Innate immunity is the first line of defense in mammals to protect the host during early infection by pathogens, including viruses, bacteria, fungi, and parasites. Different cellular receptors detect nucleic acids of pathogens and then trigger rapid innate immune responses to the pathogen in a nonspecific manner. This process is called innate immune sensing, which induces the expression of protective cytokines to inhibit pathogens. For example, viral infection-induced type I interferons (IFN-I) are important antiviral cytokines in innate immunity. However, pathogens evolve various mechanisms to avoid innate surveillance to establish successful infections in the host. Some viruses encode their own proteins or exploit host cell machinery to counteract innate immune responses.
More than 100 types of chemical modifications have been identified in cellular RNAs, which play a critical role in regulating gene expression. One of the most common RNA modifications is 2′-O-methylation (or Nm, where N can be any nucleotide) found in host tRNA, rRNA, mRNA, and small noncoding RNA.1 Eukaryote cellular mRNAs are methylated at the 2′-O positions of the 5′-guanosine cap by methyltransferases (MTases). The Nm of cellular mRNA represents a molecular mark for the host immune system to distinguish it from non-self-RNA of pathogens lacking Nm. However, some viruses (such as West Nile virus) encode their own 2′-O-MTase to modify the 5′ cap of viral RNA to subvert host innate responses mediated by IFN-induced antiviral proteins.2 Ebola virus and Flaviviruses also use their own 2′-O-MTase to methylate internal adenosines of viral RNA genomes, suggesting that the Nm of viral RNA is a conserved mechanism of virus immune evasion.
Many viruses do not encode their own 2′-O-MTase and may exploit cellular 2′-O-MTase to methylate viral RNA to escape from innate immune sensing. Human immunodeficiency virus type 1 (HIV-1) is the AIDS-causing retrovirus that contains two copies of the RNA genome. Each copy of HIV-1 RNA genome has ∼9173 nucleotides that comprise nine genes and encode 15 viral proteins. HIV-1 does not encode a 2′-O-MTase, and whether HIV-1 RNA contains Nm was previously unknown. A recent publication by Ringeard and colleagues revealed that HIV-1 recruits a cellular 2′-O-MTase named FTSJ3, leading to Nm of viral RNA and thereby avoiding innate immune sensing in host cells.3 This study identified a new mechanism by which HIV-1 hijacks cellular proteins to modify its RNA genome and hide from host immune surveillance.
Trans-activation-responsive (TAR) RNA-binding protein (TRBP) is a double-stranded RNA-binding protein that facilitates HIV-1 expression through interaction with the 5′ leader sequence TAR and the Rev response element of HIV-1 RNA. TRBP is also associated with RNA interfering machinery and interacts with Dicer and other cellular proteins in the RNA-induced silencing complex. Using tandem immunoaffinity purification, mass spectrometry, and an MTase activity assay, Ringeard et al. found that FTSJ3 is a cellular 2′-O-MTase associated with TRBP overexpressed in HeLa cells. Additional experiments showed that the FTSJ3−TRBP complex is recruited to HIV-1 viral RNA in a TRBP-dependent but Dicer-independent manner. Using RiboMethSeq analysis, they found that HIV-1 RNA isolated from viral particles is internally 2′-O-methylated by FTSJ3 at 17 high-confidence residues across the HIV-1 RNA genome, including 15 adenosine and two uracil residues. These RNA residues are highly conserved among major subtypes of HIV-1, suggesting that these 2′-O-methylated sites exist in diverse HIV-1 isolates.
The authors demonstrated that infection with HIV-1 particles produced in FTSJ3 knockdown cells induced IFN-I (IFN-α and IFN-β) mRNA expression but inhibited HIV-1 expression in myeloid cells. It is known that cytoplasmic RNA sensors, such as MDA5 (melanoma differentiation-associated protein 5) and RIG-I (retinoic acid-inducible gene I), recognize viral RNAs and induce IFN-I production to establish an antiviral state in cells. The authors silenced MDA5 or RIG-I in monocytic U937 cells and then infected cells with HIV-1 produced from FTSJ3-silenced or TRBP-silenced HEK293T cells, which induced higher levels of IFN-I expression compared to that of wild-type HIV-1 produced in control cells. The level of IFN-I induction was reduced in MDA5-silenced but not RIG-I-silenced U937 cells, suggesting that the FTSJ3−TRBP complex 2′-O-methylates HIV-1 RNA, thereby allowing HIV-1 to escape from MDA5-mediated sensing.
Myeloid cells, including monocytes and their differentiated dendritic cells or macrophages, play an important role in innate immune responses to pathogens. Thus, Ringeard et al. assessed the immunostimulatory activity of viral particles containing poorly methylated HIV-1 RNA in human primary monocyte-derived dendritic cells and macrophages. It is known that phosphorylation of interferon regulatory factor 3 (IRF3), IRF7, and signal transducer and activator of transcription 1 (STAT1) drives induction and activation of IFN-I signaling. Compared to the wild-type virus, HIV-1 produced from FTSJ3-silenced cells induced strong phosphorylation of IRF-3, IRF-7, and STAT1 and induced a significant increase in the level of induction of IFN-I in myeloid cells. HIV-1 produced from FTSJ3-silenced cells also replicated less efficiently than wild-type HIV-1 in a spreading infection as measured by viral capsid protein released from infected dendritic cells. Notably, a reduced level of HIV-1 replication was partially rescued using antibodies to block IFN-α/β receptors on dendritic cells, suggesting that unmethylated viral RNA leads to innate immune sensing of HIV-1 and inhibition of viral replication in myeloid cells (Figure 1).
Figure 1.
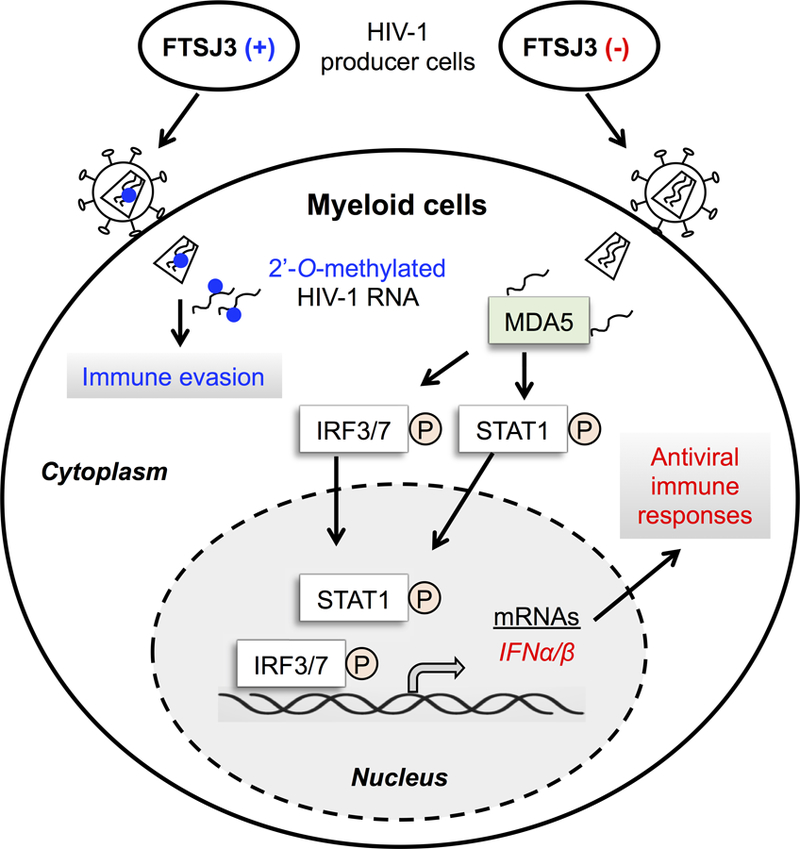
HIV-1 exploits the cellular 2′-O-methyltransferase TSJ3 to methylate viral RNA to escape from innate immune surveillance. In HIV-1 producer cells, TRBP (not shown) recruits FTSJ3 to HIV-1 RNA and leads to internal 2′-O-methylation (blue dots) of viral RNA. HIV-1 with 2′-O-methylated RNA avoids innate sensing in infected myeloid cells, thereby escaping immune surveillance. Silencing FTSJ3 (−) expression in HIV-1 producer cells yields viruses lacking 2′-O-methylation of viral RNA. When HIV-1 with unmethylated RNA infects myeloid cells, the cytoplasmic protein MDA5 recognizes unmethylated HIV-1 RNA as non-self nucleic acids and triggers phosphorylation (indicated by the letter P) of the transcription factors IRF3, IRF7, and STAT1. Phosphorylation of these transcription factors leads to IFN-α/β expression and generates antiviral innate immune responses in HIV-1-infected myeloid cells.
The discovery of HIV-1 RNA Nm by Ringeard et al.3 also raised many questions to be addressed in attempts to further understand the mechanisms. Does the Nm of HIV-1 RNA affect its nuclear export? Can the Nm of HIV-1 RNA and similar effects occur in primary CD4+ T-cells (the major target cells of HIV-1)? Can the Nm of HIV-1 RNA directly affect viral infectivity in cells or in vivo? Does Nm occur in the RNA of other retroviruses and act as an immune evasion strategy? Can unmethylated HIV-1 RNA induce expression of interferon-stimulated genes in target cells?
Additional modifications of HIV-1 RNA may exist and play a synergistic role to avoid the innate immune responses to HIV-1 in host cells. N6-Methyladenosine (m6A) modification of HIV-1 RNA regulates viral replication and protein expression. Recent studies reported that RNA m6A modification enzymes negatively regulate the innate immune response to infection of human cytomegalovirus, influenza A virus, adenovirus, or vesicular stomatitis virus by targeting IFN-I.4,5 However, whether m6A modification of HIV-1 RNA contributes to viral immune invasion remains unknown. Further studying the functions and mechanisms of HIV-1 RNA modifications will improve our understanding of virus−host interactions and potentially aid in the development of new approaches to eradicate HIV-1 infection.
ACKNOWLEDGMENTS
The author thanks Dr. Corine St. Gelais for editing the manuscript.
Funding
The author and work in the author’s lab are supported by National Institutes of Health Grants R01AI120209 and R01GM128212.
Footnotes
Notes
The author declares no competing financial interest.
REFERENCES
- (1).Dimitrova DG, Teysset L, and Carre C (2019) RNA 2’-O-Methylation (Nm) Modification in human diseases. Genes 10, No. E117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Daffis S, Szretter KJ, Schriewer J, Li J, Youn S, Errett J, Lin TY, Schneller S, Zust R, Dong H, Thiel V, Sen GC, Fensterl V, Klimstra WB, Pierson TC, Buller RM, Gale M Jr., Shi PY, and Diamond MS (2010) 2’-O methylation of the viral mRNA cap evades host restriction by IFIT family members. Nature 468, 452–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Ringeard M, Marchand V, Decroly E, Motorin Y, and Bennasser Y (2019) FTSJ3 is an RNA 2’-O-methyltransferase recruited by HIV to avoid innate immune sensing. Nature 565, 500–504. [DOI] [PubMed] [Google Scholar]
- (4).Rubio RM, Depledge DP, Bianco C, Thompson L, and Mohr I (2018) RNA m6A modification enzymes shape innate responses to DNA by regulating interferon beta. Genes Dev 32, 1472–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Winkler R, Gillis E, Lasman L, Safra M, Geula S, Soyris C, Nachshon A, Tai-Schmiedel J, Friedman N, Le-Trilling VTK, Trilling M, Mandelboim M, Hanna JH, Schwartz S, and Stern-Ginossar N (2019) m6A modification controls the innate immune response to infection by targeting type I interferons. Nat. Immunol 20, 173–182. [DOI] [PubMed] [Google Scholar]


