Abstract
IgM related AL amyloidosis is a rare disease with patients presenting with more renal and neurological involvement and less cardiac involvement compared to those with non-IgM related disease. We retrospectively reviewed 38 patients receiving autologous stem cell transplant (ASCT) for IgM related AL amyloidosis at the Mayo Clinic between May 1999 and June 2018. Median age was 61 years and 71% were male . The most common organs involved were renal (63%), neurological (32%) and cardiac (26%). The median difference between involved and uninvolved free light chains (dFLC) was 6.2 mg/dL and most patients had early mayo stage (87% Mayo Stage I 2004 and 74% Mayo Stage I 2012). The overall response rate (ORR) was 92% with 76% of patients achieving at least a very good partial response (VGPR). Renal response was seen in 65% (15/23) of patients (median time 18 months post ASCT, range 3-52 months) and cardiac response was seen in 60% (6/10) of patients (median time 12 months post ASCT, range 10-35 months). Median progression free and overall survival was 48 and 106 months respectively. Organ response predicted better PFS and OS (median PFS 93 months for organ response vs 16 months for no organ response, p=0.0006 and median OS 123 months for organ response vs 41 months for no organ response, p=0.02). Two patients died within 100 days of transplant representing a 5% 100 day mortality. ASCT is an effective therapy that can be safely delivered to carefully selected patients with IgM related AL amyloidosis.
Introduction
Amyloidosis related to an IgM monoclonal protein is a rare entity accounting for approximately 5% of patients with immunoglobulin light chain (AL) amyloidosis. 1,2 Patients with IgM related AL amyloidosis tend to present with lower levels of serum free light chains, have less cardiac involvement and more neurological involvement compared to those with non-IgM related AL amyloidosis.1-3 Therapy is generally extrapolated form regimens used for non-IgM related AL amyloidosis. However given the lymphoplasmacytic nature of the clonal disorder in these patients, regimens used to treat lymphoplasmacytic lymphoma have also been employed. In general the response rate to these therapies has been lower than reported for non IgM amyloidosis and this, coupled with the paucity of data makes it difficult to make treatment decisions. We have previously reported survival outcomes in 22 patients with IgM related AL amyloidosis treated with autologous stem cell transplantation (ASCT) showing similar survival to patients with non-IgM amyloid .2 Herein we describe 38 patients with IgM related AL amyloidosis treated with ASCT at the Mayo Clinic, presenting updated data on hematologic response, organ response and survival.
Methods
Between May of 1999 and June of 2018, 38 patients with biopsy proven IgM associated AL amyloidosis, received an autologous stem cell transplant at the Mayo Clinic Rochester. We conducted a retrospective review of patient and disease characteristics as well as transplant related outcomes in this cohort. Risk stratification was according to the 2004 and 2012 Mayo staging systems.4 Organ involvement was defined according to consensus criteria.5 Response was assessed at approximately 100 days post ASCT according to updated consensus criteria.6 One hundred day mortality was defined as death from any cause within 100 days of ASCT. Statistical analysis was performed on JMP software (SAS, Cary, NC). Survival analysis was performed using the Kaplan-Meier method. Overall survival (OS) was calculated from day zero of bone marrow transplant to death from any cause. Progression free survival (PFS) was defined as time to hematological progression, assessed by consensus criteria, or time to reinitiation of therapy or death. The study was approved by the Mayo Clinic Institutional Review Board.
Results
Baseline characteristics for the 38 patients comprising the study cohort are listed in Table 1. Median age and the proportion of males were consistent with a population of patients with AL amyloidosis. The most common organs involved were renal (63%), neurological (32%) and cardiac (26%). Very few patients had >2 organs involved (n=5). The monoclonal protein was IgM lambda (n=18), IgM kappa (n=13), IgM lambda plus IgM kappa (n=3), IgM kappa plus IgG lambda (n=2), IgM lambda plus IgG lambda (n=1) and IgM lambda plus IgA lambda (n=1). The median difference between involved and uninvolved free light chains (dFLC) was 6.2 mg/dL. The majority of patients had early mayo stage (87% Mayo Stage I 2004 and 74% Mayo Stage I 2012). Of 5 patients that were tested for the MYD88 L265P mutation, 2 were positive for the mutation. Over half (58%, n=22) the patients received therapy prior to transplantation and this varied over time ranging from corticosteroid therapy only in the earlier years to combination chemoimmunotherapy as well as proteasome inhibitors more recently. Conditioning was high dose melphalan in 84% (n=32) of patients (melphalan 200mg/m^2 in 63% n=24 and melphalan 140mg/m^2 in 21% n=8). Six patients received conditioning with a combination of carmustine, etoposide, cytarabine and melphalan (BEAM). Maintenance therapy was not routinely given to patients. Two patients received consolidation therapy post transplant, one received 4 doses of rituximab over a month and another patient was commenced on the combination of velcade revlimid and dexamethasone.
Table 1.
Patient characteristics and hematologic response.
| Variable | IgM Amyloidosis (n=38) |
|---|---|
| Age, median (range) | 61 (40-70) |
| Male | 27 (71) |
| Organ involved | |
| Cardiac | 10 (26) |
| Renal | 24 (63) |
| >2 | 5 (13) |
| Light Chain Type | |
| Lambda | 20 (53) |
| Kappa | 13 (34) |
| Biclonal | 5 (13) |
| Bone marrow findings | |
| % involvement, median (range) | 8 (1-50) |
| Lymphoplasmacytic features, n (%) | 17 (45) |
| dFLC, mg/dL, median (IQR) | 6.2 (2.3-16.1) |
| Serum M protein g/dL, median (IQR) | 0.9 (0.5-1.4) |
| Creatinine, median (IQR) | 1 (0.9-1.3) |
| Urine protein*, g/24hrs, median (range) | 5.1 (1.7-34.9) |
| NT-proBNP+, pg/ml, median (range) | 1567 (576-12983) |
| Mayo Stage 2004, % | |
| Stage I/II/III | 87/6/6 |
| Missing, n | 7 |
| Mayo Stage 2012, n (%) | |
| Stage I/II/III/IV | 74/11/7/7 |
| Missing, n | 11 |
| Prior therapy, n (%) | 22 (58) |
| Number prior therapies, median (range) | 1 (1-5) |
| Corticosteroid only | 4 (11) |
| Chemotherapy | 5 (13) |
| Proteasome inhibitor | 7 (18) |
| Chemoimmunotherapy | 8 (21) |
| Single agent Rituximab | 3 (8) |
| Conditioning | |
| Melphalan 200 | 24 (63) |
| Melphalan 140 | 8 (21) |
| BEAM | 6 (16) |
| Hematologic Response, n (%) | |
| CR | 7 (18) |
| VGPR | 22 (58) |
| PR | 6 (16) |
| NR | 3 (8) |
Abbreviations: IQR, interquartile range; dFLC, difference between involved and uninvolved light chains; BEAM, carmustine, etoposide, cytarabine and melphalan; CR, complete response; VGPR, very good partial response; PR, partial response; NR, no response.
amongst patients with renal involvement.
amongst patients with cardiac involvement.
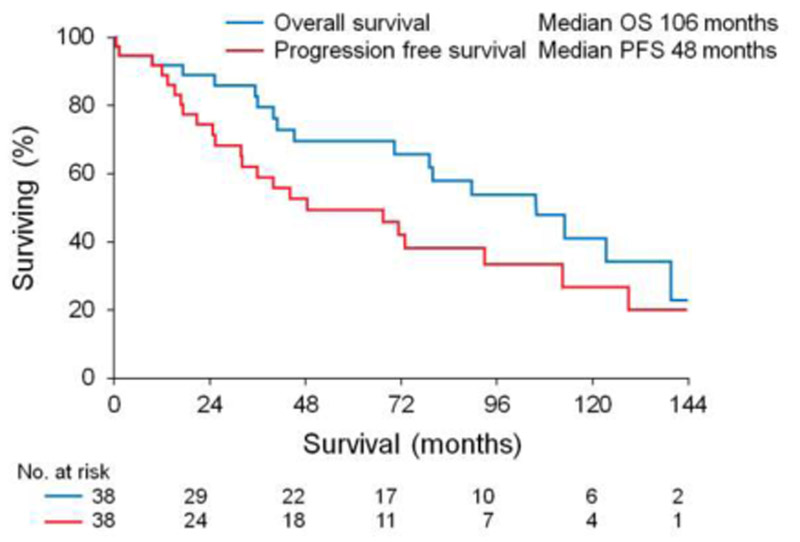
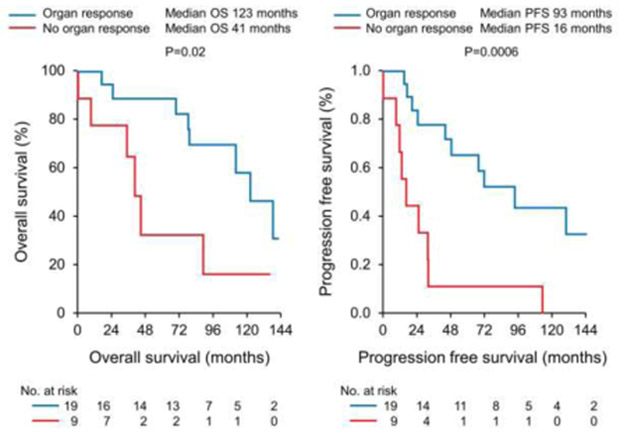
The overall response rate (ORR) was 92% with 76% of patients achieving at least a very good partial response (VGPR). Of patients receiving melphalan conditioning 22% (n=7) achieved a complete response (CR) and 50% (n=16) achieved a VGPR. All 6 patients receiving BEAM conditioning achieved a VGPR. We assessed organ response in patients with renal or cardiac involvement. Of 29 patients with renal and/or cardiac involvement 28 (97%) had data available for organ response assessment. Renal response was seen in 65% (15/23) of patients at a median time of 18 months post transplant (range 3-52 months). Cardiac response was seen in 60% (6/10) of patients at a median time of 12 months post transplant (range 10-35 months). Median progression free and overall survival was 48 and 106 months respectively, Figure 1. Organ response predicted better PFS and OS (median PFS 93 months for organ response vs 16 months for no organ response, p=0.0006 and median OS 123 months for organ response vs 41 months for no organ response, p=0.02), Figure 2. Two patients died within 100 days of transplant representing a 5% 100 day mortality. The first patient died due to multi-organ failure in the setting of major gastrointestinal bleeding 40 days post ASCT and the second had a sudden cardiac arrest in the setting of a respiratory tract infection 15 days post ASCT.
Figure 1.
Progression free and overall survival for the whole cohort.
Figure 2.
Progression free and overall survival by organ response.
Discussion
Our study shows that ASCT is an effective therapy for patients with IgM related AL amyloidosis. The overall survival and mortality rate at a hundred days compares favorably to recently published data on outcomes in the overall cohort of patients with AL amyloidosis.7,8 Our data are consistent with previous reports, showing patients with IgM related AL amyloidosis have more renal involvement, less cardiac involvement and lower levels of involved serum free light chains. The median dFLC seen in this cohort (6.2 mg/dL) is far lower than normally seen in patients with AL amyloidosis.9 In addition the rate of cardiac involvement (26%) in our cohort is lower than previous reports of patients with IgM related AL amyloidosis and this is likely to reflect our exclusion of patients with advanced cardiac disease from receiving ASCT.
Response to therapy in patients with IgM related AL amyloidosis have been less impressive to those with non-IgM related disease. The behavior of the underlying neoplastic clone in patients with IgM related AL amyloidosis varies between a low grade non Hodgkin lymphoma and multiple myeloma and this may explain the heterogeneity in therapeutic approach reported in the literature as well response depth. In a large European collaborative study of 250 patients with IgM related AL amyloidosis, 212 received treatment (various regimens), of whom 172 had available data on hematologic response.1 The hematologic response rate was 57% with the vast majority being partial responses (43% partial response (PR), 9% VGPR and 5% complete response (CR)). Organ response rates were poor with renal and cardiac response rates of 18% and 5% respectively. A smaller study of 10 patients treated with a combination of rituximab, bortezomib and dexamethasone reported responses in 7/9 (78%) evaluable patients, suggesting that plasma cell directed therapy in conjunction with B cell therapy may be a more effective approach.10 Given the effectiveness of the combination of bendamustine and rituximab in patients with low grade lymphoma this regimen has also been utilized in the setting of IgM related AL amyloidosis. In a recent publication, Manwani and colleagues reported outcomes in 27 patients treated with bendamustine and rituximab showing a hematologic response rate of 59% (11% CR, 37% VGPR and 11% PR) and renal and cardiac response rates of 18%.11 These results are encouraging and suggest this combination should be further studied in patients with IgM related AL amyloidosis. Compared to the regimens described above, our cohort of patients treated with ASCT with a hematologic response rate of 92% (18% CR) and organ responses in approximately two thirds of patients suggest that ASCT should be considered in all eligible patients with this disease. We recognize however that this population is selected, and only 20-25% of patients with AL amyloidosis are eligible for ASCT at presentation. One of the concerns with ASCT in AL amyloidosis traditionally, has been the high treatment related mortality. We have recently shown a marked reduction in early mortality (<5%) is possible with careful patient selection for ASCT in AL amyloidosis.7 Our preference for the conditioning regimen has evolved over time. High dose melphalan was the preferred conditioning regimen in earlier years, however in recent years we have moved towards utilizing BEAM conditioning in patients with lymphoplasmacytic disease identified on bone marrow or lymph node biopsy.
Our study is limited by its retrospective nature and long time period over which it was conducted. Furthermore therapy prior to transplantation was not uniform, given we are a tertiary referral center many patients present to us having already commenced therapy locally. We also included four patients who had an additional non-IgM monoclonal protein detected and determining which protein is amyloidogenic in these cases is difficult. Despite these limitations, we report the largest single center experience on a rare disease, showing that ASCT is an effective therapy for patients with IgM related AL amyloidosis.
Highlights.
Autologous stem cell transplant is efficacious in patients with IgM related AL amyloidosis
A hematologic response is seen in approximately 90% of patients with 76% of patients achieving at least a very good partial response
Organ response is seen in approximately 60% of patients and predicts both progression free and overall survival.
Acknowledgments
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sachchithanantham S, Roussel M, Palladini G, et al. European Collaborative Study Defining Clinical Profile Outcomes and Novel Prognostic Criteria in Monoclonal Immunoglobulin M-Related Light Chain Amyloidosis. J Clin Oncol. 2016;34(17):2037–2045. [DOI] [PubMed] [Google Scholar]
- 2.Gertz MA, Buadi FK, Hayman SR. IgM amyloidosis: clinical features in therapeutic outcomes. Clin Lymphoma Myeloma Leuk. 2011;11(1):146–148. [DOI] [PubMed] [Google Scholar]
- 3.Sissoko M, Sanchorawala V, Seldin D, et al. Clinical presentation and treatment responses in IgM-related AL amyloidosis. Amyloid. 2015;22(4):229–235. [DOI] [PubMed] [Google Scholar]
- 4.Kumar S, Dispenzieri A, Lacy MQ, et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. Journal of Clinical Oncology. 2012;30(9):989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gertz MA, Comenzo R, Falk RH, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am J Hematol. 2005;79(4):319–328. [DOI] [PubMed] [Google Scholar]
- 6.Palladini G, Dispenzieri A, Gertz MA, et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. Journal of Clinical Oncology. 2012;30(36):4541–4549. [DOI] [PubMed] [Google Scholar]
- 7.Sidiqi MH, Aljama MA, Buadi FK, et al. Stem Cell Transplantation for Light Chain Amyloidosis: Decreased Early Mortality Over Time. J Clin Oncol. 2018;36(13):1323–1329. [DOI] [PubMed] [Google Scholar]
- 8.D’Souza A, Dispenzieri A, Wirk B, et al. Improved Outcomes After Autologous Hematopoietic Cell Transplantation for Light Chain Amyloidosis: A Center for International Blood and Marrow Transplant Research Study. Journal of Clinical Oncology. 2015;33(32):3741–3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muchtar E, Gertz MA, Kumar SK, et al. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood. 2017;129(15):2111–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palladini G, Foli A, Russo P, et al. Treatment of IgM-associated AL amyloidosis with the combination of rituximab, bortezomib, and dexamethasone. Clin Lymphoma Myeloma Leuk. 2011;11(1):143–145. [DOI] [PubMed] [Google Scholar]
- 11.Manwani R, Sachchithanantham S, Mahmood S, et al. Treatment of IgM-associated immunoglobulin light-chain amyloidosis with rituximab-bendamustine. Blood. 2018;132(7):761–764. [DOI] [PubMed] [Google Scholar]