Abstract
There are limited data on whether to adjust high-dose chemotherapy prior to autologous hematopoietic cell transplant (autoHCT) in obese patients. This study explores the effects of dose adjustment on the outcomes of obese patients, defined as body mass index (BMI) ≥ 30 kg/m2. Dose adjustment was defined as a reduction in standard dosing of ≥ 20%, based on ideal, reported dosing and actual weights. We included two groups of US patients who had received autoHCT between 2008 and 2014. Specifically, we included patients with multiple myeloma (MM, n=1696) treated with high-dose melphalan; and we included patients with Hodgkin or non-Hodgkin lymphomas (n=781) who received carmustine, etoposide, cytarabine, and melphalan (BEAM) conditioning. Chemotherapy dose was adjusted in 1324 (78%) patients with MM and 608 (78%) patients with lymphoma. Age, sex, BMI, race, performance score, comorbidity index, and disease features (stage at diagnosis, disease status and time to transplant) were similar between dose groups. In multivariate analyses for MM, adjusting for melphalan dose and for center effect had no impact on overall survival (p=0.894) and treatment-related mortality (TRM) (p=0.62), progression (p=0.12), and progression-free survival (p=0.178). In multivariate analyses for lymphoma, adjusting chemotherapy doses did not affect survival (p=0.176), TRM (p=0.802), relapse (p=0.633) or PFS (p=0.812). No center effect was observed in lymphoma. This study demonstrates that adjusting chemotherapy dose prior to autoHCT in obese patients with MM and lymphoma does not influence mortality. These results do not support adjusting chemotherapy dose in this population.
Keywords: obesity, conditioning regimen, autologous hematopoietic cell transplantation
INTRODUCTION
Obesity incidence has been increasing steadily in recent years 1-3. The American Society of Clinical Oncology guidelines recommended chemotherapy dosing based on actual body weight for obese patients being treated with curative-intent4 chemotherapy in solid tumors. In contrast, hematopoietic cell transplantation (HCT) requires higher doses of chemotherapy and practices of adjusting the weight due to concerns of organ toxicity are common5, 6. Recently, the American Society for Blood and Marrow Transplant (ASBMT) completed an extensive review of the literature and published recommendations on chemotherapy dosing in obese patients5. That review focused on the effect of chemotherapy dosing on survival and toxicity, and it included comparisons between obese and non-obese patients. Yet, the recommendations were limited by the paucity of data in this population.
The complex clinical context of HCT, particularly allogeneic HCT, makes it difficult to isolate the impact of dose adjustment. Autologous HCT is associated with low treatment-related mortality (TRM), but relapse remains the main cause of treatment failure. The potentially lower “background noise” and more uniform conditioning regimens across transplant centers make autologous HCT a better setting for exploring the impact of adjusted doses. Additionally, chemotherapy doses in autologous HCT are generally much higher than for standard cancer treatment, so it is important to know whether obese patients have a higher toxicity when doses are based on actual body weight.
The Center for International Blood and Marrow Transplant Research (CIBMTR) data show that many, but not all, transplant centers adjust chemotherapy dose for obese patients; and dose-adjustment practices vary widely. This study compares outcomes for obese patients whose chemotherapy dose was adjusted. Specifically, we compare outcomes for patients with multiple myeloma treated with high-dose melphalan and patients with Hodgkin or non-Hodgkin lymphomas who received carmustine, etoposide, cytarabine, and melphalan (BEAM) conditioning.
PATIENTS AND METHODS
Data sources
The Center for International Blood and Marrow Transplant Research (CIBMTR) is a working group of more than 500 transplantation centers worldwide that contribute detailed data on HCT to a statistical center at the Medical College of Wisconsin. CIBMTR® is a research collaboration between the National Marrow Donor Program® (NMDP)/Be The Match® and the Medical College of Wisconsin. Participating centers are required to report all transplantations consecutively; patients are followed longitudinally, and compliance is monitored by on-site audits. Data quality is ensured, both by computerized checks for discrepancies and by physicians’ review of submitted data. CIBMTR conducts observational studies and complies with all applicable federal regulations that protect human subjects.
The CIBMTR collects data at two levels: Transplant Essential Data (TED) and Comprehensive Report Form (CRF) data. TED-level data include: disease; age; sex; pre-HCT disease stage and chemotherapy-responsiveness; date of diagnosis; graft type (bone marrow- and/or blood-derived stem cells); conditioning regimen; post-transplant disease progression and survival; development of a new malignancy; and cause of death. All CIBMTR centers contribute TED-level data. More detailed CRF-level data are collected on a subset of patients selected by a weighted randomization scheme. TED- and CRF-level data are collected pre-transplant, and then post-transplant at 100 days, at 6 months, annually until year 6, and biannually thereafter until death. Data for the current analysis were retrieved from CIBMTR (TED and CRF) report forms.
Patients
Adult (≥ 18 years) patients, with body mass index (BMI) ≥ 30 kg/m2 undergoing a first autologous HCT for myeloma or lymphoma performed in US centers, between years 2008 and 2014, were included in this study. Body mass index was calculated based on reported pre-transplant actual weight and height. Additional eligibility included only those patients with MM receiving single-drug melphalan (200mg/m2) conditioning regimen and only those with lymphoma receiving a conditioning regimen with carmustine, etoposide, cytarabine, and melphalan (BEAM). The determination of chemotherapy dose-reduction in patients with lymphoma receiving BEAM was based on the actual dose of melphalan.
Study Design
This was a retrospective, registry-based study. To determine whether the dose of chemotherapy had been adjusted, we looked at three possible measures: pre-transplant actual body weight (ABW), dosing weight, (DW) and calculated ideal body weight (IBW). We considered that the patient received an adjusted dose if the DW was < 80% of ABW. Two groups were defined:
unadjusted group, which dosed chemotherapy using actual weight
adjusted group, which dosed chemotherapy using an adjusted weight based on the difference between IBW and actual weight.
The degree of adjustment (adjusted factor β) varied across centers, and it was determined based on the formula: DW = IBW + (adjusted factor β) × (ABW – IBW). DW is the same as adjusted body weight used to calculate chemotherapy dosing. As the biology and outcomes of MM and lymphoma are significantly different, we performed the analyses for the whole group and separately for myeloma and lymphoma.
Endpoints and definitions
As a surrogate for treatment-related toxicity, we used duration of hospitalization within the first 100 days after HCT. The Hematopoietic Cell Transplant Co-Morbidity Index (HCT-CI)7 was calculated excluding obesity. Because many centers perform autologous HCT as an outpatient procedure or a hybrid, in which the hospitalization is limited to the period while patients receive chemotherapy, we considered only hospitalizations of > 7 days. Treatment-related mortality (TRM) was defined as any death in the absence of disease relapse or progression. Patients who died in the first 28 days post-transplant without reported disease relapse or progression were considered to have TRM. Relapse or progression was the competing risk for this event. Disease relapse or progression was defined by the transplant center either as morphologic or radiological relapse. For patients without relapse or progression information for whom the reported cause of death was the primary disease, we considered them to have relapse or progression, and the date was input as the day prior to the date of death. TRM was the competing risk for this event. Progression-free survival (PFS) was defined as freedom from death, relapse or progression of the disease for which the patient received the autologous HCT. Living patients were censored at last follow-up. Overall survival (OS) was defined by death from any cause after HCT; living patients were censored at last follow-up. Variables tested in the multivariate analyses were: dose adjustment (main effect), age (18-49, 50-59, > 60 years), sex, Karnofsky performance score (≥ 90% vs. < 90%), HCT-CI excluding obesity (0, 1-2 or ≥ 3), BMI (30-34, 35-39 or ≥ 40 kg/m2), year of transplant and time from diagnosis to HCT (< 6, 6-12, 13-24 or > 24 months). Additional disease-specific variables included International Staging System8 at time of diagnosis (ISS, I-II or III) and disease status at transplant (complete response, partial response or stable disease/progression) for multiple myeloma; and lymphoma subtype (non-Hodgkin lymphoma or Hodgkin lymphoma) and prior chemotherapy response (sensitive or resistant) for lymphoma.
Statistical Analysis
Patient-related and transplant-related factors were compared between dose-adjusted and unadjusted cohorts using chi-square test for categorical variables and Mann-Whitney test for continuous variables. Mann-Whitney test was selected to accommodate continuous variables without normal distribution. Probabilities for TRM and disease relapse were calculated, using cumulative incidence function accounting for competing risks, and compared using Gray’s test. PFS and OS were calculated using the Kaplan-Meier estimator and compared using log-rank test.
Multivariate analysis of the four major outcomes were done using Cox regression analysis, using the chemotherapy dose-adjustment covariate as the main effect and forced in all models. The four outcomes were: TRM, disease progression or relapse, treatment failure (1-PFS), and overall mortality (1-OS).
Each variable was tested for proportional hazard assumption. If assumption was violated, the variable was included as a time-dependent variable. To identify the significant risk factors, stepwise forward selection with a significance level of .05 was used to define variables with a significant association with the outcome. Sensitivity analysis with significance level of 0.1 was also tested, which did not change the variables selected on the final models. Interaction terms were examined between the chemotherapy dose-adjustment covariate and other significant covariates. Separate analyses were conducted for patients with MM and lymphoma. Dose-adjustment practices were done according to transplant center guidelines, which may result in a significant center effect, as individual cases cannot be considered independently. Center effects were tested using the score test and adjusted using a marginal model 9. Analysis were done using SAS, version 9.4.
RESULTS
Patients and Transplant Characteristics
Patient, disease and transplant characteristics for patients with MM (n= 1696) and lymphoma (n=781) are summarized in Table 1.
Table 1.
Patients and transplant characteristics of obese patients by dosing weight.
| Multiple myeloma N(range or %) |
Lymphoma N (range or %) |
|||||
|---|---|---|---|---|---|---|
| Variable | Adjusted weight |
Actual weight |
P value | Adjusted weight |
Actual weight |
P value |
| Number of patients | 1324 | 372 | 609 | 172 | ||
| Number of centers | 103 | 69 | 74 | 51 | ||
| Age, median, y (range) | 58 (20-76) | 58 (33-77) | 0.66 | 55(18-76) | 55(18-77) | 0.89 |
| Male sex, No. (%) | 747(56) | 210(56) | 0.99 | 380(62) | 102(59) | 0.46 |
| Race | 0.08 | 0.80 | ||||
| White | 954(72) | 245(66) | 512 (84) | 148 (86) | ||
| Black | 322(24) | 115(31) | 78(13) | 21(12) | ||
| Others | 22(2) | 5(1) | 12(2) | 2(1) | ||
| Not reported | 26(2) | 7(2) | 7(1) | 1(<1) | ||
| Karnofsky Score ≥ 90% | 753(57) | 202(54) | 0.66 | 378(62) | 111(65) | <0.001 |
| HCT-CI | 0.12 | 0.28 | ||||
| No comorbidity except obesity | 518(39) | 151(41) | 229(38) | 76(44) | ||
| 1-2 | 414(31) | 134(36) | 194(32) | 48(28) | ||
| ≥3 | 390(29) | 86(23) | 181(30) | 48(28) | ||
| Not reported | 2(<1) | 1(<1) | 5(<1) | 0 | ||
| Body mass index, kg/m2 | 0.08 | |||||
| Median (range) | 34(30-80) | 34(30-88) | 34 (30-70) | 34 (30-80) | ||
| 30-34 | 768(58) | 240(65) | 333 (55) | 102 (59) | ||
| 35-39 | 342(26) | 82(22) | 146(24) | 39(23) | ||
| ≥40 | 214(16) | 50(13) | 130(21) | 31(18) | ||
| Dosing weight adjusted factor β,%a | <0.001 | <0.001 | ||||
| Median (range) | 25 (0-79) | 100(90-100) | 26(0-80) | 100(100) | ||
| < 30% | 888 (67) | 0 | 326(54) | 0 | ||
| 30-79% | 376 (33) | 0 | 283 (46) | 0 | ||
| 80-89% | 0 | 52(14) | 0 | 0 | ||
| 90-100% | 0 | 320(86) | 0 | 172(100) | ||
| Disease | 0.70 | 0.98 | ||||
| Multiple myeloma, IgG | 784(59) | 214(58) | ||||
| Multiple myeloma, IgA | 266(20) | 78(21) | ||||
| Multiple myeloma, IgD | 7(<1) | 2(<1) | ||||
| Multiple myeloma, IgE | 1(<1) | 0 | ||||
| Multiple myeloma, IgM | 8(<1) | 4(1) | ||||
| Multiple myeloma, light chain | 238(18) | 64(17) | ||||
| Multiple myeloma, non-secretory | 20(2) | 10(3) | ||||
| DLBCL | 225 (37) | 65 (38) | ||||
| Follicular NHL | 68(11) | 21(12) | ||||
| Mantle cell NHL | 86(14) | 22(13) | ||||
| Other B cell NHL | 40(7) | 9(5) | ||||
| T cell NHL | 47(8) | 14(8) | ||||
| HL | 143(23) | 41(24) | ||||
| Multiple myeloma stage at diagnosis | 0.01 | |||||
| Stage III | 655 (49) | 185(50) | ||||
| Stage I-II | 595(45) | 180(48) | ||||
| Not reported | 74(6) | 7(2) | ||||
| Multiple myeloma status prior to HCT | 0.32 | |||||
| Complete remission | 206(16) | 69(19) | ||||
| Partial response | 966(73) | 266(72) | ||||
| Stable / relapse / progression | 152(11) | 37(10) | ||||
| Lymphoma status prior to HCT | 0.34 | |||||
| Chemotherapy-sensitiveb | 560(92) | 162(94) | ||||
| Chemotherapy-resistantc | 42(7) | 7(4) | ||||
| PIF / REL sensitivity unknown | 7(1) | 3(2) | ||||
| Conditioning regimen | ||||||
| Melphalan dose, mg/kg (range) | 4(3-5) | 4(2-5) | 0.06 | 3 (2-8) | 3 (<1-10) | 0.84 |
| Actual melphalan dose, median, mg (range) | 436(307-704) | 435(163-619) | 0.10 | 318(179-1035) | 307(26-962) | 0.004 |
| Median time from diagnosis to transplant, months (range) | 8 (<1-183) | 8(3-763) | 0.17 | 16(0-271) | 17(1-180) | 0.87 |
| Transplant year | 0.003 | 0.70 | ||||
| 2008-2010 | 541(41) | 145(39) | 316(52) | 85(49) | ||
| 2011-2012 | 405(30) | 89(24) | 114(19) | 37(22) | ||
| 2013-2014 | 378(29) | 138(37) | 179(29) | 50(29) | ||
| Median follow-up of survivors, months (range) | 52(1-103) | 59(2-101) | 55(2-110) | 59(1-101) | ||
Abbreviations: HCT-CI, hematopoietic cell transplantation-comorbidity index; DLBCL, diffuse large B-cell lymphoma; NHL, non-Hodgkin lymphoma; HL, Hodgkin lymphoma; PIF primary induction failure; REL, relapse
Chemo-sensitive: complete remission, partial remission, relapse/progression or never in remission and sensitive to prior treatment immediately prior to conditioning
Chemo-resistant: relapse/progression or never in remission and resistant to prior treatment immediately prior to conditioning
Among patients with MM, most patients had their melphalan dose reduced (78%). The median age, sex, performance score, HCT-CI, and BMI were similar between the groups that had chemotherapy adjusted or unadjusted. Disease characteristics including MM stage, disease status at transplantation, median time from diagnosis to transplantation, and year of transplantation were also similar between the two groups. The median adjustment factor for melphalan was 25%, i.e. the dosing weight was the IBW plus 25% of the difference between IBW and ABW. Cytogenetic markers and induction therapy were only available for a subgroup of patients with CRF-level data (n = 1094) and were not considered.
Among patients with lymphoma, chemotherapy adjustment was done on 78% of patients. The groups of patients whose chemo was adjusted or unadjusted were similar in terms of: median age, sex, performance score, HCT-CI, BMI, lymphoma subtype, chemotherapy sensitivity prior to transplantation, median time from diagnosis to transplantation, and year of transplantation. Median chemotherapy adjustment factor based on melphalan dosing in BEAM was 26%. In a subgroup of patients with CRF-level reporting (n = 407), the distribution of staging at diagnosis and number of prior lines of therapy was similar between dose adjustment groups.
Effect of Chemotherapy Adjustment on Length of Hospital Stay
As a surrogate measure of treatment-related toxicity, the proportion of patients hospitalized >7 days in the first 100 days post-transplantation was compared between the dose-adjustment groups. The length of hospitalization was available for 1042 patients with MM and 459 with lymphoma. Seventy-eight percent of patients received dose-adjusted chemotherapy, with a median length of hospitalization in the first 100 days of 14 days (range: 8-72), in contrast to 15 days (range: 8-70) in those with unadjusted chemotherapy dose (P = 0.20). A higher proportion of patients with MM who received unadjusted doses of chemotherapy had a hospital stay longer than 15 days (P = 0.04). The same was not observed among patients with lymphoma (P =0.59). (See Table 3s.)
Effect of Chemotherapy Adjustment on Outcomes of All Patients
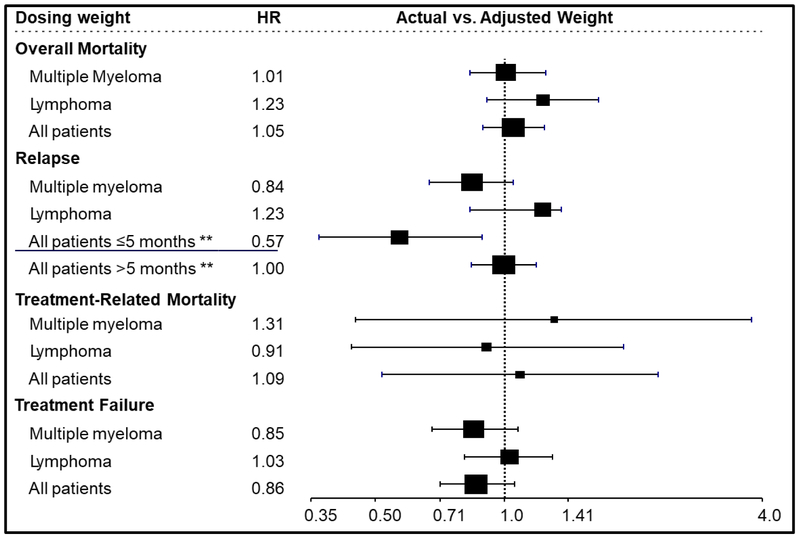
To maximize the power to detect slight differences, the impact of dose adjustment was tested on all patients with results shown in Figure 1 and summarized in Table S1. Multivariate analyses of overall mortality demonstrated a hazard ratio (HR) of 1.05 (95% Confidence Interval [CI] 0.89-1.24, P = 0.58). Younger age, KPS ≥ 90, HCT-CI < 3, and chemotherapy sensitive disease were independent predictor of lower mortality for the whole study population. There was also no effect of dose adjustment on TRM (HR = 1.09; 95% CI 0.52-2.29, P = 0.83) and treatment failure (HR=0.86; 95 CI 0.71-1.06, P = 0.16). Younger age, KPS ≥90, and a diagnosis of myeloma were independent predictors of lower risk TRM. The independent predictors of lower hazards of treatment failure were female sex, KPS ≥ 90, chemotherapy sensitive disease, and disease.. In contrast, there was a time-dependent effect of chemotherapy dose adjustment on the risk of relapse: Patients who received full-dose conditioning regimens had a 43% lower relapse hazard within 5 months post-HCT (HR=0.57; 95% CI 0.37-0.89, p=0.01) and similar relapse hazard thereafter (> 5 months HR 1.0; 95% CI 0.84-1.19, p=0.96). The impact of disease in the relapse model varied over time. The relapse hazard for both diseases was the same in the first 8 months post-HCT (HR=0.98, 95% CI 0.81-1.18, p=0.79). However, after 8 months, relapses were less common among patients with lymphoma compared to MM (HR=0.32, 95% CI 0.27-0.38, p<0.01). Female sex and chemotherapy-sensitive disease also independently predicted a lower hazard of relapse. In addition, because chemotherapy adjustment varied by center, for patients with MM, a statistically significant center effect was observed on TRM (p=0.023), PFS (p<0.001), and relapse (p<0.001), but not in survival (p=0.66). For patients with lymphoma, no center effect was observed on any outcomes. The causes of death were similar whether there were chemotherapy dose adjustments or not (Table 2).
Figure 1:
Multivariate analysis comparing unadjusted to adjusted doses of chemotherapy prior to autologous hematopoietic cell transplantation for patients with multiple myeloma, lymphoma and all patients.
** In the combined population, the effect of dose adjustment on disease relapse was significant in the first 5 months after transplant and not significant beyond 5 months.
Table 2.
Cause of death
| Variable | Adjusted body weight | Actual body weight |
|---|---|---|
| Number of patients | 1933 | 544 |
| Number of deaths | 622 (32%) | 174(32%) |
| Cause of death | ||
| Primary disease | 465 (75%) | 129 (74%) |
| Infection | 22 (4%) | 12 (7%) |
| Lung failure | 9 (1%) | 1 (<1%) |
| Other organ failure | 19 (3%) | 6 (3%) |
| Other | 57 (9%) | 18 (10%) |
| Not reported | 50 (8%) | 8 (5%) |
Effect of Chemotherapy Adjustment on Outcomes of Patients with MM
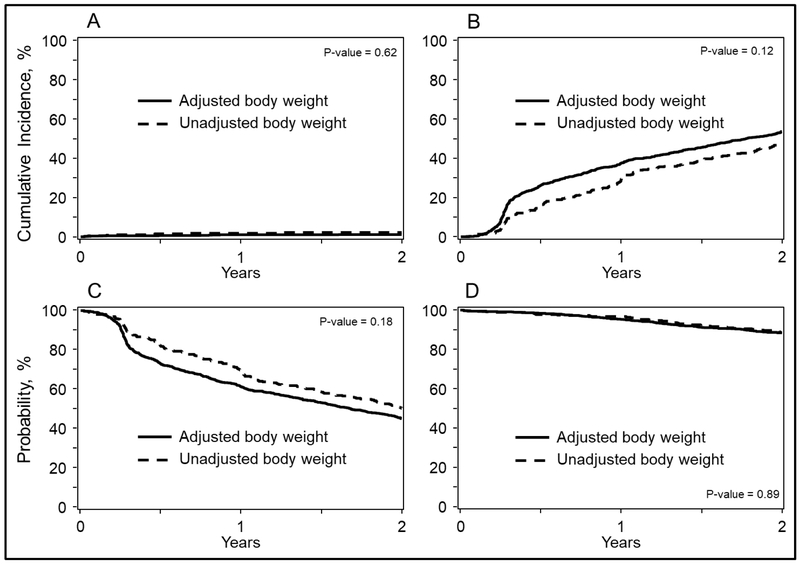
The 2-year overall survival probabilities were 88% (95% CI, 87-90%) and 89% (95% CI, 85-92%; P = 0.92) and PFS were 45% (95% CI, 42-48%) and 50% (95% CI, 45-55%; P = 0.08) for adjusted and unadjusted groups, respectively (Figure 2). Corresponding 2-year cumulative incidences of disease progression were 54% (95% CI, 51-56%) and 48% (95% CI, 43-53%; P = 0.002) and of TRM were 1% (95% CI, 1-2%) and 2% (95% CI, 1-4%; P = 0.29) (Figure 2). Multivariate analyses were carried out adjusting for center effect on overall mortality (HR= 1.01; 95% CI, 0.83-1.25; P = 0.89), treatment failure (HR 0.85; 95% CI, 0.68-1.08; P = 0.1784), disease progression (HR 0.84, 95% CI, 0.67-1.05, P = 0.12) and TRM (HR 1.31; 95% CI, 0.45-3.79; P = 0.62) of unadjusted compared to dose-adjusted chemotherapy groups (Figure 1). Additional covariates associated with these outcomes are shown on Table S2.
Figure 2:
Outcomes of patients with multiple Myeloma after autologous hematopoietic cell transplantation using actual (unadjusted) weight and adjusted weight to calculate chemotherapy doses. Log-rank P values are shown in each panel. Outcomes include:
A)Transplant-related mortality
B)Disease progression
C)Progression-free survival
D)Overall survival
Effect of Chemotherapy Adjustment on Outcomes of Patients with Lymphoma
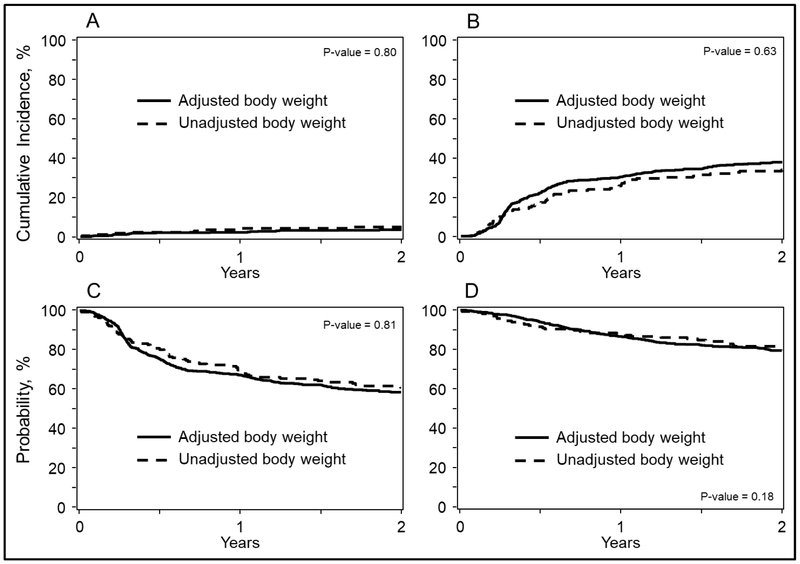
The 2-year overall survival probabilities were 79% (95% CI, 76-83%) and 82% (95% CI, 75-87%; P = 0.51) and PFS were 59% (95% CI, 55-63%) and 61% (95% CI, 54-68%; P = 0.57) for adjusted and unadjusted groups, respectively (Figure 3). Corresponding 2-year cumulative incidences of disease progression were 38% (95% CI, 34-42%) and 34% (95% CI, 27-41%; P = 0.35) and of TRM were 3% (95% CI, 2-5%) and 5% (95% CI, 2-9%; P = 0.42) (Figure 3). Multivariate analyses of overall mortality (HR 1.23; 95% CI, 0.91-1.66; P = 0.176), treatment failure (HR 1.03; 95% CI, 0.81-1.30; P = 0.812), disease progression (HR 1.06; 95% CI, 0.83-1.36; P = 0.633) and TRM (HR 0.91; 95% CI, 0.44-1.90, P = 0.802) of unadjusted compared to adjusted chemotherapy groups(Figure 1). Additional covariates associated with these outcomes are shown on Table S2.
Figure 3:
Outcomes of patients with lymphoma after autologous hematopoietic cell transplantation using actual (unadjusted) weight and adjusted weight to calculate chemotherapy doses. Log-rank P values are shown in each panel. Outcomes include:
A)Transplant-related mortality
B)Disease progression
C)Progression-free survival
D)Overall survival
DISCUSSION
High-dose chemotherapy with autologous HCT is the standard of care for patients with MM and subsets of lymphoma. The active therapy in autologous HCT is the high dose of chemotherapy, whereas the autologous graft serves as a supportive measure to accelerate hematopoietic recovery. Thus, chemotherapy dosing is critical.
Our study compares the effect of adjusted doses (or reducing the dose by using an adjusted body weight) to doses based on actual weight, among obese (BMI ≥ 30 kg/m2) patients undergoing autologous HCT for MM and lymphoma. The hypothesis of this study was that adjusting the conditioning regimen chemotherapy dose would result in reduced regimen-related toxicity and early mortality but would adversely affect long-term outcomes due to worse disease control.
The main findings were:1) most obese patients received dose-adjusted conditioning regimens, 2) adjusting doses did not appear to influence regimen-related toxicity, and 3) using acutal weight to dose high dose chemotherapy prior to HCT did not worsen overall survival in patients with either MM or lymphoma.
Single center reports also found no differences in survival when comparing obese patients with MM10 and lymphoma11 who received dose-adjusted conditioning regimens to non-obese patient who received chemotherapy based on actual body weight. In contrast, a report of BEAM dosed on actual body weight for obese patients found no adverse effect on outcomes as well12. However, these are single center studies with smaller numbers of patients and somewhat more uniform supportive care as compared to our registry-based study.
Our findings expand on a previous combined report from EBMT/IBMTR that compared the outcomes of obese patients with multiple myeloma (BMI ≥ 30; n = 323) to non-obese patients (n = 764) 13. Like in our study, most obese patients received reduced doses of chemotherapy, and there was no effect on mortality. But in contrast to our study, patients who received a melphalan-only conditioning regimen (n = 278) did not have increased rates of early relapse. Albeit a small number (n=45), obese patients who received a conditioning regimen of melphalan with total body irradiation (n=45) had lower rates of relapse and mortality.
Additionally, our study showed that most obese patients with MM or lymphoma (78%), notwithstanding ASBMT guidelines of melphalan dosing5, received adjusted doses. The prevalence of obesity (BMI ≥ 30 kg/m2) continues to increase in the general population1, 2. The Centers for Disease Control and Prevention report that 36.7% of the US population is obese3. Concern over the most appropriate dosing strategy for the obese population led ASBMT to recently issue a position paper. However, paucity of data to inform the field remains a challenge5. The American Society of Clinical Oncology also issued guidelines for dose adjustment for chemotherapeutic agents4, although these were not specific to transplant. The main concern about the ASCO guidelines was the frequent practice of under-dosing chemotherapy in obese patients, resulting in worse control of disease. Historically, the use of IBW offers a simple way to approximate to lean body, which is more cumbersome to estimate14. Also, using lean body weight, or its surrogate, could be a safer way to precisely predict the pharmacokinetics. However, pharmacokinetics and pharmacodynamics also are influenced by age, gender, type of chemotherapy, and genetics, among other factors4-6. Even within the same chemotherapy, the formulation needs to be considered, as pharmacokinetic studies of Captisolstabilized melphalan demonstrated a close to 10% increased systemic drug exposure compared to standard propylene-glycol–based melphalan15.
To better isolate the effect of chemotherapy dosing, the current study was limited to dosing strategies only in obese patients undergoing autologous HCT. The dose-adjustment strategy used varied depending on the transplant center, but most centers adjust the dose of the conditioning regimen in obese patients. This reflects the concerns of transplant physicians about the potential for increased toxicity of chemotherapy delivered based on actual body weight.
To assess the impact of conditioning regimen dose adjustment on toxicity in this study, the number of days hospitalized in the first 100 days post-HCT was used as a surrogate. As many centers only hospitalize autologous HCT recipients during the administration of the conditioning regimen and others do HCT as an outpatient procedure, we considered only patient who spent more than 7 of the first 100 days hospitalized. Overall, the length of hospitalizations was similar regardless of dose-adjustment strategy. However, when split by disease, patients with MM who received doses based on actual weight, stayed longer in the hospital, based on a higher number of patients with more than 15 days in the hospital. This was mainly driven by the subset of patients with BMI between 30 to 34 kg/m2, perhaps because this was the largest group. This was not observed among patients with lymphoma. Our finding contrasts with a single-center report on 80 patients. That report observed longer hospitalizations and higher risks of grade-3 to grade-4 mucositis in patients with lymphoma who received a melphalan dose > 3.6mg/Kg16. One caveat on the comparison of both studies is that melphalan is typically dosed in mg/m2, and when the dose is converted to mg/Kg, patients who are underweight rather than overweight are more frequently over of the proposed threshold of 3.6mg/Kg.
The ideal assessment of regimen-related toxicity in autologous HCT would include detailed gastrointestinal side effects (mucositis, diarrhea), need for total parenteral nutrition, and infections, which were not available on the current study.
Because disease biology, post-HCT treatment management, autologous HCT goals, and treatment options at the time of disease relapse are different between MM and lymphoma, the outcomes analysis was performed for all patients and then separately for each disease group. After adjusting for center effect, there was no adverse effect of dose adjusting the conditioning regimen on overall mortality, TRM, and treatment failure for the entire population of the MM and lymphoma patient group studied separately.
However, the higher risk of early relapse in those who received dose-adjusted chemotherapy possibly reflects the loss of intensity of the conditioning regimen on disease control. Considering that both MM and lymphoma have effective salvage therapies, it is not unexpected that this increased risk of early relapse does not affect overall mortality.
In summary, most obese patients undergoing autologous HCT receive a dose-adjusted conditioning regimen. The practice of reducing the dose of the conditioning regimen in obese patients did not adversely affect mortality but did result in an increased risk of early relapse after autologous HCT. Thus, our findings do not support adjusting doses of conditioning regimens for obese patients with MM or lymphoma.
Supplementary Material
Highlights:
Chemotherapy dose adjustment for obese patients is a common practice.
The adjustment factor varies across center practices.
Among patients with multiple myeloma and lymphoma, dose adjustment did not impact overall survival
Acknowledgments:
The authors thank the assistance of Jennifer Motl for the editorial review of the manuscript. The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 4U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-17-1-2388 and N0014-17-1-2850 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals, Inc.; *Amgen, Inc.; *Amneal Biosciences; *Angiocrine Bioscience, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; Atara Biotherapeutics, Inc.; Be the Match Foundation; *bluebird bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; Cerus Corporation; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Gilead Sciences, Inc.; HistoGenetics, Inc.; Immucor; *Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Juno Therapeutics; Karyopharm Therapeutics, Inc.; Kite Pharma, Inc.; Medac, GmbH; MedImmune; The Medical College of Wisconsin; *Mediware; *Merck & Co, Inc.; *Mesoblast; MesoScale Diagnostics, Inc.; Millennium, the Takeda Oncology Co.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; *Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Otsuka Pharmaceutical Co, Ltd. -Japan; PCORI; *Pfizer, Inc; *Pharmacyclics, LLC; PIRCHE AG; *Sanofi Genzyme; *Seattle Genetics; Shire; Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; Telomere Diagnostics, Inc.; and University of Minnesota. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
*Corporate Members
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Finkelstein EA, Khavjou OA, Thompson H, et al. Obesity and severe obesity forecasts through 2030. Am J Prev Med. 2012;42:563–570. [DOI] [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services. The Surgeon General’s Call To Action To Prevent and Decrease Overweight and Obesity. Rockville, MD: 2001. [Google Scholar]
- 3.Centers for Disease Control and Prevention: Obesity and Overweight. website https://www.cdc.gov/obesity/data/adult.html. Last accessed May 2018. [Google Scholar]
- 4.Griggs JJ, Mangu PB, Anderson H, et al. Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30:1553–1561. [DOI] [PubMed] [Google Scholar]
- 5.Bubalo J, Carpenter PA, Majhail N, et al. Conditioning chemotherapy dose adjustment in obese patients: a review and position statement by the American Society for Blood and Marrow Transplantation practice guideline committee. Biology of Blood and Marrow Transplantation : journal of the American Society for Blood and Marrow Transplantation. 2014;20:600–616. [DOI] [PubMed] [Google Scholar]
- 6.Jain R Implications of Obesity for Drug Therapy: Limitations and Challenges. Clinical Pharmacology Therapeutics. 2011;90:77–89. [DOI] [PubMed] [Google Scholar]
- 7.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. Journal of Clinical Oncology: official journal of the American Society of Clinical Oncology. 2005;23:3412–3420. [DOI] [PubMed] [Google Scholar]
- 9.Commenges D, Andersen PK. Score test of homogeneity for survival data. Lifetime Data Analysis 1995;1:145–156. [DOI] [PubMed] [Google Scholar]
- 10.Shultes KC, Arp C, Stockerl-Goldstein K, Trinkaus K, DeFrates S. Impact of Dose-Adjusted Melphalan in Obese Patients Undergoing Autologous Stem Cell Transplantation. Biology of Blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2018, 24:687–693. [DOI] [PubMed] [Google Scholar]
- 11.Bachanova V, Rogosheske J, Shanley R, et al. Adjusting Cyclophosphamide Dose in Obese Patients with Lymphoma Is Safe and Yields Favorable Outcomes after Autologous Hematopoietic Cell Transplantation. Biology of Blood and Marrow Transplantation : journal of the American Society for Blood and Marrow Transplantation. 2016;22:571–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fair C, Shanley R, Rogosheske J, et al. BEAM conditioning is well-tolerated and yields similar survival in obese and non-obese patients with lymphoma: no requirement for weight-based dose modifications. Bone Marrow Transplant. 2017;52:491–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogl DT, Wang T, Perez WS, et al. Effect of obesity on outcomes after autologous hematopoietic stem cell transplantation for multiple myeloma. Biology of Blood and Marrow Transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17:1765–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pai MP, Paloucek FP. The origin of the "ideal" body weight equations. Ann Pharmacother. 2000;34:1066–1069. [DOI] [PubMed] [Google Scholar]
- 15.Aljitawi OS, Ganguly S, Abhyankar SH, et al. Phase IIa cross-over study of propylene glycol-free melphalan (LGD-353) and alkeran in multiple myeloma autologous transplantation. Bone Marrow Transplant. 2014;49:1042–1045. [DOI] [PubMed] [Google Scholar]
- 16.Costa LJ, Micallef IN, Inwards DJ, et al. Effect of the dose per body weight of conditioning chemotherapy on severity of mucositis and risk of relapse after autologous haematopoietic stem cell transplantation in relapsed diffuse large B cell lymphoma. Br J Haematol. 2008;143:268–273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.