Summary:
Intraductal papillary mucinous neoplasm (IPMN) is a pancreatic cancer precursor lesion with established genetic features, but the cellular ecosystem of these tumors remains to be fully characterized. This study utilizes single cell RNA-seq to describe the dynamic landscape of epithelial, immune, and stromal cells during IPMN progression to invasive cancer.
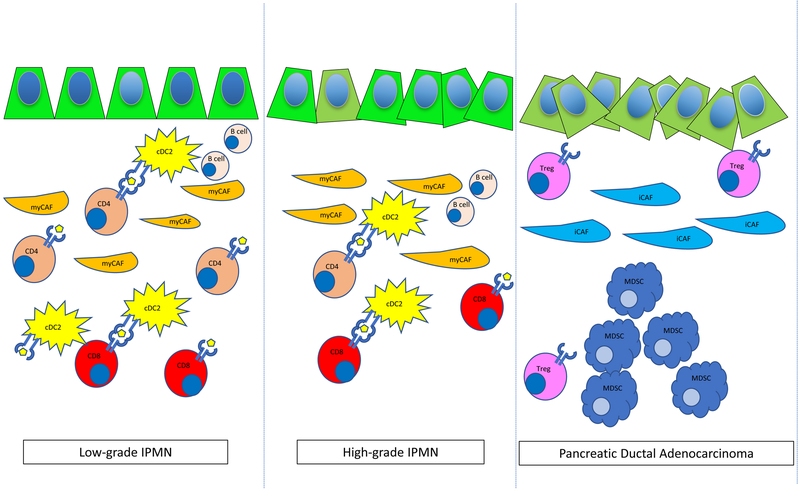
In this issue of Clinical Cancer Research, Bernard and colleagues [1] performed single cell transcriptomic analysis of low-grade, high-grade and pancreatic ductal adenocarcinoma (PDAC) subtypes and reported the heterogeneity of the epithelial, immune, and stromal cells of intraductal papillary mucinous cystic neoplasms (IPMNs), a precursor to PDAC. In their study, they analyzed 5,403 single cells from 6 patients, 2 with low grade dysplasia (LGD-IPMN), 2 with high grade dysplasia (HGD-IPMN) and 2 with invasive PDAC. Previous single cell RNA-seq work in the autochthonous pancreatic mouse model had demonstrated heterogeneity in tumor cells in advanced disease [2], and the current work now expands our understanding of the evolution of human PDAC heterogeneity during malignant progression of IPMN to invasive cancer. Figure 1 summarizes the changes in cell types from IPMN to PDAC. The authors find that there is loss of expression of tumor suppressor genes, including RAP1GAP, between LGD-IPMN compared to HGD-IPMN and PDAC. Core signaling pathways upregulated in HGD-IPMN included those involving integrins, small GTPases, Wnt/B-catenin, axonal guidance, apoptosis and G1/S phase regulation. PDAC lesions had additional enrichment of genes involved with the DNA damage response, TGF-B signaling and SAPK/JNK signaling. Comparison of LGD-IPMN, HGD-IPMN, and PDAC epithelial cells revealed a relative enrichment of G2/M and S phase proliferative cells with more advanced disease. Notably, there was a subset of LGD-IPMN epithelial cells which demonstrated increased proliferative markers that may be associated with increased risk of malignant progression, however, the clinical relevance of this finding remains to be seen in a larger independent cohort of IPMN samples.
Figure 1:
Diagram of single cells found in low-grade dysplasia IPMN, high-grade dysplasia IPMN and PDAC. Activated CD4+ and CD8+ T cells, cDC2 dendritic cells, and rare CD19+ CD20+ B cells are found in LGD-IPMN and HGD-IPMN, with transition to Tregs and MDSCs enriched in PDAC. Myofibroblasts (myCAFs) are found in LGD-IPMN and HGD-IPMN with transition to inflammatory CAFs (iCAFs) cells during malignant progression.
In addition to the heterogeneity seen in cancer cells, there is also great interest in understanding the associated immune response seen in PDAC development. The most common PDAC precursor lesion is pancreatic intraepithelial neoplasia (PanIN), which is characterized by an immunosuppressive environment with the most plentiful immune cells being myeloid derived suppressor cells (MDSCs), T regulatory (Treg) cells, tumor associated macrophages (TAMs), and γ-T cells [3]. Jang et al. have also demonstrated that Tregs promote tumor growth in pancreatic cancer with an associated lack of cytotoxic CD8+ T cell activation through direct interaction with CD11c+ dendritic cells [4]. Altogether, these features are believed to preclude efficacy of immune checkpoint blockade. Although work has been done on understanding the immune response in PanIN to PDAC progression, there has been relatively little work done in understanding these features in IPMN to PDAC. Here, the authors have identified 7 unique clusters of stromal cells, 5 immune subpopulations and 2 fibroblast populations. Unlike the PanIN-PDAC sequence, which lose CD8+ T cells at early stages, IPMNs have a high proportion of cytotoxic CD8+ T cells in LGD-IPMNs when compared to more advanced lesions. Interestingly, MDSC are virtually absent from LGD-IPMN and HGD-IPMN, though they are present in PDAC as has been previously reported. LGD-IPMN also have activated CD4+ T cells which persist through the HGD stage. Furthermore, IPMNs show a unique subclass of myeloid cells — DC2 type dendritic cells, which are characterized by CD1c, THBD and FCER1a, and are present in both LGD-IPMN and HGD-IPMN. The presence of cytotoxic CD8+ T cells, activated CD4+ T cells and dendritic cells in the microenvironment of IPMNs is clearly distinct from that of PanIN lesions. Accordingly, harnessing this knowledge may provide a unique opportunity for therapeutic interventions for a subset of patients. Most significantly, the profile of immune cells in IPMN suggest that this subset of pancreatic cancer precursor lesions may be responsive to immunotherapy or vaccine therapy as a chemoprevention strategy.
Finally, the dense desmoplastic stromal response indicative of pancreatic cancer has recently been demonstrated to be heterogeneous between tumors. Previous work by Ohlund and colleagues have described two populations of cancer associated fibroblasts (CAFs) in pancreatic cancer [5]: myofibroblasts (myCAFs) that are characterized as having high expression of smooth muscle actin (SMA) and low expression of IL-6; and inflammatory CAFs (iCAFs) that are characterized by low expression of SMA and high expression of IL-6. RNA sequencing analysis demonstrated that these CAFs have distinct transcriptional profiles with Acta2, Ctgf and Col1a1 upregulated in myCAFs and Il6, Il11, Lif, Cxcl1 and Cxcl2 upregulated in iCAFs. Of the 7 unique stromal clusters identified, Bernard and colleagues found a population of myCAFs and iCAFs. The myCAFs were clustered to LGD and HGD lesions and were characterized by reduced expression of CXCL12, ACTA2, and COL3A1, whereas iCAFs were exclusively found in the PDAC subset and were characterized by upregulation of FAP, ACTA2, COL3A1, and CXCL12, which is associated with metastatic spread in many solid cancers. Their findings suggest that similar to pancreatic cancer, iCAFs have a pro-tumorigenic role in advanced lesions.
This study characterizes the single cell phenotypes of epithelial, immune, and stromal compartments in IPMN, an important pancreatic cancer precursor lesion which accounts for ~10% of all PDAC. Prior work showed that the stroma has a complex role in PDAC pathogenesis, with the diversity of fibroblastic and immune cells having both tumor promoting and inhibiting effects depending on their activation state and the stage of tumorigenesis. In advanced tumors the stroma contributes to the resistance of PDAC to conventional chemotherapy and immunotherapy. In this study, Bernard and his group have classified a unique immune infiltrate that is not characteristic of classical PDAC. Their findings open the possibility for targeting dendritic and T cell cross talk, which has previously not been possible in other PDAC precursors. While one limitation of this study is the generalizability of these findings given the small number of samples analyzed, it provides an important starting point for studies focused on the understanding the molecular features of IPMN. Future validation studies in additional human IPMN and PDAC tumors combined with new genetically engineered mouse models of IPMN will certainly build upon this work to understand the factors that are involved in the progression of the different histological subtypes of IPMNs to PDAC. Moreover, the application of RNA and protein based in situ based methods to determine the spatial context of these cell types in IPMN and PDAC will likely uncover new relationships of epithelial, immune, and stromal cells that cannot be resolved with single cell RNA-seq. In summary, this study carries great impact in the field as it establishes that there are marked differences in the tumor microenvironment between pancreatic cancer precursor lesions, and it points to the potential of using immunotherapeutic approaches to target IPMN.
Acknowledgments
Funding: This work is supported by the NIH T32DK007191–43S1 to Y.G. H-B; the Granara-Skerry Trust, the Begg Family, and grants from the NIH (P01 CA117969, R01 CA215498–01) to N.B.; SU2C-Lustgarten 2015–002, the NSF PHY-1549535, the Warshaw Institute for Pancreatic Cancer to D.T.T.; and the Linda J. Verville Foundation to N.B. and D.T.T.
Footnotes
Conflicts of Interest and Disclosures:
Y. G. H-B and N.B. have no conflicts to disclose.
D.T.T. receives sponsored research support from ACD-Biotechne. D.T.T. has performed consulting for Millipore-Sigma, Ventana-Roche, and Merrimack Pharmaceuticals. D.T.T. is a founder and has equity in PanTher Therapeutics. None of the above relationships is believed to be in conflict with this work.
References:
- 1).Bernard V, Semaan A, Huang J, Anthony San Lucas F, Mulu FC, Stephens BM, et al. Single Cell Transcriptomics of Pancreatic Cancer Precursors Demonstrates Epithelial and Microenvironmental Heterogeneity as an Early Event in Neoplastic Progression. Clin Cancer Res. 2018; November 1 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Ting DT, Wittner BS, Ligorio M, Jordan NV, Shah AM, Miyamoto DT, et al. Single-Cell RNA Sequencing Identifies Extracellular Matrix Gene Expression by Pancreatic Circulating Tumor Cells. Cell Rep. 2014; 8: 1905–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Daley D, Zambirinis CP, Seifert L, Akkad N, Mohan N, Werba G, et al. γδ T cells Support Pancreatic Oncogenesis by Restraining αβ T cell activation. Cell. 2016; 166: 1485–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Jang JE, Hajdu CH, Liot C, Miller G, Dustin ML, Bar-Sagi D. Crosstalk between Regulatory T Cells and Tumor-Associated Dendritic Cells Negates Anti-tumor Immunity in Pancreatic Cancer. Cell Rep; 20: 558–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Ohlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017. 214: 579–596. [DOI] [PMC free article] [PubMed] [Google Scholar]