Abstract
The introduction of HER2-targeted therapy for breast and gastric patients with ERBB2(HER2) amplification/overexpression has led to dramatic improvements in oncologic outcomes. In the past 20 years, five HER2-targeted therapies have been FDA approved, four in the past 8 years. HER2-targeted therapy similarly was found to improve outcomes in HER2-positive gastric cancer. Over the past decade, with the introduction of next generation sequencing into clinical practice, our understanding of HER2 biology has dramatically improved. We have recognized that HER2 amplification is not limited to breast and gastric cancer, but is also found in a variety of tumor types such as colon cancer, bladder cancer, and biliary cancer. Further, HER2-targeted therapy has signal of activity in several tumor types. In addition to HER2 amplification and overexpression, there is also increased recognition of activating HER2 mutations and their potential therapeutic relevance. Further, there is a rapidly growing number of new therapeutics targeting HER2 including small molecule inhibitors, antibody-drug conjugates and bispecific antibodies. Taken together, an increasing number of patients are likely to benefit from approved and emerging HER2-targeted therapies.
Keywords: HER2, ERBB2, precision oncology, pertuzumab, trastuzumab
Introduction:
HER2 (ERBB2) is emerging as a promising target for genomically-informed therapy across a variety of tumor types. For HER2, gene amplification (increased copy number) is by far the most common genomic alteration and is generally, although not always, associated with protein overexpression (1–3). HER2 overexpression drives tumorigenesis through the creation of spontaneous receptor homodimers (4,5), or heterodimers with other ERBB family members (6) resulting in activated oncogenic downstream signaling, such as PI3K/Akt/mTOR and MAPK, promoting cellular proliferation, survival and angiogenesis (6–8). (6–8). In particular, HER2-HER3 heterodimers transduce PI3K signaling via direct binding between HER3 and the p85 subunit of PI3K (9). Spontaneous formation of these heterodimers increases with amplification of the HER2 gene (10).
Algorithms for HER2 classification have been evolving. For example, for breast cancer, 3+ HER2 protein overexpression by immunohistochemistry, or HER2 amplification assessed by in situ hybridization (ISH) have been considered HER2-positive, and detailed guidelines for interpretation (11) have been developed by the American Society of Clinical Oncology and College of American Pathologists and are regularly updated.
Approved Indications for HER2 targeted Therapy
HER2-targeted therapy has transformed outcomes for HER2-amplified/overexpressing (HER2-positive) breast and gastric/gastroesophageal cancer. Several therapies are approved for HER2-positive breast cancer in the adjuvant and metastatic setting: trastuzumab (metastatic and adjuvant), pertuzumab (metastatic and adjuvant), lapatinib (metastatic), ado-trastuzumab emtansine (metastatic), and neratinib (adjuvant). Trastuzumab is also approved for HER2-positive metastatic gastric/gastroesophageal junction cancers, in combination with cisplatin and a fluoropyrimidine (capecitabine or 5-fluorouracil). Further, Trastuzumab-dkst (Ogivri), a trastuzumab biosimilar, was approved for all indications included in the label of trastuzumab. FDA approved indications are detailed in Supplementary Table 1.
Targeting HER2 Overexpression/Amplification Beyond Breast and Gastric cancer with Agents Approved For Breast/Gastric Cancer
With increased genomic profiling of many types of tumor, there is growing recognition that HER2 amplification occurs in in several tumor types including salivary(3.9%), vaginal(3.6%), bladder(3.6%), endometrial(3.4%), cervical(2.2%), and colorectal cancer (CRC; 1.3%) (Figure 1A) (12).
Figure 1. HER2 alterations across tumor types.
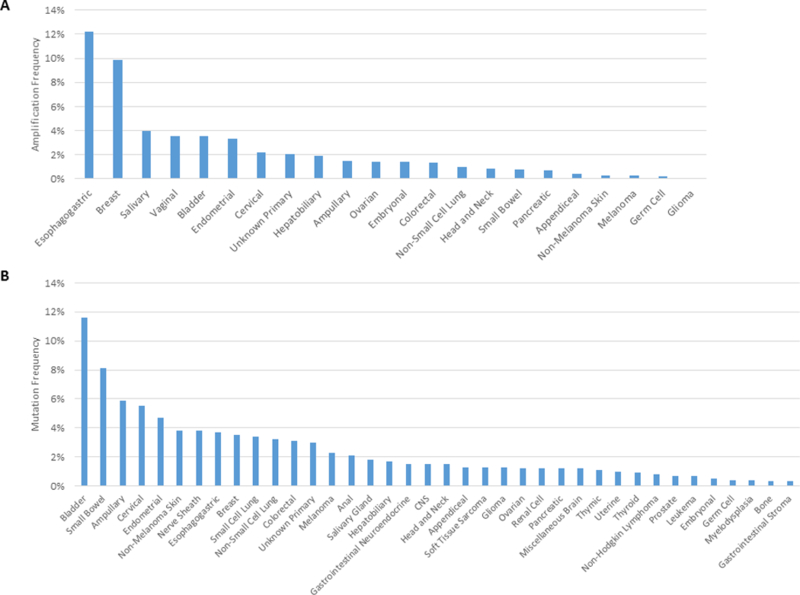
A.Prevalence of HER2 amplifications across diverse cancer types in a cohort of 37,436 sequenced cases extracted from AACR Project GENIE (version 3.0.0, accessed on 16th July 2018) (12).. B. Prevalence of HER2 mutations across diverse cancer types (version 3.0.0, accessed on 16th July 2018).
The efficacy of pertuzumab and trastuzumab was tested for HER2-positive tumors in the MY PATHWAY basket trial (13). Thirty of 114 patients (26%; 95% CI, 19% to 35%) with HER2 amplification/overexpression had objective responses (two CR, 28 PR). Responses were seen in nine tumor types: CRC (38% [14/37] objective response rate, ORR; 95% confidence interval [CI]:23–55%), bladder (33% ORR[3/9]; 95%CI:8–70), biliary/gallbladder (29% ORR[2/7]; 95%CI:4–71), salivary gland (80% ORR[4/5];95%CI:28->99), NSCLC (13% ORR[2/16]; 95%CI:2–38), pancreas (22% ORR[2/9]; 95%CI:3–60), ovary (13% ORR[1/8]; 95%CI:0–53), as well as one patient each with prostate, and skin cancer (apocrine). Further data on efficacy of pertuzumab and trastuzumab is expected from the MY PATHWAY trial as well as the ASCO TAPUR and NCI-MATCH trials; all three trials are being conducted in treatment-refractory patients.
Supporting the efficacy signal with HER2-targeted therapy seen in CRC in the MY PATHWAY trial, several lines of evidence point to the importance of HER2 in CRC biology. Bertotti et al. used a patient-derived xenograft(PDX) platform to identify genotype-response correlations with cetuximab, and found HER2 amplification in cetuximab-resistant, KRAS/NRAS/BRAF/PIK3CA wild-type (WT) PDXs (14). HER2 amplification was also enriched in clinically nonresponsive KRAS WT patients. Further, Raghav et al. reported that HER2 amplification is associated with resistance to anti-EGFR therapy (cetuximab/panitumumab) and shorter progression-free survival (PFS)(15). Taken together, this data suggests that HER2 amplification may not only be a potential target, but also a resistance marker for EGFR inhibitors.
The role of HER2 as a target for metastatic CRC was also assessed in the HERACLES trial, which enrolled patients with KRAS exon 2 WT patients that were HER2-positive as defined as HER2 3+ overexpression in over 50% of tumor cells by IHC or 2+ IHC and a HER2/CEP17 ratio greater than 2 in more than 50% of cells by FISH (16). Eight(30%) of 27 patients treated with dual-targeted therapy, trastuzumab and lapatinib, achieved an objective response, (one complete response, seven partial responses). Together, the HERACLES and MY PATHWAY studies demonstrate that HER2-targeted therapy is effective in CRC. An ongoing Phase II trial, the SWOG S1613 study (NCT03365882), is comparing the efficacy of trastuzumab and pertuzumab to cetuximab and irinotecan. The trial is accruing patients with metastatic or locally advanced, unresectable CRC who have not received prior epidermal growth factor or HER2 inhibitors and have HER2-amplified tumors that are KRAS/NRAS/BRAF WT. Further study is needed to determine the optimal therapeutic regimens and treatment sequencing.
Recently two multihistology basket trials using ado-trastuzumab emtansine(TDM1) for HER2-amplified disease were also reported. Li et al. reported a trial conducted at Memorial Sloan Kettering Cancer Center (MSKCC) that demonstrated an ORR of 28%, with responses in lung cancer(43% ORR), endometrial cancer(25% ORR), salivary cancer(100% ORR), as well as in ovarian cancer and biliary cancer, but not in CRC(17). In the NCI-MATCH trial, ORR was 8%, with responses seen in 2 of 3 salivary cancers (18). This data raises some interesting points. Although the differences in ORR may simply be due to small study size, there appear to be important differences in ORR between the two trials with similar study designs, using the same drug. It will be important to review the differences in patient populations when details are available. In NCI-MATCH, 33% of patients had received >3 lines of prior therapy, thus it is possible that NCI-MATCH patients were more heavily treated, limiting therapeutic efficacy. In contrast, it is likely that in the MSKCC series, patients were offered genomic testing, and genomically-matched therapy earlier in their disease course. If so, the differences in ORR would support earlier genomic testing to allow for greater benefit of genomically-informed therapy. Another interesting finding was that in the MKSCC series there was efficacy in several tumor types, but there was also variability in sensitivity by tumor type, with no responses observed in CRC. This highlights that for antibody drug conjugates, in addition to expression of the marker, sensitivity to the specific conjugate needs to be taken into consideration.
HER2 Mutations
Somatic mutations can also drive HER2 signaling. While activating mutations have been best characterized within breast and lung cancers(19–21), mutations are reported in a variety of other tumor types(22) . A query of the 214 tumor-based (non-cell line) studies within the cBIOPortal reveals a HER2 mutation frequency (mutations or fusions) of 2.7%(23) Figure 1B demonstrates the frequency of mutations across tumor types in the American Association for Cancer Research (AACR) GENIE dataset: tumor types with the highest frequency of mutations in HER2 include bladder(11.6%), small bowel(8.1%), and ampullary(5.9%) (12).
However, not all HER2 mutations are activating. Most of the known activating HER2 mutations are shown in Figure 2 and detailed in Supplementary Table 2. While mutations have been reported across the entire gene, they primarily localize within two regions: the extracellular domain (ECD) and the kinase domain (KD)(19,24). Within the KD, both missense mutations and in-frame insertions lead to increased kinase activity and promote tumorigenesis(19,25,26). Activating mutations have also been reported in the transmembrane domain(27,28), with enhanced protein stabilization being one proposed mechanism for gain-of-function(28).
Figure 2. Activating HER2 mutations.

Mutations defined as ‘activating’ based on published data demonstrating that the alteration increases the activity, expression, or stability of the encoded protein. Additionally, alterations shown to enhance downstream signaling or increase tumorigenic properties when expressed are shown (56). Mutation Mapper from cBIO portal was used to visualize mutations (57,58).
Types of mutations vary with tumor type. In non-small cell lung cancers(NSCLC), mutations are most frequent in patients that are Asian, female, never-smokers, and in adenocarcinomas(29–31). Mutations are generally exon 20 in-frame insertions within the KD(20,21,29,32). These insertions/duplications occur at the same codons identified in EGFR, indicative of a similar mechanism of activation(29). Indeed, functional studies have shown that HER2YVMA insertion/duplications increase auto-catalytic activity, leading to increased auto-phosphorylation and phosphorylation of its dimerization partners, and increased survival, proliferation, and tumorigenesis(25,26). In NSCLC, HER2 mutations are mutually exclusive with activating EGFR and KRAS mutations(21,29,30).
In breast cancer, primarily missense mutations are detected. A meta-analysis of 12,905 breast cancer patients published across 31 papers revealed a mutation frequency of 2.7%(33). Greater than 50% of mutations were located in the ECD(S310/Y) or the KD(33). The most frequently reported mutations in the KD include L755S, V777L, D769H/Y, and L755_T759del(33,34). While these and other HER2 mutations activate downstream signaling and/or increase colony formation(19), not all are tumor-promoting in vivo. The V777L, D769H, and G309A mutants induced tumor growth in xenograft models; however, the L755S, V842I, and R678Q mutants performed similar to WT, and the L755_T759del tumors grew slower than WT(19). Moreover, a study investigating the effects of HER2 mutants expressed at endogenous levels found none induced tumor formation in vivo(35). However, HER2 mutations may cooperate with other oncogenic events(e.g. PIK3CA activation).
Although rare, HER2 gene fusions have also been reported. Within the TCGA PanCancer Atlas study, 1.7% of esophagus, 1.4% of breast, and 1.4% of cervical samples contained a HER2 fusion(23). Three novel fusions were reported in gastric cancer(36). Two (NOS-HER2 and ZNF207-HER2), were further characterized and found to induce auto-phosphorylation and cellular transformation(36). Thus, fusions are another potential therapeutic target.
Targeting HER2 Mutations
Neratinib
Bose et al. reported that many activating HER2 mutations are resistant to HER2 inhibitor lapatinib, but sensitive to ireversible inhibitor neratinib (19). Ma et al. evaluated efficacy of neratinib in HER2-mutant, nonamplified breast cancer (37). The clinical benefit ratio (CBR) was 31%. Upon longitudinal circulating free DNA analysis, the mutant HER2 allele decreased with treatment and increased with progression in 9 of 11 patients.
The efficacy of neratinib in HER2-mutant tumors was also tested in the SUMMIT trial(38). Neratinib demonstrated the greatest activity in breast cancer patients(ORR at 8 weeks 32%), all with HER2-nonamplifiedtumors. Responses were observed in both ER+ and ER- tumors and in patients with mutations in the ECD,KD as well as KD insertions. In lung cancer, only one objective response (in a patient with L755S mutation) was observed among the 26 patients enrolled; but progression free-survival was 5.5 months. Responses were also observed in biliary tumors and cervical cancer, while none were seen among the 16 bladder cancer and 12 CRC patients enrolled, suggesting that histology may impact neratinib’s efficacy.
Pertuzumab/trastuzumab
Although preclinical data have suggested that monoclonal antibodies may not be as effective as irreversible kinase inhibitors for HER2 mutations, there have been anectodal reports of responses with trastuzumab-based therapy. In the MYPATHWAY trial, among the initial 36 patients who received trastuzumab plus pertuzumab for tumors with HER2 mutations without amplification/overexpression; four patients (11%) had objective responses: three patients (out of 14) with NSCLC and one with biliary cancer (13). The efficacy of pertuzumab/trastuzumab for HER2 mutations is also being explored in the ASCO TAPUR trial.
TDM1
Li et al. tested TDM1’s efficacy in HER2 mutant lung tumors (39). In patients with HER2-nonamplified lung cancer with HER2 mutations, the ORR was 33%. Scientifically this result is somewhat surprising, but the efficacy, at least in part, is being attributed to HER2 internalization with ADC treatment.
Mechanisms of Intrinsic and Acquired Resistance to HER2-targeted therapy and Rational Combinations to Overcome Resistance
There is increasing understanding of mechanisms of intrinsic and acquired resistance to HER2-targeted therapy (Table 1). Much of this data has emerged from breast cancer studies so more study is needed to determine if they are extrapolatable to other tumor types and whether additional mechanisms of resistance are identified in other histologies. Intratumoral HER2 heterogeneity (some cells having HER2 overexpression/amplification and others not) as well as genomic evolution with loss of HER2 amplification are concerns that support the use of pre-treatment biopsies for confirmation of HER2 status, and exploration of the role of liquid biopsies and functional imaging with HER2-PET for assessment of HER2 status.
Table 1.
Mechanisms of intrinsic and acquired resistance for HER2 targeted therapy
| HER2 Targeted Therapy | Mechanisms of Resistance | Potential Strategy to Overcome Resistance |
|---|---|---|
| Trastuzumab/pertuzumab/T-DM1 (59–61) | Intrinsic/Acquired: Co-expression of EGFR or HER3 | Dual inhibition of EGFR and HER2 |
| Intrinsic/Acquired: Overexpression of IGF1R | Treatment with mTOR/IGF1R inhibitors, inhibition of HER2 kinase activity | |
| Intrinsic/Acquired: Overexpression of MET or HGF | MET inhibitors, MET ADC | |
| Intrinsic/Acquired: Overexpression of EphA2 | Treatment with an Epha2 neutralizing antibody | |
| Intrinsic/Acquired: PIK3CA activation | Inhibition of PI3K or mTOR pathways | |
| Intrinsic/Acquired: PTEN loss | Inhibition of PI3K or mTOR pathways | |
| Intrinsic: High levels of cathecolamines in TM | Use of ß-blockers | |
| Intrinsic: Expression of estrogen receptor | Blockade of ER signaling | |
| Intrinsic: High p95 HER2 expression or high p95/HER2 ratio | Inhibition of HER2 kinase activity | |
| Intrinsic: D16 HER2 expression | Inhibition of Src kinase | |
| Intrinsic: HER2 exon 20 insertion | HER2 exon 20 inhibitors | |
| Intrinsic: Higher expression of PD-L1 | Combination with a checkpoint inhibitor | |
| Intrinsic: FcgRIII deficiency/polymorphism (deficiency in NK cells and macrophages capable of binding to Fc region of trastuzumab) | Inhibition of HER2 kinase activity | |
| Acquired: Overexpression of AXL | AXL inhibitor or AXL ADC | |
| Acquired: constitutive activation of Src kinase | Src kinase inhibitors | |
| Acquired: upregulation of survivin and Mcl-1 | Broad spectrum kinase inhibitors or Mcl1 inhibitors | |
| Acquired: upregulation of cyclin E | Treatment with CDK2 inhibitors | |
| Acquired: downregulation of p27KIP1 | Treatment with CDK2 inhibitors | |
| Acquired: downregulation of HER2 expression | Treatment HER2-targeted therapies with efficacy in lower HER2-expressing tumors | |
| T-DM1 (61) | Intrinsic: poor internalization of HER2-T-DM1 complexes/increased recycling/defective trafficking to lysosomes | |
| Acquired: upregulation of ABCC1 (MRP1) | Modification of linker | |
| Lapatinib/neratinib (59,60), (41) (42) | Acquired: Increase in HER3 transcription and phosphorylation | Inhibition of HER3, and HER3 ADC |
| Acquired: Overexpression of AXL | Combination with an AXL inhibitor or AXL ADC | |
| Acquired: increased activation of Src family kinase activity | Combination with Src inhibitors | |
| Acquired: Increased signaling through PI3K and AKT/ mTOR pathways | Inhibition of PI3K or mTOR pathways | |
| Intrinsic/Acquired: low/downregulation of BIM levels | ||
| Acquired: upregulation of ERα, leading to FoxO3a-mediated transcription of survivin | Treatment with ER antagonists | |
| Acquired: HER2 gatekeeper mutations (L755S, T862A, T798M for lapatinib; HER2 T798I for neratinib | Treatment with Afatinib |
In spite of years of study of mechanisms of sensitivity and resistance, HER2 aberrations remain the primary biomarker for patient selection. HER2 copy number, including HER2 copy number in cfDNA and HER2-enriched subtype by RNA-seq analysis(40) are associated with sensitivity to HER2-targeted therapy. However, there is evidence for intrinsic mechanisms of resistance including PI3K pathway alterations (mainly PTEN loss and PIK3CA mutations), and emerging data that activating receptor tyrosine kinases/RAS/RAF may limit the efficacy of anti-HER2 therapy(38), making these survival pathways appealing targets for combination therapy. Small molecule inhibitors of HER2, have been association with the emergence of gatekeeper HER2 mutations (L755S, T862A, T798M for lapatinib; HER2 T798I for neratinib) that may still be sensitive to alternate small molecule inhibitors (e.g. afatinib) (41,42).
Combination therapy trials have focused on several approaches: a) combinations of HER2-targeted therapies (doublets and triplets), b) combination with chemotherapy, c) overcoming ER and HER2 cross-talk via combination with endocrine therapy, d) combination with CDK4/6 inhibitors, and e) targeting intrinsic and acquired resistance mechanisms with cell signaling inhibitors such as PI3K/Akt/mTOR pathway inhibitors. A summary of targeted therapies combined with HER2-targeted agents are listed in Supplementary Table 3.
Novel Her2-targeted Therapies
Antibody Drug Conjugates (ADC)
DS-8201a is a novel anti-HER2 ADC, with a derivative of DX-8951(DXd), a topoisomerase I inhibitor(43) with a drug to antibody ratio (DAR) of approximately 8. Pre-clinically, DS-8201a was effective in a T-DM1-insensitive HER2-positive PDX model. DS-8201a, but not T-DM1, also demonstrated efficacy against HER2-low breast cancer models. In 2017, FDA granted DS-8201 breakthrough designation for HER2-positive breast cancer. DS-8201a demonstrated remarkable activity in heavily pretreated patients, with a confirmed ORR of 54.5%(54/99) and disease control rate(DCR) of 93.9% (93/99) and with preliminary signal of activity in HER2-low tumors: ORR 50%(17/34) and DCR 85.3% (29/34)(44). However, cases of fatal pneumonitis have also been reported(44), highlighting that greater efficacy in HER2-low cells may have a safety trade-off. Studies are ongoing to better define the efficacy as well as safety of DS-8201a.
Additional HER2 ADCs are in development, varying in antibody and linker-payload. The use of antibodies with greater affinity for HER2 or ADCs with higher DAR may overcome resistance due to decreased HER2 expression. Use of toxins with greater bystander effect may help overcome resistance due to tumor heterogeneity and may prove to be effective even in HER2-low cancers, but therapeutic window will need to be assessed.
Bispecific Antibodies
ZW25 is biparatropic antibody that simultaneously binds two HER2 epitopes, extracellular domain 4 (the trastuzumab binding domain) and extracellular domain 2 (the pertuzumab binding domain). Pre-clinically, its unique binding facilitates increased tumor cell binding, ZW25-HER2 clustering and enhanced internalization (including in the setting of lower HER2 concentrations). In a Phase I trial, ZW25 led to objective responses in heavily pretreated patients with HER2-amplified/overexpressing breast cancer (33% ORR), gastroesophegeal cancer (44% ORR) as well as other HER2- amplified/overexpressing tumor types (33% ORR), including CRC and gallbladder cancer (45). MCLA-128 targets both HER2 and HER3, enhancing antibody-dependent cell mediated cytotoxicity, with clinical benefit rate of 70% in heavily pretreated patients (46). Other bispecific antibodies of special interest include antibodies that also directly engage immune mediators discussed below.
Small molecule inhibitors
Several new HER2 tyrosine kinase inhibitors are in development, including tucatinib, poziotinib and pyrotinib. Tyrosine kinase inhibitors are being explored in multiple tumor types and for tumors with HER2-amplified/overexpression as well as activating mutations. Tucatinib was granted fast track designation by the FDA in 2016 for treatment of HER2-positive metastatic breast cancer and orphan drug status in 2017 for treatment of HER2-positive CNS metastasis. Tucatinib as a single agent, as well as in combination with standard-of-care therapies, demonstrated significant growth inhibition in HER2-positive xenografts, including breast cancer models(47,48). Tucatinib also significantly enhanced survival in intracranial tumor xenograft models(49). In the Phase I trial, tucatinib monotherapy had activity in heavily pretreated patients: ORR was 14% and the clinical benefit rate (CBR, PR + stable disease ≥ 24 weeks) was 27% (50). Efficacy of tucatinib has also been tested in combination studies, for example, in combination with TDM1, 68% patients treated with the maximum tolerated dosage had ORR of 47% and CBR was 58% (51). Further, it has been explored in combination with capecitabine (ORR 83%), trastuzumab (ORR 40%), and with trastuzumab and capecitabine (ORR 61%) (52). In both phase 1b combination studies (NCT01983501 and NCT02025192), clinical benefit has been noted in patients with CNS disease at similar rates compared to those without CNS disease and patients were allowed to continue on study with isolated CNS progression following local directed therapy which allowed patients to remain on study longer(52). Validation of overall efficacy and further evaluation of the latter approach will be determined in the ongoing pivotal double-blinded randomized registration study, HER2CLIMB(NCT02614794), which is enrolling patients with and without CNS metastases. Tucatinib is also being evaluated in combination with trastuzumab in HER2-positive, RAS WT CRC.
Poziotinib is a small molecule irreversible inhibitor of EGFR, HER2 and HER4. Poziotinib has shown clinical activity in HER2-positive breast cancer and is being developed in combination therapy. Robichaux et al. reported that poziotinib is a potent inhibitor of exon 20 mutations in EGFR and HER2, pre-clinically. This is notable as exon 20 mutations have been intrinsically resistant to approved targeted therapies(53). Preliminary data from a Phase II trial demonstrated ORR of 64% in lung cancer patients with exon 20 EGFR mutations. Clinical activity was also reported in a lung cancer patient with exon 20 HER2 mutation, and this efficacy for HER2 mutations is being better defined in ongoing trials. Several other small molecule inhibitors are in development, some with proposed greater selectivity for HER2, or for selected HER2 mutations (e.g., exon 20) (Figure 3).
Figure 3. Approved and emerging HER2 targeted therapies in clinical development.

HER2 targeted therapies approved by the United States Food and Drug Administration (FDA) for HER2-positive cancer or currently in clinical trials (from www.clinicaltrials.gov, last accessed July 11th 2018). “FDA” = the drug is approved by the FDA.
DARPins
MP0274 is a proprietary, designed ankyrin repeat protein (DARPin)-based agent targeting HER2. MP0274 binds to two distinct non-overlapping epitopes on HER2, inhibiting the activity of HER2 and promoting internalization. MP0274 is now in Phase I clinical trials.
HER2-targeted immunotherapy
PANACEA, the first Phase Ib/II trial evaluating the antitumor efficacy of immunotherapy in combination with HER2-targeted therapy (pembrolizumab and trastuzumab), reported a ORR of 15% and DCR of 25% in PDL1-positive patients, and no responses in PDL1-negative patients (54). Although there was modest benefit in the PDL1+ cohort, the disease control in those who responded was durable for one year without chemotherapy, which is notable. Heavily pretreated metastatic breast tumors are thought to be poorly immunogenic and there is much interest in assessing the association between immune function and benefit from trastuzumab in earlier settings. Many other HER2-immunotherapy combinations are ongoing (Supplementary Table 3). There is also great interest in combinations ofHER2 ADCs and immunotherapy, as ADCs elicit immune responses and have enhanced efficacy in combination with checkpoint inhibitors pre-clinically(44). In addition, bispecifics are being explored targeting HER2 and immune components. For example, GBR1302 is proposed to direct HER2 and CD3 redirecting cytotoxic T cells onto HER2+ cells, while PRS-343 increases tumor lymphocyte infiltration via bispecific targeting of 41BB(CD137) and HER2. In addition, HER2 vaccines are in clinical trials and CAR-T approaches are still being explored(55).
Conclusions
Outside of breast and gastric cancer, in what diseases, and in what clinical setting HER2 testing for amplification/overexpression should be initiated remains controversial. However emerging data suggests efficacy of HER2-targeted therapy for HER2 amplified/overexpressing tumors across a variety of tumor types. Thus, genomic testing or specifically HER2 testing by IHC and/or ISH should be considered for advanced/metastatic disease for tumor types where HER2 is known to be amplified (Figure 1A). HER2 also represents an important opportunity for seeking histology-agnostic approvals. It should be noted that most NGS platforms call HER2 amplification at 6–7 copies. Additional studies are needed to validate the efficacy of HER2-targeted therapy across tumor types and to determine whether efficacy can be extended to patients with lower levels of amplification detectable by ISH, and patients with overexpression of RNA or protein in the absence of amplification.
With increasing number of HER2-targeted therapies, we will likely be able to truly personalize therapy selection, offering targeted therapies with greatest expected efficacy based on mutation type and expression status, as well as expected adverse events. Newer therapies may allow us to offer HER2 targeted therapies to patients with lower HER2 expression, leading to a redefinition of “HER2-negative”. For HER2 mutations, evolving data will likely allow us to select optimal therapies individualizing therapy based on variant type.
For advanced disease, more efficacious therapy could improve outcomes. Additionally, transitioning new agents to early stage disease may allow us to offer targeted therapies alone, sparing patients the toxicity of chemotherapy. Finally, increasing the efficacy of neoadjuvant therapy in breast cancer may increase breast-conserving surgery and spare patients axillary lymph nodal dissections, and further strengthen the evolving paradigm of avoiding surgery altogether in exceptional responders. In conclusion, greater awareness of the HER2 status of patients can enhance incorporation of HER2-targeted therapy into the multidisciplinary care across tumor types.
Supplementary Material
Acknowledgments
Funding
This work was supported by The Cancer Prevention and Research Institute of Texas RP150535 (FMB, AJ, KB), Sheikh Khalifa Al Nahyan Ben Zayed Institute for Personalized Cancer Therapy (FMB), Nellie B. Connally Breast Cancer Research Endowment (FMB), and the MD Anderson Cancer Center Support grant (NIH/NCI P30 CA016672; FMB). The authors would like to also acknowledge the American Association for Cancer Research and its financial and material support in the development of the AACR Project GENIE registry, as well as members of the consortium for their commitment to data sharing. Interpretations are the responsibility of study authors.
Footnotes
Conflict of Interests:
Drs. Balaji, Dumbrava, Johnson, Raghav, and Michelle Bhatt have nothing to disclose.
Dr. Murthy reports research funding from Roche/Genentech, Cascadian Therapeutics, Pfizer/Alliance, Daiichi Sankyo, and EMD-Serono.
Dr. Piha-Paul reports research funding from GlaxoSmithKline, AbbVie, XuanZhu, Incyte, Principia, Pieris, Novartis, FivePrime, Pfizer, Puma, Helix Biopharma, Curis, Newlink Genetics, Medimmune, Medivation, Taiho, and Tesaro.
Dr. Rodon reports research funding from Bayer and Novartis as well as personal fees from Servier, Peptomyc, and Lilly.
Dr. Meric-Bernstam reports funding from Novartis, AstraZeneca, Taiho Pharmaceuticals, Genentech, Calithera Biosciences, Debiopharm Group, Bayer, Aileron Therapeutics, PUMA Biotechnology, CytomX Therapeutics, Zymeworks, Curis, Pfizer, eFFECTOR Therapeutics, and AbbVie as well as personal fees from Sumitomo Group, Dialectica, Genentech, Inflection Biosciences, Pieris Pharmaceuticals, Darwin Health, Samsung Bioepis, Spectrum Pharmaceuticals, OrigiMed, Xencor, and Aduro.
References
- 1.Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer 2004;5(1):63–9. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235(4785):177–82. [DOI] [PubMed] [Google Scholar]
- 3.Yaziji H, Goldstein LC, Barry TS, Werling R, Hwang H, Ellis GK, et al. HER-2 testing in breast cancer using parallel tissue-based methods. JAMA 2004;291(16):1972–7 doi 10.1001/jama.291.16.1972. [DOI] [PubMed] [Google Scholar]
- 4.Di Fiore PP, Pierce JH, Fleming TP, Hazan R, Ullrich A, King CR, et al. Overexpression of the human EGF receptor confers an EGF-dependent transformed phenotype to NIH 3T3 cells. Cell 1987;51(6):1063–70. [DOI] [PubMed] [Google Scholar]
- 5.Hendriks BS, Opresko LK, Wiley HS, Lauffenburger D. Quantitative analysis of HER2-mediated effects on HER2 and epidermal growth factor receptor endocytosis: distribution of homo- and heterodimers depends on relative HER2 levels. J Biol Chem 2003;278(26):23343–51 doi 10.1074/jbc.M300477200. [DOI] [PubMed] [Google Scholar]
- 6.Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 2007;26(45):6469–87 doi 10.1038/sj.onc.1210477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001;2(2):127–37 doi 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 8.Benlimame N, He Q, Jie S, Xiao D, Xu YJ, Loignon M, et al. FAK signaling is critical for ErbB-2/ErbB-3 receptor cooperation for oncogenic transformation and invasion. J Cell Biol 2005;171(3):505–16 doi 10.1083/jcb.200504124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF, 3rd, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci U S A 2003;100(15):8933–8 doi 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dey N, Williams C, Leyland-Jones B, De P. A critical role for HER3 in HER2-amplified and non-amplified breast cancers: function of a kinase-dead RTK. Am J Transl Res 2015;7(4):733–50. [PMC free article] [PubMed] [Google Scholar]
- 11.Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2018;36(20):2105–22 doi 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 12.AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer discovery 2017;7(8):818–31 doi 10.1158/2159-8290.cd-17-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hainsworth JD, Meric-Bernstam F, Swanton C, Hurwitz H, Spigel DR, Sweeney C, et al. Targeted Therapy for Advanced Solid Tumors on the Basis of Molecular Profiles: Results From MyPathway, an Open-Label, Phase IIa Multiple Basket Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2018;36(6):536–42 doi 10.1200/JCO.2017.75.3780. [DOI] [PubMed] [Google Scholar]
- 14.Bertotti A, Migliardi G, Galimi F, Sassi F, Torti D, Isella C, et al. A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer discovery 2011;1(6):508–23 doi 10.1158/2159-8290.CD-11-0109. [DOI] [PubMed] [Google Scholar]
- 15.Raghav KPS, Overman MJ, Yu R, Meric-Bernstam F, Menter D, Kee BK, et al. HER2 amplification as a negative predictive biomarker for anti-epidermal growth factor receptor antibody therapy in metastatic colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2016;34(Suppl; abstra 3517). [Google Scholar]
- 16.Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F, et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. The Lancet Oncology 2016;17(6):738–46 doi 10.1016/S1470-2045(16)00150-9. [DOI] [PubMed] [Google Scholar]
- 17.Li BT VM, Buonocore DJ, Offin MD, Olah ZT, Panora E, et al. A multi-histology basket trial of ado-trastuzumab emtansine in patients with HER2 amplified cancers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2018;36(Suppl, abstr 2502). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jhaveri KL, Makker V, Wang XV, Chen AP, Flaherty K, Conley BA, et al. Ado-trastuzumab emtansine (T-DM1) in patients (pts) with HER2 amplified (amp) tumors excluding breast and gastric/gastro-esophageal junction (GEJ) adenocarcinomas: Results from the National Cancer Institute (NCI) Molecular Analysis for Therapy Choice (MATCH) trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2018;36(Suppl, abstr 100). [Google Scholar]
- 19.Bose R, Kavuri SM, Searleman AC, Shen W, Shen D, Koboldt DC, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer discovery 2013;3(2):224–37 doi 10.1158/2159-8290.CD-12-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazieres J, Peters S, Lepage B, Cortot AB, Barlesi F, Beau-Faller M, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2013;31(16):1997–2003 doi 10.1200/JCO.2012.45.6095. [DOI] [PubMed] [Google Scholar]
- 21.Arcila ME, Chaft JE, Nafa K, Roy-Chowdhuri S, Lau C, Zaidinski M, et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res 2012;18(18):4910–8 doi 10.1158/1078-0432.CCR-12-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loree JM, Bailey AM, Johnson AM, Yu Y, Wu W, Bristow CA, et al. Molecular Landscape of ERBB2/ERBB3 Mutated Colorectal Cancer. Journal of the National Cancer Institute 2018. doi 10.1093/jnci/djy067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.cBIOPortal. cBIOPortal for Cancer Genomics. Accessed June 8, 2018.
- 24.Mishra R, Hanker AB, Garrett JT. Genomic alterations of ERBB receptors in cancer: clinical implications. Oncotarget 2017;8(69):114371–92 doi 10.18632/oncotarget.22825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang SE, Narasanna A, Perez-Torres M, Xiang B, Wu FY, Yang S, et al. HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell 2006;10(1):25–38 doi 10.1016/j.ccr.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 26.Perera SA, Li D, Shimamura T, Raso MG, Ji H, Chen L, et al. HER2YVMA drives rapid development of adenosquamous lung tumors in mice that are sensitive to BIBW2992 and rapamycin combination therapy. Proc Natl Acad Sci U S A 2009;106(2):474–9 doi 10.1073/pnas.0808930106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akiyama T, Matsuda S, Namba Y, Saito T, Toyoshima K, Yamamoto T. The transforming potential of the c-erbB-2 protein is regulated by its autophosphorylation at the carboxyl-terminal domain. Mol Cell Biol 1991;11(2):833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto H, Higasa K, Sakaguchi M, Shien K, Soh J, Ichimura K, et al. Novel germline mutation in the transmembrane domain of HER2 in familial lung adenocarcinomas. Journal of the National Cancer Institute 2014;106(1):djt338 doi 10.1093/jnci/djt338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shigematsu H, Takahashi T, Nomura M, Majmudar K, Suzuki M, Lee H, et al. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res 2005;65(5):1642–6 doi 10.1158/0008-5472.CAN-04-4235. [DOI] [PubMed] [Google Scholar]
- 30.Li C, Sun Y, Fang R, Han X, Luo X, Wang R, et al. Lung adenocarcinomas with HER2-activating mutations are associated with distinct clinical features and HER2/EGFR copy number gains. J Thorac Oncol 2012;7(1):85–9 doi 10.1097/JTO.0b013e318234f0a2. [DOI] [PubMed] [Google Scholar]
- 31.Buttitta F, Barassi F, Fresu G, Felicioni L, Chella A, Paolizzi D, et al. Mutational analysis of the HER2 gene in lung tumors from Caucasian patients: mutations are mainly present in adenocarcinomas with bronchioloalveolar features. Int J Cancer 2006;119(11):2586–91 doi 10.1002/ijc.22143. [DOI] [PubMed] [Google Scholar]
- 32.Stephens P, Hunter C, Bignell G, Edkins S, Davies H, Teague J, et al. Lung cancer: intragenic ERBB2 kinase mutations in tumours. Nature 2004;431(7008):525–6 doi 10.1038/431525b. [DOI] [PubMed] [Google Scholar]
- 33.Petrelli F, Tomasello G, Barni S, Lonati V, Passalacqua R, Ghidini M. Clinical and pathological characterization of HER2 mutations in human breast cancer: a systematic review of the literature. Breast Cancer Res Treat 2017;166(2):339–49 doi 10.1007/s10549-017-4419-x. [DOI] [PubMed] [Google Scholar]
- 34.Wen W, Chen WS, Xiao N, Bender R, Ghazalpour A, Tan Z, et al. Mutations in the Kinase Domain of the HER2/ERBB2 Gene Identified in a Wide Variety of Human Cancers. J Mol Diagn 2015;17(5):487–95 doi 10.1016/j.jmoldx.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zabransky DJ, Yankaskas CL, Cochran RL, Wong HY, Croessmann S, Chu D, et al. HER2 missense mutations have distinct effects on oncogenic signaling and migration. Proc Natl Acad Sci U S A 2015;112(45):E6205–14 doi 10.1073/pnas.1516853112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu DH, Tang L, Dong H, Dong Z, Zhang L, Fu J, et al. Oncogenic HER2 fusions in gastric cancer. J Transl Med 2015;13:116 doi 10.1186/s12967-015-0476-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma CX, Bose R, Gao F, Freedman RA, Telli ML, Kimmick G, et al. Neratinib Efficacy and Circulating Tumor DNA Detection of HER2 Mutations in HER2 Nonamplified Metastatic Breast Cancer. Clin Cancer Res 2017;23(19):5687–95 doi 10.1158/1078-0432.CCR-17-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hyman DM, Piha-Paul SA, Won H, Rodon J, Saura C, Shapiro GI, et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 2018;554(7691):189–94 doi 10.1038/nature25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li BT, Shen r, Buonocore D, Olah ZT, Ni A, Ginsberg MS, et al. Ado-trastuzumab emtansine in patients with HER2 mutant lung cancers: Results from a phase II basket trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2017;35(Suppl; abstract 8510). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lesurf R, Griffith OL, Griffith M, Hundal J, Trani L, Watson MA, et al. Genomic characterization of HER2-positive breast cancer and response to neoadjuvant trastuzumab and chemotherapy—results from the ACOSOG Z1041 (Alliance) trial. Annals of Oncology 2017;28(5):1070–7 doi 10.1093/annonc/mdx048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kancha RK, von Bubnoff N, Bartosch N, Peschel C, Engh RA, Duyster J. Differential sensitivity of ERBB2 kinase domain mutations towards lapatinib. PLoS One 2011;6(10):e26760 doi 10.1371/journal.pone.0026760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanker AB, Brewer MR, Sheehan JH, Koch JP, Sliwoski GR, Nagy R, et al. An Acquired HER2(T798I) Gatekeeper Mutation Induces Resistance to Neratinib in a Patient with HER2 Mutant-Driven Breast Cancer. Cancer discovery 2017;7(6):575–85 doi 10.1158/2159-8290.CD-16-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin Cancer Res 2016;22(20):5097–108 doi 10.1158/1078-0432.CCR-15-2822. [DOI] [PubMed] [Google Scholar]
- 44.Iwata H, Tamura K, Doi T, Tsurutani J, Modi S, Park H, et al. Trastuzumab deruxtecan (DS-8201a) in subjects with HER2-expressing solid tumors: Long-term results of a large phase 1 study with multiple expansion cohorts. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2018;36(Suppl; abstr 2501). [Google Scholar]
- 45.Meric-Bernstam F, Beeram M, Mayordomo JI, Hanna DL, Ajani JA, Murphy MAB, et al. Single agent activity of ZW25, a HER2‑targeted bispecific antibody, in heavily pretreated HER2‑expressing cancers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2018;36(Suppl; abstr 2500). [Google Scholar]
- 46.Alsina M, Boni V, Schellens JHM, Moreno V, Bol K, Westendorp M, et al. First-in-human phase 1/2 study of MCLA-128, a full length IgG1 bispecific antibody targeting HER2 and HER3: Final phase 1 data and preliminary activity in HER2+ metastatic breast cancer (MBC). Journal of Clinical Oncology 2017;35(15_suppl):2522– doi 10.1200/JCO.2017.35.15_suppl.2522. [DOI] [Google Scholar]
- 47.Pheneger T, Bouhana K, Anderson D, Garrus J, Ahrendt K, Allen S, et al. Abstract #1795: In Vitro and in vivo activity of ARRY-380: A potent, small molecule inhibitor of ErbB2. Cancer Research 2009;69(9 Supplement):1795–. [Google Scholar]
- 48.Lee P, Napier C, Garrus J, Avrustkaya A, White A, Winkler J. In vivo activity of ARRY-380: A potent, small molecule inhibitor of ErbB-2 in combination with trastuzumab or docetaxel in a BT-474 human breast carcinoma xenograft model. Presented at the American Association of Cancer Research 100th Annual Meeting, Denver CO, Apr 18–22 2009; Cancer Res 69 (abstr 5581). [Google Scholar]
- 49.Dinkel V, Anderson D, Winski S, Winkler J, Koch K, Lee PA. Abstract 852: ARRY-380, a potent, small molecule inhibitor of ErbB2, increases survival in intracranial ErbB2+ xenograft models in mice. Cancer Research 2012;72(8 Supplement):852– doi 10.1158/1538-7445.am2012-852. [DOI] [Google Scholar]
- 50.Moulder SL, Borges VF, Baetz T, McSpadden T, Fernetich G, Murthy RK, et al. Phase I Study of ONT-380, a HER2 Inhibitor, in Patients with HER2(+)-Advanced Solid Tumors, with an Expansion Cohort in HER2(+) Metastatic Breast Cancer (MBC). Clin Cancer Res 2017;23(14):3529–36 doi 10.1158/1078-0432.CCR-16-1496. [DOI] [PubMed] [Google Scholar]
- 51.Borges VF, Ferrario C, Aucoin N, Falkson C, Khan Q, Krop I, et al. Tucatinib Combined With Ado-Trastuzumab Emtansine in Advanced ERBB2/HER2-Positive Metastatic Breast Cancer: A Phase 1b Clinical Trial. JAMA oncology 2018. doi 10.1001/jamaoncol.2018.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murthy R, Borges VF, Conlin A, Chaves J, Chamberlain M, Gray T, et al. Tucatinib with capecitabine and trastuzumab in advanced HER2-positive metastatic breast cancer with and without brain metastases: a non-randomised, open-label, phase 1b study. The Lancet Oncology 2018. doi 10.1016/S1470-2045(18)30256–0. [DOI] [PubMed] [Google Scholar]
- 53.Robichaux JP, Elamin YY, Tan Z, Carter BW, Zhang S, Liu S, et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nature medicine 2018;24(5):638–46 doi 10.1038/s41591-018-0007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loi S, Giobbe-Hurder A, Gombos A, Bachelot T, Hui R, Curigliano G, et al. Abstract GS2–06: Phase Ib/II study evaluating safety and efficacy of pembrolizumab and trastuzumab in patients with trastuzumab-resistant HER2-positive metastatic breast cancer: Results from the PANACEA (IBCSG 45–13/BIG 4–13/KEYNOTE-014) study. Cancer Research 2018;78(4 Supplement):GS2–06-GS2- doi 10.1158/1538-7445.sabcs17-gs2-06. [DOI] [Google Scholar]
- 55.Ahmed N, Brawley V, Hegde M, et al. Her2-specific chimeric antigen receptor–modified virus-specific t cells for progressive glioblastoma: A phase 1 dose-escalation trial. JAMA oncology 2017;3(8):1094–101 doi 10.1001/jamaoncol.2017.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.MD Anderson Cancer Center Knowledge Base for Precision Oncology. <https://pct.mdanderson.org/>. Accessed 7/6/2018.
- 57.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Science signaling 2013;6(269):pl1 doi 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer discovery 2012;2(5):401–4 doi 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luque-Cabal M, Garcia-Teijido P, Fernandez-Perez Y, Sanchez-Lorenzo L, Palacio-Vazquez I. Mechanisms Behind the Resistance to Trastuzumab in HER2-Amplified Breast Cancer and Strategies to Overcome It. Clin Med Insights Oncol 2016;10(Suppl 1):21–30 doi 10.4137/CMO.S34537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rexer BN, Arteaga CL. Intrinsic and acquired resistance to HER2-targeted therapies in HER2 gene-amplified breast cancer: mechanisms and clinical implications. Crit Rev Oncog 2012;17(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barok M, Joensuu H, Isola J. Trastuzumab emtansine: mechanisms of action and drug resistance. Breast Cancer Res 2014;16(2):209 doi 10.1186/bcr3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


