Abstract
Glioblastoma (GBM) is a devastating disease with an extremely poor prognosis. Immune therapy via adoptive cell transfer (ACT), especially with T cells engineered to express chimeric antigen receptors (CARs), represents a particularly promising approach. Despite the recent success of CAR T cells for blood cancers, the question remains whether this powerful anti-cancer therapy will ultimately work for brain tumors, and if the primary immunologic challenges in this disease—which include antigenic heterogeneity, immune suppression and T-cell exhaustion—can be adequately addressed. Here, we contextualize these concepts by reviewing recent developments in ACT for GBM, with a special focus on pioneering clinical trials of CAR T-cell therapy.
Keywords: Glioblastoma, Central nervous system neoplasms, Immunotherapy, T-lymphocytes, Chimeric antigen receptor
Introduction
Glioblastoma (GBM) is the most common and most aggressive primary malignant brain tumor and represents an unmet clinical need. Contrary to conventional notions of central nervous system (CNS) immune privilege, there are now well-described mechanisms by which the immune system interfaces with tumors in the brain. Several first-in-class immune-based therapies for various cancers outside the CNS have been approved by the Food and Drug Administration (FDA) in the last decade. These include a dendritic cell vaccine for prostate cancer in 2010 (1), monoclonal antibody-based immune checkpoint blockade for metastatic melanoma and other cancers beginning in 2011 (2), bispecific T-cell engagers (BiTEs) for acute lymphoblastic leukemia (ALL) in 2014 (3), and oncolytic viral therapy for melanoma in 2015 (4). Perhaps the most promising T-cell technology in development is the chimeric antigen receptor (CAR), which received accelerated approval for hematological malignancies in 2017 (5). In this setting, CAR T cells were directed against a single molecule that is universally expressed on the surface of ALL cells called CD19. By contrast, aggressive solid tumors, including those arising in the brain, are inherently heterogenous (6). This makes targeting them through a single antigen less likely to yield durable, complete remissions. Despite this, early clinical trials of CARs for GBM have reported exciting results, and in at least one case demonstrated complete regression of bulky, multifocal cancer in the brain and spinal canal following intraventricular infusion (7). Here, we discuss recent groundbreaking advances in the development of adoptive T-cell therapy for brain tumors, and summarize emerging opportunities for further investigation.
Immune Biology of Brain Tumors
Despite state-of-the-art advances in treatment for GBM, including a combination of maximal surgical resection, radiation therapy, chemotherapy, anti-angiogenic agents and alternating electrical fields, the prognosis for patients with GBM remains exceedingly grim, with 5-year survival rates of less than 10% (8). In addition, currently available treatments are limited by adverse effects on normal, healthy tissues. As an alternative, immune-based therapies are rapidly evolving, and T cells in particular are thought to be an essential component of an effective antitumor response. Indeed, it has been acknowledged for decades that gliomas are infiltrated by lymphocytes and that their presence in GBM may correlate with improved prognosis (9,10). However, such naturally occurring T cells are observed less frequently than in other tumor types (11,12) and are ostensibly incapable of potentiating tumor regression on their own, due in part to mechanisms of T-cell dysfunction, several of which are idiosyncratic to the natural history of GBM.
In the context of glioma immune biology, T cells face an exceptionally hostile tumor microenvironment. Anatomically, the CNS is considered an immunologically “distinct” site given the absence of conventional lymphatic structures and dearth of resident professional antigen-presenting cells (13). In the healthy brain, specialized tight junctions between endothelial cells also exist, which impede communication with the systemic circulation (14); however, in the setting of various pathologies including tumors and inflammatory disease, the integrity of the blood-brain barrier (BBB) becomes compromised (15). In addition, nonclassical lymphatic vessels have recently been described along dural venous sinuses, providing a putative gateway for immune cells to travel through the meninges (16). Recent evidence suggests that tumors in the CNS may uniquely potentiate large-scale sequestration of immune effectors in the bone marrow, thus crippling antitumor immunity by harboring T cells in an agnostic anatomical compartment (17). Compounding this GBM-related T-cell deficiency, standard-of-care treatment with temozolomide chemotherapy and high-dose corticosteroids also leads to profound lymphopenia and immune suppression, further depressing the development of effective antitumor immune responses.
Functionally, the immune system in patients with GBM is suppressed by a network of tightly intertwined, dynamic elements. These include counterproductive cytokine skew (e.g., IL-10, TGF-β), direct inhibition of T cells via cell-surface molecules on tumor or extracellular vesicles (e.g., PD-L1, CD95) (18), elaboration of suppressive immune populations (e.g., regulatory T cells [Tregs], tumor associated macrophages [TAMs], myeloid-derived suppressor cells [MDSCs]), and production of additional modulatory factors (e.g., indolamine 2,3-dioxygenase). Many of these phenomena are shared observations with other cancers and have been reviewed in greater detail elsewhere (19,20). Notable for GBM, however, is the relative paucity of available tumor-specific and tumor-associated neoantigens that are frequently and homogenously expressed, especially when compared to melanoma and cancers of the lung and colon (21). Perhaps most reflective of the notoriously “cold” immunological milieu associated with GBM is that—despite promising single-arm studies of vaccines and immune checkpoint inhibitors—a randomized trial of immune based therapy for GBM has yet to successfully demonstrate a survival advantage (22,23).
The Promise of Cellular Therapy
Since its introduction in animal models of tumor over 60 years ago (24), adoptive cell transfer (ACT) strategies have emerged as a bona fide treatment for cancer, most recently evidenced by FDA approval of CAR T-cell therapy for acute lymphoblastic leukemia in 2017, which also represents the first ACT approved for cancer of any type. Unlike vaccines and immunomodulatory agents that rely on in vivo priming of endogenous tumor-reactive cells, ACT introduces an ability to optimally select or genetically engineer cells with specificity for tumor antigens, and then provide appropriate stimulation to promote proliferation, expansion, and maintenance of potent effector functions to achieve therapeutic goals. In addition, ACT permits favorable manipulation of host immunity prior to cell transfer (i.e., lymphodepletion and removal of Tregs or endogenous lymphocytes that may compete with transferred cells for homeostatic cytokines), which may be exploited to create a suitable environment for fostering antitumor immune responses. Indeed, the plasticity of the “living” cellular response, and its ability to both sense and adapt to surroundings, represents one of the most intriguing aspects of this approach. Highlighting this is a recent report of a complete regression achieved in a patient with chronic lymphocytic leukemia, whose treatment response was, remarkably, attributed to the in vivo expansion of a single CAR T-cell clone (25).
ACT therapies for GBM have evolved considerably over time (Summarized in Table 1). Earlier work focused on less specific approaches utilizing natural killer (NK) or lymphokine activated killer (LAK) cells, neither of which rely on human leukocyte antigen (HLA)-restricted mechanisms of killing. Clinical trials for these platforms have become less favored over the past decades, in part due to Phase III evidence from solid tumor settings wherein infusion of cells with IL-2 was not found to be superior to IL-2 alone (26). Local application of allogeneic cytotoxic T lymphocytes (CTLs) for GBM has also been attempted with hopes of redirecting the effects of histocompatibility mismatch against tumor cells. However, as has been characterized in hematological disease, donor lymphocyte infusion typically requires allogeneic hematopoietic stem cell transplantation to prevent elimination by host immunity and ultimately carries with it the potential for graft-versus-host toxicity. To avoid these drawbacks, several strategies based on autologous cells have been conceived and are in active development.
Table 1:
Adoptive T-cell Therapies for Glioblastoma in Development
| Type | Mechanism | Sponsor Institution: Identifier |
|---|---|---|
| Lymphokine-activated killer cells | Unknown, likely MHC-independent | Hoag Memorial Hospital Presbyterian: NCT00331526 |
| Allogeneic donor lymphocyte infusion | Graft versus tumor HLA-mismatch, may include viral antigens (CMV) | City of Hope Medical Center: NCT01082926 Jonsson Comprehensive Cancer Center: NCT01144247 Milton S. Hershey Medical Center: NCT00990496 University of Colorado: NCT00002572 |
| Autologous lymphocytes | MHC-restricted TCR recognition, may include viral antigens (CMV) | Baylor College of Medicine: NCT01205334 CytoVac A/S: NCT01588769 Duke University Medical Center: NCT00693095 Green Cross Cell Corporation: NCT00807027 Huashan Hospital: NCT03347097 M.D. Anderson Cancer Center: NCT02661282 TVAX Biomedical: NCT01290692, NCT01081223 |
| Tumor infiltrating lymphocytes | MHC-restricted TCR recognition | National Cancer Institute: NCT01174121 |
| Transgenic T-cell receptor T cells | MHC-restricted TCR recognition | National Cancer Institute: NCT03412877 |
| Antibody-armed T cells | MHC-independent antigen recognition | Barbara Ann Karmanos Cancer Institute: NCT02521090 University of Virginia: NCT03344250 |
| Chimeric antigen receptor T cells | MHC-independent antigen recognition coupled with intracellular signaling domains | Baylor College of Medicine: NCT01109095, NCT02442297 Beijing Sanbo Brain Hospital: NCT02844062, NCT02937844 City of Hope Medical Center: NCT00730613, NCT03389230, NCT02208362 Duke University Medical Center: NCT02664363, NCT03283631 Fuda Cancer Hospital: NCT02575261 National Cancer Institute: NCT01454596 RenJi Hospital: NCT02331693 Shenzhen Geno-Immune Medical Institute: NCT03170141 University of Pennsylvania: NCT02209376 |
Abbreviations: MHC = major histocompatibility complex; HLA = human leukocyte antigen; CMV = cytomegalovirus, TCR = T-cell receptor
Unlike NK, LAK and allogeneic CTLs, ACT with autologous TILs relies on specific HLA-restricted tumor antigen recognition via T-cell receptors (TCRs). Adoptive transfer with TILs targeting tumor-associated somatic mutations has resulted in significant clinical regressions in both primary and metastatic cancers (27–30). Indeed, local infusion of autologous TILs with concomitant recombinant IL-2 has also been attempted in patients with malignant glioma (31), and favorable responses have been achieved with systemic TIL therapy for metastatic melanoma in the brain (32,33). Although providing evidence that peripherally administered lymphocytes may traffic to and effect measurable antitumor activity in the CNS, technical factors—including the ability to isolate and expand TILs from primary brain tumors—have limited the utility of this approach for GBM. Interestingly, a recent analysis demonstrated that TILs may in fact lack specificity for the tumor in which they are found, a phenomenon that appears to be consistent across various types of cancer (34).
Alternative methods of obtaining tumor-specific lymphocytes from draining lymph nodes, or cultivating these cells by ex vivo sensitization with autologous tumor tissue, have also been stymied by inadequate specimens. As such, one intriguing proposal has been ACT with autologous cytomegalovirus (CMV)-specific T cells, which can be readily isolated from peripheral blood or expanded in vitro using synthetic peptide epitopes (35,36). Rationale for this approach stems from the discovery of CMV antigen expression in GBM and its absence in normal tissues (37). Although the reliable detection of CMV viral proteins in brain tumors has been confirmed by nine different laboratories, it has been disputed by some when using different techniques than originally published (38–42).
Gene-engineered Cell Therapies
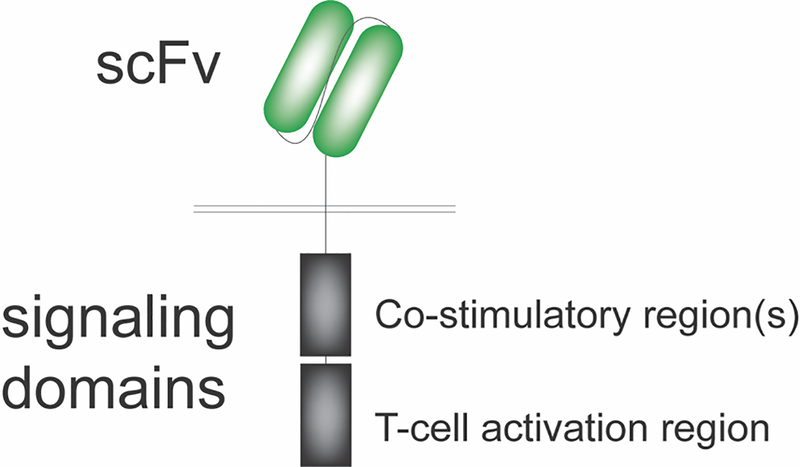
Of all ACT-based immune therapies currently in development for GBM, gene-engineered CAR T cells are at the forefront, with encouraging results reported from several recent clinical trials (43). Structurally, CAR molecules consist of an extracellular, antigen-binding domain translated in tandem with assorted intracellular signaling regions that have differential effects on T-cell proliferation, effector function and survival (Fig. 1). While first-generation CAR constructs contained CD3ζ in isolation, second- and third-generation constructs included CD3ζ as well as one or two co-stimulatory domains (e.g., CD28, OX40, 4–1BB), respectively. Unlike other gene-engineered ACT platforms such as transgenic TCR, the extracellular portion of CAR T cells is typically composed of an antibody-derived single-chain variable fragment (scFv). This design not only enables recognition of a broad array of antigens (e.g., proteins, carbohydrates) but also obviates the need for presentation in the context of major histocompatibility complex (MHC), downregulation of which represents a well-characterized mechanism of tumor immune escape.
Figure 1.
Chimeric antigen receptors are composed of an extracellular antigen-binding domain, typically in the form of an antibody-derived single-chain variable fragment (scFv), translated in tandem with assorted intracellular signaling regions that have differential effects on T-cell proliferation, effector function and survival.
Treatment with CAR T cells directed at the B-cell antigen, CD19, has resulted in remarkable and durable remissions in patients with hematological cancers (5,44), even in cases of extensive disease involvement in the CNS (45). While this certainly provides robust proof-of-concept, CAR T cells have yet to be successfully translated in parallel for solid tumors. One major barrier has been antigenic heterogeneity and the challenge associated with identifying targets that are consistently expressed on cancer cells of interest. To date, four antigens have been pursued in CAR clinical trials for GBM. These include epidermal growth factor receptor variant III (EGFRvIII) (46), human epidermal growth factor receptor 2 (HER2) (47), and interleukin receptor 13Rα2 (IL-13Rα2) (7,48). There is also interest in erythropoietin-producing hepatocellular carcinoma A2 (EphA2), though results from clinical trials investigating this target have not yet been released (NCT02575261).
EGFRvIII
Tumor-specific antigens are those that are present in cancer cells but completely absent from normal tissues. This pattern of expression is ideal, as it confers the immune system with the theoretical capacity to eliminate tumors while minimizing toxicity and leaving healthy cells intact. A classic antigen matching this profile is EGFRvIII. EGFRvIII is a constitutively-activated, mutated form of the wild-type receptor that was first identified in primary human GBM, where its incidence is approximately 30% (49). The EGFRvIII deletion-mutation results in the translation of a novel glycine residue in the extracellular domain, flanked by normally disparate portions of the receptor, thus providing an ideal epitope for surface recognition by CAR T cells.
In 2017, O’Rourke and colleagues reported a series of 10 patients with recurrent GBM who had been treated with a single-dose of intravenous, second-generation (i.e., 4–1BB, CD3ζ) EGFRvIII CAR T cells (NCT02209376) (46). All patients had EGFRvIII-positive tumors confirmed by next-generation sequencing assay. Notably, 7 out of 10 patients in the study underwent post-treatment surgical intervention, which provided the opportunity to directly evaluate the tumor microenvironment following CAR T-cell infusion. Molecular and histopathological analyses of these specimens demonstrated trafficking of peripherally administered CAR T cells to tumors in the brain. Interestingly, this was accompanied by a striking degree of infiltration by new immigrant, unmodified T cells, although a substantial proportion of these were identified as immune suppressive Tregs. Post-treatment tumor revealed decreased levels of EGFRvIII expression, consistent with successful elimination of EGFRvIII-positive tumor. Compared to pre-treatment disease, residual and recurrent tumor displayed enhancement of several immune suppressive pathways, including increased expression of PD-L1, IDO1 and IL-10.
HER2
Another member of the epidermal growth factor receptor family, HER2, has well-documented overexpression in a wide variety of cancer types and is also found in approximately 80% of GBMs (50). However, this antigen is also present in healthy epithelial cells; thus, CAR T cells specific for HER2 carry a theoretical risk of on-target, off-tumor autoimmune effects. Indeed, this phenomenon was manifest in a 2010 case report of fatal toxicity in a patient with metastatic colon cancer, with signs of severe multiple organ dysfunction occurring just minutes after a single intravenous infusion of third-generation HER2 CAR (51).
Nonetheless, in 2017, Ahmed and colleagues performed a subsequent study which successfully demonstrated safety without dose-limiting toxicity, this time with an alternative HER2 CAR T-cell product in a trial of 17 patients with GBM (NCT01109095) (47). Important differences that may have contributed to a more favorable toxicity profile included the use of a second-generation (i.e., CD28, CD3ζ) CAR with a different scFv, as well as the absence of concomitant IL-2 and lymphodepletive chemotherapy, both of which were administered in the aforementioned case report. Of the 17 patients treated with peripheral infusions of HER2 CAR T cells, three had stable disease for 24 months, and one patient with an unresectable thalamic GBM was noted to have a partial response.
IL-13Rα2
Unlike mutations in somatic genes or overexpression of otherwise normal proteins, cancer-germline antigens represent a class of immunogenic targets best described as having shared expression in both normal gametogenic cells as well as in human cancer. One such cancer-germline antigen is a cytokine receptor known as IL-13Rα2, which is found in both glioma cells and the testes. As a caveat, reports of its detection in several normal adult human tissues suggest that IL-13Rα2 may in fact be more accurately categorized as a tumor-associated antigen. It is estimated that IL-13Rα2 has relative overexpression in approximately 50% of GBMs, although its presence can vary somewhat within individual tumors (52).
A distinguishing feature of IL-13Rα2 CARs to date has been the use of a membrane-tethered, mutated IL-13 ligand for target antigen recognition, rather than a traditional scFv as has been typical with other constructs. Employing this design, data from two clinical trials studying IL-13Rα2 CAR T cells in patients with GBM have been published. In 2015, Brown and colleagues published first-in-human experience with a first-generation (i.e., CD3ζ) IL-13Rα2 CAR in three patients with recurrent GBM (NCT00730613) (48). Patients received multiple intracavitary infusions via indwelling catheter, a strategy that was found to be both feasible and safe. Tumor specimen was available for analysis after therapy and in one instance demonstrated evidence of reduced IL-13Rα2 expression. These findings led to a subsequent study of a second-generation (i.e., 4–1BB, CD3ζ) IL-13Rα2 CAR, during which the authors observed an extraordinary response to treatment after serial intraventricular administration in one patient with multifocal GBM (NCT02208362) (7). In this case, IL-13Rα2 CAR was noted to mediate the complete regression of several bulky lesions in the brain and spinal canal. Given this response, perhaps most intriguing is that the patient’s GBM did not homogeneously express the target antigen, with no verified staining of IL-13Rα2 in approximately 30% of the original tumor. These results raise the possibility that CAR T cells successfully targeted cells with low levels of IL-13Rα2 or triggered immunity to other targets through epitope spreading.
Future Challenges and Opportunities
Over the past decades, CAR technology has become an especially intriguing area of research in GBM. Clinical experience with ACT frequently provides new insights that have broader implications for general principles in brain tumor immune therapy. For example, a common theme presented throughout CAR T-cell trials is a repeated account of CNS immune access—namely that peripherally administered, tumor-specific T cells have the capacity to infiltrate lesions beyond the BBB. Moreover, mounting data support that introduction of these activated cells into the CNS can be accomplished with acceptable safety, even when administered directly into the brain. Certainly, there is some evidence that route-of-delivery may significantly impact outcome, since, in at least one case, infusion into the intraventricular space appeared to be necessary for tumor regression, whereas prior attempts by intracavitary administration in the same patient led to disease progression (7). While intraventricular approaches may theoretically increase the risk of neurosurgical complications such as hydrocephalus (53), potential advantages include enhanced access to multifocal disease throughout the CNS as well as the ability to achieve increased effector-to-target ratios at the tumor bed. Furthermore, local administration may also prove to mitigate off-tumor toxicity attributable to first-pass clearance in the lung, a mechanism which was implicated in the case of lethal toxicity following systemic infusion with HER2 CAR (51).
Despite this promise, substantial gaps in our understanding remain. Although CAR T cells efficiently eliminate cancer cells expressing their cognate antigen of interest, whether this approach will ultimately treat tumors that heterogeneously express these targets is unknown. Indeed, reports of CD19-negative escape variants in the setting of ALL suggest that CAR T cells may be limited in their ability to efficiently prime the immune response and protect against antigen loss. At least one contributing factor in the resistance to therapy is T-cell exhaustion, related in part to excessive CD3ζ phosphorylation within nonnative, CAR-mediated signaling complexes. Lastly, it is still unclear how to best optimize ACT in general, and conceptualize this therapy in the context of aforementioned suppressive glioma biology and iatrogenic effects of chemotherapy or steroid use. Ongoing advances in preclinical modeling have provided some insight into these issues. Although used less frequently than immune-compromised rodents bearing xenografted tumors, syngeneic systems have also been developed in order to better capture phenomena such as epitope-spreading via endogenous immunity (54) and the potential to enhance ACT responses through lymphodepletive host-conditioning (55). More recently, robust humanized models have offered an exciting alternative for screening ACT products, and have even been used to successfully recapitulate the complexities of CAR-mediated cytokine release syndrome (56).
CAR T cells are not only the first ACT treatment approved by the FDA, but they also represent the first gene-modified therapy made available for any indication. Now more than ever, the manipulation of genetic material has taken its place at the forefront of viable options in the battle against cancer. In fact, further modification of CAR T cells for GBM is already underway and has yielded a wide range of permutations; these include changes that allow CAR T cells to target multiple surface antigens at once (57–60), to be used as allogeneic products “off-the-shelf,” or to alter expression of cytokines and other immune-modulating molecules (61). The prospect of combination therapy has also been suggested between ACT and other treatments currently under investigation for GBM such as immune checkpoint blockade or oncolytic viruses (62,63). Localized treatment including radiosurgery, laser ablation, and that of various therapeutic devices also offer potential synergy with immune therapy (64), and the degree to which these interventions may be used to enhance adoptive therapy has yet to be seen.
Given the potential for significant crosstalk between cellular effectors, tumor cells, and accompanying treatment modalities, it will become increasingly important to implement appropriate clinical tools to assess surrogates of response to ACT, such as in vivo engraftment, trafficking and persistence. Regardless of route-of-delivery, advances in molecular imagining may offer noninvasive techniques to monitor the location and viability of adoptively transferred cells, as has been demonstrated in GBM through positron emission tomography (PET) (NCT00730613, NCT01082926) (65). These efforts, in conjunction with guidelines proposed by the response and assessment in neuro-oncology (RANO) criteria, will be vital in defining accurate endpoints for future clinical trials, given that local inflammation triggered by ACT may be indistinguishable from tumor progression using standard radiographic assessment (66).
Conclusions
Results from clinical trials for ACT immune therapy—particularly with CAR T cells—suggest a safe and feasible strategy for eliciting effective immune responses in GBM. The introduction of CAR T cells for the treatment of hematological malignancies has been transformative in the field of cancer immune therapy; however, as detailed here, GBM poses a unique set of challenges that must be addressed before the full potential of ACT can be realized. Continued familiarity with ACT may offer significant insight into general mechanisms of cellular immunity and their role in GBM. Given early successes in translation, there appears to be a bright future with abundant opportunity for research.
Acknowledgments
Funding: This work was supported by grants from the National Institutes of Health: R01-NS099463 (Sampson), P50-CA190991 (Sampson), P30-CA14236 (Sampson), R01-CA177476 (Sampson), U01-NS090284 (Sampson), R01-NS085412 (Sampson), and R25-NS065743 (Choi). Additional support was provided by the Neurosurgery Research & Education Foundation and B*Cured Research Fellowship Grant (Choi), and the Society for Immunotherapy of Cancer—AstraZeneca Postdoctoral Cancer Immunotherapy in Combination Therapies Clinical Fellowship Award (Choi). The funders had no role in the preparation of the manuscript or decision to publish.
Footnotes
Conflicts of Interest: M. V. Maus reports receiving commercial research grants from Kite Pharma, TCR2, Agentus, and Crispr Therapeutics, is an inventor on patents related to the use of engineered cell therapies for GBM and other cancers (owned by University of Pennsylvania and Massachusetts General Hospital), and is a consultant/advisory board member for Adaptimmune, Adaptive Biotechnologies, Agentus, Cellectis, Crispr Therapeutics, Kite Pharma, Novartis, Takeda, TCR2, and Windmil. C. H. June reports receiving commercial research grants from Novartis and Tmunity Therapeutics, is listed as co-inventor on patents in the area of CAR T cells that are owned by the University of Pennsylvania and licensed to Novartis and Tmunity Therapeutics, and is a consultant/advisory board member for Tmunity Therapeutics. J. H. Sampson holds ownership interest (including patents) in Annias Immunotherapeutics (which has licensed intellectual property from Duke University related to the use of the pepCMV vaccine in the treatment of glioblastoma multiforme) and Istari Oncology (which has licensed intellectual property from Duke University related to the use of poliovirus and D2C7 in the treatment of glioblastoma), and is an inventor on patents related to PEP-CMV DC vaccine with tetanus, as well as poliovirus vaccine and D2C7 in the treatment of glioblastoma. No potential conflicts of interest were disclosed by the other author.
REFERENCES
- 1.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363(5):411–22 doi 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 2.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363(8):711–23 doi 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kantarjian H, Stein A, Gokbuget N, Fielding AK, Schuh AC, Ribera JM, et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N Engl J Med 2017;376(9):836–47 doi 10.1056/NEJMoa1609783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J Clin Oncol 2015;33(25):2780–8 doi 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 5.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med 2018;378(5):439–48 doi 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 2014;344(6190):1396–401 doi 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, et al. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N Engl J Med 2016;375(26):2561–9 doi 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10(5):459–66 doi 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 9.Palma L, Di Lorenzo N, Guidetti B. Lymphocytic infiltrates in primary glioblastomas and recidivous gliomas. Incidence, fate, and relevance to prognosis in 228 operated cases. J Neurosurg 1978;49(6):854–61 doi 10.3171/jns.1978.49.6.0854. [DOI] [PubMed] [Google Scholar]
- 10.Brooks WH, Markesbery WR, Gupta GD, Roszman TL. Relationship of lymphocyte invasion and survival of brain tumor patients. Ann Neurol 1978;4(3):219–24 doi 10.1002/ana.410040305. [DOI] [PubMed] [Google Scholar]
- 11.Berghoff AS, Kiesel B, Widhalm G, Rajky O, Ricken G, Wohrer A, et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol 2015;17(8):1064–75 doi 10.1093/neuonc/nou307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berghoff AS, Kiesel B, Widhalm G, Rajky O, Ricken G, Wöhrer A, et al. IB-02ROLE OF TUMOR INFILTRATING LYMPHOCYTES AND PDL1 EXPRESSION IN GLIOBLASTOMA (GBM) AND BRAIN METASTASES (BM): COMPARATIVE ANALYSIS. Neuro Oncol 2014;16(Suppl 5):v107 doi 10.1093/neuonc/nou257.2. [DOI] [Google Scholar]
- 13.Hart DN, Fabre JW. Demonstration and characterization of Ia-positive dendritic cells in the interstitial connective tissues of rat heart and other tissues, but not brain. J Exp Med 1981;154(2):347–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banks WA. From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov 2016;15(4):275–92 doi 10.1038/nrd.2015.21. [DOI] [PubMed] [Google Scholar]
- 15.de Vries HE, Kuiper J, de Boer AG, Van Berkel TJ, Breimer DD. The blood-brain barrier in neuroinflammatory diseases. Pharmacol Rev 1997;49(2):143–55. [PubMed] [Google Scholar]
- 16.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015;523(7560):337–41 doi 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woroniecka KI, Rhodin KE, Chongsathidkiet P, Keith KA, Fecci PE. T-cell Dysfunction in Glioblastoma: Applying a New Framework. Clin Cancer Res 2018. doi 10.1158/1078-0432.CCR-18–0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ricklefs FL, Alayo Q, Krenzlin H, Mahmoud AB, Speranza MC, Nakashima H, et al. Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci Adv 2018;4(3):eaar2766 doi 10.1126/sciadv.aar2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol 2015;35 Suppl:S185–S98 doi 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Fecci PE, Brastianos PK, Goodwin CR, Anders CK, Pendergast AM, Sampson JH. Mechanistic cause of brain and spine metastasis. Clin Cancer Res 2018. [Google Scholar]
- 21.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015;348(6230):69–74 doi 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 22.Reardon DA, Omuro A, Brandes AA, Rieger J, Wick A, Sepulveda J, et al. OS10.3 Randomized Phase 3 Study Evaluating the Efficacy and Safety of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma: CheckMate 143. Neuro-Oncology 2017;19(suppl_3):iii21–iii doi 10.1093/neuonc/nox036.071. [DOI] [Google Scholar]
- 23.Weller M, Butowski N, Tran DD, Recht LD, Lim M, Hirte H, et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol 2017;18(10):1373–85 doi 10.1016/S1470-2045(17)30517-X. [DOI] [PubMed] [Google Scholar]
- 24.Mitchison NA. Studies on the immunological response to foreign tumor transplants in the mouse. I. The role of lymph node cells in conferring immunity by adoptive transfer. J Exp Med 1955;102(2):157–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fraietta JA, Nobles CL, Sammons MA, Lundh S, Carty SA, Reich TJ, et al. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature 2018;558(7709):307–12 doi 10.1038/s41586-018-0178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Law TM, Motzer RJ, Mazumdar M, Sell KW, Walther PJ, O’Connell M, et al. Phase III randomized trial of interleukin-2 with or without lymphokine-activated killer cells in the treatment of patients with advanced renal cell carcinoma. Cancer 1995;76(5):824–32. [DOI] [PubMed] [Google Scholar]
- 27.Zacharakis N, Chinnasamy H, Black M, Xu H, Lu YC, Zheng Z, et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat Med 2018;24(6):724–30 doi 10.1038/s41591-018-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tran E, Ahmadzadeh M, Lu YC, Gros A, Turcotte S, Robbins PF, et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science 2015;350(6266):1387–90 doi 10.1126/science.aad1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014;344(6184):641–5 doi 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran E, Robbins PF, Lu YC, Prickett TD, Gartner JJ, Jia L, et al. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N Engl J Med 2016;375(23):2255–62 doi 10.1056/NEJMoa1609279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quattrocchi KB, Miller CH, Cush S, Bernard SA, Dull ST, Smith M, et al. Pilot study of local autologous tumor infiltrating lymphocytes for the treatment of recurrent malignant gliomas. J Neurooncol 1999;45(2):141–57. [DOI] [PubMed] [Google Scholar]
- 32.Hong JJ, Rosenberg SA, Dudley ME, Yang JC, White DE, Butman JA, et al. Successful treatment of melanoma brain metastases with adoptive cell therapy. Clin Cancer Res 2010;16(19):4892–8 doi 10.1158/1078-0432.CCR-10-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehta GU, Malekzadeh P, Shelton T, White DE, Butman JA, Yang JC, et al. Outcomes of Adoptive Cell Transfer With Tumor-infiltrating Lymphocytes for Metastatic Melanoma Patients With and Without Brain Metastases. J Immunother 2018;41(5):241–7 doi 10.1097/CJI.0000000000000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simoni Y, Becht E, Fehlings M, Loh CY, Koo SL, Teng KWW, et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 2018;557(7706):575–9 doi 10.1038/s41586-018-0130-2. [DOI] [PubMed] [Google Scholar]
- 35.Schuessler A, Smith C, Beagley L, Boyle GM, Rehan S, Matthews K, et al. Autologous T-cell therapy for cytomegalovirus as a consolidative treatment for recurrent glioblastoma. Cancer Res 2014;74(13):3466–76 doi 10.1158/0008-5472.CAN-14-0296. [DOI] [PubMed] [Google Scholar]
- 36.Reap EA, Suryadevara CM, Batich KA, Sanchez-Perez L, Archer GE, Schmittling RJ, et al. Dendritic Cells Enhance Polyfunctionality of Adoptively Transferred T Cells That Target Cytomegalovirus in Glioblastoma. Cancer Res 2018;78(1):256–64 doi 10.1158/0008-5472.CAN-17-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cobbs CS, Harkins L, Samanta M, Gillespie GY, Bharara S, King PH, et al. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res 2002;62(12):3347–50. [PubMed] [Google Scholar]
- 38.Prins RM, Cloughesy TF, Liau LM. Cytomegalovirus immunity after vaccination with autologous glioblastoma lysate. N Engl J Med 2008;359(5):539–41 doi 10.1056/NEJMc0804818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheurer ME, Bondy ML, Aldape KD, Albrecht T, El-Zein R. Detection of human cytomegalovirus in different histological types of gliomas. Acta Neuropathol 2008;116(1):79–86 doi 10.1007/s00401-008-0359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchell DA, Xie W, Schmittling R, Learn C, Friedman A, McLendon RE, et al. Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro Oncol 2008;10(1):10–8 doi 10.1215/15228517-2007-035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lucas KG, Bao L, Bruggeman R, Dunham K, Specht C. The detection of CMV pp65 and IE1 in glioblastoma multiforme. J Neurooncol 2011;103(2):231–8 doi 10.1007/s11060-010-0383-6. [DOI] [PubMed] [Google Scholar]
- 42.Soroceanu L, Matlaf L, Bezrookove V, Harkins L, Martinez R, Greene M, et al. Human cytomegalovirus US28 found in glioblastoma promotes an invasive and angiogenic phenotype. Cancer Res 2011;71(21):6643–53 doi 10.1158/0008-5472.CAN-11-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi BD, Curry WT, Carter BS, Maus MV. Chimeric antigen receptor T-cell immunotherapy for glioblastoma: practical insights for neurosurgeons. Neurosurg Focus 2018;44(6):E13 doi 10.3171/2018.2.FOCUS17788. [DOI] [PubMed] [Google Scholar]
- 44.Park JH, Riviere I, Gonen M, Wang X, Senechal B, Curran KJ, et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med 2018;378(5):449–59 doi 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abramson JS, McGree B, Noyes S, Plummer S, Wong C, Chen YB, et al. Anti-CD19 CAR T Cells in CNS Diffuse Large-B-Cell Lymphoma. N Engl J Med 2017;377(8):783–4 doi 10.1056/NEJMc1704610. [DOI] [PubMed] [Google Scholar]
- 46.O’Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med 2017;9(399) doi 10.1126/scitranslmed.aaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahmed N, Brawley V, Hegde M, Bielamowicz K, Kalra M, Landi D, et al. HER2-Specific Chimeric Antigen Receptor-Modified Virus-Specific T Cells for Progressive Glioblastoma: A Phase 1 Dose-Escalation Trial. JAMA Oncol 2017;3(8):1094–101 doi 10.1001/jamaoncol.2017.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown CE, Badie B, Barish ME, Weng L, Ostberg JR, Chang WC, et al. Bioactivity and Safety of IL13Ralpha2-Redirected Chimeric Antigen Receptor CD8+ T Cells in Patients with Recurrent Glioblastoma. Clin Cancer Res 2015;21(18):4062–72 doi 10.1158/1078-0432.CCR-15-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wikstrand CJ, McLendon RE, Friedman AH, Bigner DD. Cell surface localization and density of the tumor-associated variant of the epidermal growth factor receptor, EGFRvIII. Cancer Res 1997;57(18):4130–40. [PubMed] [Google Scholar]
- 50.Mineo JF, Bordron A, Baroncini M, Maurage CA, Ramirez C, Siminski RM, et al. Low HER2-expressing glioblastomas are more often secondary to anaplastic transformation of low-grade glioma. J Neurooncol 2007;85(3):281–7 doi 10.1007/s11060-007-9424-1. [DOI] [PubMed] [Google Scholar]
- 51.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 2010;18(4):843–51 doi 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jarboe JS, Johnson KR, Choi Y, Lonser RR, Park JK. Expression of interleukin-13 receptor alpha2 in glioblastoma multiforme: implications for targeted therapies. Cancer Res 2007;67(17):7983–6 doi 10.1158/0008-5472.CAN-07-1493. [DOI] [PubMed] [Google Scholar]
- 53.Mount CW, Majzner RG, Sundaresh S, Arnold EP, Kadapakkam M, Haile S, et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M(+) diffuse midline gliomas. Nat Med 2018;24(5):572–9 doi 10.1038/s41591-018-0006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sampson JH, Choi BD, Sanchez-Perez L, Suryadevara CM, Snyder DJ, Flores CT, et al. EGFRvIII mCAR-modified T-cell therapy cures mice with established intracerebral glioma and generates host immunity against tumor-antigen loss. Clin Cancer Res 2014;20(4):972–84 doi 10.1158/1078-0432.CCR-13-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suryadevara CM, Desai R, Abel ML, Riccione KA, Batich KA, Shen SH, et al. Temozolomide lymphodepletion enhances CAR abundance and correlates with antitumor efficacy against established glioblastoma. Oncoimmunology 2018;7(6):e1434464 doi 10.1080/2162402X.2018.1434464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med 2018;24(6):739–48 doi 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- 57.Bielamowicz K, Fousek K, Byrd TT, Samaha H, Mukherjee M, Aware N, et al. Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma. Neuro Oncol 2018;20(4):506–18 doi 10.1093/neuonc/nox182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Genssler S, Burger MC, Zhang C, Oelsner S, Mildenberger I, Wagner M, et al. Dual targeting of glioblastoma with chimeric antigen receptor-engineered natural killer cells overcomes heterogeneity of target antigen expression and enhances antitumor activity and survival. Oncoimmunology 2016;5(4):e1119354 doi 10.1080/2162402X.2015.1119354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Han J, Chu J, Keung Chan W, Zhang J, Wang Y, Cohen JB, et al. CAR-Engineered NK Cells Targeting Wild-Type EGFR and EGFRvIII Enhance Killing of Glioblastoma and Patient-Derived Glioblastoma Stem Cells. Sci Rep 2015;5:11483 doi 10.1038/srep11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hegde M, Corder A, Chow KK, Mukherjee M, Ashoori A, Kew Y, et al. Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Mol Ther 2013;21(11):2087–101 doi 10.1038/mt.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kosaka A, Ohkuri T, Ikeura M, Kohanbash G, Okada H. Transgene-derived overexpression of miR-17–92 in CD8+ T-cells confers enhanced cytotoxic activity. Biochem Biophys Res Commun 2015;458(3):549–54 doi 10.1016/j.bbrc.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wing A, Fajardo CA, Posey AD Jr., Shaw C, Da T, Young RM, et al. Improving CART-Cell Therapy of Solid Tumors with Oncolytic Virus-Driven Production of a Bispecific T-cell Engager. Cancer Immunol Res 2018;6(5):605–16 doi 10.1158/2326-6066.CIR-17-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rafiq S, Yeku OO, Jackson HJ, Purdon TJ, van Leeuwen DG, Drakes DJ, et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol 2018;36(9):847–56 doi 10.1038/nbt.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer 2018;18(5):313–22 doi 10.1038/nrc.2018.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Keu KV, Witney TH, Yaghoubi S, Rosenberg J, Kurien A, Magnusson R, et al. Reporter gene imaging of targeted T cell immunotherapy in recurrent glioma. Sci Transl Med 2017;9(373) doi 10.1126/scitranslmed.aag2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Okada H, Weller M, Huang R, Finocchiaro G, Gilbert MR, Wick W, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol 2015;16(15):e534–e42 doi 10.1016/S1470-2045(15)00088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]