Abstract
Previous studies have reported single B cell related chronic graft-versus-host disease diagnostic (cGvHD) biomarkers such as B cell activating factor (BAFF), CD21low and immature B cells, but research on the performance of biomarker combinations and the covariate effect of steroids is lacking. The primary objective of this study was to determine the most accurate combination of B cell populations using cell surface staining flow cytometry in an independent cGvHD cohort. Secondary objectives included assessing the effect of corticosteroid use at sample collection on the make-up and accuracy of the diagnostic panel and identifying the mechanism underlying low surface expression of BAFF receptor (BAFF-R) on B cells in cGvHD. Flow cytometric analysis was performed in an adult cohort of post-HCT patients with cGvHD onset (n = 44) and time-matched recipients without cGvHD (n = 63). We confirmed that the onset of cGvHD was associated with higher soluble BAFF (sBAFF) levels, elevated numbers of CD27−CD10−CD21low CD19+ B cells and classical switched memory B cells, and reduced numbers of transitional and naïve B cells. The highest single B cell population area under the Receiver Operator Characteristic (ROC) curve (AUC) was 0.72 for transitional type 1 CD21low B cells. We also showed a significant inverse relationship between sBAFF and surface BAFF-R expression caused by sBAFF modulation of BAFF-R. Steroid use at sample collection influenced the significance of the sBAFF: B cell ratio, naïve and marginal zone-like B cells. The optimal combination of B cell subsets most significantly associated with cGvHD onset with or without concurrent corticosteroid use resulted in ROC AUC of 0.87 and 0.84 respectively. Transitional and CD21low B cells were the only populations present in both panels; however only analyzing them resulted in ROC AUC of 0.79 and 0.78 respectively. This suggests that the inclusion of other populations and use of different panels depending on steroid use is necessary to achieve better accuracy. Soluble BAFF was not part of either panel. These novel B cell profiles could be prospectively tested in patients post-HSCT and lead to focused mechanistic studies.
INTRODUCTION
Chronic graft-versus-host disease (cGvHD) is now the leading cause of late non-relapse mortality and morbidity following allogeneic hematopoietic cell transplantation (HCT) [1]. The pathogenic role of B cells in cGvHD was first identified in murine models in 1995 [2]. The role of abnormalities in B cell function have been confirmed in humans due to the successful use of B cell directed therapies such as rituximab [3] and approval of the first FDA approved drug for cGvHD therapy, ibrutinib [4].
One of the most consistent B cell associated abnormalities that has been identified in cGvHD is high levels of soluble B-cell activating factor (BAFF) which are correlated inversely with BAFF receptor expression, its principal receptor, on B cells [5,6]. BAFF is a critical soluble factor necessary for normal peripheral B-cell maturation and survival [7]. The importance of BAFF and its principal receptor, BAFF-R to peripheral B-cell maturation and survival is demonstrated by presence of arrested B cell development at the stage of transitional B cells and severe reduction of all subsequent B cell stages in mice lacking BAFF [8] or BAFF-R [9] and humans lacking BAFF-R [10]. In contrast, transgenic mice overexpressing BAFF develop lymphocytic disorders with autoimmune manifestations and have vastly increased numbers of mature B cells [11]. It has previously been shown that B cells in cGvHD express lower levels of surface BAFF receptor, in contrast to patients post-HCT without cGvHD who have supranormal B cell numbers with higher levels of surface BAFF-R that could act as a sink for excessive soluble BAFF (sBAFF). It has been hypothesized that this leads to higher levels of circulating sBAFF in cGvHD which rescues autoreactive B cell clones [12]. It has been shown that increased BAFF occupancy of BAFF-R accounted for low BAFF-R detection by antibody on CD19+ B cells in cGvHD [13].
Chronic GvHD is also associated with defects in B cell development including decreased numbers of naïve B cells and increasing numbers of antigen-experienced CD27+ B cells in the setting of total B cell lymphopenia [14]. A B cell subpopulation that has received considerable attention is CD21low B cells [15,16]. In normal B cell development, the differential expression of CD21 identifies 2 subsets of CD10+ transitional B cells. CD21low transitional B cells (T1 B cells) are less mature than the CD21+/hi subset (T2 B cells) [17]. Low CD21 expression also defines CD19lowCD27− plasmablasts. In contrast, a recent report characterized the expanded CD21low population in cGvHD as CD10−, CD27− and CD20hi with features of exhaustion including increased expression of inhibitory receptors, altered expression of chemokine and adhesion molecules, and poor proliferative response and calcium flux in response to B cell receptor triggering [18].
In routine clinical practice, the disease manifestations of cGvHD are diverse and result from inflammatory changes in a variety of organs. Sometimes patients with clinically suspected cGvHD begin first-line treatment with corticosteroids before the diagnosis is confirmed and potentially before peak grade is achieved. Furthermore, many patients have already experienced acute GvHD and are continuing corticosteroid therapy. Therefore, it is crucial to better understand how corticosteroid use at the time of potential cGvHD diagnosis influences the significance of B cell-related biomarkers.
We took an integrative approach using blood samples from patients with cGVHD to better characterize the relationship between the different B cell factors and populations in cGvHD. We evaluated the relative relationship of CD21lowB cells, memory and immature B cells, sBAFF, and BAFF-R and found unique combinations of B cell markers with and without concomitant steroid use that were strongly associated with cGvHD. We hypothesized that our study establishes a framework from which further mechanistic studies could be pursued.
METHODS
Sample collection and processing protocol
Patient plasma samples were collected as part of the Chronic GvHD Consortium Protocol 6501 cohort study as previously described with appropriate consent [19]. The remaining white blood cells were separated by density gradient using Ficoll-Paque PLUS (GE Healthcare®). Following platelet depletion, cells were slowly frozen in 10% dimethyl sulfoxide (DMSO)/fetal bovine serum (FBS) at −80°C and stored in the vapor phase of liquid nitrogen until analysis. Blood was also collected from healthy donors at BC Children’s Hospital as part of a local research study approved by our Institutional Review Board.
Reagents
The recombinant human soluble BAFF was an unmodified 152 amino acid polypeptide (aa 134–285 of full length BAFF) produced in E. coli with molecular weight of 17.0 kDa (PeproTech, catalog # 310–13). Mass spectrometry confirmed the molecular weight. The human 697 relapsed pre-B ALL cell line was purchased from DSMZ (Braunschweig, Germany). Belimumab (GSK Canada) was purchased from our hospital pharmacy.
Isolation of Healthy Donor CD19+ B cells
Human B cells were purified from freshly isolated peripheral blood mononuclear cells using the StemSep® Human B Cell Enrichment kit according to the manufacturer’s instructions (StemCell Technologies). The purity of the CD19+ fraction was typically >95% and was determined by CD19 expression by FACS.
BAFF enzyme-linked immunosorbent assay
Measurements of soluble BAFF were done in thawed plasma using a quantitative sandwich enzyme immunoassay according to the manufacturer’s instructions (Quantikine Human BAFF/BLyS kit, R&D Systems Cat. SBLYS0).
Flow cytometric analysis of peripheral B cells
Flow cytometric analysis was performed on thawed cells. Antibodies used for flow cytometry were as follows: IgD-BV510 (clone IA6–2), CD21-FITC (clone Bu32), CD19-Pacific Blue (clone HIB19), CD27-BV785 (clone O323), CD38-PE (clone HIT2), CD10-APC (clone HI10), CD5-PE-Cy7 (clone UCH2), CD24-PE-DAZZLE 594 (clone ML5), BAFF-R-APC-Cy7 (clone 11C1) and 7AAD (All are from BioLegend). We choose to use the 11C1 clone for the anti-BAFF-R antibody as it has previously been shown not to be sensitive to receptor occupancy with BAFF allowing a proper determination of surface BAFF-R expression [20–22]. Gating strategy is shown in supplemental Figure 1. The lymphocyte gate was established using forward and side scatter. Only CD19+ B cells were analyzed to ensure that CD3+ cells were not included in the analysis of CD38 or CD27 expression.
Statistical methods
Data were analyzed both as actual values and after log-transformation. The latter approach is sensitive to effects that are associated with proportional changes rather than absolute changes, and we had no basis to assume which was more appropriate for any given variable. For ease of interpretation, mean differences on the log scale data were re-expressed as fold changes. Differences between cGvHD case and control groups were assessed by 2-sample t-test. With cGvHD status as the dependent variable, logistic regression was used with forward selection to define optimal combinations of B cell variables associated with cGvHD. Results adjusted for any steroid use at sample collection are from a multivariate regression model that includes cGvHD status and steroid use. The Spearman rank test was used for correlation analysis. All p-values are 2-sided and unadjusted for multiple comparisons.
RESULTS
Study population
We analyzed B cell related parameters in samples from 107 patients consisting of 44 with cGvHD onset (median of 207 days post-HCT; range 83–424 days) and 63 patients without cGvHD with sample collection a median of 194 days post-HCT (range 153–430 days). Onset cGvHD samples were taken a median of 6 days post diagnosis of cGvHD (range of −12 to 29 days). There were no significant differences in the patients with cGvHD versus those that did not in patient or transplantation characteristics except stem cell source and usage of steroids at sample collection (Table 1). The cGvHD cohort had a significantly higher frequency of peripheral blood stem cells as a graft source (84% vs. 51%), less cord blood (9% vs. 43%) and a higher percentage of patients were taking steroids at sample collection (43% vs. 10%). Our initial analyses were performed on a sub-cohort of patients who were not taking steroids at sample collection (25 cGvHD onset and 57 controls). A second analysis was performed in the total cohort after adjusting for the effect of steroids. There were no statistically significant differences in absolute lymphocyte count (ALC), % CD19+ of ALC or absolute CD19+ counts between patients with cGvHD and patients post-HCT without cGvHD in both the no steroid and total (included those who received steroids) cohorts.
Table 1.
Patient and transplantation characteristics.
| Characteristics | cGvHD (n=44) | No cGvHD (n=63) |
|---|---|---|
| Time from HCT to sample collection | ||
| Early (<9 months) | 29 (66) | 36 (57) |
| Late (>9 months) | 15 (34) | 27 (43) |
| Prior acute GvHD | ||
| No | 22 (50) | 27 (43) |
| Yes | 22 (50) | 36 (57) |
| Age | ||
| <50 years | 16 (36) | 25 (40) |
| ≥50 years | 28 (64) | 38 (60) |
| Donor | ||
| Matched related | 19 (43) | 17 (27) |
| Matched unrelated | 14 (32) | 17 (27) |
| Mismatched | 11 (25) | 29 (46) |
| Stem cell source § | ||
| PBSC | 37 (84) | 32 (51) |
| BM | 3 (7) | 4 (6) |
| Cord | 4 (9) | 27 (43) |
| Conditioning | ||
| Myeloablative w/o TBI | 8 (18) | 8 (13) |
| Myeloablative with TBI | 10 (23) | 19 (30) |
| Non-myeloablative | 26 (59) | 36 (57) |
| GvHD prophylaxis | ||
| CNI + MTX/MMF ± Sirolimus | 38 (86) | 48 (76) |
| CNI ± Sirolimus | 6 (14) | 10 (16) |
| Other | 0 | 5 (8) |
| Gender | ||
| Male | 26 (59) | 34 (54) |
| Female | 18 (41) | 29 (46) |
| Female donor to male patient | ||
| No | 34 (77) | 49 (78) |
| Yes | 10 (23) | 14 (22) |
| CMV serostatus | ||
| Negative | 20 (45) | 26 (41) |
| Positive | 24 (55) | 37 (59) |
| Disease diagnosis | ||
| AML | 11 (25) | 25 (40) |
| ALL | 7 (16) | 9 (14) |
| CML | 1 (2) | 4 (6) |
| CLL | 2 (5) | 2 (3) |
| MDS | 12 (27) | 9 (14) |
| HL/NHL | 7 (16) | 10 (16) |
| MM | 3 (7) | 2 (3) |
| Other | 1 (2) | 2 (3) |
| On steroids at sample collection § | ||
| No | 25 (57) | 57 (90) |
| Yes | 19 (43) | 6 (10) |
| Days from HCT to cGvHD, median (range) | 207 (83 – 424) | |
| Days from cGvHD to sample, median (range) | 6 (−12 – 29) | |
| Skin involvement | 61% | |
| Oral involvement | 66% | |
| GI involvement | 23% | |
| Eye involvement | 45% | |
| Joint involvement | 15% | |
| Lung involvement | 47% | |
| Liver involvement | 51% | |
| Genital involvement | 16% | |
| NIH overall severity: mild / moderate / severe | 16% / 29% / 25% | |
PBSC, peripheral blood stem cells; BM, bone marrow; TBI, total body irradiation; CNI, calcineurin inhibitor; MTX, methotrexate; MMF, mycophenolate mofetil; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; CML, chronic myeloid leukemia; CLL, chronic lymphocytic leukemia; MDS, myelodysplastic syndrome; HL, Hodgkin’s lymphoma; NHL, non-Hodgkin’s lymphoma; MM, multiple myeloma
- P < .01; the difference between groups is considered to be statistically significant
BAFF receptor surface expression is associated with soluble BAFF levels in cGvHD in the absence of steroids
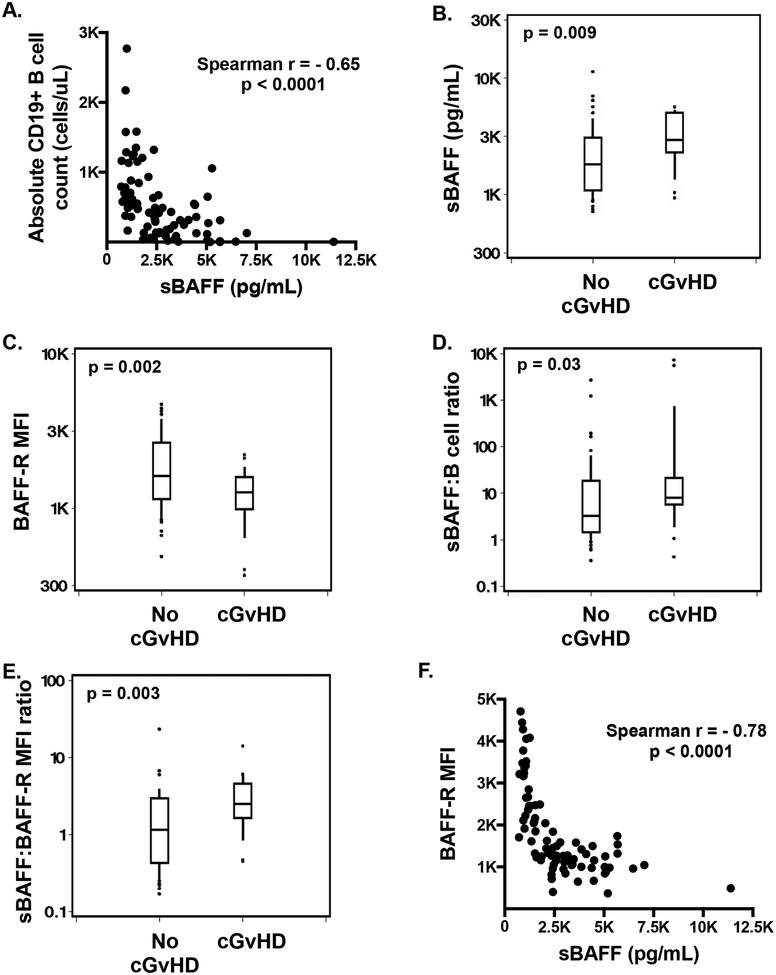
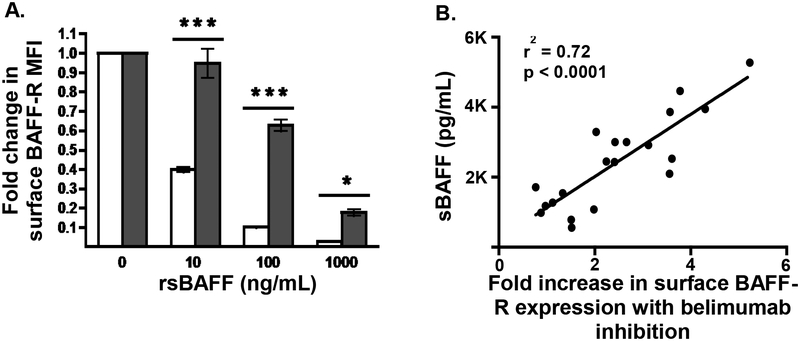
Since steroids have been shown to have a significant effect on B cell associated factors after HCT [6], our initial analyses were limited to patients who were not receiving steroids at the onset of cGvHD compared to a non cGvHD cohort without steroids. Similar to previously reported [19], we found a strong inverse relationship between sBAFF and the CD19+ cell counts (cells/uL) (r= −0.65, p<0.0001; Figure 1A) and found significantly higher sBAFF levels (1.5 fold, p=0.009; Figure 1B) in patients with cGvHD. In addition, we found significantly lower (0.7 fold, p=0.002; Figure 1C) mean fluorescence intensity (MFI) of BAFF-R surface expression on CD19+ B cells from patients with cGvHD. There was also a significantly higher ratio of sBAFF to CD19+ B cells (2.9 fold, p=0.03; Figure 1D) and BAFF-R surface expression (2.2 fold, p=0.003; Figure 1E) in cGvHD patients. Soluble BAFF levels had a strong inverse relationship with BAFF-R MFI (r= −0.78, p<0.0001; Figure 1F), but not because of competition between sBAFF and the monoclonal antibody (11C1) detecting surface BAFF-R (Figure 2A). Plasma from patients post-HCT decreased the surface expression of BAFF-R on the pre-B acute lymphoblastic cell line, 697, an effect mediated by sBAFF, as demonstrated by increased expression of BAFF-R when sBAFF was neutralized with belimumab. The effect of blocking sBAFF with belimumab on surface BAFF-R expression was strongly correlated to sBAFF concentration (r=0.72, Figure 2B).
Figure 1. Relationships of sBAFF and BAFF-R expression.
A. Correlation between CD19+ B cell count and sBAFF, B-E. Differences in sBAFF, BAFF-R MFI, sBAFF:B cell and sBAFF:BAFF-R ratios between patients with cGvHD onset and no cGvHD post-HCT, F. Correlation between BAFF-R MFI and sBAFF.
Figure 2. Effect of sBAFF on surface BAFF-R expression.
A. Healthy donor isolated CD19+ B cells (n=5) were incubated overnight in complete media (10% FCS in 1640 RPMI) at 37°C with stated concentrations of recombinant soluble BAFF and surface expression of BAFF-R was measured by flow cytometry and compared to complete media alone (white bars). To measure the effect of receptor occupancy on flow cytometry antibody binding caused by rsBAFF binding to BAFF-R, the same concentrations of rsBAFF were incubated with the same healthy donor CD19+ B cells at 4°C (gray bars). The bars represent means ± SEM compared using paired T test. MFI, mean fluorescence intensity. *P < .05 ***P < .001, B. Plasma from patients post-HCT down-modulates surface expression of BAFF-R on pre-B ALL 697 cells. 697 cells were incubated with a 1:1 mix of complete media and patient plasma (n=20) with or without belimumab (10 ug/mL) overnight and surface expression of BAFF-R was measured by flow cytometry. The MFI was compared between both conditions for each sample and expressed as fold increase in BAFF-R due to sBAFF inhibition by belimumab. This was then correlated to soluble BAFF levels.
Evaluation of CD19+21low B cell subsets associated with cGvHD onset in the absence of steroids
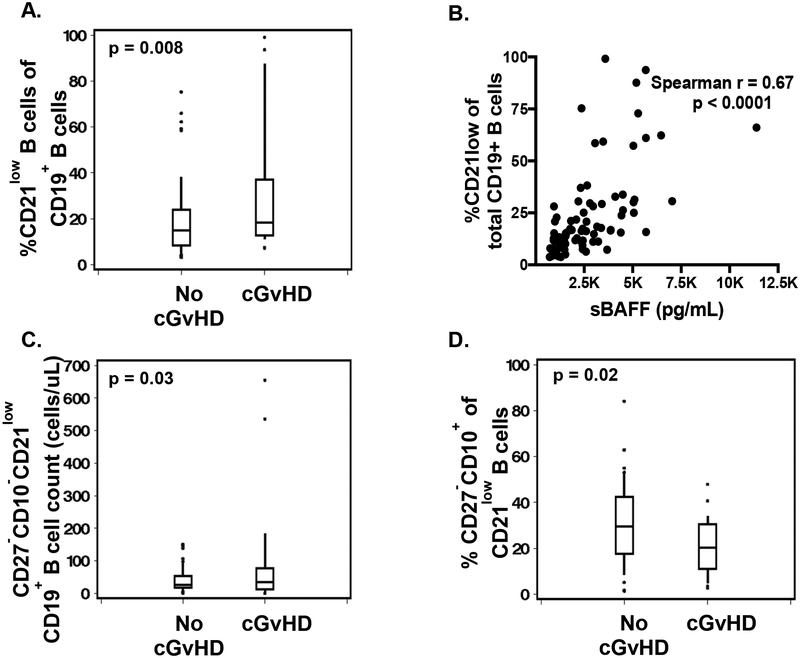
We evaluated whether the CD27−CD10− subpopulation of CD21lowCD19+ B cells was associated with cGvHD in our study population. We found significantly higher percentages of CD21low cells within the total CD19+ B cell pool (+14%, p = 0.008; Figure 3A) in patients with cGvHD as compared to post HCT non-cGvHD controls. Soluble BAFF levels had a strong positive relationship to the percentage of CD21low B cells (r=0.67, p<0.0001; Figure 3B). The absolute number of CD27−CD10−CD21low CD19+ B cells was significantly higher in cGvHD patients (+48 cells/μL, p = 0.03; Figure 3C). Further analysis of this subpopulation showed that they expressed intermediate to high levels of IgD and CD38. CD21low CD19+ B cells also contained significantly lower percentages of T1 transitional CD27−CD10+ B cells in patients with cGvHD (−9%, p=0.02; Figure 3D).
Figure 3. CD21low B cell populations.
A, C and D. Differences in %CD21low B cells, CD27-CD10−CD21lowCD19+ cells and %CD27−CD10+ of CD19+CD21low B cells between patients with cGvHD onset and no cGvHD, B. Correlation between %CD21low of total CD19+ B cells and sBAFF.
Evaluation for other B cell subsets associated with cGvHD onset in the absence of steroids
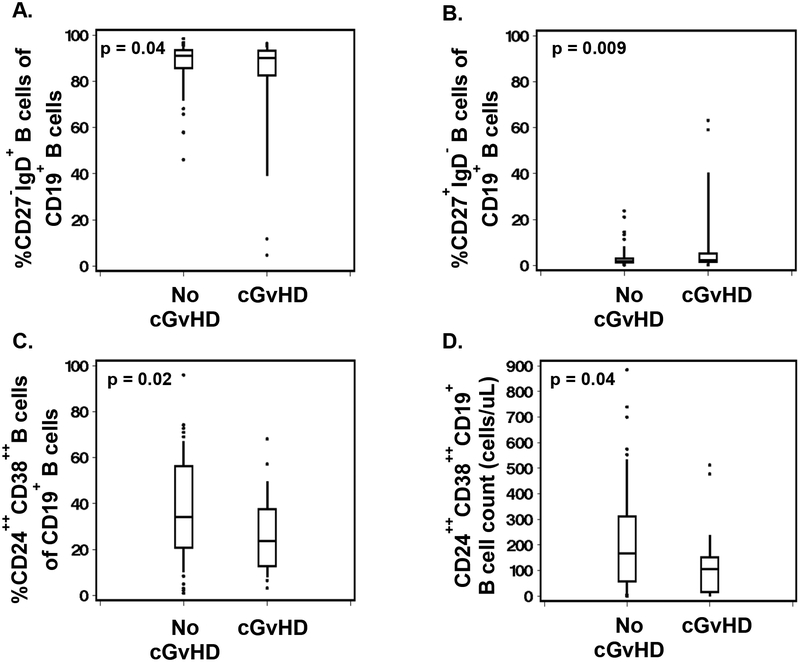
We further evaluated other B cell subsets associated with cGvHD including: a) naïve B cells (CD27−IgD+); classical switched memory B cells (CD27+IgD−); non-switched memory or marginal zone-like B cells (CD27+IgD+), double negative memory B cells (CD27−IgD−), and transitional B cells (CD19+CD24highCD38high). There was a significantly lower percentage of naïve B cells (−9%, p=0.04; Figure 4A) and higher percentage of classical switched memory B cells (+7%, p=0.009; Figure 4B) in cGvHD patients. Finally, cGVHD was associated with lower percentages and absolute numbers of transitional cells (−11%, p = 0.02; −93 cells/μL, p = 0.04; Figures 4C and D).
Figure 4. Other B cell subsets associated with cGvHD onset.
A-D. Differences in naïve B cells (CD27−IgD+), classical switched memory B cells (CD27+IgD−) and transitional B cells (CD19+CD24highCD38high) between patients with cGvHD onset and no cGvHD.
The impact of steroids on B cell populations at cGvHD onset
The significant B cell populations identified in the no steroid sub-cohort were analyzed in the entire cohort after adjusting for the effect of steroid use (Table 2). There were no longer any significant differences in sBAFF: B cell ratio and naïve B cells between patients with cGvHD onset and controls. All other differences remained significant.
Table 2.
B cell variables considering steroid use at sample collection
| B cell variable | 25 cases and 57 controls – no steroid patients included (univariate AUC) | 44 cases and 63 controls including steroid use patients (univariate AUC) | Steroid effect* | |
|---|---|---|---|---|
| sBAFF/BAFF receptor pathway | Fold change in sBAFF (pg/mL) | 1.5x, p=0.009 (0.69) | 1.3x, p=0.02 (0.63) | NS |
| Fold change in BAFF-R MFI on CD19+ B cells | 0.7x, p=0.002 (0.68) | 0.7x, p=0.004 (0.68) | NS | |
| Fold change in sBAFF:B cell ratio | 2.9x, p=0.03 (0.64) | NS | 8.2x, p=0.0003 | |
| Fold change in sBAFF:BAFF-R MFI ratio | 2.2x, p=0.003 (0.70) | 1.9x, p=0.004 (0.66) | NS | |
| CD21low | Absolute change in %CD21low of CD19+ | +14%, p=0.008 (0.66) | +13%, p=0.01 (0.66) | +25%, p<0.0001 |
| CD21lowCD19+ (cells/uL) | NS | NS | −52, p=0.05 | |
| cGvHD associated CD21low | Absolute change in % CD27−CD10− of CD21lowCD19+ | NS | +8%, p=0.05 (0.65) | NS |
| CD27−CD10− CD21lowCD19+ (cells/uL) | +48, p=0.03 (0.53) | +37, p=0.04 (0.50) | NS | |
| Transitional type 1(T1) CD21low | Absolute change in %CD27−CD10+ of CD21lowCD19+ | −9%, p=0.02 (0.65) | −13%, p<0.0001 (0.72) | −9%, p=0.02 |
| CD27−CD10+ CD21lowCD19+ (cells/uL) | NS | NS | −15, p=0.003 | |
| Naïve | Absolute change in %CD27−IgD+of CD19+ | −9%, p=0.04 (0.58) | NS | −21%, p=0.0003 |
| CD27−IgD+CD19+ (cells/uL) | NS | NS | −285, p=0.01 | |
| Classical switched memory | Absolute change in %CD27+IgD− of CD19+ | +7%, p=0.009 (0.58) | +6%, p=0.05 (0.59) | +10%, p=0.007 |
| CD27+IgD−CD19+ (cells/uL) | NS | NS | −5, p=0.02 | |
| Marginal-zone like | Absolute change in %CD27+IgD+ of CD19+ | NS | −1%, p=0.04 (0.59) | +1%, p=0.004 |
| CD27+IgD+CD19+ (cells/uL) | −4, p=0.04 (0.63) | −5, p=0.004 (0.64) | NS | |
| Transitional | Absolute change in %CD24++CD38++ of CD19+ | −11%, p=0.02 (0.65) | −14%, p=0.0004 (0.69) | −15%, p=0.002 |
| CD24++CD38=CD19+ (cells/uL) | −93, p=0.04 (0.64) | −110, p=0.002 (0.67) | −128, p=0.003 | |
* This column shows the difference between patients on steroids at sample collection versus patients without steroid use at sample collection regardless of GvHD status.
Combination of B cell subsets as cGvHD diagnostic profiles inclusive of corticosteroid use
We identified a number of B cell populations and sBAFF that were either higher or lower at the onset of cGvHD in patients who were not on steroid treatment. We evaluated the association of each of these cell populations and sBAFF with the onset of cGvHD through multivariate analyses in order to identify the strongest associations. The strongest markers were a combination of the a) BAFF-R surface expression, b) CD27−CD10−CD21low CD19+ B cell count, c) CD19+CD24highCD38high cell count and d) percentage of classical switched memory B cells (CD27+IgD−) (area under the Receiver Operator Characteristic curve, 0.85) (Table 3). Analysis of the entire cohort, including patients who were on corticosteroids at sample collection, revealed a different B cell panel consisting of a) percentage of CD27−CD10+ of CD21lowCD19+ B cells, b) CD27−CD10−CD21low CD19+ B cell count, c) CD19+CD24highCD38high cell count and percentage and d) percentage of naïve and marginal-zone like B cells with an AUC of 0.87 (Table 4). Measurements related to sBAFF concentrations did not add information to either multivariate analysis.
Table 3.
Optimal combination of B cell subsets diagnostic of cGvHD onset without steroid use
| Cell Variable | Phenotyping | Multivariate ROC AUC |
|---|---|---|
| Increased cGvHD associated CD21low* | CD27−CD10−CD21lowCD19+ (cells/uL) | 0.84 |
| Decreased transitional* | CD24hiCD38hiCD19+ (cells/uL) | |
| BAFF-R surface expression | BAFF-R MFI | |
| Classical switched memory | %CD27+IgD− of CD19+ |
only combining these two populations gives a ROC AUC of 0.79
Table 4.
Optimal combination of B cell subsets diagnostic of cGvHD onset regardless of steroid use
| Cell Variable | Phenotyping | Multivariate ROC AUC |
|---|---|---|
| Increased cGvHD associated CD21low* | %CD27−CD10+ of CD21lowCD19+ | 0.87 |
| CD27−CD10−CD21lowCD19+ (cells/uL) | ||
| Decreased transitional* | % CD24hiCD38hi of CD19+ | |
| CD24hiCD38hiCD19+ (cells/uL) | ||
| Naïve | %CD27−IgD+ of CD19+ | |
| Marginal-zone like | %CD27+IgD+ of CD19+ |
only combining these two populations gives a ROC AUC of 0.78
DISCUSSION
We confirmed in our cohort of adult HCT patients, that the onset of cGvHD is associated with significantly higher plasma concentrations of sBAFF. In the case of cGvHD, it initially appears that sBAFF levels are higher than in controls due to lower numbers of CD19+ B cells and associated BAFF receptors, because we found an inverse correlation between sBAFF levels and CD19+ B cell counts and BAFF-R surface expression. However, our data also suggests that the decreased surface expression of the primary BAFF receptor, BAFF-R, results from higher circulating sBAFF concentrations, rather than an intrinsic B cell defect. Consistent with previous studies in other disorders [20–24], binding of sBAFF from post-HSCT plasma decreases surface BAFF receptor expression and this was inhibited with belimumab, a fully human IgG1γ recombinant monoclonal antibody against soluble BAFF. Therefore, the reduced surface expression of BAFF-R could be considered as a surrogate marker of BAFF activation. This notion is supported by work showing that B cells from patients with cGvHD are activated and primed for survival via BAFF-mediated pathways [25]. Our data also support the hypothesis that BAFF regulates surface BAFF-R expression through BAFF-R internalization in response to ligand binding.
The addition of sBAFF to our multivariate analysis did not improve the model suggesting that alterations in the numbers of B cells or their subsets represent the primary driver associated with cGvHD and that the higher sBAFF concentrations may represent a secondary effect.
We also independently confirmed an association of an elevated percentage of CD21low B cells with the presence of cGvHD [15,16]. Our data suggest that CD10−CD27− B cells represent the majority of this CD21low B cell subset, similar to observations in Sjögren’s syndrome, rheumatoid arthritis, and common variable immunodeficiency [26]. One of the consistent findings in autoimmune and inflammatory diseases is that this population remains responsive to TLR-9 stimulation [27]. Previously, our group showed that cGvHD is associated with a B cell population highly responsive to TLR-9 ligation [28]. High SYK expression is a common feature of CD21low B cells, and this high expression is sufficient to drive constitutive phosphorylation of SYK and its immediate targets Bruton’s tyrosine kinase (BTK) and phospholipase Cγ2. The high SYK expression is induced by CpG deoxynucleotides (CpG ODN), a TLR9 agonist, but not by BCR stimulation [29]. We hypothesize that the presence of increased CD21low B cells may result from chronic activation caused by inflammation. For example, it has been shown that treatment of chronic hepatitis C infection rescues normal CD21 expression in a CD21low B cell population [30].
We show that cGvHD is associated with a lower frequency of transitional B cells [18]. We hypothesize that the smaller pool of transitional B cells overall in cGvHD could be explained by increased inflammatory effects on B lymphopoiesis. In mice, inflammation diverts lymphoid progenitors away from the B lineage, and mobilizes developing B cells to the spleen and away from other secondary lymphoid organs [31–33]. TLR ligation induces the differentiation of common lymphoid progenitors into innate immune cells such as dendritic cells [34]. Redirected differentiation of common lymphoid progenitors as a result of TLR ligation could also directly reduce the B-cell progenitor pool. Decreased numbers of CD19+ B cells were observed in association with osteoblast destruction in the bone marrow biopsies of human cGvHD cases [35], and significantly greater numbers of bone marrow B cell precursors were found in patients who did not develop cGvHD compared with those who developed cGvHD [36,37].
Our study highlights the importance of considering steroid use when studying abnormal B cell populations associated with cGvHD onset as potential biomarkers because our results indicate that steroid usage regardless of cGvHD status has a stronger impact on certain B cell populations. This does not necessary indicate that patients on steroids have different cGvHD mechanisms instead demonstrating how steroids may secondarily alter the B cell profile generated by cGvHD. The transitional CD27−CD10+ and CD27−CD10−CD21lowCD19+ B cell populations remained significantly associated with cGvHD onset after adjustment for steroid use. These findings provide candidate B cell populations that could be potential therapeutic targets in steroid resistance cGvHD patients. It has previously been shown in systemic lupus erythematosus that plasmacytoid dendritic cells (pDCs) drive the differentiation of immature regulatory B cells and these interactions are normalized in patients responding to rituximab therapy [38]. In HCT, increased pDC cells in bone marrow allografts are associated with less cGvHD [39] and rituximab has successfully been used as salvage therapy for steroid refractory cGvHD [40].
Limitations of our study include that our results were not replicated in a separate cohort or applied prospectively to patients post-HCT and we did not perform any mechanistic investigations on the abnormal B cell subsets in order to determine whether they are pathogenic and not simply reactive changes. These are important research directions to pursue in future studies.
Our comprehensive analysis of B cell subpopulations has provided a clearer picture of the dysregulated B cell lymphopoiesis associated with the onset of cGvHD. We hypothesize that the CD27−CD10−CD21lowCD19+ B cell population may be uniquely selected at the expense of normal B cell development and transitional B cell populations in a persistent inflammatory setting such as cGvHD. Furthermore, the presence of elevated sBAFF in an overall B cell lymphopenic environment may further promote the survival of B cells in the circulation. This profile of B cell dysregulation strengthens the notion that inflammation serves as an important risk factor for cGvHD in HCT patients. Therefore, therapies could be aimed at dampening pro-inflammatory signals post-HCT by neutralizing IL-6 or boosting counter-regulatory responses through the expansion of immuno-modulatory cell populations such as regulatory NK cells. Finally, our comprehensive B cell profile could serve as an improved diagnostic tool for cGvHD in prospective clinical trials.
Supplementary Material
HIGHLIGHTS.
The onset of cGvHD is associated with significantly higher CD19+CD10−CD27−CD21low B cells and lower T1 transitional CD27−CD10+ B cells.
These B cell populations remain significant regardless of steroid use at cGvHD diagnosis.
Soluble BAFF down-regulates B cell surface BAFF-R expression.
ACKNOWLEDGMENTS
This work was supported by a CIHR project grant (KRS), Vanier Canada Graduate Scholarship (JR), project grant from the Regensburg Institute of Interventional Immunology (DW) and grant CA163438 from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services (SJL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE OF CONFLICTS OF INTEREST: There are no conflicts of interest to declare
REFERENCES
- 1.Socie G, Ritz J. Current issues in chronic graft-versus-host disease. Blood. 2014;124:374–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schultz KR, Paquet J, Bader S, Hayglass KT. Requirement for B cells in T cell priming to minor histocompatibility antigens and development of graft-versus-host disease. Bone Marrow Transplant. 1995;16:289–295. [PubMed] [Google Scholar]
- 3.Cutler C, Kim HT, Bindra B, et al. Rituximab prophylaxis prevents corticosteroid-requiring chronic GVHD after allogeneic peripheral blood stem cell transplantation: results of a phase 2 trial. Blood. 2013;122:1510–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miklos D, Cutler CS, Arora M, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood. 2017;130:2243–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujii H, Cuvelier G, She K, et al. Biomarkers in newly diagnosed pediatric-extensive chronic graft-versus-host disease: a report from the Children’s Oncology Group. Blood. 2008;111:3276–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarantopoulos S, Stevenson KE, Kim HT, et al. High levels of B-cell activating factor in patients with active chronic graft-versus-host disease. Clin Cancer Res. 2007;13:6107–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackay F, Browning JL. BAFF: a fundamental survival factor for B cells. Nat Rev Immunol. 2002;2:465–75 [DOI] [PubMed] [Google Scholar]
- 8.Schiemann B, Gommerman JL, Vora K, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–4 [DOI] [PubMed] [Google Scholar]
- 9.Sasaki Y, Casola S, Kutok JL, Rajewsky K, Schmidt-Supprian M. TNF family member B cell-activating factor (BAFF) receptor-dependent and independent roles for BAFF in B cell physiology. J Immunol. 2004;173:2245–52 [DOI] [PubMed] [Google Scholar]
- 10.Warnatz K, Salzer U, Rizzi M, et al. B-cell activating factor receptor deficiency is associated with an adult-onset antibody deficiency syndrome in humans. Proc Natl Acad Sci USA. 2009;106:13945–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackay F, Woodcock SA, Lawton P, et al. Mice transgenic for Baff develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarantopoulos S, Blazar BR, Cutler C, Ritz J. B cells in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21:16–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarantopoulos S, Stevenson KE, Kim HT, et al. Recovery of B-cell homeostasis after rituximab in chronic graft-versus-host disease. Blood. 2011;117(7):2275–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarantopoulos S, Stevenson KE, Kim HT, et al. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood. 2009;113:3865–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greinix HT, Kuzmina Z, Weigl R, et al. CD19+CD21low B cells and CD4+CD45RA+CD31+ T cells correlate with first diagnosis of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21:250–8 [DOI] [PubMed] [Google Scholar]
- 16.Kuzmina Z, Krenn K, Petkov V, et al. CD19(+)CD21(low) B cells and patients at risk for NIH-defined chronic graft-versus-host disease with bronchiolitis obliterans syndrome. Blood. 2013;121:1886–95 [DOI] [PubMed] [Google Scholar]
- 17.Suryani S, Fulcher DA, Santner-Nanan B, et al. Differential expression of CD21 identifies developmental and functionally distinct subsets of human transitional B cells. Blood. 2010;115:519–29 [DOI] [PubMed] [Google Scholar]
- 18.Khoder A, Alsuliman A, Basar R, et al. Evidence for B cell exhaustion in chronic graft-versus-host disease. Front Immunol. 2017;8:1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kariminia A, Holtan SG, Ivison S, et al. Heterogeneity of chronic graft-versus-host disease biomarkers: association with CXCL10 and CXCR3+ NK cells. Blood. 2016;127:3082–3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carter RH, Zhao H, Liu X, et al. Expression and occupancy of BAFF-R on B cells in systemic lupus erythematosus. Arthritis Rheum. 2005;52(12):3943–3954 [DOI] [PubMed] [Google Scholar]
- 21.Barbosa RR, Silva SL, Silva SP, et al. Reduced BAFF-R and increased TACI expression in common variable immunodeficiency. J Clin Immunol. 2014;34:573–83 [DOI] [PubMed] [Google Scholar]
- 22.Sellam J, Miceli-Richard C, Gottenberg JE, et al. Decreased B cell activating factor receptor expression on peripheral lymphocytes associated with increased disease activity in primary Sjögren’s syndrome and systemic lupus erythematosus. Ann Rheum Dis. 2007;66:790–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kreuzaler M, Rauch M, Salzer U, et al. Soluble BAFF levels inversely correlate with peripheral B cell numbers and the expression of BAFF receptors. J Immunol. 2012;188:497–503 [DOI] [PubMed] [Google Scholar]
- 24.Smulski CR, Kury P, Seidel LM, et al. BAFF- and TACI-dependent processing of BAFFR by ADAM proteases regulates the survival of B cells. Cell Rep. 2017;18:2189–2202 [DOI] [PubMed] [Google Scholar]
- 25.Allen JL, Fore MS, Wooten J, et al. B cells from patients with chronic GVHD are activated and primed for survival via BAFF-mediated pathways. Blood. 2012;120:2529–2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thorarinsdottir K, Camponeschi A, Gjertsson I, Martensson IL. CD21-/low B cells: A snapshot of a unique B cell subset in health and disease. Scand J Immunol. 2015;82:254–61 [DOI] [PubMed] [Google Scholar]
- 27.Saadoun D, Terrier B, Bannock J, et al. Expansion of autoreactive unresponsive CD21-/low B cells in Sjögren’s syndrome-associated lymphoproliferation. Arthritis Rheum. 2013;65:1085–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.She K, Gilman AL, Aslanian S, et al. Altered Toll-like receptor 9 responses in circulating B cells at the onset of extensive chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13:386–97 [DOI] [PubMed] [Google Scholar]
- 29.Keller B, Stumpf I, Strohmeier V, et al. High SYK expression drives constitutive activation of CD21low B cells. J Immunol. 2017;198:4285–4292 [DOI] [PubMed] [Google Scholar]
- 30.Del Padre M, Todi L, Mitrevski M, et al. Reversion of anergy signatures in clonal CD21low B cells of mixed cryoglobulinemia after clearance of HCV viremia. Blood. 2017;130:35–38 [DOI] [PubMed] [Google Scholar]
- 31.Nagaoka H, Gonzalez-Aseguinolaza G, Tsuji M, Nussenzweig MC. Immunization and infection change the number of recombination activating gene (RAG)-expressing B cells in the periphery by altering immature lymphocyte production. J Exp Med. 2000;191:2113–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ueda Y, Yang K, Foster SJ, Kondo M, Kelsoe G. Inflammation controls B lymphopoiesis by regulating chemokine CXCL12 expression. J Exp Med. 2004;199:47–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med. 2005;201:1771–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagai Y, Garrett KP, Ohta S, et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shono Y, Shiratori S, Kosugi-Kanaya M, et al. Bone marrow graft-versus-host disease: evaluation of its clinical impact on disrupted hematopoiesis after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20:495–500 [DOI] [PubMed] [Google Scholar]
- 36.Storek J, Wells D, Dawson MA, Storer B, Maloney DG. Factors influencing B lymphopoiesis after allogeneic hematopoietic cell transplantation. Blood. 2001;98:489–91 [DOI] [PubMed] [Google Scholar]
- 37.Fedoriw Y, Samulski TD, Deal AM, et al. Bone marrow B cell precursor number after allogeneic stem cell transplantation and GVHD development. Biol Blood Marrow Transplant. 2012;18:968–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menon M, Blair PA, Isenberg DA, Mauri C. A regulatory feedback between plasmacytoid dendritic cells and regulatory B cells is aberrant in systemic lupus erythematosus. Immunity. 2016;44:683–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Auletta JJ, Devine SM, Waller EK. Plasmacytoid dendritic cells in allogeneic hematopoietic cell transplantation: benefit or burden? Bone Marrow Transplant. 2016;51:333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clavert A, Chevallier P, Guillaume T, et al. Safety and efficacy of rituximab in steroid-refractory chronic GVHD. Bone Marrow Transplant. 2013;48:734–736. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.