Abstract
Cancer tumor cell dormancy is a significant clinical problem in breast cancer (BrCa). We used a 3D in vitro model of the endosteal bone niche (EN), consisting of endothelial, bone marrow stromal cells and fetal osteoblasts in a 3D collagen matrix (GELFOAM™), to identify genes required for dormancy. Human triple-negative MDA-MB-231 BrCa cells, but not the bone-tropic metastatic variant, BoM1833, established dormancy in 3D-EN cultures in a p38-MAPK-dependent manner, whereas both cell types proliferated on 2D plastic or in 3D collagen alone. “Dormancy-reactivation suppressor genes” (DRSG) were identified using a genomic shRNA screen in MDA-MB-231 cells for gene knockdowns that induced proliferation in the 3D-EN. DSRG candidates enriched for genes controlling stem cell biology, neurogenesis, MYC targets, ribosomal structure and translational control. Several potential DRSG were confirmed using independent shRNAs, including BHLHE41, HBP1 and WNT3. Overexpression of the WNT3/a antagonists, secreted frizzled-related protein 2 or 4 (SFRP2/4), induced MDA-MB-231 proliferation in the EN. In contrast, overexpression of SFRP3, known not to antagonize WNT3/a, did not induce proliferation. Decreased WNT3 or BHLHE41 expression was found in clinical BrCa metastases compared to primary-site lesions, and the loss of WNT3 or BHLHE41 or gain of SFRP1, 2 and 4 in the context of TP53 loss/mutation correlated with decreased progression-free and overall survival.
Keywords: breast cancer, dormancy, bone endosteal niche, shRNA screen, p38-MAPK
INTRODUCTION
The progression of breast cancer (BrCa) at metastatic sites continues to be the largest contributor to patient mortality (1, 2). There has been renewed focus on identifying mechanisms governing the establishment of dormancy in specific peripheral sites and the reawakening of dormant cells as major contributors of this cancer’s lethal phenotype (3), especially because only a fraction of disseminated tumor cells (DTC), and even fewer circulating tumor cells (CTC), give rise to clinical macrometastases (4-6). Both estrogen receptor (ER)-positive and triple-negative BrCa (“TNBC”: ER−/PR−/Her2−) metastasize to and enter dormancy in the bone, with ER+ tumors often exhibiting a longer-lasting dormancy (7). Importantly, dormant vs. active growth depends on which bone niche is colonized (8). Colonization of the endosteal niche (EN), enriched in osteoblasts and marked by low oxygen and high calcium levels, results in dormancy, whereas colonization of the perivascular niche, enriched in hematopoietic stem cells, results in active proliferation and formation of macrometastases (7, 9). Signaling pathways in tumor cells define whether DTCs will remain dormant or become proliferative: activation of the p38 MAP-Kinase (MAPK) pathway in the absence of ERK1/2 MAPK activity favors quiescence (10). Additionally, long-term survival of dormant cells is likely to require adoption of stem-like quiescence properties through increased activity of lineage plasticity pathways. Although several groups identified genes and pathways differentially-regulated in dormant vs. proliferating BrCa metastatic cells (8, 11-16), few studies have addressed possible causative roles for these genes, especially in the context of p38 control (16, 17), confounding attempts at therapeutic targeting.
Using a novel 3D-EN culture system developed by Marlow et al. (18), in which otherwise aggressive MDA-MB-231 human BrCa cells become dormant for up to 60 days due to direct contact with EN environmental cells, we show here that knockdown of p38-MAPK induced proliferation, confirming the notion that dormancy is p38-dependent. In order to identify genes that promote or maintain dormancy, we used a high-stringency genomic shRNA screen to identify gene knockdowns that induced proliferation of MDA-MB-231 in 3D-EN cultures. Identification of the top hits, BHLHE41, a known p38 target required for induction of quiescence (19), and LSP1, a suppressor of ERK1/2 activity (20) increased confidence in the screen’s validity. Other gene hits grouped into several regulatory categories not previously identified as suppressors of dormancy. These include genes involved in neurogenesis, translation and non-canonical WNT signaling- all of which play roles in regulating the maintenance of cancer stem cells. These data strengthen the notion that BrCa dormancy in the EN is promoted by p38-MAPK-controlled stem cell pathways.
MATERIALS AND METHODS
Cell culture:
MDA-MB-231 (ATCC HTB-26), MDA-MB-231 BoM 1833 (gift from Joan Massague, Memorial Sloan Kettering Cancer Center (21), HEK293T (ATCC CRL-11268), Phoenix 293T (ATCC CRL-3213) and HS-5 (ATCC CRL-11882) were cultured in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin (Pen/Strep) and incubated at 37°C and 5% CO2. hFOB (human fetal osteoblasts; ATCC CRL-11372) were cultured with DMEM/F12 (1:1) media without phenol red and supplemented with 15% FBS and G418 (0.3mg/ml) and incubated at 32°C and 5% CO2. HUVEC (Lonza C2517A) were cultured to no more than 5 passages with EBM-2 (Lonza CC-3156) supplemented with SingleQuot supplement pack (Lonza CC-4176) and grown at 37°C and 5% CO2.
3D cultures:
3D cultures recapitulating growth in the endosteal niche (EN) or control 3D collagen matrix growth were produced as described (18). Briefly, GELFOAM™ (Pfizer) discs were cut (5mm diameter and 3mm thickness) using a sterile hole punch and scalpel and then UV treated for two hours (1.2 × 10⁵ μ J/CM²) using a UV cross linker. GELFOAM™ discs were placed into a low attachment 96-well plate (Eppendorf Microplate 96/F-PP, Sigma-Aldrich #C150179G) using sterile forceps. Discs were incubated in 200 μl of 1X Dulbecco’s PBS (DPBS) at 37°C/5% CO₂ for 20 min. EN cultures were produced by seeding HS-5, hFOB and HUVEC onto GELFOAM discs (4×104 cells/5μl each) via capillary action, incubating for 2h and then topped off with 200 μl niche media. After 24 h, media was removed and MDA-MB-231 cells (transduced with RFP-expressing shRNA libraries) were seeded onto EN cultures (104 cells/10μl) and grown for 7d, with niche media (100μl) replaced daily. MDA-MB-231 cells grown on tissue culture plates (2D) or in GELFOAM alone (3D) served as negative controls. Bone-tropic MDA-MB-231[BoM1833] cells, infected with pGIPZ (GFP-expressing) lentivirus vector, served as a positive control for 3D-EN growth. MDA MB-231 growth was monitored every other day via fluorescence microscopy using a Nikon Eclipse TS100 inverted microscope and SPOT Insight Fire Wire Camera and SPOT 5.2 software. Cell numbers were quantified using ImageJ software (NIH) from five different fields containing >100 cells/field.
shRNA and expression vectors:
Lentivirus shRNA clones (pGIPZ-based; Supplementary Table S2), Cellecta Human DECIPHER™ screening libraries (Table 1), and DNA expression vectors (ORFeome 8.1) were provided by the Roswell Park Comprehensive Cancer Center Gene Modulation Core (Irwin H. Gelman, Director). Plasmids were propagated in Stbl3 bacteria (ThermoFisher) in LB media supplemented with 100 μg/ml ampicillin for 18 hours at 37°C at a constant speed of 200 rpm. Plasmids were extracted using QIAprep Spin Miniprep Kit (Qiagen cat# 27104) according to protocol. Relative plasmid concentrations were quantified using a Nanodrop 2000 (ThermoFisher Scientific).
TABLE 1:
shRNA library and screening criteria
| Human Module | Gene Targets | # of target mRNAs |
shRNA complexity |
|---|---|---|---|
| 1 | Signaling pathway targets | 5,043 | 27,500 |
| 2 | Disease-associated targets | 5,412 | 27,500 |
| 3 | Cell surface, extracellular, DNA-binding targets | 4,922 | 27,500 |
| Hit Criteria | Definition | Module # | # of hits | |
|---|---|---|---|---|
| Fold increase | ≥1.55 | 1 | 65 | |
| # clones | ≥3 | 2 | 139 | |
| Replicates | ≥2 of 3 | 3 | 198 | |
| p38 regulated | Up- or down-regulated | |||
| Metastasis | Involved? |
Transfection:
HEK293T cells were transfected with LipoD293/DNA mixtures as described previously (22).
Lentivirus packaging and infection:
Polytropic lentiviruses were packaged in HEK293T cells using psPAX2 and pMD2.G packaging constructs as described previously (23). For infection of target cells plated the previous day at confluency in 6-well dishes, 150 μl of lentiviral supernatant and 4μg/ml polybrene (Sigma) was added to the cells along with 1-2 ml DMEM (10% FBS, 1% Pen/Strep) until cells were completely covered. Cells were then incubated for 30 min at 37°C with 5% CO2, centrifuged at 1800rpm for 45 min, and then incubated overnight again at 37°C/5% CO2. The cultures were split at a 1:5 ratio in DMEM (10% FBS, 1% Pen/Strep, 2mg/ml puromycin).
PCR:
RNA was extracted using TRIzol (Life Technologies) according to the manufacturer’s protocol. One μg of total RNA/reaction was used in qRT-PCR reactions (50 μl total volume) containing the High Capacity cDNA Reverse Transcriptase Kit reagents (Life Technologies/Applied Biosystems #4368814). qPCR was performed using Power SYBR green PCR Mastermix (Life Technologies #4367659) on a Step One Plus thermocycler (Applied Biosystems). GAPDH housekeeping gene was used as the loading control. qRT-PCR reactions were performed in triplicate and analysis was determined using the 2−ΔΔCt method (StepOne™ software).
Immunoblot analysis:
Immunoblot analysis was performed as described previously (24) using the following antibodies: primary- V5 tag (ThermoFisher #37-7500), GAPDH (Santa Cruz #sc-25778), α-tubulin (Santa Cruz, #sc-5286), p38-MAPK (Cell Signaling, #9212), p38poT180/Y182 (Cell Signaling, #9211), ERK1/2 (Cell Signaling, #9102S), ERK1/2poT202/Y204 (Cell Signaling, #9101S); secondary- AlexaFluor700 anti-mouse Ig (1:1000) or AlexaFluor800 anti-rabbit Ig (1:10,000).
High-throughput sequencing and gene identification:
DNA was isolated from 7d 2D and 3D-EN cultures using phenol/chloroform/isoamyl (25:24:1). Cells in the 3D culture were isolated by removing GELFOAM™ discs with cells and incubating on a rotator for 1h at 37°C in 5 ml 1X collagenase/hyaluronidase solution (STEMCELL Technologies). After vigorous mixing, cells were pelleted and washed three times with PBS. First-round and nested PCR were performed according to the Cellecta shRNA library manual (http://www.cellecta.com/resources/product-manuals-and-certificates/) as we described previously (23) using primers described in Supplementary Table S3. All experiments were conducted in triplicate. The PCR products were cleaned using QIAquick Gel Extraction Kits, and then subjected to for single-end Rapid Mode sequencing on an Illumina HiSeq2500 as performed by the Roswell Park Comprehensive Cancer Center Genomics Shared Resource (Sean Glenn, Director). Using FASTQ sequencing data files, barcodes were trimmed from flanking sequence using the ShortRead package from Bioconductor (25). The isolated barcode sequences were aligned to a reference file matching shRNA clones to gene targets using the DECIPHER BarCode Deconvoluter program (Cellecta), that allows for up to 2 incorrect base changes for accurate barcode identification. Individual sequence read counts were normalized by total reads sequenced, and top hits were filtered based on a threshold determined by luciferase shRNA negative controls (21 clones). An analysis of row sums was performed to identify genes targeted by multiple shRNA clones and across replicates.
Statistical analysis:
Statistical analysis was performed on the fold change between the cell counts from Day 1 to Day 7 using the student’s two-tailed t test. Error bars indicate standard error of the mean (S.E.M.). Significant differences between experimental groups had a p value lower than 0.05.
RESULTS and DISCUSSION
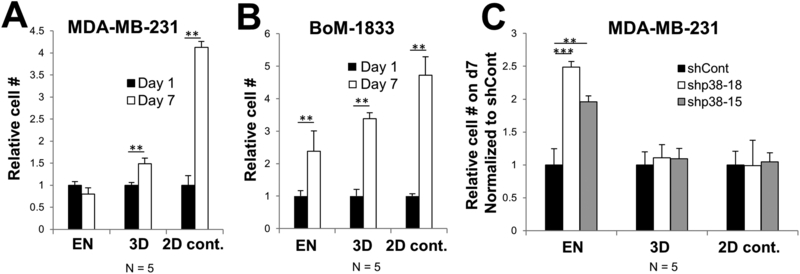
Using a novel 3D model of dormancy for bone metastatic BrCa (18), we endeavored to identify genes that suppress tumor cell quiescence in a cultured microenvironment recapitulating bone EN. In this model, the human TNBC cell line MDA-MB-231 proliferates in a GELFOAM™ biomatrix whereas it is growth-arrested in EN conditions (human hFOB osteoblasts, HUVEC endothelial cells and HS-5 diploid fibroblasts in GELFOAM™)(Fig. 1A). Importantly, the inclusion of bone marrow origin fibroblasts (HS-5) and human endothelial cells (HUVEC) promoted the long-term survival of hFOB osteoblasts even after these cells reached initial confluence after 24 h of growth. This EN culture condition was previously shown to induce growth arrest of ER-positive (MCF7, T47D, ZR75-1, and BT474) and ER-negative (SUM149, SUM159, MDA-MB-231, and MDA-MB-453) human BrCa cell lines, whereas these lines could proliferate in either GELFOAM™ alone, or in GELFOAM™ seeded with primary human bone marrow stem cells, representing a perivascular niche (18). In contrast, the bone-metastatic MDA-MB-231 variant, BoM1833, which was selected in vivo for increased bone growth (26), proliferates in either niche (Fig. 1B). Consistent with the notion that activated p38 MAPK in the absence of MEK-ERK activation favors dormancy, we showed that the knockdown of p38 by shRNA (shRNA clones #15 and #18) also induced MDA-MB-231 proliferation in the EN (Fig. 1C), consistent with previous data (18) using the p38 kinase inhibitor, SB203580.
Figure 1. Dormancy induction in 3D-EN is p38-MAPK-dependent.
Relative cell numbers of MDA-MB-231 (A), MDA-MB-231[BoM1833] (B) or MDA-MB-231 cells with p38 knockdown (vs. shCont.) (C) grown for either 1 or 7 d in 3D-EN or 3D, or in 2D (control) conditions. N = independent replicates; error bars, SEM; **, p <0.001.
To identify suppressors of tumor cell proliferation in a bone niche, MDA-MB-231 cells were transduced with a genomic shRNA library (Cellecta DECIPHER® library covering 15,377 human genes with 82,500 independent shRNA clones, divided into 3 modules; Table 1) and clones that proliferated in EN cultures were enriched. Genes that are potentially required for MDA-MB-231 dormancy within the EN were identified by performing next-gen-sequencing (NGS) of shRNA clone barcodes from DNA taken from triplicate screen aliquots of freshly infected cells (24h) and from infected cells incubated for 7d in 3D-EN. The barcode sequences were trimmed from flanking sequences and shRNA-targeted genes then identified using Cellecta’s BarCode Deconvoluter software. We selected gene targets (shRNA bar-codes) that were found in ≥2 of 3 independent screens, identified by ≥3 independent shRNA clones/gene, each at >1.5-fold increase over background (normalized against the relative abundance of each clone in the library) (Table 1). This analysis identified 416 potential “dormancy-reactivation suppressor” genes (DRSG) in the 3 shRNA clone modules (Table 1).
One of the ways we established statistical significance for potential DSRG candidates was to compare the relative frequency of shRNA clones to the 16 luciferase shRNA controls (shLuc) contained within each module. For example, two module-2 genes, DOLK and MICALL2, had at least 2 independent shRNA clones with fold-change sequence reads over the 1.55-fold shLuc threshold (Supplementary Fig. S1A), yet when compared between screening replicates, only the MICALL2 clones showed consistent statistical significance over the luciferase clones (Supplementary Fig. S1B) in ≥2 replicates. Indeed, the knockdown of DOLK using independent shRNAs failed to induce increase MDA-MB-231 proliferation in 3D-EN cultures (Table 2).
TABLE 2:
DSRG candidates subjected to secondary validation
| Gene Targets | Human Module |
Fold increase in proliferation at d7 |
P value |
|---|---|---|---|
| MAPK14 | -- | 2.4 +/− 0.4 | <0.001 |
| HBP1 | 1 | 1.8 +/− 0.16 | <0.01 |
| WNT3 | 1 | 2.9 +/− 0.4 | <0.001 |
| NES | 1 | 0.9 +/− 0.2 | N.S. |
| BHLHE41 | 1 | 2.2 +/− 0.2 | <0.001 |
| TIAL1 | 2 | 1.1 +/− 0.2 | N.S. |
| HTATIP2 | 2 | 1.06 +/− 0.032 | N.S. |
| DOLK | 2 | 1.04 +/− 0.06 | N.S. |
Many studies have shown that metastatic dormancy is controlled by the simultaneous upregulation of p38MAPK and downregulation of ERK activation (27), yet little is known about mediators of dormancy downstream of p38MAPK. Thus, DSRG were subjected to Ingenuity pathway analysis and Pubmed search to bin them based on a predicted or known relationship to p38MAPK signaling and metastasis. An example is shown in Table 3 for the 65 potential DSRG from module-1, relative to their relationship to p38MAPK signaling and metastasis. Of these candidates, 14 genes were associated with both p38MAPK signaling and metastasis (ADRB2, BHLHE41, CASR, CD63, CDC2L1, FLT1, HBP1, KEAP1, LSP1, NOB1, NRG1, P11, TTF1, WNT3), 12 genes were associated with metastasis but not with p38MAPK signaling (BIK, BRD4, CLDN2, EIF4A1, FUBP1, HSPD1, KIF11, NES, NOX1, RELN, SERPIN1, WNT8A), and 5 genes were associated with p38MAPK signaling alone (CACNB3, EHF, NNAT, OASL, TAC4). As a whole, there was a selection for DSRG candidates in module-1 that are involved in the regulation of neurogenesis or stem cell biology (Table 3), and/or protein translation (Tables 2&3). Loss of differentiation (neurogenesis) or stem cell induction genes resulting in active BrCa cell growth in the 3D-EN is consistent with the notion that disseminated tumor cells exhibit stem cell-like properties (28, 29). Differential expression of genes controlling ribosome biogenesis are known to control stem cell homeostasis (30), and indeed, antagonism of this process was shown to inhibit tumor formation induced by CD44+/CD24− human BrCa stem cells (31).
TABLE 3:
Module-1 DSRG candidates
| Gene | Fold increase* |
# of hits |
p38- related** |
metastasis | pathway |
|---|---|---|---|---|---|
| ADCY8 | 1.81 | 5 | no | no | neuro/stem |
| ADK | 1.90 | 4 | no | no | neuro/stem |
| ADRB2 | 2.19 | 5 | yes | yes | neuro/stem |
| ARG1 | 1.64 | 4 | no | no | neuro/stem |
| ATG2A | 2.00 | 4 | no | no | autophagy |
| ATP2B1 | 1.91 | 4 | no | no | Ca transport |
| BHLHE41 ‡ | 2.3 | 5 | yes | yes | p38/dormancy |
| BIK | 1.56 | 4 | no | yes | apoptosis |
| BRD4 | 1.78 | 7 | no | yes | neuro/stem |
| CACNA1B | 1.75 | 4 | no | no | neuro/stem |
| CACNB3 | 2.70 | 5 | yes | no | neuro/stem |
| CASR | 1.76 | 5 | yes | yes | neuro/stem |
| CD63 | 1.62 | 4 | yes | yes | β-catenin signal |
| CDC2L1 | 1.84 | 5 | yes | yes | translation |
| CFD | 2.23 | 5 | no | no | ? |
| CHRND | 1.80 | 4 | no | no | neuro/stem |
| CIP29 | 2.31 | 6 | no | no | translation |
| CLDN2 | 1.88 | 4 | no | yes | neuro/stem |
| CLK3 | 1.64 | 4 | no | no | translation |
| EHF/ESE3 | 2.33 | 4 | yes | no | neuro/stem |
| EIF2S3 | 2.45 | 4 | no | no | translation |
| EIF4A1 | 1.82 | 4 | no | yes | translation |
| EME2 | 1.71 | 5 | no | no | DNA repair |
| F10 | 1.68 | 4 | no | no | coagulation |
| FLT1 (VEGFR1) | 1.74 | 4 | yes | yes | met. promoter |
| FUBP1 | 2.39 | 6 | no | yes | translation |
| GABRG3 | 1.70 | 5 | no | no | neuro/stem |
| GCGR | 1.60 | 4 | no | no | neuro/stem |
| GFPT2 | 1.82 | 5 | no | no | metabolism |
| GHRL | 1.70 | 4 | no | no | metabolism |
| GPD2 | 1.85 | 4 | no | no | metabolism |
| HBP1 ‡ | 1.85 | 5 | yes | yes | neuro/stem |
| HMGB2 | 1.84 | 4 | no | no | neuro/stem |
| HSPD1 | 1.71 | 4 | no | yes | chaperone |
| IL2RG | 1.84 | 4 | no | no | survival |
| IREB2 | 1.60 | 4 | no | no | Iron metabolism |
| KEAP1 | 2.26 | 5 | yes | yes | neuro/stem |
| KIF11 | 2.22 | 11 | no | yes | met. promoter |
| KREMEN1 | 1.69 | 5 | no | no | survival |
| LSP1 | 2.20 | 5 | yes | yes | ERK1/2 supp |
| NEDD8 | 1.81 | 4 | no | no | neuro/stem |
| NES | 1.76 | 5 | no | yes | neuro/stem |
| NNAT | 2.63 | 7 | yes | no | neuro/stem |
| NOB1 | 2.67 | 6 | yes | yes | translation |
| NOX1 | 1.86 | 7 | no | yes | neuro/stem |
| NRG1 | 1.97 | 4 | yes | yes | neuro/stem |
| OASL | 2.84 | 3 | yes | no | translation |
| OGG1 | 1.85 | 6 | no | no | DNA repair |
| P11 (s100A10) | 1.79 | 4 | yes | yes | BrCa adhesion |
| PDE6H | 1.91 | 4 | no | no | cAMP metabol |
| PLLP | 2.20 | 4 | no | no | metabolism |
| PSMB4 | 2.03 | 4 | no | no | neuro/stem |
| PSMC6 | 2.29 | 4 | no | no | neuro/stem |
| PSMD7 | 1.68 | 6 | no | no | neuro/stem |
| RELN | 1.96 | 4 | no | yes | neuro/stem |
| SCN7A | 1.63 | 4 | no | no | Na channel |
| SERPIN1 | 1.55 | 4 | no | yes | invasion |
| SHC3 | 2.02 | 6 | no | no | neuro/stem |
| SUOX | 1.98 | 4 | no | no | metabolism |
| TAC4 | 2.27 | 7 | yes | no | neuro/stem |
| TTF1 | 2.26 | 5 | yes | yes | neuro/stem |
| VARS | 2.02 | 5 | no | no | translation |
| WNT3 ‡ | 1.91 | 5 | yes | yes | β-catenin signal |
| WNT8A | 2.23 | 4 | no | yes | β-catenin signal |
| YARS | 1.62 | 4 | no | no | translation |
Confirmed by independent shRNA knockdown
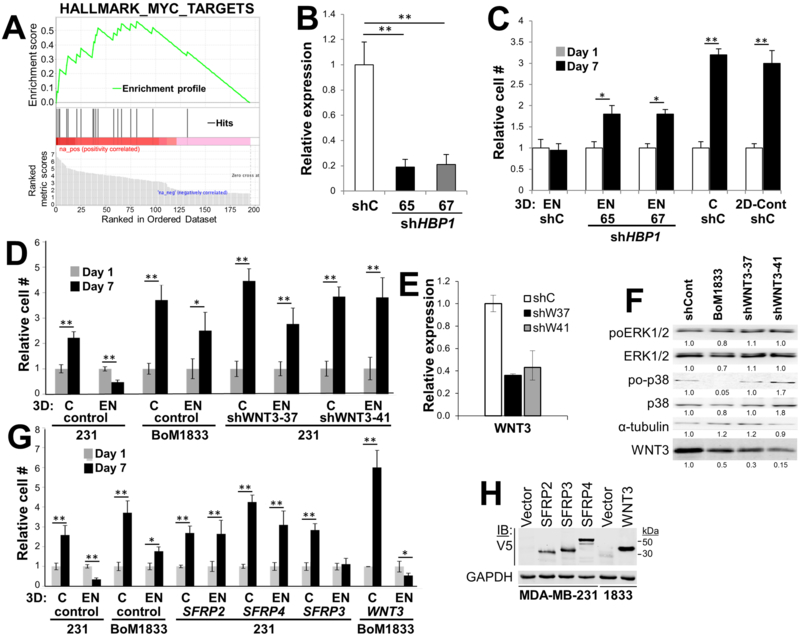
In contrast, several had roles that might directly control known dormancy functions. For example, BHLHE41 has been reported to play a role in p38MAPK-mediated dormancy (19), ADRB2 suppresses prostate cancer proliferation in bones by downregulating osteoblast-expressed GAS6 (32), LSP1 negatively controls ERK signaling (20), and P11 (S110A10) controls breast cancer adhesion to endothelial cells in the metastatic niche (33). Lastly, Gene Set Enrichment Analysis of all the module-1 genes showed that 18/65 genes (27.7%) were likely MYC targets (Fig. 2A). Although a role of MYC as a driver of dormancy reawakening has not been addressed, MYC amplification is associated with high-grade BrCa and worse prognosis (34), and in several non-BrCa models, the forced re-expression of MYC rescues proliferation in dormant tumor cells (35).
Figure 2. Analysis of HBP1 and WNT3 as potential DSRG.
(A) Gene Set Enrichment Analysis of module-1 DSRG candidates identified 18 of 65 hits as being MYC target genes. (B) qRT-PCR showing knockdown of HBP1 in MDA-MB-231 cells. (C and D) Knockdown of HBP1 (C) or WNT3 (D) induces proliferation in 3D-EN vs. 3D (“C”) or 2D cultures. Error bars, SEM of three independent replicates; *, p < 0.01; **, p <0.001. (E) Confirmation of WNT3 knockdown by qRT-PCR. Error bars, SEM of three independent replicates. (F) Immunoblot of lysates of MDA-MB-231 or BoM1833 transduced with scramble shRNA (“shCont”), or WNT3-knockdown MDA-MB-231 cells probed for total or activated (poT202/Y204) ERK1/2, total or activated (poT180/Y182) p38-MAPK or α-tubulin (as a loading control). Digital quantifications are shown as normalized to the shControl. This blot is typical of three independent experiments. (G) Overexpression of SFRP2 or 4, but not SFRP3, in MDA-MB-231 induces proliferation in 3D-EN cultures, whereas the overexpression of WNT3 in BoM1833 suppresses 3D-EN proliferation. Error bars, SEM of three independent replicates; *, p < 0.01; **, p <0.001. (H) Immunoblot of MDA-MB-231 lysates transduced with lentivirus expressing V5-tagged SFRP2, 3, or 4 (or empty vector), or BoM1833 (“1833”) cells transduced with WNT3 (or empty vector), probed for V5 or GAPDH. Molecular weight markers are at right.
We then sought to independently confirm that the downregulation of several DSRG candidates leads to MDA-MB-231 proliferation in 3D-EN cultures. Thus, MDA-MB-231 cells were transduced with two independent shRNA clones/gene, and following confirmation of gene knockdown by either qRT-PCR or immunoblotting (IB), the cells were assessed for proliferation (vs. scrambled shRNA controls) in 3D-EN cultures as in Fig. 1. For this analysis, we chose three predicted DSRG from module-1, BHLHE41, HBP1 and WNT3, which were both p38- and metastasis-associated (Table 3), and one gene, NES, not known to be p38-regulated. As well, we chose two negative controls (not predicted to be DSRG): DOLK, that was neither p38- nor metastasis-associated (Supplementary Table S1) and that was likely not significant due to lack of replicate hits (Supplementary Fig. S1B), and HTATIP2, a module-2 gene that failed to make the cut because it had only 2 shRNA hits in one of three replicates. BHLHE41, HBP1 and WNT3 were validated as DSRG, i.e.- knockdown resulted in significantly increased proliferation in EN over controls (BHLHE41: Table 2, HBP1 and WNT3: Fig. 2C&D), whereas DOLK, NES and HTATIP2 knockdown failed to induce MDA-MB-231 proliferation in the 3D-EN (Table 3).
WNT signaling largely has been linked to metastatic progression, especially in models of TNBC (36). However, recent data suggest that specific WNT family members, such as WNT5A, might promote either metastatic progression or dormancy, depending on whether signaling is through canonical or non-canonical pathways (37). Therefore, we sought to follow-up our finding that WNT3 knockdown induced MDA-MB-231 proliferation in our 3D-EN assay. First, we confirmed that WNT deficiency caused proliferation (Fig. 2D), using two independent WNT3-specific shRNAs, which knocked down WNT3 expression in MDA-MB-231 cells ~2.5-fold over scrambled controls (“shCont”; Fig. 2E). Interestingly, WNT3 levels were relatively decreased in BoM1833 cells, consistent with the notion that WNT3 loss facilitates proliferation in the 3D-EN (Fig. 2F). However, WNT3 knockdown in MDA-MB-231 cells had no effect on relative p38poT180/Y182 levels, indicating that the increased proliferation of MDA-MB-231 cells after WNT3 deficiency was not a result of loss of p38 activation. Although this finding would be consistent with WNT3 being a downstream mediator of p38 signaling, we cannot rule out that the WNT3 effect is p38-independent. In contrast, the BoM1833 variant, which failed to growth-arrest in the 3D-EN cultures (Fig. 1B), exhibited decreased relative p38 activation levels.
Secreted forms of Frizzled-related proteins (SFRP) are thought to antagonize WNT/β-catenin signaling by directly binding WNT members (38), and more specifically, SFRP2 and 4, but not SFRP3, are known to bind WNT3 at high affinity (39). We therefore transduced MDA-MB-231 cells with V5-epitope tagged SFRP2, 3 or 4, confirmed ectopic expression vs. an empty vector control (Fig. 2H), and tested these cells for proliferation in 3D-EN cultures. Fig. 2G shows that SFRP2 and 4, but not 3, could release MDA-MB-231 cells from dormancy. Similarly, the over-expression of WNT3 in BoM1833 suppressed proliferation in 3D-EN, but not in 3D-control cultures (Fig. 2G). Taken together, these data strongly suggest that WNT3 promotes dormancy in our 3D-EN model. This is consistent with a report showing that increased Hedgehog-mediated Sfrp1 expression in liver stroma increased the metastatic potential of human Capan-1 pancreatic tumor cells through the suppression of WNT3 signaling, and that the overexpression of WNT3A in Capan-1 cells decreased experimental metastasis formation (40).
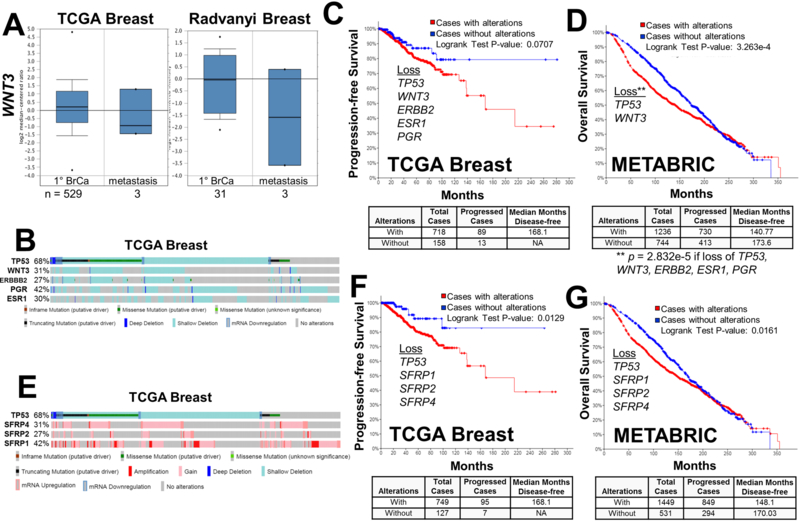
To address this in a clinical context, we compared relative WNT3 expression levels in primary-site vs. metastatic breast cancers in TCGA and Radvanyi Oncomine datasets (41). These data show lower levels of WNT3 in clinical macrometastases compared to primary-site BrCa (Fig. 3A), suggesting that WNT3 deficiency promotes active metastatic progression. One drawback, however, is that both studies have very few metastatic cases (3 each), with none derived from bone, confounding determination of statistical significance. We then analyzed how the loss of WNT3 correlates with either progression-free or overall survival using the TCGA Breast and METABRIC (42, 43) datasets in cBioPortal (http://www.cbioportal.org). We noted that WNT3 loss, either due to gene deletion or transcriptional downregulation, occurred in 31% and 21% of all BrCa cases in TCGA and METABRIC, respectively, and that these cases showed strong co-occurrence with the loss/mutation of TP53 (q-values of 1.36e-21 and 2.98e-58, respectively). Indeed, clinical cases of TNBC are marked by specific TP53 mutations (44). We then sought to determine if the loss of WNT3 and/or TP53 predicted poorer clinical survival, or whether any survival correlation associated with WNT3 loss was potentiated in a background of ER, PR and HER2 loss (encoded by ESR1, PGR and ERBB2, respectively), reflecting TNBC. Indeed, a large portion of the WNT3-deficient cases in the TCGA Breast dataset had coincident losses of ESR1, PGR and ERBB2 (Fig. 3B). Fig 3C shows that in the TCGA dataset, poorer progression-free survival was only detected in cases with combined losses of WNT3, TP53, ESR1, PGR and ERBB2; the loss of any of these genes alone or the combined loss of ESR1, PGR and ERBB2 did not affect survival (WNT3 loss alone: p = 0.652; TP53 loss alone: p = 0.125; WNT3 plus TP53 loss: p = 0.0855; ESR1, PGR and ERBB2 combined loss: p = 0.300). It is noteworthy that BRCA1 mutational status, which represents fewer than 3% of all the WNT3-deficient cases in the TGCA Breast database, has no effect on survival (WNT3 loss + BRCA1 mutation: p = 0.693). In contrast, loss of only WNT3 and TP53 in the METABRIC dataset showed poorer survival (Fig. 3D). Taken together, these data show that WNT3 loss contributes to poorer survival, especially in the context of TP53 loss. The superior powering of the METABRIC dataset, and the fact that it includes many more cases of disease recurrence/progression associated with metastasis, allows for the conclusion that WNT3/TP53 loss is sufficient for poorer survival, a value that worsens in the context of what are likely triple-negative cases (ESR1, PGR and ERBB2 loss). These data correlate with the fact that TNBC dormancy in the bones is shorter in duration than that of ER-positive BrCa (7), suggesting that the combined loss of ER, PR and HER2 might sensitize dormant BrCa cells towards WNT3 loss. It is important to note that we only studied the role of WNT3 signaling in tumor cells in the context of a 3D-EN microenvironment. Although secreted factors are likely to play an important role, it should be noted that cell-cell contact was required for MDA-MB-231 dormancy in this 3D-EN (18). The exact roles played by each EN niche cell type, whether in its direct interaction to BrCa cells or through its secretome, remains to be elucidated. Additionally, the role of WNT3 in controlling tumor dormancy in the bone may be cancer type-dependent because Nandana et al. (45) showed that prostate cancer invasiveness and bone colonization required TBX2-regulated WNT3A expression.
Figure 3. WNT3 expression in clinical BrCa datasets and correlation with survival.
(A) Oncomine TCGA Breast and Radvanyi datasets showing relative WNT3 expression in primary (1°) vs. metastatic BrCa cases. N = number of cases. (B) Copy number variations, mutations and expression changes of TP53, WNT3, ERBB2, PGR and ESR1 in the TCGA Breast dataset produced through cBioPortal, with vertical bars representing a single patient, and the percentages representing the total changes for a given gene. (C and D) Progression-free (C) and overall survival (D) for TGCA Breast and METABRIC datasets, respectively, based on combined TP53, WNT3, ERBB2, PGR and ESR1 losses in the TGCA data, and TP53 and WNT3 losses in the METABRIC dataset. The number of cases with or without these gene changes, as well as the median number of disease-free months, are shown below. (E) Copy number variations, mutations and expression changes of TP53, SFRP1, 2 and 4 in the TCGA Breast dataset produced through cBioPortal. (F and G) Progression-free (F) and overall survival (G) for TGCA Breast and METABRIC datasets, respectively, based on combined TP53, SFRP1, 2 and 4 losses, with numbers of cases with or without changes (below).
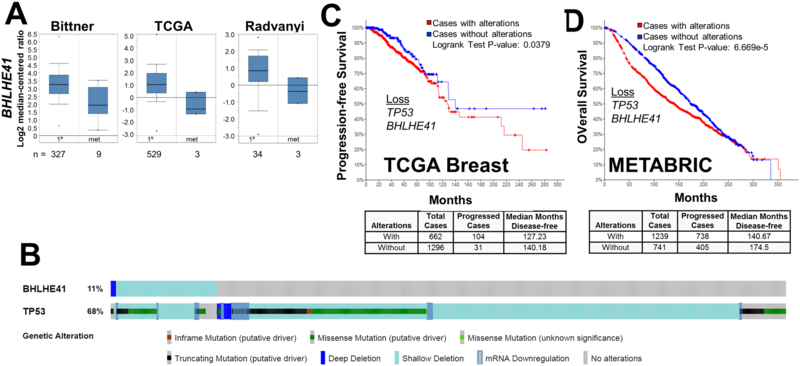
We performed a similar analysis on the two other validated DSRG, which indicated that BHLHE41, but not HBP1, exhibited decreased expression in BrCa metastases compared to levels in primary tumors (Fig. 4A; Supplementary Fig. S1C). In the TCGA Breast dataset, 11% of cases exhibited partial or full loss of BHLHE41 (Fig. 4B). As with WNT3 loss, the loss of BHLHE41 had a statistical co-occurrence with mutation/loss of TP53 (q = 0.0242) in this dataset. Moreover, the combined loss of BHLHE41 and TP53 correlated with decreased progression-free or overall survival, respectively, in the TCGA Breast and METABRIC datasets (TCGA: p = 0.0379; METABRIC: p = 6.669e-5). In contrast, loss of BHLHE41 alone did not correlate with decreased survival in TCGA Breast (p = 0.568). Interestingly, the combined loss of WNT3, BHLHE41 and TP53 did significantly change the rate of progression-free survival in TCGA Breast cases, strongly suggesting that the WNT3/TP53 and BHLHE41/TP53 loss cohorts were independent groups, and that either loss of WNT3 or BHLHE41 was individually capable of initiating reawakening in the context of TP53 loss. This also suggests that TP53 loss is the main driver of the poorer prognosis. In regards to possible mechanisms underlying BHLHE41 as a DSRG, the gene product, BHLHE41 (a.k.a.- DEC2 and SHARP1), functions as a transcriptional repressor of epithelial-to-mesenchymal transition and invasion factors, SNAI1, SNAI2 and TWIST (46). Additionally, Adorno et al. (47) showed that BrCa cases with higher levels of BHLHE41 and CCNG2, two p63-induced genes, correlated with lower metastatic risk. Interestingly, specific sets of p53 mutations abrogate p63 activity, likely leading to BHLHE41 loss (48).
Figure 4. BHLHE41 expression in clinical BrCa datasets and correlation with survival.
(A) Oncomine Bittner, TCGA Breast and Radvanyi datasets showing relative BHLHE41 expression in primary (1°) vs. metastatic BrCa cases. N = number of cases. (B) Copy number variations, mutations and expression changes of TP53 and BHLHE41 in the TCGA Breast dataset produced through cBioPortal, with vertical bars representing a single patient, and the percentages representing the total changes for a given gene. (C and D) Progression-free (C) and overall survival (D) for TGCA Breast and METABRIC datasets, respectively, based on combined TP53 and BHLHE41 losses in the METABRIC dataset. The number of cases with or without these gene changes, as well as the median number of disease-free months, are shown below.
In conclusion, the current study marks a novel method to identify and validate potential DSRG based on an in vitro 3D-EN dormancy model for BrCa. Our data suggest several therapeutic avenues, but these would likely be divided into treatments that either secure dormancy, i.e.- antagonize reawakening, or that induce large scale reawakening in a neoadjuvant setting, linked to standard chemotherapies prescribed for TNBC. Examples of reawakening suppressors might include small molecule inhibitors of MYC (49) or Nutlin-3a to normalize mutant p53 function (50), whereas inducers of reawakening might include inhibitors of WNT3 signaling (51, 52) or p38 kinase activity (53).
Supplementary Material
Table 4:
Translation-controlling potential DSRG
| Gene | Gene Name | Function |
|---|---|---|
| 60S subunit | ||
| RPL3 | Ribosomal protein L3 | Ribosome, structural |
| RPL4 | Ribosomal protein L4 | Ribosome, structural |
| RPL6 | Ribosomal protein L6 | Ribosome, structural |
| RPL7 | Ribosomal protein L7 | Ribosome, structural |
| RPL8 | Ribosomal protein L8 | Ribosome, structural |
| RPL10 | Ribosomal protein L10 | Ribosome, structural |
| RPL12 | Ribosomal protein L12 | Ribosome, structural |
| RPL13A | Ribosomal protein L13A | Ribosome, structural |
| RPL14 | Ribosomal protein L14 | Ribosome, structural |
| RPL21 | Ribosomal protein L21 | Ribosome, structural |
| RPL23 | Ribosomal protein L23 | Ribosome, structural |
| RSL24 | Ribosomal protein L24 | Ribosome, structural |
| RPL32 | Ribosomal protein L32 | Ribosome, structural |
| RPL37A | Ribosomal protein L37A | Ribosome, structural |
| 40S subunit | ||
| RPS4X | Ribosomal protein S4X | Ribosome, structural |
| RPS14 | Ribosomal protein S14 | Ribosome, structural |
| RPS15AP17 | Ribosomal protein S15a pseudogene 17 | |
| RPS20 | Ribosomal protein S20 | Ribosome, structural |
| RPS26 | Ribosomal protein S26 | Ribosome, structural |
| RPS27AP5 | Ribosomal protein S27a pseudogene 5 | |
| RPL36P14 | Ribosomal protein L36 pseudogene 14 | |
| Elongation factors | ||
| EIF2B5 | Eukaryotic translation initiation factor 2B subunit ε | Elongation factor |
| EIF2S2 | Eukaryotic translation initiation factor 2B subunit β | Elongation factor |
| EIF2S3 | Eukaryotic translation initiation factor 2B subunit γ | Elongation factor |
| EIF3A | Eukaryotic translation initiation factor 3 subunit A | Elongation factor |
| EIF4A1 | Eukaryotic translation initiation factor 4A1 | Elongation factor |
| Misc. regulators | ||
| CLK3 | CDC-like kinase 3 | Regulates splicing factors |
| FCF1 | rRNA-processing protein | Ribosome formation |
| KARS | Lysyl tRNA synthase | Codon usage |
| NARS2 | Asparaginyl-tRNA synthetase 2 | Codon usage |
| NOB1 | NIN1 binding protein 1 | rRNA processing |
| TSFM | Ts translation elongation factor, mitochondrial | Mitochondrial translation |
| VARS | Valyl-tRNA synthetase | Codon usage |
| YARS | Tyrosyl-tRNA synthetase | Codon usage |
Implications:
These data describe several novel, potentially targetable pathways controlling BrCa dormancy in the EN.
ACKNOWLEDGEMENTS
We thank G. Dontu for critical discussion regarding the 3D bone growth models. This work was supported by the Roswell Park Alliance Foundation and by National Cancer Institute (NCI) grant P30-CA016056 involving the use of Roswell Park Comprehensive Cancer Center’s Genomics, Bioinformatics and Gene Modulation Shared Resource.
FUNDING: This work was supported by grants CA94108 (National Institutes of Health/National Cancer Institute) and by an Alliance Foundation grant (IHG), and in part, through National Cancer Institute Comprehensive Cancer funds (P30-CA016056) involving the use of Roswell Park Comprehensive Cancer Center’s Genomics and Gene Modulation Shared Resources.
ABBREVIATIONS
- BrCa
breast cancer
- EN
endosteal niche
- FBS
fetal bovine serum
- GFP
green fluorescent protein
- HUVEC
human umbilical vascular endothelial cells
- Luc
luciferase
- RT-PCR
reverse transcriptase-polymerase chain reaction
- SEM
standard error of the mean
- TCGA
The Cancer Gene Atlas
- TNBC
triple-negative breast cancer
- WT
wild-type
Footnotes
CONFLICTS OF INTEREST: The authors declare that they have no conflicts of interest with the contents of this article.
REFERENCES
- (1).Zhang XH, Giuliano M, Trivedi MV, Schiff R, Osborne CK, Metastasis dormancy in estrogen receptor-positive breast cancer. Clin Cancer Res 2013;19:6389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Banys M, Hartkopf AD, Krawczyk N, Kaiser T, Meier-Stiegen F, Fehm T, et al. , Dormancy in breast cancer. Breast Cancer (Dove Med Press) 2012;4:183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Sosa MS, Bragado P, Aguirre-Ghiso JA, Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer 2014;14:611–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, et al. , Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 2013;339:580–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Broersen LH, van Pelt GW, Tollenaar RA, Mesker WE, Clinical application of circulating tumor cells in breast cancer. Cell Oncol (Dordr) 2014;37:9–15. [DOI] [PubMed] [Google Scholar]
- (6).Banys M, Krawczyk N, Fehm T, The role and clinical relevance of disseminated tumor cells in breast cancer. Cancers (Basel) 2014;6:143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Ottewell PD, O’Donnell L, Holen I, Molecular alterations that drive breast cancer metastasis to bone. Bonekey Rep 2015;4:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Kim RS, Avivar-Valderas A, Estrada Y, Bragado P, Sosa MS, Aguirre-Ghiso JA, et al. , Dormancy signatures and metastasis in estrogen receptor positive and negative breast cancer. PLoS ONE 2012;7:e35569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Chen YC, Sosnoski DM, Mastro AM, Breast cancer metastasis to the bone: mechanisms of bone loss. Breast Cancer Res 2010;12:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Sosa MS, Avivar-Valderas A, Bragado P, Wen HC, Aguirre-Ghiso JA, ERK1/2 and p38α/β Signaling in Tumor Cell Quiescence: Opportunities to Control Dormant Residual Disease. Clin Cancer Res 2011;17:5850–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, et al. , The perivascular niche regulates breast tumour dormancy. Nat Cell Biol 2013;15:807–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Wang N, Docherty F, Brown HK, Reeves K, Fowles A, Lawson M, et al. , Mitotic quiescence, but not unique “stemness,” marks the phenotype of bone metastasis-initiating cells in prostate cancer. FASEB J 2015;29:3141–50. [DOI] [PubMed] [Google Scholar]
- (13).Patel P, Chen EI, Cancer stem cells, tumor dormancy, and metastasis. Front Endocrinol (Lausanne) 2012;3:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Ogba N, Manning NG, Bliesner BS, Ambler SK, Haughian JM, Pinto MP, et al. , Luminal breast cancer metastases and tumor arousal from dormancy are promoted by direct actions of estradiol and progesterone on the malignant cells. Breast Cancer Res 2014;16:489–0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Lawson DA, Bhakta NR, Kessenbrock K, Prummel KD, Yu Y, Takai K, et al. , Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature 2015;526:131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Gao H, Chakraborty G, Lee-Lim AP, Mavrakis KJ, Wendel HG, Giancotti FG, Forward genetic screens in mice uncover mediators and suppressors of metastatic reactivation. Proc Natl Acad Sci U S A 2014;111:16532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Sosa MS, Parikh F, Maia AG, Estrada Y, Bosch A, Bragado P, et al. , NR2F1 controls tumour cell dormancy via SOX9- and RARbeta-driven quiescence programmes. Nat Commun 2015;6:6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Marlow R, Honeth G, Lombardi S, Cariati M, Hessey S, Pipili A, et al. , A novel model of dormancy for bone metastatic breast cancer cells. Cancer Res 2013;73:6886–99. [DOI] [PubMed] [Google Scholar]
- (19).Adam AP, George A, Schewe D, Bragado P, Iglesias BV, Ranganathan AC, et al. , Computational identification of a p38SAPK-regulated transcription factor network required for tumor cell quiescence. Cancer Res 2009;69:5664–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Zhang H, Wang Y, Liu Z, Yao B, Dou C, Xu M, et al. , Lymphocyte-specific protein 1 inhibits the growth of hepatocellular carcinoma by suppressing ERK1/2 phosphorylation. FEBS Open Bio 2016;6:1227–37. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- (21).Zhang XH, Jin X, Malladi S, Zou Y, Wen YH, Brogi E, et al. , Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell 2013;154:1060–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Su B, Zheng Q, Vaughan MM, Bu Y, Gelman IH, SSeCKS metastasis-suppressing activity in MatLyLu prostate cancer cells correlates with VEGF inhibition. Cancer Res 2006;66:5599–607. [DOI] [PubMed] [Google Scholar]
- (23).Su B, Gao L, Baranowski C, Gillard B, Wang J, Ransom R, et al. , A Genome-Wide RNAi Screen Identifies FOXO4 as a Metastasis-Suppressor through Counteracting PI3K/AKT Signal Pathway in Prostate Cancer. PLoS ONE 2014;9:e101411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Akakura S, Nochajski P, Gao L, Sotomayor P, Matsui S, Gelman IH, Rb-dependent cellular senescence, multinucleation and susceptibility to oncogenic transformation through PKC scaffolding by SSeCKS/AKAP12. Cell Cycle 2010;9:4656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Morgan M, Anders S, Lawrence M, Aboyoun P, Pages H, Gentleman R, ShortRead: a bioconductor package for input, quality assessment and exploration of high-throughput sequence data. Bioinformatics 2009;25:2607–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Zhang XH, Wang Q, Gerald W, Hudis CA, Norton L, Smid M, et al. , Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell 2009;16:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Aguirre-Ghiso JA, Bragado P, Sosa MS, Metastasis awakening: targeting dormant cancer. Nat Med 2013;19:276–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Muzes G, Sipos F, Metastatic cell dormancy and re-activation: an overview on series of molecular events critical for cancer relapse. Anticancer Agents Med Chem 2016;17:472–82. [DOI] [PubMed] [Google Scholar]
- (29).Linde N, Fluegen G, Aguirre-Ghiso JA, The Relationship Between Dormant Cancer Cells and Their Microenvironment. Adv Cancer Res 2016;132:45–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Brombin A, Joly JS, Jamen F, New tricks for an old dog: ribosome biogenesis contributes to stem cell homeostasis. Curr Opin Genet Dev 2015;34:61–70. [DOI] [PubMed] [Google Scholar]
- (31).Erol A, Acikgoz E, Guven U, Duzagac F, Turkkani A, Colcimen N, et al. , Ribosome biogenesis mediates antitumor activity of flavopiridol in CD44(+)/CD24(−) breast cancer stem cells. Oncol Lett 2017;14:6433–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Decker AM, Jung Y, Cackowski FC, Yumoto K, Wang J, Taichman RS, Sympathetic Signaling Reactivates Quiescent Disseminated Prostate Cancer Cells in the Bone Marrow. Mol Cancer Res 2017;15:1644–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Myrvang HK, Guo X, Li C, Dekker LV, Protein interactions between surface annexin A2 and S100A10 mediate adhesion of breast cancer cells to microvascular endothelial cells. FEBS Lett 2013;587:3210–5. [DOI] [PubMed] [Google Scholar]
- (34).Blancato J, Singh B, Liu A, Liao DJ, Dickson RB, Correlation of amplification and overexpression of the c-myc oncogene in high-grade breast cancer: FISH, in situ hybridisation and immunohistochemical analyses. Br J Cancer 2004;90:1612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Bellovin DI, Das B, Felsher DW, Tumor dormancy, oncogene addiction, cellular senescence, and self-renewal programs. Adv Exp Med Biol 2013;734:91–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Pohl SG, Brook N, Agostino M, Arfuso F, Kumar AP, Dharmarajan A, Wnt signaling in triple-negative breast cancer. Oncogenesis 2017;6:e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Asem MS, Buechler S, Wates RB, Miller DL, Stack MS, Wnt5a Signaling in Cancer. Cancers (Basel) 2016;8:E79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Taciak B, Pruszynska I, Kiraga L, Bialasek M, Krol M, Wnt signaling pathway in development and cancer. J Physiol Pharmacol 2018;69:10. [DOI] [PubMed] [Google Scholar]
- (39).Wawrzak D, Metioui M, Willems E, Hendrickx M, de GE, Leyns L, Wnt3a binds to several sFRPs in the nanomolar range. Biochem Biophys Res Commun 2007;357:1119–23. [DOI] [PubMed] [Google Scholar]
- (40).Huelsken J, Tumor-stroma crosstalk vis Hedgehog and Wnt pathways bypasses resident immune cells to enable liver metastases. Cancer Res 2016;76:IA60. [Google Scholar]
- (41).Radvanyi L, Singh-Sandhu D, Gallichan S, Lovitt C, Pedyczak A, Mallo G, et al. , The gene associated with trichorhinophalangeal syndrome in humans is overexpressed in breast cancer. Proceedings of the National Academy of Science, U S A 2005;102:11005–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. , The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 2012;486:346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Pereira B, Chin SF, Rueda OM, Vollan HK, Provenzano E, Bardwell HA, et al. , The somatic mutation profiles of 2,433 breast cancers refines their genomic and transcriptomic landscapes. Nat Commun 2016;7:11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Curigliano G, Goldhirsch A, The triple-negative subtype: new ideas for the poorest prognosis breast cancer. J Natl Cancer Inst Monogr 2011;2011:108–10. [DOI] [PubMed] [Google Scholar]
- (45).Nandana S, Tripathi M, Duan P, Chu CY, Mishra R, Liu C, et al. , Bone metastasis of prostate cancer can be therapeutically targeted at the TBX2-WNT signaling axis. Cancer Res 2017;%20. pii: canres.0497.2016. doi: 10.1158/0008-5472.CAN-16-0497.:canres-5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Asanoma K, Liu G, Yamane T, Miyanari Y, Takao T, Yagi H, et al. , Regulation of the Mechanism of TWIST1 Transcription by BHLHE40 and BHLHE41 in Cancer Cells. Mol Cell Biol 2015;35:4096–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Adorno M, Cordenonsi M, Montagner M, Dupont S, Wong C, Hann B, et al. , A Mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell 2009;137:87–98. [DOI] [PubMed] [Google Scholar]
- (48).Piccolo S, Enzo E, Montagner M, p63, Sharp1, and HIFs: master regulators of metastasis in triple-negative breast cancer. Cancer Res 2013;73:4978–81. [DOI] [PubMed] [Google Scholar]
- (49).Chen H, Liu H, Qing G, Targeting oncogenic Myc as a strategy for cancer treatment. Signal Transduct Target Ther 2018;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Yee-Lin V, Pooi-Fong W, Soo-Beng AK, Nutlin-3, A p53-Mdm2 Antagonist for Nasopharyngeal Carcinoma Treatment. Mini Rev Med Chem 2018;18:173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Zheng S, Liu J, Wu Y, Huang TL, Wang G, Small-molecule inhibitors of Wnt signaling pathway: towards novel anticancer therapeutics. Future Med Chem 2015;7:2485–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Lee HJ, Bao J, Miller A, Zhang C, Wu J, Baday YC, et al. , Structure-based Discovery of Novel Small Molecule Wnt Signaling Inhibitors by Targeting the Cysteine-rich Domain of Frizzled. J Biol Chem 2015;290:30596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Igea A, Nebreda AR, The Stress Kinase p38alpha as a Target for Cancer Therapy. Cancer Res 2015;75:3997–4002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.