMT- A and B -WAIS -BNT -RAVLT |
Abbreviations: DA: Dopamine, MAO: Monoamine oxidase, GDS: Global Deterioration Scale, MMSE: Mini-Mental State Examination, MoCA: Montreal Cognitive Assessment, NPI: Neuropsychiatric Inventory, WMS-R: Wechsler Memory Scale-Revised, WAIS: Wechsler Adult Intelligence Scale-Revised, HDRS: Hamilton Depression Rating Scale, RAVLT: Rey Auditory Verbal Learning Test, CAMCOG: Cambridge Cognitive Examination, FAB: Frontal Assessment Battery, CVLT: California Verbal Learning Test, BVMT: Brief Visuospatial Memory Test, TMT: Trail Making Test, ONT: Object Naming Test, ACE-R: Addenbrooke’s Cognitive Examination-Revised, BNT: Boston Naming Test
PD-NC: Parkinson’s Disease Normal Cognition, PD-MCI : PD Mild Cognitive Impairment, PDD- PD Dementia, CDR: The Clinical Dementia Rating, DSM-IV: Diagnostic and Statistical Manual, NINCD: National Institute of Neurologic and Communicative Disorder, SADRDA: Stroke/Alzheimer’s Disease and Related Disorders Association, MDS-TF: Movement Disorders Task Force, 123I FP-CIT SPECT: 123 55 I-Fluoropropyl-2-beta-carbomethoxy-3-beta(4-iodophenyl) nortropane Single Photon Emission Computed Tomography
Medication not specified
2.3. Hippocampal subfield atrophy in Parkinson’s disease
The PD-related changes that occur in different hippocampal subfields can impact the episodic memory impairments experienced by these patients and warrant further study (Table 3). For example, autopsy studies have shown that dementia-related alpha-synuclein and Lewy-body pathology preferentially affect some subfields more than others. Lewy-body pathology is most prominent in the CA2–3 subfields (Dickson et al 1991, Dickson et al 1994) but is also found throughout CA1 in PD (Churchyard & Lees 1997). A few structural MRI studies have begun to explore these relationships. Foo and colleagues (Foo et al 2017) measured hippocampal subfield volumes in PD and correlated the volumes with cognitive and motor decline at two time points over 18 months. At baseline, they found lower volumes in right CA1, right hippocampal-amygdala-transition-area and left fimbria. These were accompanied by lower global cognition scores in PD-MCI as compared to PD-NC. At 18 months, a volume reduction was noted in right CA2–3 along with significant decline in episodic memory and executive function in PD-converters (who converted from PD-NC to PD-MCI) as opposed to PD-stable (who did not convert).
Table 3.
Hippocampal subfield atrophy studies in PD
| Study | Results | No. of Participants: Mean Age | Mean Disease Duration (years) | Parkinson’s and Dementia Diagnosis | Medication state | Major Clinical Assessment Tools |
|---|---|---|---|---|---|---|
| (Churchyard& Lees,1997) | Degree of cognitive impairment measured with MMSE, which correlated with Lewy neurites in CA2 | -10 PD-NC: 75.8 -7 PD-mild to moderate dementia: 75.9 -10 PD-severe dementia: 75.4 |
15.9 14.3 13.4 |
-PD: UK Parkinson’s Disease Society Brain Bank Criteria (Daniel & Lees, 1993) -Dementia: DSM-III criteria |
On dopaminergic medication | MMSE done before death |
| (Beyer et al.,2013) | Atrophy in hippocampus associated with verbal memory recall and recognition scores | -114 PD-NC: 65.8 -29 PD-MCI:70.6 |
Not specified | -PD: NINDS criteria (Gelb, Oliver, & Gilman, 1999) -Dopamine transporter imaging for differential diagnosis -Criteria for dementia diagnosis not specified -Dementia: exclusion criteria |
Most On Dopaminergic medications, but some drug-naive | MMSE CVLT VOSP |
| (Pereira etal., 2013) | Atrophy in CA2–3-4, DG correlated with learning performance. Atrophy in CA2–3-4, DG and subiculum associated with hallucinations in PD | 18 PD: 73.8 18 PD with visual hallucination: 73.8 |
12.8 12.9 |
-PD: UK Parkinson’s Disease Society Brain Bank Criteria -Dementia: DSM-IV-TR |
On dopaminergic medications | RAVLT HDRS |
| (Stav et al.,2016) | CA1, presubiculum and subiculum volumes and CSF biomarker amyloid β levels correlated with visuospatial learning and cognitive deficits | 33 non-demented PD: 64.9 | 2.7** | -PD: UK Parkinson’s Disease Society Brain Bank Criteria -DaTSCAN to support diagnosis of PD -Dementia (tested using cognitive screening): exclusion criteria |
On dopaminergic medications | RAVLT TMT-B |
| (Gyorfi etal., 2017) | A2–3 atrophy observed in early stage of PD | -35 PD-NC: 51.9 | Not specified | -PD: UK Parkinson’s Disease Society Brain Bank Criteria -MCI excluded based on MDS criteria |
-Baseline testing in non-medicated drug naive state -levodopa started after baseline testing |
MoCA RAVLT HDRS HARS |
| (Foo et al., 2017) | Volumes of DG, right CA4, left parasubiculum and hippocampal-amygdala transition area predictive of conversion from normal cognition to MCI | -54 PD-NC: 63.39 -11 PD-MCI: 69.45 |
4.74 4.09 |
-PD: NINDS criteria (Gelb et al., 1999) -Dementia diagnosis using ADAS-Cog: exclusion criteria |
On dopaminergic medications | MoCA ADAS-Cog FAB Digit Span |
Abbreviations: DA: Dopamine, MAO: Monoamine oxidase, MMSE: Mini-Mental State Examination, CVLT: California Verbal Learning Test, RAVLT: Rey’s Auditory Verbal Learning Test, HDRS: Hamilton Depression Rating Scale, HARS: Hamilton Anxiety Rating Scale, TMT: Trail Making Test, ADAS-Cog: Alzheimer’s Disease Assessment Scale-cognitive subscale, FAB: Frontal Assessment Battery, VOSP: Visual Object and Space Perception Battery
PD-NC: Parkinson’s Disease Normal Cognition, PD-MCI: PD Mild Cognitive Impairment, DSM: Diagnostic and Statistical Manual, NINDS criteria: National Institute of Neurological Disorders and Stroke criteria, MDS-TF: Movement Disorders Task Force
Motor symptom duration
Another study demonstrated a correlation between atrophy in hippocampal subfields and cognitive impairment (Beyer et al 2013). In this study, California Verbal Learning Test 2 (CVLT-2) recognition scores in PD patients were associated with atrophy in right CA1 and subiculum. Recently, atrophy in hippocampus and surrounding regions were also linked to CSF biomarker Aβ and cognition in early PD (Stav et al 2016). Patients had significantly smaller volumes of total hippocampus, presubiculum, subiculum, CA2–3, CA4-DG and hippocampal tail. Specifically, lower total hippocampus, presubiculum and subiculum predicted poorer visual copying; lower CSF Aβ38 and Aβ42 predicted overall thinner perirhinal cortex and lower verbal learning and recall. Hence, smaller hippocampal volumes and Aβ levels were associated with higher cognitive deficits.
In addition to having effects on cognition, decreased CA2–3 volumes have also been associated with concomitant depressive symptoms in non-medicated PD (Gyorfi et al 2017). CA2–3 has been implicated in formation of new memories (Jones & McHugh 2011, Lisman 1999) suggested to be an interface between emotional processing and higher-level cognition (Chevaleyre & Piskorowski 2016), therefore believed to play an important role in depression as well. In another study, PD patients with hallucinations also demonstrated higher levels of atrophy in CA2–3, CA4-DG and the degeneration extended up to subiculum (Pereira et al 2013) and volume decreases in CA2–3 and CA4-DG correlated with verbal learning.
High resolution fMRI studies have been done, focusing on functions of the hippocampal subfields during performance of episodic memory tasks in healthy humans. In one study, CA2–3, subiculum and CA1 were shown to be activated in an fMRI task of recollection (Suthana et al 2015), especially subiculum was shown to have consistent activation during successful recollection at short and long delays after learning (Viskontas et al 2009). Furthermore, CA3 and quite possibly CA2 and DG (it is not possible to delineate these three subfields using an MRI study) were found to represent similar events (Chadwick et al 2014) and similar objects with different environmental context (pattern separation) (Dimsdale-Zucker et al 2018). CA3 probably contributes to both pattern completion and pattern separation during memory recollection, as has also been observed in electrophysiological studies in rodents (Guzowski et al 2004, Lee et al 2004, Leutgeb et al 2004, Vazdarjanova & Guzowski 2004).
Therefore, studies in healthy humans demonstrate crucial roles of CA1, CA2–3 and subiculum in episodic memory recollection; and volumetric hippocampal studies in PD exhibit consistent smaller volumes of the same subfields, namely CA1, CA2–3 and subiculum that are highly correlated with learning and recognition scores as well. As these same subfields have been established as crucial for memory recollection in a non-disease state, the evidence clearly reveals an association between smaller volumes of subiculum, CA2–3 and CA1 and impaired recollection process in PD; plausibly contributing towards cognitive decline. Since, CA3 has also been implicated in pattern completion and pattern separation, thus degeneration of CA3 in PD plausibly affects process of recollection all the more, by impacting pattern completion and pattern separation sub-processes in PD and hence unsuccessful recollection. Although recollection could possibly happen in presence or absence of pattern separation, but recollection is more accurate when pattern separation happens successfully (Kim & Yassa 2013). Thus, degeneration of hippocampal subfields in PD, inevitably takes a toll on the crucial process of episodic memory recollection that is a dominant aspect of cognition.
In summary, the preponderance of evidence suggests that medial temporal lobe atrophy can be found in PD patients, albeit to a lesser degree than what is seen in even early AD patients. Further, it appears this atrophy is associated with the development of cognitive impairments as patients decline to dementia. There is also evidence to suggest that the relationship between atrophy and PD memory impairments could be subfield specific, with CA1, CA2–3, CA4-DG and subiculum correlating with poorer neurocognitive scores. Since CA3 is involved in both pattern completion and pattern separation and is crucial for recollection, atrophy in CA3 subregion would be expected to impact episodic memory recollection process in PD as well.
Concluding remarks
In this review, we assimilated the current literature of episodic recognition memory in PD. Based on current literature, we conclude that the hippocampus is critical for episodic recollection, specifically subfields CA2–3 and subiculum. Further, we conclude that these same subfields undergo conspicuous neurodegeneration in PD and that smaller volumes of CA2–3 and subiculum are highly correlated with cognitive decline in PD. Since, CA3 has also been implicated in pattern completion and pattern separation, therefore in addition to subiculum, degeneration of CA3 in PD affects process of recollection, as both pattern completion and pattern separation are crucial features of overall successful recollection. Finally, the role of CA1 in PD cognitive impairment has been suggested by autopsy (Adamowicz et al 2017) and imaging studies (Stav et al 2016, Suthana et al 2015), but the exact role of this subfield is unclear and thus warrants further investigation.
Figure 2.
Schematic of Hippocampus
Acknowledgements
We would like to acknowledge Prof Craig Stark and Prof Charan Ranganath for providing with the hippocampus and ROC images respectively.
Funding
This work was supported by the NIH (P50 AG047366, P50 NS062684), the Michael J. Fox Foundation for Parkinson’s Research, and the Seiger Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. 2003. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol 60: 387–92 [DOI] [PubMed] [Google Scholar]
- Aarsland D, Bronnick K, Williams-Gray C, Weintraub D, Marder K, et al. 2010. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology 75: 1062–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarsland D, Kurz MW. 2010. The epidemiology of dementia associated with Parkinson’s disease. Brain Pathol 20: 633–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarsland D, Larsen JP, Karlsen K, Lim NG, Tandberg E. 1999. Mental symptoms in Parkinson’s disease are important contributors to caregiver distress. Int J Geriatr Psychiatry 14: 866–74 [PubMed] [Google Scholar]
- Aarsland D, Larsen JP, Tandberg E, Laake K. 2000. Predictors of nursing home placement in Parkinson’s disease: a population-based, prospective study. J Am Geriatr Soc 48: 938–42 [DOI] [PubMed] [Google Scholar]
- Aarsland D, Londos E, Ballard C. 2009. Parkinson’s disease dementia and dementia with Lewy bodies: different aspects of one entity. Int Psychogeriatr 21: 216–9 [DOI] [PubMed] [Google Scholar]
- Adamowicz DH, Roy S, Salmon DP, Galasko DR, Hansen LA, et al. 2017. Hippocampal alpha-Synuclein in Dementia with Lewy Bodies Contributes to Memory Impairment and Is Consistent with Spread of Pathology. J Neurosci 37: 1675–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP, Brown MW. 1999. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci 22: 425–44; discussion 44–89 [PubMed] [Google Scholar]
- Aggleton JP, McMackin D, Carpenter K, Hornak J, Kapur N, et al. 2000. Differential cognitive effects of colloid cysts in the third ventricle that spare or compromise the fornix. Brain 123 ( Pt 4): 800–15 [DOI] [PubMed] [Google Scholar]
- Algarabel S, Rodriguez LA, Escudero J, Fuentes M, Peset V, et al. 2010. Recognition by familiarity is preserved in Parkinson’s without dementia and Lewy-Body disease. Neuropsychology 24: 599–607 [DOI] [PubMed] [Google Scholar]
- Apaydin H, Ahlskog JE, Parisi JE, Boeve BF, Dickson DW. 2002. Parkinson disease neuropathology: later-developing dementia and loss of the levodopa response. Arch Neurol 59: 102–12 [DOI] [PubMed] [Google Scholar]
- Armstrong MJ, Gruber-Baldini AL, Reich SG, Fishman PS, Lachner C, Shulman LM. 2014. Which features of Parkinson’s disease predict earlier exit from the workforce? Parkinsonism Relat Disord 20: 1257–9 [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. 2004. Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8: 170–7 [DOI] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. 2005. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron 47: 907–18 [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. 2007. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia 45: 2883–901 [DOI] [PubMed] [Google Scholar]
- Bekris LM, Tsuang DW, Peskind ER, Yu CE, Montine TJ, et al. 2015. Cerebrospinal fluid Abeta42 levels and APP processing pathway genes in Parkinson’s disease. Mov Disord 30: 936–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, et al. 2010. Coherent theta oscillations and reorganization of spike timing in the hippocampal- prefrontal network upon learning. Neuron 66: 921–36 [DOI] [PubMed] [Google Scholar]
- Beyer MK, Bronnick KS, Hwang KS, Bergsland N, Tysnes OB, et al. 2013. Verbal memory is associated with structural hippocampal changes in newly diagnosed Parkinson’s disease. J Neurol Neurosurg Psychiatry 84: 23–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird CM. 2017. The role of the hippocampus in recognition memory. Cortex 93: 155–65 [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C. 2006. Dorsolateral prefrontal cortex promotes long-term memory formation through its role in working memory organization. J Neurosci 26: 916–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard TP, Malykhin N, Martin WR, Hanstock CC, Emery DJ, et al. 2008. Age and dementia-associated atrophy predominates in the hippocampal head and amygdala in Parkinson’s disease. Neurobiol Aging 29: 1027–39 [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. 2003. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24: 197–211 [DOI] [PubMed] [Google Scholar]
- Braak H, Rub U, Jansen Steur EN, Del Tredici K, de Vos RA. 2005. Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology 64: 1404–10 [DOI] [PubMed] [Google Scholar]
- Brandt KR, Eysenck MW, Nielsen MK, von Oertzen TJ. 2016. Selective lesion to the entorhinal cortex leads to an impairment in familiarity but not recollection. Brain Cogn 104: 82–92 [DOI] [PubMed] [Google Scholar]
- Breen EK. 1993. Recall and recognition memory in Parkinson’s disease. Cortex 29: 91–102 [DOI] [PubMed] [Google Scholar]
- Breen KC, Drutyte G. 2013. Non-motor symptoms of Parkinson’s disease: the patient’s perspective. J Neural Transm (Vienna) 120: 531–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. 2000. Hippocampal volume reduction in major depression. Am J Psychiatry 157: 115–8 [DOI] [PubMed] [Google Scholar]
- Brockmann MD, Poschel B, Cichon N, Hanganu-Opatz IL. 2011. Coupled oscillations mediate directed interactions between prefrontal cortex and hippocampus of the neonatal rat. Neuron 71: 332–47 [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. 2001. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci 2: 51–61 [DOI] [PubMed] [Google Scholar]
- Brown MW, Banks PJ. 2015. In search of a recognition memory engram. Neurosci Biobehav Rev 50: 12–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruck A, Kurki T, Kaasinen V, Vahlberg T, Rinne JO. 2004. Hippocampal and prefrontal atrophy in patients with early non-demented Parkinson’s disease is related to cognitive impairment. J Neurol Neurosurg Psychiatry 75: 1467–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton EJ, Barber R, Mukaetova-Ladinska EB, Robson J, Perry RH, et al. 2009. Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain 132: 195–203 [DOI] [PubMed] [Google Scholar]
- Caetano SC, Hatch JP, Brambilla P, Sassi RB, Nicoletti M, et al. 2004. Anatomical MRI study of hippocampus and amygdala in patients with current and remitted major depression. Psychiatry Res 132: 141–7 [DOI] [PubMed] [Google Scholar]
- Camicioli R, Moore MM, Kinney A, Corbridge E, Glassberg K, Kaye JA. 2003. Parkinson’s disease is associated with hippocampal atrophy. Mov Disord 18: 784–90 [DOI] [PubMed] [Google Scholar]
- Chadwick MJ, Bonnici HM, Maguire EA. 2014. CA3 size predicts the precision of memory recall. Proc Natl Acad Sci U S A 111: 10720–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahine LM, Weintraub D, Hawkins KA, Siderowf A, Eberly S, et al. 2016. Cognition in individuals at risk for Parkinson’s: Parkinson associated risk syndrome (PARS) study findings. Mov Disord 31: 86–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles DP, Browning PG, Gaffan D. 2004. Entorhinal cortex contributes to object-in-place scene memory. Eur J Neurosci 20: 3157–64 [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Piskorowski RA. 2016. Hippocampal Area CA2: An Overlooked but Promising Therapeutic Target. Trends Mol Med 22: 645–55 [DOI] [PubMed] [Google Scholar]
- Cholerton B, Johnson CO, Fish B, Quinn JF, Chung KA, et al. 2018. Sex differences in progression to mild cognitive impairment and dementia in Parkinson’s disease. Parkinsonism Relat Disord [DOI] [PMC free article] [PubMed]
- Cholerton BA, Zabetian CP, Quinn JF, Chung KA, Peterson A, et al. 2013. Pacific Northwest Udall Center of excellence clinical consortium: study design and baseline cohort characteristics. J Parkinsons Dis 3: 205–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchyard A, Lees AJ. 1997. The relationship between dementia and direct involvement of the hippocampus and amygdala in Parkinson’s disease. Neurology 49: 1570–6 [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Squire LR. 1980. Preserved learning and retention of pattern-analyzing skill in amnesia: dissociation of knowing how and knowing that. Science 210: 207–10 [DOI] [PubMed] [Google Scholar]
- Cohn M, Emrich SM, Moscovitch M. 2008. Age-related deficits in associative memory: the influence of impaired strategic retrieval. Psychol Aging 23: 93–103 [DOI] [PubMed] [Google Scholar]
- Cohn M, Giannoylis I, De Belder M, Saint-Cyr JA, McAndrews MP. 2016. Associative reinstatement memory measures hippocampal function in Parkinson’s Disease. Neuropsychologia 90: 25–32 [DOI] [PubMed] [Google Scholar]
- Cohn M, Moscovitch M, Davidson PS. 2010. Double dissociation between familiarity and recollection in Parkinson’s disease as a function of encoding tasks. Neuropsychologia 48: 4142–7 [DOI] [PubMed] [Google Scholar]
- Cohn M, Moscovitch M, Lahat A, McAndrews MP. 2009. Recollection versus strength as the primary determinant of hippocampal engagement at retrieval. Proc Natl Acad Sci U S A 106: 22451–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compta Y, Parkkinen L, O’Sullivan SS, Vandrovcova J, Holton JL, et al. 2011. Lewy- and Alzheimer-type pathologies in Parkinson’s disease dementia: which is more important? Brain 134: 1493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin-Golomb A, Braun AE. 1997. Visuospatial dysfunction and problem solving in Parkinson’s disease. Neuropsychology 11: 44–52 [DOI] [PubMed] [Google Scholar]
- Curran HV, Mintzer MZ. 2006. Psychopharmacology of memory. Psychopharmacology (Berl) 188: 393–6 [DOI] [PubMed] [Google Scholar]
- Dalton MA, Maguire EA. 2017. The pre/parasubiculum: a hippocampal hub for scene-based cognition? Curr Opin Behav Sci 17: 34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel SE, & Lees AJ (1993). Parkinson’s Disease Society Brain Bank, London: overview and research. J Neural Transm Suppl, 39, 165–172. [PubMed] [Google Scholar]
- Danti S, Toschi N, Diciotti S, Tessa C, Poletti M, et al. 2015. Cortical thickness in de novo patients with Parkinson disease and mild cognitive impairment with consideration of clinical phenotype and motor laterality. Eur J Neurol 22: 1564–72 [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza R. 2006. Triple dissociation in the medial temporal lobes: recollection, familiarity, and novelty. J Neurophysiol 96: 1902–11 [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. 2003. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci U S A 100: 2157–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PS, Anaki D, Saint-Cyr JA, Chow TW, Moscovitch M. 2006. Exploring the recognition memory deficit in Parkinson’s disease: estimates of recollection versus familiarity. Brain 129: 1768–79 [DOI] [PubMed] [Google Scholar]
- Dickson DW, Ruan D, Crystal H, Mark MH, Davies P, et al. 1991. Hippocampal degeneration differentiates diffuse Lewy body disease (DLBD) from Alzheimer’s disease: light and electron microscopic immunocytochemistry of CA2–3 neurites specific to DLBD. Neurology 41: 1402–9 [DOI] [PubMed] [Google Scholar]
- Dickson DW, Schmidt ML, Lee VM, Zhao ML, Yen SH, Trojanowski JQ. 1994. Immunoreactivity profile of hippocampal CA2/3 neurites in diffuse Lewy body disease. Acta Neuropathol 87: 269–76 [DOI] [PubMed] [Google Scholar]
- Dimsdale-Zucker HR, Ritchey M, Ekstrom AD, Yonelinas AP, Ranganath C. 2018. CA1 and CA3 differentially support spontaneous retrieval of episodic contexts within human hippocampal subfields. Nat Commun 9: 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y 2004. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol 55: 51–86 [DOI] [PubMed] [Google Scholar]
- Dujardin K, Degreef JF, Rogelet P, Defebvre L, Destee A. 1999. Impairment of the supervisory attentional system in early untreated patients with Parkinson’s disease. J Neurol 246: 783–8 [DOI] [PubMed] [Google Scholar]
- Dulas MR, Duarte A. 2014. Aging affects the interaction between attentional control and source memory: an fMRI study. J Cogn Neurosci 26: 2653–69 [DOI] [PubMed] [Google Scholar]
- Dulas MR, Duarte A. 2016. Age-related changes in overcoming proactive interference in associative memory: The role of PFC-mediated executive control processes at retrieval. Neuroimage 132: 116–28 [DOI] [PubMed] [Google Scholar]
- Edelstyn NM, John CM, Shepherd TA, Drakeford JL, Clark-Carter D, et al. 2015. Evidence of an amnesia-like cued-recall memory impairment in nondementing idiopathic Parkinson’s disease. Cortex 71: 85–101 [DOI] [PubMed] [Google Scholar]
- Edelstyn NM, Mayes AR, Condon L, Tunnicliffe M, Ellis SJ. 2007. Recognition, recollection, familiarity and executive function in medicated patients with moderate Parkinson’s disease. J Neuropsychol 1: 131–47 [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. 2007. The medial temporal lobe and recognition memory. Annu Rev Neurosci 30: 123–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, … Dubois B (2007). Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord, 22(12), 1689–1707; quiz 1837. doi: 10.1002/mds.21507 [DOI] [PubMed] [Google Scholar]
- Foo H, Mak E, Chander RJ, Ng A, Au WL, et al. 2017. Associations of hippocampal subfields in the progression of cognitive decline related to Parkinson’s disease. Neuroimage Clin 14: 37–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffan D 1974. Recognition impaired and association intact in the memory of monkeys after transection of the fornix. J Comp Physiol Psychol 86: 1100–9 [DOI] [PubMed] [Google Scholar]
- Galvin JE, Uryu K, Lee VM, Trojanowski JQ. 1999. Axon pathology in Parkinson’s disease and Lewy body dementia hippocampus contains alpha-, beta-, and gamma-synuclein. Proc Natl Acad Sci U S A 96: 13450–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb DJ, Oliver E, & Gilman S (1999). Diagnostic criteria for Parkinson disease. Arch Neurol, 56(1), 33–39 [DOI] [PubMed] [Google Scholar]
- Gold JJ, Smith CN, Bayley PJ, Shrager Y, Brewert JB, et al. 2006. Item memory, source memory, and the medial temporal lobe: Concordant findings from fMRI and memory-impaired patients. P Natl Acad Sci USA 103: 9351–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalves BD, Kahn I, Curran T, Norman KA, Wagner AD. 2005. Memory strength and repetition suppression: multimodal imaging of medial temporal cortical contributions to recognition. Neuron 47: 751–61 [DOI] [PubMed] [Google Scholar]
- Gratwicke J, Jahanshahi M, Foltynie T. 2015. Parkinson’s disease dementia: a neural networks perspective. Brain 138: 1454–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Knierim JJ, Moser EI. 2004. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron 44: 581–4 [DOI] [PubMed] [Google Scholar]
- Gyorfi O, Nagy H, Bokor M, Moustafa AA, Rosenzweig I, et al. 2017. Reduced CA2-CA3 Hippocampal Subfield Volume Is Related to Depression and Normalized by l-DOPA in Newly Diagnosed Parkinson’s Disease. Front Neurol 8: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday G, Hely M, Reid W, Morris J. 2008. The progression of pathology in longitudinally followed patients with Parkinson’s disease. Acta Neuropathol 115: 409–15 [DOI] [PubMed] [Google Scholar]
- Hely MA, Morris JG, Reid WG, Trafficante R. 2005. Sydney Multicenter Study of Parkinson’s disease: non-L-dopa-responsive problems dominate at 15 years. Mov Disord 20: 190–9 [DOI] [PubMed] [Google Scholar]
- Hely MA, Morris JG, Traficante R, Reid WG, O’Sullivan DJ, Williamson PM. 1999. The sydney multicentre study of Parkinson’s disease: progression and mortality at 10 years. J Neurol Neurosurg Psychiatry 67: 300–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. 2008. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord 23: 837–44 [DOI] [PubMed] [Google Scholar]
- Henson RN, Shallice T, Dolan RJ. 1999. Right prefrontal cortex and episodic memory retrieval: a functional MRI test of the monitoring hypothesis. Brain 122 ( Pt 7): 1367–81 [DOI] [PubMed] [Google Scholar]
- Higginson CI, Wheelock VL, Carroll KE, Sigvardt KA. 2005. Recognition memory in Parkinson’s disease with and without dementia: evidence inconsistent with the retrieval deficit hypothesis. J Clin Exp Neuropsychol 27: 516–28 [DOI] [PubMed] [Google Scholar]
- Irwin DJ, Lee VM, Trojanowski JQ. 2013. Parkinson’s disease dementia: convergence of alpha-synuclein, tau and amyloid-beta pathologies. Nat Rev Neurosci 14: 626–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin DJ, White MT, Toledo JB, Xie SX, Robinson JL, et al. 2012. Neuropathologic substrates of Parkinson disease dementia. Ann Neurol 72: 587–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, et al. 2018. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14: 535–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., Knopman DS, Jagust WJ, Petersen RC, Weiner MW, et al. 2013. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 12: 207–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby LL, Dallas M. 1981. On the relationship between autobiographical memory and perceptual learning. J Exp Psychol Gen 110: 306–40 [DOI] [PubMed] [Google Scholar]
- Jellinger KA. 2007. Morphological substrates of parkinsonism with and without dementia: a retrospective clinico-pathological study. J Neural Transm Suppl: 91–104 [DOI] [PubMed]
- Jellinger KA, Attems J. 2008. Prevalence and impact of vascular and Alzheimer pathologies in Lewy body disease. Acta Neuropathol 115: 427–36 [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Seppi K, Wenning GK, Poewe W. 2002. Impact of coexistent Alzheimer pathology on the natural history of Parkinson’s disease. J Neural Transm (Vienna) 109: 329–39 [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Wenning GK, Seppi K. 2007. Predictors of survival in dementia with lewy bodies and Parkinson dementia. Neurodegener Dis 4: 428–30 [DOI] [PubMed] [Google Scholar]
- Jokinen P, Bruck A, Aalto S, Forsback S, Parkkola R, Rinne JO. 2009. Impaired cognitive performance in Parkinson’s disease is related to caudate dopaminergic hypofunction and hippocampal atrophy. Parkinsonism Relat Disord 15: 88–93 [DOI] [PubMed] [Google Scholar]
- Jones MW, McHugh TJ. 2011. Updating hippocampal representations: CA2 joins the circuit. Trends Neurosci 34: 526–35 [DOI] [PubMed] [Google Scholar]
- Junque C, Ramirez-Ruiz B, Tolosa E, Summerfield C, Marti MJ, et al. 2005. Amygdalar and hippocampal MRI volumetric reductions in Parkinson’s disease with dementia. Mov Disord 20: 540–4 [DOI] [PubMed] [Google Scholar]
- Kalaitzakis ME, Graeber MB, Gentleman SM, Pearce RK. 2008. Striatal beta-amyloid deposition in Parkinson disease with dementia. J Neuropathol Exp Neurol 67: 155–61 [DOI] [PubMed] [Google Scholar]
- Kandiah N, Zainal NH, Narasimhalu K, Chander RJ, Ng A, et al. 2014. Hippocampal volume and white matter disease in the prediction of dementia in Parkinson’s disease. Parkinsonism Relat Disord 20: 1203–8 [DOI] [PubMed] [Google Scholar]
- Karlsson MP, Frank LM. 2009. Awake replay of remote experiences in the hippocampus. Nat Neurosci 12: 913–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehagia AA, Barker RA, Robbins TW. 2010. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurol 9: 1200–13 [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. 2006. Amygdala activity is associated with the successful encoding of item, but not source, information for positive and negative stimuli. Journal of Neuroscience 26: 2564–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP. 2013. A process analysis of the CA3 subregion of the hippocampus. Front Cell Neurosci 7: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Hunsaker MR, Warthen MW. 2008. The CA3 subregion of the hippocampus is critical for episodic memory processing by means of relational encoding in rats. Behav Neurosci 122: 1217–25 [DOI] [PubMed] [Google Scholar]
- Kesner RP, Rolls ET. 2001. Role of long-term synaptic modification in short-term memory. Hippocampus 11: 240–50 [DOI] [PubMed] [Google Scholar]
- Kim J, Yassa MA. 2013. Assessing recollection and familiarity of similar lures in a behavioral pattern separation task. Hippocampus 23: 287–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Kim DH, Lee Y, Park SJ, Ryu JH. 2014. Distinct roles of the hippocampus and perirhinal cortex in GABAA receptor blockade-induced enhancement of object recognition memory. Brain Res 1552: 17–25 [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Stark CE. 2004. Medial temporal lobe activation during encoding and retrieval of novel face-name pairs. Hippocampus 14: 919–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotzbauer PT, Cairns NJ, Campbell MC, Willis AW, Racette BA, et al. 2012. Pathologic accumulation of alpha-synuclein and Abeta in Parkinson disease patients with dementia. Arch Neurol 69: 1326–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovari E, Gold G, Herrmann FR, Canuto A, Hof PR, et al. 2003. Lewy body densities in the entorhinal and anterior cingulate cortex predict cognitive deficits in Parkinson’s disease. Acta Neuropathol 106: 83–8 [DOI] [PubMed] [Google Scholar]
- Labutta RJ, Miles RB, Sanes JN, Hallett M. 1994. Motor program memory storage in Parkinson’s disease patients tested with a delayed response task. Mov Disord 9: 218–22 [DOI] [PubMed] [Google Scholar]
- Lavenex P, Banta Lavenex P, Amaral DG. 2007. Postnatal development of the primate hippocampal formation. Dev Neurosci 29: 179–92 [DOI] [PubMed] [Google Scholar]
- Leaver K, Poston KL. 2015. Do CSF Biomarkers Predict Progression to Cognitive Impairment in Parkinson’s disease patients? A Systematic Review. Neuropsychol Rev 25: 411–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Kesner RP. 2003. Differential roles of dorsal hippocampal subregions in spatial working memory with short versus intermediate delay. Behav Neurosci 117: 1044–53 [DOI] [PubMed] [Google Scholar]
- Lee I, Yoganarasimha D, Rao G, Knierim JJ. 2004. Comparison of population coherence of place cells in hippocampal subfields CA1 and CA3. Nature 430: 456–9 [DOI] [PubMed] [Google Scholar]
- Lepage M, Ghaffar O, Nyberg L, Tulving E. 2000. Prefrontal cortex and episodic memory retrieval mode. Proc Natl Acad Sci U S A 97: 506–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK. 2007. Pattern separation, pattern completion, and new neuronal codes within a continuous CA3 map. Learn Mem 14: 745–57 [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI. 2004. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science 305: 1295–8 [DOI] [PubMed] [Google Scholar]
- Levy G, Schupf N, Tang MX, Cote LJ, Louis ED, et al. 2002. Combined effect of age and severity on the risk of dementia in Parkinson’s disease. Ann Neurol 51: 722–9 [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Cools R, Robbins TW, Dove A, Barker RA, Owen AM. 2003. Using executive heterogeneity to explore the nature of working memory deficits in Parkinson’s disease. Neuropsychologia 41: 645–54 [DOI] [PubMed] [Google Scholar]
- Lisman JE. 1999. Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate-CA3 interactions. Neuron 22: 233–42 [DOI] [PubMed] [Google Scholar]
- Litvan I, Goldman JG, Troster AI, Schmand BA, Weintraub D, Petersen RC, … Emre M (2012). Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord, 27(3), 349–356. doi: 10.1002/mds.24893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak E, Su L, Williams GB, Firbank MJ, Lawson RA, et al. 2015. Baseline and longitudinal grey matter changes in newly diagnosed Parkinson’s disease: ICICLE-PD study. Brain 138: 2974–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malapani C, Deweer B, Gibbon J. 2002. Separating storage from retrieval dysfunction of temporal memory in Parkinson’s disease. J Cogn Neurosci 14: 311–22 [DOI] [PubMed] [Google Scholar]
- Malchow B, Strocka S, Frank F, Bernstein HG, Steiner J, et al. 2015. Stereological investigation of the posterior hippocampus in affective disorders. J Neural Transm (Vienna) 122: 1019–33 [DOI] [PubMed] [Google Scholar]
- Marr D 1971. Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci 262: 23–81 [DOI] [PubMed] [Google Scholar]
- Massman PJ, Delis DC, Butters N, Levin BE, Salmon DP. 1990. Are all subcortical dementias alike? Verbal learning and memory in Parkinson’s and Huntington’s disease patients. J Clin Exp Neuropsychol 12: 729–44 [DOI] [PubMed] [Google Scholar]
- Mata IF, Leverenz JB, Weintraub D, Trojanowski JQ, Hurtig HI, et al. 2014. APOE, MAPT, and SNCA genes and cognitive performance in Parkinson disease. JAMA Neurol 71: 1405–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathuranath PS, Nestor PJ, Berrios GE, Rakowicz W, & Hodges JR (2000). A brief cognitive test battery to differentiate Alzheimer’s disease and frontotemporal dementia. Neurology, 55(11), 1613–1620. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, … Perry RH (1996). Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology, 47(5), 1113–1124. [DOI] [PubMed] [Google Scholar]
- Melton AW. 1963. Memory. Science 140: 82–6 [DOI] [PubMed] [Google Scholar]
- Melzer TR, Watts R, MacAskill MR, Pitcher TL, Livingston L, et al. 2012. Grey matter atrophy in cognitively impaired Parkinson’s disease. J Neurol Neurosurg Psychiatry 83: 188–94 [DOI] [PubMed] [Google Scholar]
- Merkow MB, Burke JF, Kahana MJ. 2015. The human hippocampus contributes to both the recollection and familiarity components of recognition memory. Proc Natl Acad Sci U S A 112: 14378–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolaenko I, Pletnikova O, Kawas CH, O’Brien R, Resnick SM, et al. 2005. Alpha-synuclein lesions in normal aging, Parkinson disease, and Alzheimer disease: evidence from the Baltimore Longitudinal Study of Aging (BLSA). J Neuropathol Exp Neurol 64: 156–62 [DOI] [PubMed] [Google Scholar]
- Mishkin M 1978. Memory in monkeys severely impaired by combined but not by separate removal of amygdala and hippocampus. Nature 273: 297–8 [DOI] [PubMed] [Google Scholar]
- Montaldi D, Mayes AR. 2010. The role of recollection and familiarity in the functional differentiation of the medial temporal lobes. Hippocampus 20: 1291–314 [DOI] [PubMed] [Google Scholar]
- Montaldi D, Spencer TJ, Roberts N, Mayes AR. 2006. The neural system that mediates familiarity memory. Hippocampus 16: 504–20 [DOI] [PubMed] [Google Scholar]
- Mormino EC. 2014. The relevance of beta-amyloid on markers of Alzheimer’s disease in clinically normal individuals and factors that influence these associations. Neuropsychol Rev 24: 300–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormino EC, Betensky RA, Hedden T, Schultz AP, Amariglio RE, et al. 2014. Synergistic effect of beta-amyloid and neurodegeneration on cognitive decline in clinically normal individuals. JAMA Neurol 71: 1379–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG. 2001. Perspectives on object-recognition memory following hippocampal damage: lessons from studies in rats. Behav Brain Res 127: 159–81 [DOI] [PubMed] [Google Scholar]
- Muslimovic D, Post B, Speelman JD, Schmand B. 2005. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology 65: 1239–45 [DOI] [PubMed] [Google Scholar]
- Myskiw JC, Rossato JI, Bevilaqua LR, Medina JH, Izquierdo I, Cammarota M. 2008. On the participation of mTOR in recognition memory. Neurobiol Learn Mem 89: 338–51 [DOI] [PubMed] [Google Scholar]
- Ozer F, Meral H, Hanoglu L, Ozturk O, Aydemir T, et al. 2007. Cognitive impairment patterns in Parkinson’s disease with visual hallucinations. J Clin Neurosci 14: 742–6 [DOI] [PubMed] [Google Scholar]
- Pereira JB, Junque C, Bartres-Faz D, Ramirez-Ruiz B, Marti MJ, Tolosa E. 2013. Regional vulnerability of hippocampal subfields and memory deficits in Parkinson’s disease. Hippocampus 23: 720–8 [DOI] [PubMed] [Google Scholar]
- Pillon B, Deweer B, Agid Y, Dubois B. 1993. Explicit memory in Alzheimer’s, Huntington’s, and Parkinson’s diseases. Arch Neurol 50: 374–9 [DOI] [PubMed] [Google Scholar]
- Pitarque A, Melendez JC, Sales A, Satorres E, Escudero J, Algarabel S. 2017. The Role of Recollection and Familiarity in Nondemented Parkinson’s Patients. J Gen Psychol 144: 230–43 [DOI] [PubMed] [Google Scholar]
- Preston AR, Eichenbaum H. 2013. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol 23: R764–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusky GT, Douglas RM, Nelson L, Shabanpoor A, Sutherland RJ. 2004. Visual memory task for rats reveals an essential role for hippocampus and perirhinal cortex. Proc Natl Acad Sci U S A 101: 5064–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Blumenfeld RS, Ramsay IS, Yonelinas A, Yoon J, et al. 2012. Neural correlates of relational and item-specific encoding during working and long-term memory in schizophrenia. Neuroimage 59: 1719–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Ruiz B, Junque C, Marti MJ, Valldeoriola F, Tolosa E. 2006. Neuropsychological deficits in Parkinson’s disease patients with visual hallucinations. Mov Disord 21: 1483–7 [DOI] [PubMed] [Google Scholar]
- Ranganath C, Cohen MX, Dam C, D’Esposito M. 2004. Inferior temporal, prefrontal, and hippocampal contributions to visual working memory maintenance and associative memory retrieval. J Neurosci 24: 3917–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Johnson MK, D’Esposito M. 2003. Prefrontal activity associated with working memory and episodic long-term memory. Neuropsychologia 41: 378–89 [DOI] [PubMed] [Google Scholar]
- Ratcliff R, Sheu CF, Gronlund SD. 1992. Testing global memory models using ROC curves. Psychol Rev 99: 518–35 [DOI] [PubMed] [Google Scholar]
- Reagh ZM, Watabe J, Ly M, Murray E, Yassa MA. 2014. Dissociated signals in human dentate gyrus and CA3 predict different facets of recognition memory. J Neurosci 34: 13301–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rektorova I, Biundo R, Marecek R, Weis L, Aarsland D, Antonini A. 2014. Grey matter changes in cognitively impaired Parkinson’s disease patients. PLoS One 9: e85595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Ortiz CJ, Bermudez-Rattoni F. 2007. Memory Reconsolidation or Updating Consolidation? In Neural Plasticity and Memory: From Genes to Brain Imaging, ed. Bermudez-Rattoni. F Boca Raton (FL) [Google Scholar]
- Rodriguez LA, Algarabel S, Escudero J. 2014. Exploring recollection and familiarity impairments in Parkinson’s disease. J Clin Exp Neuropsychol 36: 494–506 [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kesner RP. 2006. A computational theory of hippocampal function, and empirical tests of the theory. Prog Neurobiol 79: 1–48 [DOI] [PubMed] [Google Scholar]
- Rubin RD, Schwarb H, Lucas HD, Dulas MR, Cohen NJ. 2017. Dynamic Hippocampal and Prefrontal Contributions to Memory Processes and Representations Blur the Boundaries of Traditional Cognitive Domains. Brain Sci 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Yonelinas AP. 2003. Human recognition memory: a cognitive neuroscience perspective. Trends Cogn Sci 7: 313–19 [DOI] [PubMed] [Google Scholar]
- Rutishauser U, Mamelak AN, Schuman EM. 2006. Single-trial learning of novel stimuli by individual neurons of the human hippocampus-amygdala complex. Neuron 49: 805–13 [DOI] [PubMed] [Google Scholar]
- Sabbagh MN, Adler CH, Lahti TJ, Connor DJ, Vedders L, et al. 2009. Parkinson disease with dementia: comparing patients with and without Alzheimer pathology. Alzheimer Dis Assoc Disord 23: 295–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheltens P, Weinstein HC, Leys D. 1992. Neuro-imaging in the diagnosis of Alzheimer’s disease. I. Computer tomography and magnetic resonance imaging. Clin Neurol Neurosurg 94: 277–89 [DOI] [PubMed] [Google Scholar]
- Schwarb H, Watson PD, Campbell K, Shander CL, Monti JM, et al. 2015. Competition and Cooperation among Relational Memory Representations. PLoS One 10: e0143832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura B, Baggio HC, Marti MJ, Valldeoriola F, Compta Y, et al. 2014. Cortical thinning associated with mild cognitive impairment in Parkinson’s disease. Mov Disord 29: 1495–503 [DOI] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. 1996. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A 93: 3908–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stav AL, Johansen KK, Auning E, Kalheim LF, Selnes P, et al. 2016. Hippocampal subfield atrophy in relation to cerebrospinal fluid biomarkers and cognition in early Parkinson’s disease: a cross-sectional study. NPJ Parkinsons Dis 2: 15030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield C, Junque C, Tolosa E, Salgado-Pineda P, Gomez-Anson B, et al. 2005. Structural brain changes in Parkinson disease with dementia: a voxel-based morphometry study. Arch Neurol 62: 281–5 [DOI] [PubMed] [Google Scholar]
- Suthana NA, Donix M, Wozny DR, Bazih A, Jones M, et al. 2015. High-resolution 7T fMRI of Human Hippocampal Subfields during Associative Learning. J Cogn Neurosci 27: 1194–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. 2003. Perirhinal and parahippocampal cortices of the macaque monkey: cytoarchitectonic and chemoarchitectonic organization. J Comp Neurol 463: 67–91 [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Westman E, Ballard C, Aarsland D. 2012. Cognitive impairment in patients with Parkinson’s disease: diagnosis, biomarkers, and treatment. Lancet Neurol 11: 697–707 [DOI] [PubMed] [Google Scholar]
- Tam CW, Burton EJ, McKeith IG, Burn DJ, O’Brien JT. 2005. Temporal lobe atrophy on MRI in Parkinson disease with dementia: a comparison with Alzheimer disease and dementia with Lewy bodies. Neurology 64: 861–5 [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Stan AD, Wagner AD. 2010. The hippocampal formation in schizophrenia. Am J Psychiatry 167: 1178–93 [DOI] [PubMed] [Google Scholar]
- Thoma RJ, Monnig M, Hanlon FM, Miller GA, Petropoulos H, et al. 2009. Hippocampus volume and episodic memory in schizophrenia. J Int Neuropsychol Soc 15: 182–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsivilis D, Vann SD, Denby C, Roberts N, Mayes AR, et al. 2008. A disproportionate role for the fornix and mammillary bodies in recall versus recognition memory. Nat Neurosci 11: 834–42 [DOI] [PubMed] [Google Scholar]
- Tsuang D, Leverenz JB, Lopez OL, Hamilton RL, Bennett DA, et al. 2013. APOE epsilon4 increases risk for dementia in pure synucleinopathies. JAMA Neurol 70: 223–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi Y, Uchikado H, Dickson DW. 2007. Neuropathology of Parkinson’s disease dementia and dementia with Lewy bodies with reference to striatal pathology. Parkinsonism Relat Disord 13 Suppl 3: S221–4 [DOI] [PubMed] [Google Scholar]
- Uncapher MR, Rugg MD. 2005. Encoding and the durability of episodic memory: a functional magnetic resonance imaging study. J Neurosci 25: 7260–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kesteren MT, Ruiter DJ, Fernandez G, Henson RN. 2012. How schema and novelty augment memory formation. Trends Neurosci 35: 211–9 [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, Guzowski JF. 2004. Differences in hippocampal neuronal population responses to modifications of an environmental context: evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles. J Neurosci 24: 6489–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viskontas IV, Carr VA, Engel SA, Knowlton BJ. 2009. The neural correlates of recollection: hippocampal activation declines as episodic memory fades. Hippocampus 19: 265–72 [DOI] [PubMed] [Google Scholar]
- Ward CD, & Gibb WR (1990). Research diagnostic criteria for Parkinson’s disease. Adv Neurol, 53, 245–249. [PubMed] [Google Scholar]
- Warden C, Hwang J, Marshall A, Fenesy M, Poston KL. 2016. The effects of dopamine on digit span in Parkinson’s disease. J Clin Mov Disord 3: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson GS, Cholerton BA, Gross RG, Weintraub D, Zabetian CP, et al. 2013. Neuropsychologic assessment in collaborative Parkinson’s disease research: a proposal from the National Institute of Neurological Disorders and Stroke Morris K. Udall Centers of Excellence for Parkinson’s Disease Research at the University of Pennsylvania and the University of Washington. Alzheimers Dement 9: 609–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiermann B, Stephan MA, Kaelin-Lang A, Meier B. 2010. Is there a recognition memory deficit in Parkinson’s disease? Evidence from estimates of recollection and familiarity. Int J Neurosci 120: 211–6 [DOI] [PubMed] [Google Scholar]
- Weintraub D, Burn DJ. 2011. Parkinson’s disease: the quintessential neuropsychiatric disorder. Mov Disord 26: 1022–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D, Doshi J, Koka D, Davatzikos C, Siderowf AD, et al. 2011. Neurodegeneration across stages of cognitive decline in Parkinson disease. Arch Neurol 68: 1562–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D, Moberg PJ, Culbertson WC, Duda JE, Stern MB. 2004. Evidence for impaired encoding and retrieval memory profiles in Parkinson disease. Cogn Behav Neurol 17: 195–200 [PubMed] [Google Scholar]
- Weintraub D, Simuni T, Caspell-Garcia C, Coffey C, Lasch S, et al. 2015. Cognitive performance and neuropsychiatric symptoms in early, untreated Parkinson’s disease. Mov Disord 30: 919–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis S, Klaver P, Reul J, Elger CE, Fernandez G. 2004a. Neural correlates of successful declarative memory formation and retrieval: the anatomical overlap. Cortex 40: 200–2 [DOI] [PubMed] [Google Scholar]
- Weis S, Specht K, Klaver P, Tendolkar I, Willmes K, et al. 2004b. Process dissociation between contextual retrieval and item recognition. Neuroreport 15: 2729–33 [PubMed] [Google Scholar]
- Winer JR, Maass A, Pressman P, Stiver J, Schonhaut DR, et al. 2018. Associations Between Tau, beta-Amyloid, and Cognition in Parkinson Disease. JAMA Neurol 75: 227–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. 2004. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J Neurosci 24: 5901–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Yu S. 2017. Synucleinopathies: common features and hippocampal manifestations. Cell Mol Life Sci 74: 1485–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnall AJ. 2014. What are the priorities in Parkinson’s disease clinical research? A focus on motor complications, gait and cognition. Neurodegener Dis Manag 4: 127–36 [DOI] [PubMed] [Google Scholar]
- Yarnall AJ, Breen DP, Duncan GW, Khoo TK, Coleman SY, et al. 2014. Characterizing mild cognitive impairment in incident Parkinson disease: the ICICLE-PD study. Neurology 82: 308–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark CE. 2011. Pattern separation in the hippocampus. Trends Neurosci 34: 515–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. 1994. Receiver-operating characteristics in recognition memory: evidence for a dual-process model. J Exp Psychol Learn Mem Cogn 20: 1341–54 [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. 2001. Components of episodic memory: the contribution of recollection and familiarity. Philos Trans R Soc Lond B Biol Sci 356: 1363–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Dobbins I, Szymanski MD, Dhaliwal HS, King L. 1996. Signal-detection, threshold, and dual-process models of recognition memory: ROCs and conscious recollection. Conscious Cogn 5: 418–41 [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Jacoby LL. 1994. Dissociations of processes in recognition memory: effects of interference and of response speed. Can J Exp Psychol 48: 516–35 [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Levy BJ. 2002. Dissociating familiarity from recollection in human recognition memory: different rates of forgetting over short retention intervals. Psychon Bull Rev 9: 575–82 [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. 2005. Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci 25: 3002–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeithamova D, Maddox WT, Schnyer DM. 2008. Dissociable prototype learning systems: evidence from brain imaging and behavior. J Neurosci 28: 13194–201 [DOI] [PMC free article] [PubMed] [Google Scholar]


