Abstract
Intra-articular (IA) and peri-articular (PA) tumors of the knee are frequently encountered by orthopaedic surgeons. Nonetheless, due to the possibility of great morbidity and potential mortality, it is important to recognize and differentiate between benign and malignant lesions in a timely manner. Therefore, the purpose of this article is to provide a concise, practical, and updated review of commonly encountered IA and PA tumors including intratendinous gout, synovial chondromatosis, schwannoma, pigmented villonodular synovitis, and synovial sarcoma, and a detailed description of differentiating features to include various imaging modalities.
Keywords: musculoskeletal oncology, intra-articular, tumor, review, orthopaedics
Intra-articular (IA) and peri-articular (PA) pathology of the knee can be multifactorial and difficult to diagnose. Nonetheless, a comprehensive history and physical examination can be extremely useful in narrowing down possible culprits of knee pain. While the majority of IA and PA pathologies that cause pain are benign, they can lead to significant morbidity and functional disability if there is an initial delay in diagnosis or mismanagement.
In this review, we will focus on the following diseases, as these represent some of the most frequent referrals to orthopaedic oncology specialists: intratendinous gout, synovial chondromatosis, schwannoma, pigmented villonodular synovitis (PVNS), and synovial sarcomas. Briefly, each of these masses will be reviewed to provide an updated review for surgeons and other medical care providers alike.
Epidemiological data regarding IA/PA tumors as a whole have not been previously reported in the literature. Nonetheless, primary malignant bone and connective tissue tumors have most recently been reported as 2.2% of all annual cancer patients from 2006 to 2010, which amounts to approximately 43,000 cases.1,2 These data from the National Cancer Institute are the most robust but fail to properly depict the prevalence of IA and PA tumors and tumorlike conditions.
Due to the possibility of high morbidity with these tumors and tumorlike conditions, it is important that clinicians are aware of the presentation and radiographic findings associated with these processes. Thus, the purpose of this study was to perform a comprehensive review of tumors and tumorlike conditions within and around the knee joint providing a detailed radiographic interpretation of each.
Intratendinous Gout
Gout is a disease of purine metabolism, which results in increased serum levels of uric acid and deposition of monosodium urate (MSU) crystals in the joint and soft tissues. This deposition is a well-recognized complication of chronic gout and often lacks symptoms. It occurs most commonly in the fourth through sixth decades of life and has a 9:1 male-to- female predominance.3 Gout is most often found in the first metatarsophalangeal joint; however, extra-articular manifestations may also occur. The Achilles and triceps tendons are the most often involved intratendinous locations, whereas tophi deposition within the tendons of the knee occurs at a much less common rate. Within the knee, the popliteus and quadriceps are the most commonly involved tendon sheaths; however, rare involvement of the patellar tendon has been described.4
Although most patients with intratendinous gouty tophi are often asymptomatic, there have been few reports of painful intratendinous gout mimicking patellar tendonitis, and these cases have been associated with prior injury; that is, partial tendon tear, enthesopathy. Gouty tophi have also frequently been mistaken for a neoplastic process. Histological examination is consistent with granulomatous inflammation associated with crystalline material and positive for urate crystals.5
Treatment of gout flares is primarily medical and involves the use of nonsteroidal anti-inflammatory drugs, colchicine, allopurinol, and other uricosurics. Although most gouty tophi are without symptoms and can easily be observed, surgical treatment may be indicated in patients with loss of function, severe disability, or persistent pain in spite of medical treatments that have minimal effect on tophaceous deposits.6 As with any mass excision, intraoperative cultures should be taken in addition to surgical pathology. If tophaceous gout is considered, the specimen should be transferred in ethanol rather than formalin as the latter has been shown to degrade MSU crystals.5
On radiographs, gouty arthropathy is classically described as para-articular erosions with sclerotic margins, resulting in overhanging edges and relative preservation of the joint space. Gouty tophi appear as nodular soft tissue attenuation masses that may have faint or coarse calcifications.7 The radiographic findings of gout are a marker of disease chronicity becoming apparent after 5 to 10 years.7 Earlier manifestations are more readily assessed with cross-sectional imaging. Tophaceous gout appears intermediate to low signal intensity on T1 and heterogeneous low signal intensity on T2-weighted images, and enhances with the addition of gadolinium.8–10
The signal intensity of gouty tophi in isolation is often nonspecific and may be confused with a neoplastic or infectious process. The distribution of involvement in combination with the imaging features leads to the diagnosis of gout8 (►Fig. 1).
Fig. 1.
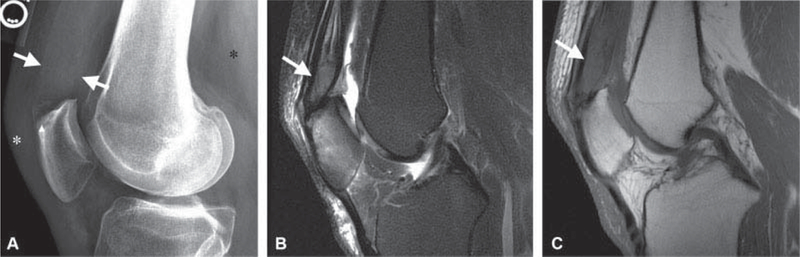
A 50-year-old male with 3-weeks’ history of knee pain following a basketball injury referred to orthopaedic oncology for the evaluation of mass identified on magnetic resonance imaging (MRI) performed to assess for internal derangement. (A) Lateral radiographs demonstrate thickening of the quadriceps tendon (white arrows), erosion of a superior patellar enthesophyte (arrowhead), and faint mineralization in Hoffa’s fat (asterisks). (B) Sagittal T2-weighted image with fat suppression and (C) T1-weighted image demonstrates a mass infiltrating the quadriceps tendon (white arrows in B and C) with intermediate T2 and hypointense T1 signal and reactive marrow edema in the patella compatible with the gout. The diagnosis was confirmed on subsequent ultrasound-guided needle biopsy.
Computed tomography (CT) typically demonstrates soft tissue masses with a density of 160 to 170 HU. Dual-energy CT uses the differential absorption of different energy X-ray beams by a material to determine its composition.11 This technology was initially used clinically to examine the composition of renal stones, but its use has been expanded to assess gout, with a diagnostic sensitivity and specificity of 88 and 90%, respectively, compared with aspiration or biopsy based on recent meta-analysis.12
Synovial Chondromatosis
Synovial chondromatosis is a benign process that most commonly affects the knee joint (70%) and can result in significant functional disability. It is characterized by proliferative meta- plasiaof synovial membrane into chondrocytes, resulting in the formation of multiple cartilaginous nodules. These nodules enlarge and can detach from the synovium to become multiple loose IA bodies, ranging from soft tissue cartilaginous bodies to firm calcified nodules.13,14 It occurs most commonly in the third to fifth decades of life and predominantly affects males. The cause of primary synovial chondromatosis has yet to be determined; however, secondary chondromatosis occurs in association with an inflammatory or degenerative joint pathology and, therefore, usually affects the older population.13
Patients with synovial chondromatosis present with pain and swelling of the knee with or without mechanical symptoms. It is most often monoarticular, with the anterior knee affected most frequently. Pain may worsen with activity but is often present even at rest.15 Histological examination of the nodules will reveal benign hyaline cartilage with mild-to- moderate cellularity, varying degrees of calcification, and a synovial tissue lining.16 It is important to note that although rare, there have been reported cases of synovial chondrosarcomas arising from primary chondromatosis associated with recurrence in spite of initial treatment.17
Primary synovial chondromatosis was described by Milgram in 1977 to occur in three phases. Phase I includes active intrasynovial disease with no free bodies, phase II involves transitional lesions with osteochondral nodules in the synovium and free osteochondral bodies within thejoint, and phase III occurs when there are multiple IA loose bodies produced by now dormant intrasynovial disease18 (►Figs. 2 and 3).
Fig. 2.
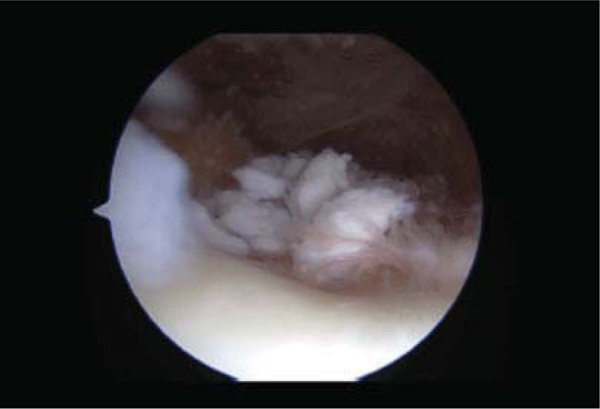
Arthroscopic image displaying pedunculated nature of synovial chondromatosis seen in phase I and II.
Fig. 3.
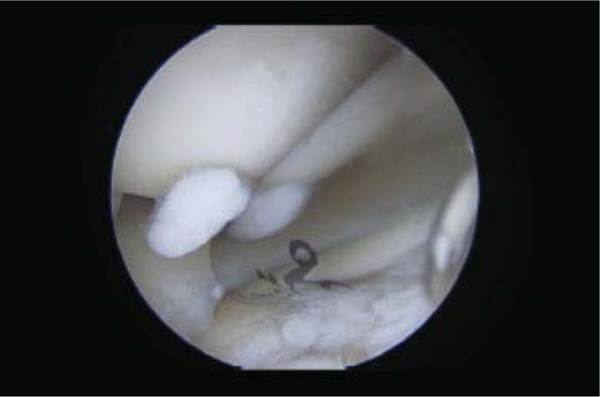
Arthroscopic image showing loose osteochondral bodies representative of synovial chondromatosis.
Treatment for synovial chondromatosis usually involves surgery, although cases with minimal symptoms may be managed conservatively especially since, on rare occasions, synovial chondromatosis can be self-limiting.19 Arthroscopic versus open removal of nodules with or without partial synovectomy prevents further articular destruction, improvement of pain and swelling, and resolution of mechanical symptoms in most cases.20
The imaging patterns, categorized as A, B, and C, of primary synovial chondromatosis mirror the three phases described by Milgram in 1977.18 Pattern A corresponds to phase I. Pattern A, seen in 14% of cases, is characterized by lobular thickening of the synovium without IA bodies. The thickened synovium has hypointense septations and is typically isointense to joint fluid, hypointense onT1, and hyperintense on T2 relative to skeletal muscle on all sequences.18 Radiographs are not very useful in the diagnosis due to the lack of calcifications.20 Pattern B, corresponding to Milgram phase II, is demonstrated in 77% of cases at magnetic resonance imaging (MRI).20 Pattern B is similar to pattern A, with the distinction of having multiple foci of signal void, isointense to the cortical bone on T1- and T2-weighted images, in the thickened synovium corresponding to calcifi- cations.20 The calcifications are usually more conspicuous on gradient echo images and can be seen on radiographs.20 Pattern C, corresponding to Milgram phase III, is characterized by foci of fat in the synovium corresponding to the marrow space in the ossified IA bodies20 ( ► Fig. 4). Bone erosion can be seen on all imaging modalities and ranges from 30 to 50% of cases.21,22 Bone erosion is theorized to be more common in joints with tightly adherent joint capsules, that is, the hip, and is less common in the knee joint.
Fig. 4.
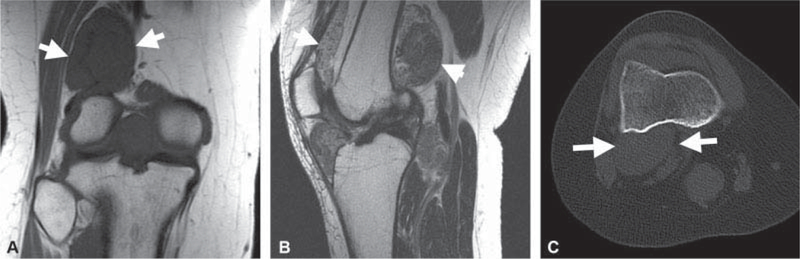
A 30-year-old female presenting with a 3-year history of progressive knee pain. (A) Coronal T1-weighted and (B) sagittal T2-weighted images without fat suppression demonstrate a heterogeneous synovial based mass (white arrows in A-C) distending thejoint space without bone erosion. There were no foci of signal void or intralesional fat, as demonstrated onT1-weighted image. No calcified or ossified mass was seen on the subsequent axial computed tomography (CT) in bone window (C), indicative of primary synovial chondromatosis pattern A.
The differential diagnosis when no calcification or ossification is seen in the masslike enlarged synovium includes rheumatoid arthritis, PVNS, and chronic indolent infection.
Schwannoma
Schwannomas (neurilemmomas) are benign peripheral nerve sheath tumors that most commonly occur in the third to fifth decades of life. They do not appear to have a race or gender preference.23,24 While schwannomas are usually solitary in nature, reports of multiple extremity schwannomas have been reported.24 The upper extremity is more often involved than the lower extremity, with a predilection for the median nerve. While malignant transformation of a benign schwannoma is rare, malignant peripheral nerve sheath tumors do exist and represent approximately 5% of all soft tissue sarcomas.23
Patients with nerve sheath tumors often present with symptoms consistent with compression of the associated nerve fascicles and may have a positive Tinel's sign on examination with symptoms in the distribution of the affected nerve. Grossly, schwannomas are well encapsulated and displace the nerve fascicles, differentiating them from neurofibromas that envelop the fascicles.24 Histologically, they are composed of Schwann cells with two very distinct areas. The Antoni A pattern involved a more cellular and ordered appearance of the cells with palisading nuclei and Verocay bodies. The Antoni B pattern is more loosely arranged with fewer cells and a myxoidlike matrix25 ( ►Fig. 5). Diffuse homogenous uptake when stained with S100 is also noted during histological workup (►Fig. 6).
Fig. 5.
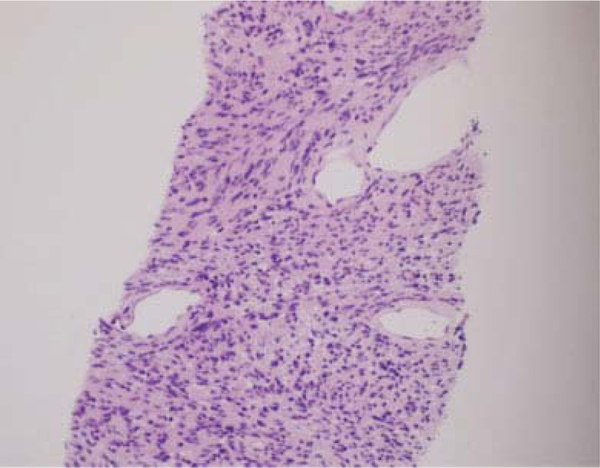
Biopsy specimen demonstrating Antoni A and B areas consistent with nerve sheath tumor.
Fig. 6.
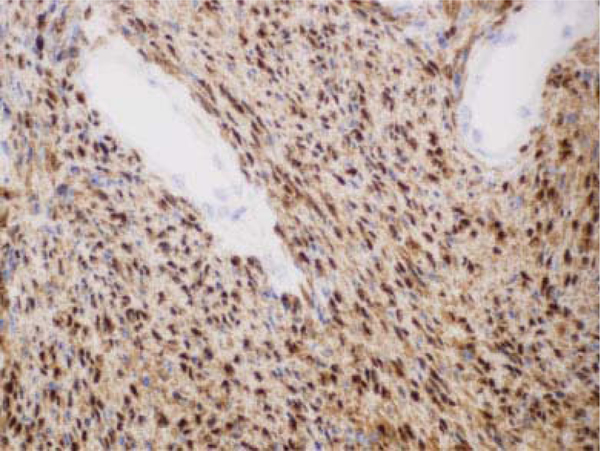
Diffuse uptake for S100 consistent with schwannoma.
If these tumors are symptomatic or enlarging, treatment involves surgical resection of the mass with maintenance of the associated nerve.26 Given the noninfiltrating growth of the tumor to the adjacent fascicles permanent neurologic damage is uncommon. However, the incidence of transient neurologic deficits, most often resulting in worsening of paresthesias, ranges from 1.5 to 80%, with an average of 32.4% documented in the literature.26 This suggests a high incidence of iatrogenic nerve injury necessitating meticulous dissection of the epineurium without fascicular damage to allow the tumor to be removed in its entirety.24 Studies have shown that the risk for neurologic deficits increases for tumors larger than 3 cm and patient age > 50 years,26 as well as a longer history of symptoms.27 Recurrence following complete surgical excision is rare.
The diagnosis of nerve sheath can be made by MRI when an intermuscular soft tissue mass is inseparable from a nerve and is accompanied by additional imaging signs.9,28 Nerve sheath tumors at the knee are most commonly present in the popliteal fossa where the sciatic nerve branches into the tibial and peroneal nerves. Identifying a mass as arising between the muscles rather than within the muscle helps to narrow the imaging differential diagnosis. This can be challenging, particularly in patients or locations with minimal fat; however, it can be aided by identification of the “split-fat” sign. The “split-fat” sign refers to the identification of fat on T1-weighted images at the proximal and distal aspects of the mass between the muscles that are splayed by an intermuscular space-occupying lesion.28,29 Another helpful sign is the “target sign,” which is more frequently seen with neurofibromas; however, it is identified in approximately 50% of schwannomas.9,28 The “target sign” refers to a relatively decreased T2 signal in the central portion of the lesion with increased T2 signal peripherally.30 Identification of the “target sign” in a peripheral nerve sheath tumor suggests benignity29,31 (►Fig. 7 ). The “string sign” is often present on MRI as well and demonstrates continuity of the tumor with the neurovascular structure proximal and distal to the mass. In large peripheral nerves, the eccentric location of the tumor relative to the nerve favors schwannoma over neurofibroma. Features that are suggestive of malignant peripheral nerves sheath tumor include an increase in size, peripheral enhancement pattern, peritumoral soft tissue edema, intratumoral cystic change, and increased metabolic activity on FDG-PET (fluoro-2-deoxy-D-glucose positron emission tomography) scan.32,33
Fig. 7.
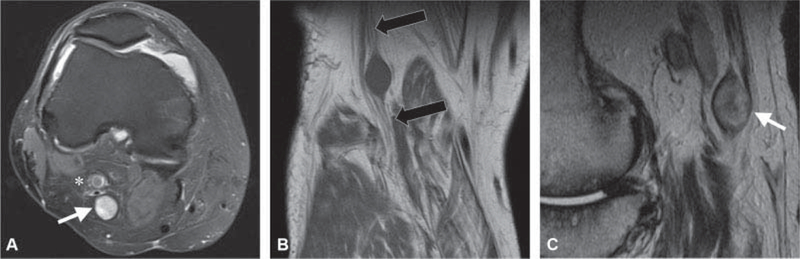
A 50-year-old man underwent knee magnetic resonance imaging (MRI) for anterior knee pain and was referred for the evaluation of incidental mass in the popliteal fossa. (A) Axial T2-weighted image with fat suppression shows an encapsulated T2 hyperintense mass (white arrow) with smooth margins posterior to the popliteal artery (asterisks) inseparable from the tibial nerve and compatible with a schwannoma. (B) Coronal T1-weighted image shows the hypointense mass with the fascicular appearance of the tibial nerve at the proximal and distal aspects of the mass. (C) Sagittal T2-weighted image shows the “target sign,” (white arrow) low central signal intensity and high signal intensity in the periphery, an indicator of a benign peripheral nerve sheath tumor.
Pigmented Villonodular Synovitis
Tenosynovial giant cell tumors are a group of usually benign IA and soft tissue tumors that share common histological features. This group includes PVNS, which is a rare synovial proliferative process with an incidence reported at 1.8 casesper million.34 It may involve any synovial-lined structure; however, IA occurrence is the most common. PNVS usually presents in a monoarticular fashion, with the anterior compartment of the knee most affected.34 There seems to be no gender predilection, and it most commonly affects patients in their twenties to forties.35,36 PVNS is described as two different types: the localized form (LPVNS) is a solitary pedunculated lesion, also referred to as focal nodular synovitis, whereas the diffuse form (DPVNS) involves the entire joint.34 PVNS is considered a benign but locally aggressive process that can lead to significant joint destruction over time.
Patients often present with symptoms of diffuse swelling, pain, locking, and other manifestations related to cartilage erosions/degenerative changes. This nonspecific presentation commonly leads to a delay in diagnosis.37 PVNS has defining features on MRI; however, definitive diagnosis depends on histological interpretation of the synovial specimen. Few processes can mimic PVNS, and tuberculosis has been reported to present in similar fashion.37 Histologically, one will see hemosiderin deposition, multinucleate giant cells, and lipid-laden macrophages.34
The aim of treatment is removal of all involved abnormal tissue to relieve pain, decrease recurrence risk, and diminish joint destruction.35 Surgical resection remains the mainstay of treatment, and although radiation has been shown to diminish recurrence in refractory disease,34 it alone is not superior to surgery.38 LPVNS is often treated with arthroscopic excision, with reports of 0 to 8% recurrence.35 Many studies have examined open versus arthroscopic synovectomy for DPVNS. It was previously felt that open synovectomy decreased recurrence risk; however, recent literature shows that combined arthroscopic and open synovectomy can reduce recurrence rates of DPVNS from 8 to 70% to less than 20%.38 Auregan et al reported a reduced rate of complications with arthroscopic techniques; however, no reduction in recurrence risk.35 Colman et al described a lower rate of recurrence when open posterior synovectomy was combined with anterior arthroscopic techniques, 9% versus approximately 60% using either all open or all arthroscopic means.38 Systemic therapy has been described for patients with unresectable or multiply recurrent disease to help stabilize disease. Small molecule inhibitors of CSF1R (colony stimulating factor 1 receptor), such as imatinib or sunitinib, can result in radiographic and symptomatic improvement.35 Likewise, IA isotope injection, known as radiosynovectomy, has been used after surgical excision with improved functional scores. Yittrium-90 is the radionuclide most often used in the knee as it emits p particles that cause fibrosis and sclerosis of the synovial membrane.39 Regardless, total joint arthroplasty remains the best option for those patients who have significant degenerative joint destruction or multiple recurrences despite other treatments.34
Radiographs in patients with PVNS may be normal or show a combination of effusion and extrinsic bone erosion with normal joint space and mineralization.40,41 Approximately 26 to 32% of patients with knee involvement will have well-defined erosions with sclerotic margins involving both sides of thejoint, a finding less frequently seen in the knee compared with other joints and theorized to be due to the capacious capsule.40,41 A minority of cases, 6%, will have calcifications.41 MRI evaluation captures DPVNS as a nodular plaque-like synovial thickening. LPVNS appears as a well-defined soft tissue mass with lobular margins, typically in Hoffa's fat.41 PVNS is iso- to hypointense to skeletal muscle onT1- and T2- weighted images with heterogeneous enhancement and may erode the bone.41,42 The most distinctive feature of PVNS or focal nodular synovitis is identification of blooming. Blooming artifact, also referred to as susceptibility artifact, occurs due to the presence of hemosiderin in the tumor. The hemosiderin induces changes in the magnetic field that make the lesion appear darker and larger on gradient echo sequences as compared with traditional T1 and T2 spin-echo images.43 Low T2 signal intensity in combination with blooming artifact is essentially pathognomonic for focal nodular synovitis41(►Fig. 8 ). Recurrent hemarthrosis due to hemophilia may mimic PVNS on MRI; however, the disease is readily excluded by clinical assessment.
Fig. 8.
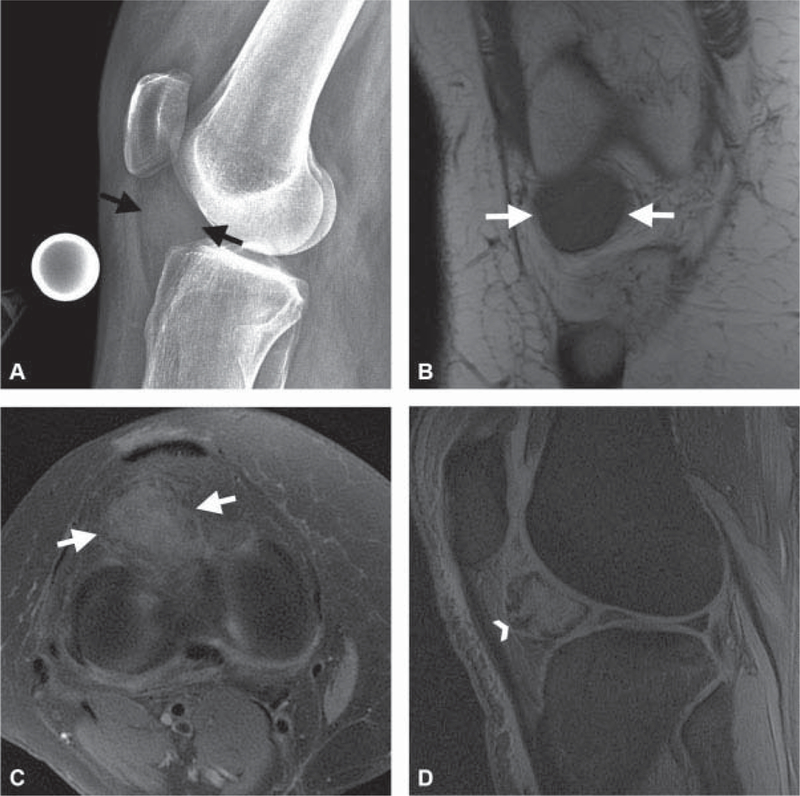
A 34-year-old with knee mass identified on magnetic resonance imaging (MRI) performed for pain following a twisting injury. (A) Lateral knee radiograph shows a soft tissue attenuation mass (black arrows) in Hoffa’s fat without calcification. (B) Axial T2-weighted image with fat suppression and (C) coronal T1-weighted image through the anterior knee show a mass (white arrows in B and C) that has heterogeneous T2 signal hypointensity and homogeneous T1 signal hypointensity. (D) Sagittal gradient echo image shows “blooming artifact” (white arrowhead) at the periphery of the mass, diagnostic of focal nodular synovitis. The “blooming” at the margins is due to local changes in the magnetic field induced by the ferromagnetic effects of hemosiderin deposited in the tumor.
Synovial Sarcoma
Synovial cell sarcoma represents approximately 9% of all adult sarcomas and 15% of all extremity sarcomas, making them the third most common extremity soft tissue sarcoma. Eighty percent of synovial sarcomas arise in the extr.emities, with equal gender distribution and median age of 35 versus 50 for most other soft tissue sarcomas. There has not been an identified genetic condition or etiological agent to date.44 The majority of lower extremity synovial sarcomas arise in a PA location.45 Three percent have been found to localize within the joint.46 When they are discovered in an IA location, they can be difficult to distinguish from other masses including LPVNS. The most consistent distinguishing features between the two processes have been tumor size greater than 3 cm, absence of effusion, and male predominance seen in synovial sarcomas over patients with PVNS.46 There have also been reports of synovial sarcoma mistaken for a parameniscal cyst, resulting in unplanned excision and need for further interventions.47 Therefore, it is an important tumor to keep within the differential for the treating surgeons when evaluating patients with IA and PA masses.
The majority of patients present with an enlarging mass that may or may not be painful. It is important to note that the tumor may remain stable in size for some time prior to any growth. Unlike most soft tissue sarcomas, synovial sarcoma has a higher incidence of nodal metastases (10–12% vs. 3–5%), and attention to nodal examination should be considered.44
Definitive diagnosis requires a biopsy with histological and often immunochemistry/molecular analysis of the specimen. Contrary to its name, synovial sarcoma is not derived from synovial tissue. Historically, it was given this name based on its often PA nature and resemblance to synovium microscopically. The tumor cells have since been identified as epithelial in origin.44 Three types of synovial sarcoma have been identified: monophasic, consisting of ovoid spindlecells; biphasic, consisting of both spindle and epithelial cells; and poorly differentiated, exhibiting necrosis, mitoses, and atypia.48 The tissue is often evaluated for the t(X;18) translocation with the SYT/SSX fusion protein.44
Surgery with wide resection remains the cornerstone of treatment. Adjuvant radiation is often used to improve local control, and chemotherapy can be considered especially on larger (>5 cm) and deep tumors. Prognosis depends on size, location (extremities faring better than central), and tumor type (undifferentiated having the worst prognosis). Targeted therapy trials have recently been ongoing with some early promise.48
At imaging, synovial sarcoma appears as a PA, not 1A mass, with a predilection for the popliteal fossa.9,49,50 Radiographs are typically normal or nonspecific but may show a calcified PA mass in 30% of cases. Indolent appearing bone erosions may be seen in 11 to 20% of cases.50 At MR1 evaluation, synovial sarcomas are usually isointense or slightly hyperintense to muscle on Tl-weighted images. Smaller lesions are typically homogeneously hyperintense on T2 sequences.50 Larger lesions maybe heterogeneous onT2-weighted images with areas of low, intermediate, and high signal intensity, referred to as the triple signal pattern.49,50 Fluid-fluid levels may also be present,50 and the margins are typically well defined in smaller lesions. 1rregular margins are more commonly seen in larger lesions, and heterogeneous enhancement postcontrast is typical50 (►fig. 9). The combination of slow growth, sometimes stable over an extended period of time, well-defined smooth margins, and homogeneous T2 signal hyperintensity in some smaller synovial sarcomas may lead to misdiagnosis as a benign cyst.9,49 The key to avoiding this pitfall is recognizing that a cystlike lesion, isointense to muscle on T1 and hyperintense to muscle on T2-weighted images, is occurring in a location atypical for a cyst, for example, not adjacent to a torn meniscus, such as a parameniscal cyst, and does not have a stalk with definitive connection to the joint, as seen with a ganglion cyst.
Fig. 9.
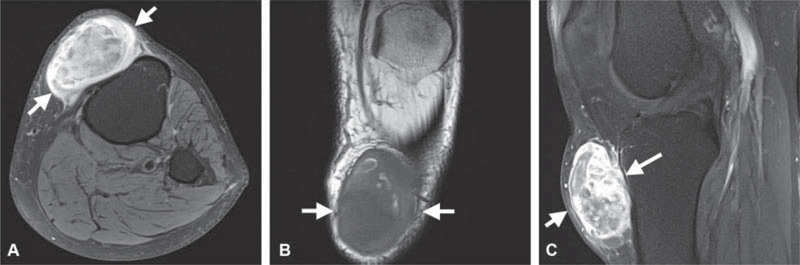
A 58-year-old man presented with enlarging anterior knee mass following impact injury 1 to 2 years prior. Mass was previously stable for many years and initially mistaken for a cyst. (A) Axial T2-weighted image with fat suppression demonstrates a large juxta-articular mass (white arrows in A-C) with triple signal intensity, areas of high, and intermediate and low signal intensity. (B) On coronal T1-weighted image, the mass is sharply marginated with smooth round contours and heterogeneous signal. (C) The mass enhances heterogeneously post contrast on sagittal T1-weighted image with fat suppression excluding cyst from the differential diagnosis. The diagnosis of synovial sarcoma was confirmed on computed tomography guided biopsy.
Discussion
The purpose of this article was to review some of the most common 1A and PA tumors and tumorlike conditions of the knee. The aforementioned descriptions provide direction for clinicians to use when identifying patients with knee masses to help determine which patients are at an increased risk of disabling sequelae versus misdiagnosis.
The aforementioned imaging descriptions also provide key imaging findings, which, in combination with history and physical examination, can aid in the correct diagnosing of patients who present with these types of tumors. By highlighting these conditions, we also give clinicians some guidance on which patients may require expedited referral to orthopaedic oncology specialists and/or further imaging work-up.
1t is important to understand the risk of recurrence with some of these 1A and PA masses. As highlighted in previous work, that patients with suspected or diagnosed masses should undergo continued follow-up to ensure appropriate diagnosis as well as provide the ability to intervene prior to significant morbidity.51
Additionally, by promptly and appropriately diagnosing patients, it is possible to avoid added risk to the patient with unplanned excisions of malignant tumors resulting in further unnecessary surgical intervention and overall negative effect on patient disease-free survival rate. Furthermore, use of appropriate resources in a timely manner prevents unnecessary testing and leads to improved patient care, providing overall cost savings to the system, which is an important factor in today's health care environment.
In conclusion, 1A and PA tumors of the knee occur infrequently but may have dire consequences if mismanaged. It is important that medical practitioners differentiate between benign, malignant, and indeterminate 1A and PA masses to effectively and efficiently treat them, decreasing morbidity and possibly mortality.
Footnotes
Conflict of Interest
None.
References
- 1.Cronin KA, Lake AJ, Scott S, et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 2018;124(13):2785–2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith E, Hoy DG, Cross M, et al. The global burden of other musculoskeletal disorders: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis 2014;73(08):1462–1469 [DOI] [PubMed] [Google Scholar]
- 3.Rodas G, Pedret C, Catala J, Soler R, Orozco L, Cusi M. Intratendi- nous gouty tophus mimics patellar tendonitis in an athlete. J Clin Ultrasound 2013;41(03):178–182 [DOI] [PubMed] [Google Scholar]
- 4.Colberg RE, Henderson RG. Diagnosis and treatment of gouty tophi in the patellar tendon using ultrasound-guided needle barbotage: a case presentation. PM R 2017;9(09):938–942 [DOI] [PubMed] [Google Scholar]
- 5.Gililland JM, Webber NP, Jones KB, Randall RL, Aoki SK. Intraten- dinous tophaceous gout imitating patellar tendonitis in an athletic man. Orthopedics 2011;34(03):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li TJ, Lue KH, Lin ZI, Lu KH. Arthroscopic treatment for gouty tophi mimicking an intra-articular synovial tumor of the knee. Arthroscopy 2006;22(08):910.e1–910.e3 [DOI] [PubMed] [Google Scholar]
- 7.Bloch C, Hermann G, Yu TF. A radiologic reevaluation of gout: a study of 2,000 patients. Am J Roentgenol 1980;134(04):781–787 [DOI] [PubMed] [Google Scholar]
- 8.Girish G, Glazebrook KN, Jacobson JA. Advanced imaging in gout. Am J Roentgenol 2013;201(03):515–525 [DOI] [PubMed] [Google Scholar]
- 9.Kransdorf MJ, Murphey MD. Imaging of Soft Tissue Tumors. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkens; 2006 [Google Scholar]
- 10.Yu KH, Lien LC, Ho HH. Limited knee joint range of motion due to invisible gouty tophi. Rheumatology (Oxford) 2004;43(02): 191–194 [DOI] [PubMed] [Google Scholar]
- 11.Coursey CA, Nelson RC, Boll DT, et al. Dual-energy multidetector CT: how does it work, what can it tell us, and when can we use it in abdominopelvic imaging? Radiographics 2010;30(04):1037–1055 [DOI] [PubMed] [Google Scholar]
- 12.Yu Z, Mao T, Xu Y, et al. Diagnostic accuracy of dual-energy CT in gout: a systematic review and meta-analysis. Skeletal Radiol 2018. Doi: 10.1007/s00256-018-2948-y [DOI] [PubMed] [Google Scholar]
- 13.Singh S, Neelakandan K, Sood C, Krishnan J. Disseminated synovial chondromatosis of the knee treated by open radical synovectomy using combined anterior and posterior approaches. J Clin Orthop Trauma 2014;5(03):157–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larbi A, Viala P, Cyteval C, et al. Imaging of tumors and tumor-like lesions of the knee. Diagn Interv Imaging 2016;97(7–8):767–777 [DOI] [PubMed] [Google Scholar]
- 15.Habusta SF, Tuck JA. Synovial chondromatosis In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2018 [PubMed] [Google Scholar]
- 16.Ho YY, Choueka J. Synovial chondromatosis of the upper extremity. J Hand Surg Am 2013;38(04):804–810 [DOI] [PubMed] [Google Scholar]
- 17.Yao MS, Chang CM, Chen CL, Chan WP. Synovial chondrosarcoma arising from synovial chondromatosis of the knee. JBR-BTR 2012; 95(06):360–362 [DOI] [PubMed] [Google Scholar]
- 18.Milgram JW. Synovial osteochondromatosis: a histopathological study of thirty cases. J Bone Joint Surg Am 1977;59(06):792–801 [PubMed] [Google Scholar]
- 19.Neumann JA, Garrigues GE, Brigman BE, Eward WC. Synovial chondromatosis. JBJS Rev 2016;4(05):01874474–201605000- 00005 [DOI] [PubMed] [Google Scholar]
- 20.Kramer J, Recht M, Deely DM, et al. MR appearance of idiopathic synovial osteochondromatosis. J Comput Assist Tomogr 1993;17 (05):772–776 [DOI] [PubMed] [Google Scholar]
- 21.Wittkop B, Davies AM, Mangham DC. Primary synovial chondro¬matosis and synovial chondrosarcoma: a pictorial review. Eur Radiol 2002;12(08):2112–2119 [DOI] [PubMed] [Google Scholar]
- 22.Norman A, Steiner GC. Bone erosion in synovial chondromatosis. Radiology 1986;161(03):749–752 [DOI] [PubMed] [Google Scholar]
- 23.Albert P, Patel J, Badawy K, et al. Peripheral nerve schwannoma: a review of varying clinical presentations and imaging findings. J Foot Ankle Surg 2017;56(03):632–637 [DOI] [PubMed] [Google Scholar]
- 24.Kim SM, Seo SW, Lee JY, Sung KS. Surgical outcome of schwannomas arising from major peripheral nerves in the lower limb. Int Orthop 2012;36(08):1721–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gosk J, Gutkowska O, Urban M, Wnukiewicz W, Reichert P, Ziolkowski P. Results of surgical treatment of schwannomas arising from extremities. BioMed Res Int 2015;2015:547926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siqueira MG, Socolovsky M, Martins RS, et al. Surgical treatment of typical peripheral schwannomas: the risk of new postoperative deficits. Acta Neurochir (Wien) 2013;155(09):1745–1749 [DOI] [PubMed] [Google Scholar]
- 27.Oberle J, Kahamba J, Richter HP. Peripheral nerve schwannomas-an analysis of 16 patients. Acta Neurochir (Wien) 1997;139(10):949–953 [DOI] [PubMed] [Google Scholar]
- 28.Beaman FD, Kransdorf MJ, Menke DM. Schwannoma: radiologic- pathologic correlation. Radiographics 2004;24(05):1477–1481 [DOI] [PubMed] [Google Scholar]
- 29.Murphey MD, Smith WS, Smith SE, Kransdorf MJ, Temple HT. From the archives of the AFIP. Imaging of musculoskeletal neurogenic tumors: radiologic-pathologic correlation. Radiographics 1999;19(05):1253–1280 [DOI] [PubMed] [Google Scholar]
- 30.Banks KP. The target sign: extremity. Radiology 2005;234(03): 899–900 [DOI] [PubMed] [Google Scholar]
- 31.Bhargava R, Parham DM, Lasater OE, Chari RS, Chen G, Fletcher BD. MR imaging differentiation of benign and malignant peripheral nerve sheath tumors: use of the target sign. Pediatr Radiol 1997; 27(02):124–129 [DOI] [PubMed] [Google Scholar]
- 32.Broski SM, Johnson GB, Howe BM, et al. Evaluation of (18)F-FDG PET and MRI in differentiating benign and malignant peripheral nerve sheath tumors. Skeletal Radiol 2016;45(08):1097–1105 [DOI] [PubMed] [Google Scholar]
- 33.Warbey VS,Ferner RE,Dunn JT,Calonje E,O'Doherty MJ. [18F]FDG PET/CT in the diagnosis of malignant peripheral nerve sheath tumours in neurofibromatosis type-1. Eur J Nucl Med Mol Imaging 2009;36(05):751–757 [DOI] [PubMed] [Google Scholar]
- 34.Mollon B, Lee A, Busse JW, et al. The effect of surgical synovectomy and radiotherapy on the rate of recurrence of pigmented villo- nodular synovitis of the knee: an individual patient meta-analysis. Bone Joint J 2015;97-B(04):550–557 [DOI] [PubMed] [Google Scholar]
- 35.Aurégan JC, Klouche S, Bohu Y, Lèfevre N, Herman S, Hardy P. Treatment of pigmented villonodular synovitis of the knee. Arthroscopy 2014;30(10):1327–1341 [DOI] [PubMed] [Google Scholar]
- 36.Ravi V, Wang WL, Lewis VO. Treatment of tenosynovial giant cell tumor and pigmented villonodular synovitis. Curr Opin Oncol 2011; 23(04):361–366 [DOI] [PubMed] [Google Scholar]
- 37.Gu HF, Zhang SJ, Zhao C, Chen Y, Bi Q. A comparison of open and arthroscopic surgery for treatment of diffuse pigmented villo- nodular synovitis of the knee. Knee Surg Sports Traumatol Arthrosc 2014;22(11):2830–2836 [DOI] [PubMed] [Google Scholar]
- 38.Colman MW, Ye J, Weiss KR, Goodman MA, McGough RL III. Does combined open and arthroscopic synovectomy for diffuse PVNS of the knee improve recurrence rates? Clin Orthop Relat Res 2013; 471(03):883–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atilgan HI, Sadic M, Ozsoy A, et al. Evaluation of patients with diffuse pigmented villonodular synovitis of knee with functional scoring systems after surgery and radiosynovectomy. Nucl Med Biomed Imaging 2016. Doi: 10.15761/NMBI.1000107 [DOI] [Google Scholar]
- 40.Dorwart RH, Genant HK, Johnston WH, Morris JM. Pigmented villonodular synovitis of synovial joints: clinical, pathologic, and radiologic features. Am J Roentgenol 1984;143(04):877–885 [DOI] [PubMed] [Google Scholar]
- 41.Murphey MD, Rhee JH, Lewis RB, Fanburg-Smith JC, Flemming DJ, Walker EA. Pigmented villonodular synovitis: radiologic-pathologic correlation. Radiographics 2008;28(05):1493–1518 [DOI] [PubMed] [Google Scholar]
- 42.Jelinek JS, Kransdorf MJ, Utz JA, et al. Imaging of pigmented villonodular synovitis with emphasis on MR imaging. Am J Roentgenol 1989;152(02):337–342 [DOI] [PubMed] [Google Scholar]
- 43.Bitar R, Leung G, Perng R, et al. MR pulse sequences: what every radiologist wants to know but is afraid to ask. Radiographics 2006;26(02):513–537 [DOI] [PubMed] [Google Scholar]
- 44.Eilber FC, Dry SM. Diagnosis and management of synovial sarcoma. J Surg Oncol 2008;97(04):314–320 [DOI] [PubMed] [Google Scholar]
- 45.Gresswell SD, Corsini AA, Balsamo LH, Miles EF. Intra-articular synovial sarcoma treated with a transfemoral amputation: a case report and review of the literature. Mil Med 2013;178(08): e956–e962 [DOI] [PubMed] [Google Scholar]
- 46.Nordemar D, Oberg J, Brosjo O, Skorpil M. Intra-articular synovial sarcomas: incidence and differentiating features from localized pigmented villonodular synovitis. Sarcoma 2015;2015:903873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jamshidi K, Yahyazadeh H, Bagherifard A. Unusual presentation of synovial sarcoma as meniscal cyst: a case report. Arch Bone Jt Surg 2015;3(04):296–299 [PMC free article] [PubMed] [Google Scholar]
- 48.El Beaino M, Araujo DM, Lazar AJ, Lin PP. Synovial sarcoma: advances in diagnosis and treatment identification of new biologic targets to improve multimodal therapy. Ann Surg Oncol 2017;24(08):2145–2154 [DOI] [PubMed] [Google Scholar]
- 49.Bakri A, Shinagare AB, Krajewski KM, et al. Synovial sarcoma: imaging features of common and uncommon primary sites, metastatic patterns, and treatment response. Am J Roentgenol 2012;199(02):W208–15 [DOI] [PubMed] [Google Scholar]
- 50.Murphey MD, Gibson MS, Jennings BT, Crespo-Rodríguez AM, Fanburg-Smith J, Gajewski DA. From the archives of the AFIP: imaging of synovial sarcoma with radiologic-pathologic correlation. Radiographics 2006;26(05):1543–1565 [DOI] [PubMed] [Google Scholar]
- 51.Emory CL. CORR Insights®: Developing an Evidence-Based Fol- lowup Schedule for Bone Sarcomas Based on Local Recurrence and Metastatic Progression. Clin Orthop Relat Res 2017;475(03): 839–841 [DOI] [PMC free article] [PubMed] [Google Scholar]


