Abstract
Native tissues possess unparalleled physiochemical and biological functions, which can be attributed to their hybrid polymer composition and intrinsic bioactivity. However, there are also various concerns or limitations over the use of natural materials derived from animals or cadavers, including the potential immunogenicity, pathogen transmission, batch to batch consistence and mismatch in properties for various applications. Therefore, there is an increasing interest in developing degradable hybrid polymer biomaterials with controlled properties for highly efficient biomedical applications. There have been efforts to mimic the extracellular protein structure such as nanofibrous and composite scaffolds, to functionalize scaffold surface for improved cellular interaction, to incorporate controlled biomolecule release capacity to impart biological signaling, and to vary physical properties of scaffolds to regulate cellular behavior. In this review, we highlight the design and synthesis of degradable hybrid polymer biomaterials and focus on recent developments in osteoconductive, elastomeric, photoluminescent and electroactive hybrid polymers. The review further exemplifies their applications for bone tissue regeneration.
1. Introduction
The extracellular matrix (ECM) of native tissues is composed of a hybrid polymer nanostructure at the molecular level, organized with different biopolymers and nanocrystallites [1]. Due to their hybrid and well-organized structure, both hard and soft native tissues demonstrate excellent physicochemical properties including viscoelasticity and strength. They also demonstrate excellent biological activity including cellular biocompatibility and tissue-inductive ability [2]. Development of new biodegradable biomaterials by mimicking the physicochemical properties and biological activity has therefore gained increasing attention in recent years [3]. Biomimetic polymer hybrid biomaterials play an important role because they can be synthesized with highly tailored physicochemical properties and bioactivity, through combining different polymers and inorganic phases at the multiple levels [4]. In past decades, biodegradable natural-based polymers (collagen, silk, alginate, chitosan, hyaluronic acid) and synthetic polymers (poly(lactic acid):PLA, poly(glycolic acid):PGA, poly(lactic-co-glycolide):PLGA, Poly(ε-caprolactone):PCL, Polyhydroxyalkanoates: PHA) have been widely studied and their promising biomedical applications are also well demonstrated [5–8]. These polymers have been hybridized in many forms including 3D scaffolds, hydrogels, microspheres, and their composites.[9–14] Hybrid hydrogel-microsphere polymers with osteoconductive properties have also been synthesized.[15–17]
In addition to the hybrid structure, osteocondutive property and electroactive ability are also very important for the application of hybrid polymers to regenerate bone [18]. Regeneration of bone can be accomplished by a combination of osteoinductive materials, regenerative cells and osteogenic growth factors. Local and long-term treatment with bone morphogenetic protein 7 (BMP-7) was accomplished by encapsulation of bioactive protein in PLGA microspheres. In combination with a nanofibrous and porous scaffold, treatment with BMP-7 significantly enhanced in vitro osteogenic differentiation and in vivo bone regeneration [19].
Pure biomedical polymers such as those listed above cannot mimic the mechanical properties of native tissues especially the strength, elasticity and modulus, due to intrinsic shortcomings. Nevertheless, they provide certain advantages. It is possible to use these polymers to design precise micro and nanoscale environments that are beneficial for cell attachment, proliferation and differentiation. They can also be tailored for tunable drug delivery. Because of these advantages, they are being developed widely for tissue regeneration. To improve their mechanical and osteogenic properties, bioactive ceramic-based nanophases (bioactive glass and calcium phosphate) and various polymers (natural and synthetic polymers) have been hybridized [20–27]. To induce elastomeric behavior, highly elastomeric hybrid polymers were also synthesized through incorporating inorganic phase into biodegradable elastomers [28]. In particular, siloxane-based biodegradable hybrid polymer elastomers were developed with significantly enhanced mechanical properties and biocompatibility [29–31]. In recent years, electric stimulation has been shown to exhibit a positive effect on tissue regeneration through enhancing cell proliferation and differentiation[32]. Therefore, conductive components such as carbon-based materials and polymer semiconductors were added to fabricate electroactive hybrid polymer biomaterials for tissue regeneration applications [33].
This work reviews the design, fabrication, and properties of biodegradable hybrid polymers with a focus on their osteoconductive functions, elastomeric property, and electroactivity. The prospective application of hybrid materials for bone tissue regeneration is also covered in this review.
2. Synthesis and properties of hybrid polymers
2.1. Osteoconductive hybrid polymers
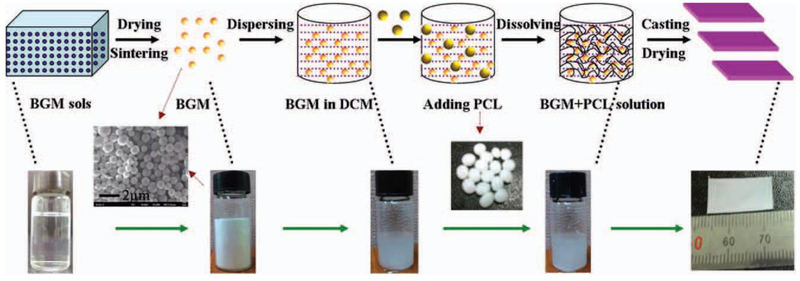
Osteoconductive hybrid polymer biomaterials can be fabricated by incorporating osteoconductive materials into biodegradable polymers. Biodegradable polymers typically have low elastic modulus and poor osteoconductive activity [34]. Bioactive inorganic biomaterials including bioactive glass (BG) and calcium phosphate (CP) have high conductive activity and bone-bonding ability, and their enhanced potential for bone regeneration have been well described in the literature [35–39]. Therefore, BG and CP-based nanoparticles have been added into various polymers to fabricate osteoconductive hybrid polymers for bone tissue regeneration[40–43]. CP-based polymer hybrid biomaterials have been fabricated successfully by melting, solvent-casting and in situ precipitation [44]. Most reports showed that addition of low content-CP-based nanoparticles can efficiently improve the mechanical strength and modulus of polymers and improve osteoconductive bioactivity [44]. Bioactive glass nanoparticles (BGN) have an amorphous structure and typical chemical composition of SiO2-CaO-P2O5 that enable the controlled biodegradation and high bone-bonding activity for in vivo implanting applications [45]. By the facile solvent-casting method, BGNs with different morphology and size were added into various polymers including gelatin, chitosan, PLA, PCL, PLGA [41,46–48]. These hybrid BGN-polymers significantly enhanced compressive strength, tensile strength, elastic modulus, biominerialization, and osteoblast biocompatibility (Figure 1). Although BGN-polymer nanocomposites have been developed well in past years, the nanoparticle-based polymer composites still showed uncontrolled biodegradation and mechanical properties in vivo due to the low interface strength between nanoparticles and polymers. These are known challenges associated with certain BGN-polymer nanocomposites.
Figure 1.
Bioactive glass particles reinforced PCL osteoconductive hybrid polymers. Reproduced from Ref.[41]
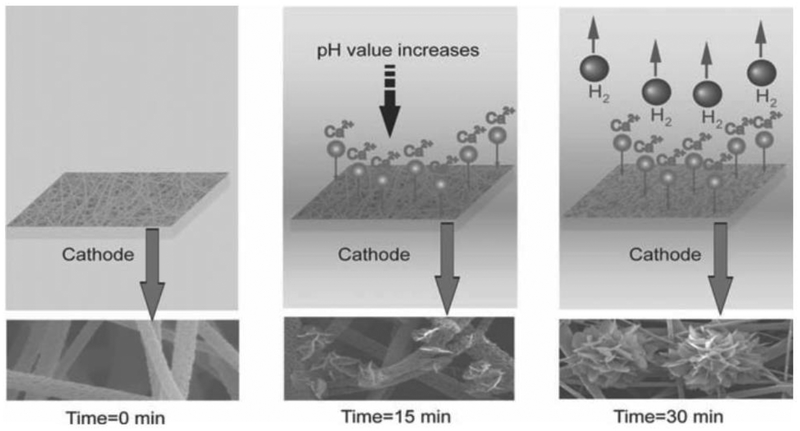
Advances have been made in hybrid polymer materials to maintain controlled degradation and mechanical properties while also enhancing in vitro osteoconductive activity [49]. Gelatin-apatite hybrid nanofibrous scaffolds fabricated by thermally induced phase separation were evaluated for biominerialization in simulated body fluid (SBF) [49]. The gelatin-apatite hybrid scaffolds demonstrated significantly enhanced mechanical strength and enhanced expression of osteogenic genes in cells. Additionally, the hybrid scaffold was coated with biological apatite nanocrystals through an electrochemical deposition technology (Figure 2) [50]. The apatite layer thickness could be tailored efficiently by the electrochemical parameters. The deposited hybrid polymer scaffolds also showed enhanced physiochemical properties and osteoconductive activity.
Figure 2.
Schematic illustration of a hypothesized mechanism for the growth of calcium phosphate crystals over time. When a deposition voltage is applied, pH in the vicinity of electrode increases, and some calcium phosphate crystals deposited onto the surface of PLLA nanofibers. Further increase of deposition time leads to the generation of hydrogen bubbles and larger flower-like crystals. Reproduced from Ref.[50]
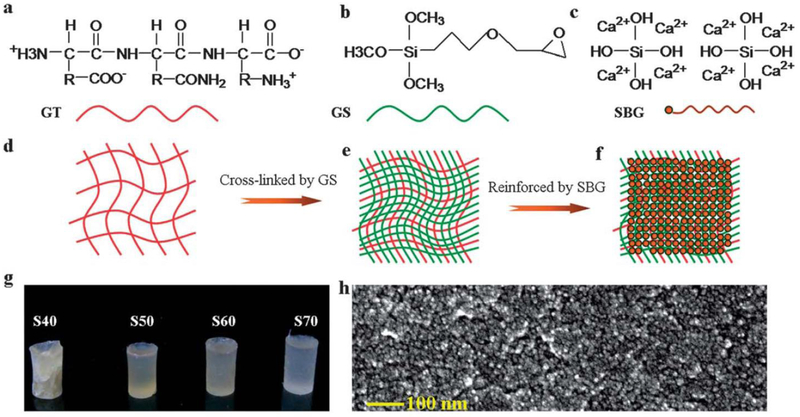
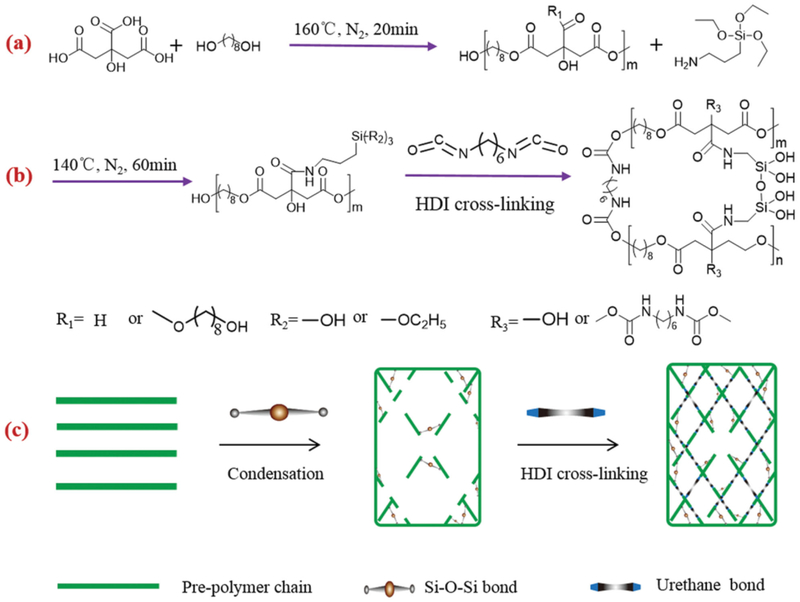
Agglomeration of BGNs within the polymer matrix is a challenge associated with hybrid polymers, as these materials may exhibit unfavorable mechanical and physiochemical properties [43]. To overcome this limitation, silica-based bioactive glass sol (SBGS) at the molecular level has been used to develop hybrid polymer biomaterials for applications in tissue regeneration. For example, SBGS-reinforced gelatin, chitosan, polyethylene glycol (PEG) and PCL hybrid polymers have been fabricated successfully through one-step hybridization process [51–57]. SBGS reinforced hybrid polymers showed significantly improved mechanical properties including strength, toughness, controlled biodegradation and biominerialization, as well as high osteoblastic activity. The SBGS-reinforced gelatin hybrid polymer was synthesized through typical sol-gel process, and the interface strength between organic and inorganic phase was controlled by siloxane coupling agents (Figure 3). The resulting SBGS-gelatin hybrid showed strong compressive strength, mimicking native bone tissue and providing evidence for its potential application in bone fixation and repair [51]. SBGS-based gelatin hybrid scaffolds and nanofibrous scaffolds were fabricated through alkaline treatment technology and thermal-induced phase separation (Figure 4–Figure 6). Significantly improved mechanical properties and biocompatibility of SBGS-gelatin hybrids were observed[41,43,51,58–60]. The SBGS-based hybrid polymer biomaterials have shown promise for bone tissue regeneration.
Figure 3.
Formation mechanism of the biomimetic siloxane-gelatin (SGT) hybrid bone implants. (a–c) Molecular structure and composition of gelatin (GT) (a), siloxane (GS), silicate bioactive glass sol (S); (d–f) GT (d) polymer matrix was cross-linked by GS (e), and then hybridized with the SBG sol at the molecular and nanoscale levels (f); (g and h) semi-transparent SGT hybrid implants with different SBG weight percent, formed after condensation and drying. Reproduced from Ref.[51] with permission from the Royal Society of Chemistry.
Figure 4.
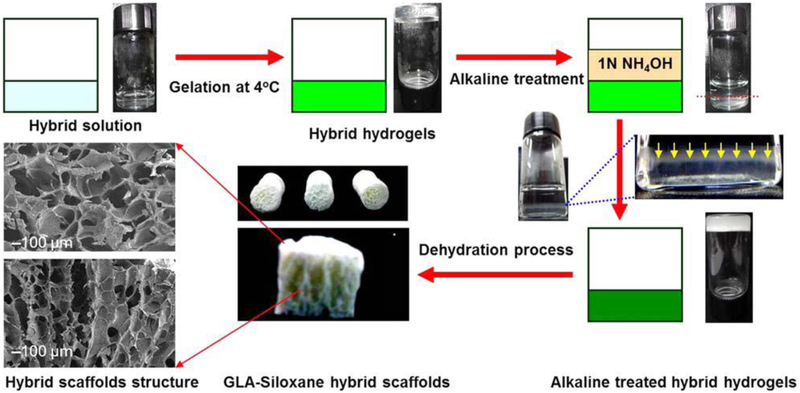
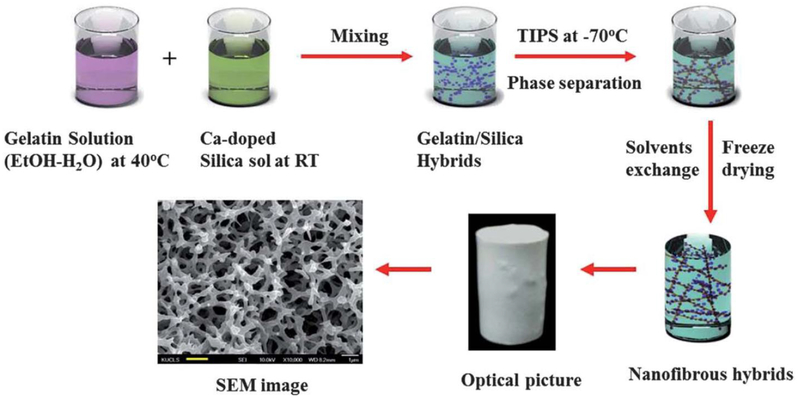
Schematic diagram showing an experimental procedure for producing anisotropic porous gelatin-silica hybrid polymer scaffolds by ammonium hydroxide treatment. Reproduced from Ref.[68]
Figure 6.
Schematic diagram showing an experimental procedure for producing nanofibrous gelatin–silica hybrid scaffolds by the thermally induced phase separation (TIPS) technique using the mixtures of the gelatin solution and sol–gel derived silica sol. Reproduced from Ref.[59]with permission from the Royal Society of Chemistry.
Additional advances have been reported in the use of carbon biomaterial-polymer hybrids as osteoconductive scaffolds for bone regeneration. Carbon nanomaterials are often synthesized as single sheets, referred to as graphene, or hollow structures referred to as carbon nanotubes (CNTs). CNTs can be single-walled or multi-walled, consisting of concentric tubular layers of graphene. One study compared CNT-PLLA scaffolds with graphene-PLLA scaffolds and reported that both carbon nanomaterial hybrids enhanced in vivo bone regeneration but graphene-PLLA scaffolds showed more osteoconductive capacity than CNT-PLLA scaffolds.[61] Polymeric scaffolds reinforced with ultrashort (US)-single walled CNTs enhanced both ectopic and in situ bone regeneration in rabbit subcutaneous and femoral condyle models.[62] Adsorption of ampiphilic comb-like polymer (APCLP) to CNTs allowed for more homogenous integration of carbon nanotubes into a bacterial cellulose (BC) scaffold. [63] In a mouse calvarial defect model, this hybrid CNT-BC scaffold improved bone formation and expression of osteocalcin. Vertically aligned CNTs combined with hydroxyapatite were made to be superhydrophilic and subsequently dispersed in poly (D, L, lactic acid) (PDLLA). The resulting hybrid scaffold showed suitable mineralization and cytocompatibility in vitro and demonstrated enhanced in vivo bone regeneration capacity in a rat calvarial defect model.[64] In addition to increasing hydrophilicity, functionalization of CNT-polymer hybrids may improve mechanical characteristics and cytocompatibility of scaffolds and was reported to enhance in vitro and in vivo bone regeneration.[65–67]
2.2. Elastomeric hybrid polymers
Many tissues in the body possess elastomeric properties. Therefore, the development of biomaterials that demonstrate highly elastomeric behavior has garnered much attention. Elastomeric materials are of particular interest because of their biomimetic mechanical properties, which enable their use in the complicated in vivo load environment [69]. Current biodegradable elastomers include physically crosslinked polymers such as polyurethanes and polyesters, chemically crosslinked polymers such as poly(glycerol sebacate) (PGS) and poly(citrate diol) (PCD) [70]. These biodegradable elastomers have shown highly tunable degradation, moderate biocompatibility and good elastomeric mechanical behavior [70]. They have demonstrated promising applications in regeneration of soft tissue due to their low mechanical strength or poor bioactivity [70]. To make these elastomers effective for a wider number of biomedical applications, developing hybrid polymers has become an attractive option to obtain biodegradable elastomers with optimized properties to meet different tissue-specific requirements.
PGS-PCL hybrid elastomers have been developed successfully by solvent electrospinning. The incorporation of PCL significantly enhanced formation of the nanofibrous structure and the hybrid materials showed mechanical properties in the range of human aortic valve tissues [71]. Gelatin was also added into PGS elastomer to fabricate hybrid polymers for tissue regeneration. The addition of gelatin significantly enhanced the mechanical properties and bioactivity of PGS elastomers [72]. Although polymer-polymer hybrid elastomers have been well developed, their limited elastomeric behavior and mechanical strength still provent their wide application in bone tissue regeneration.
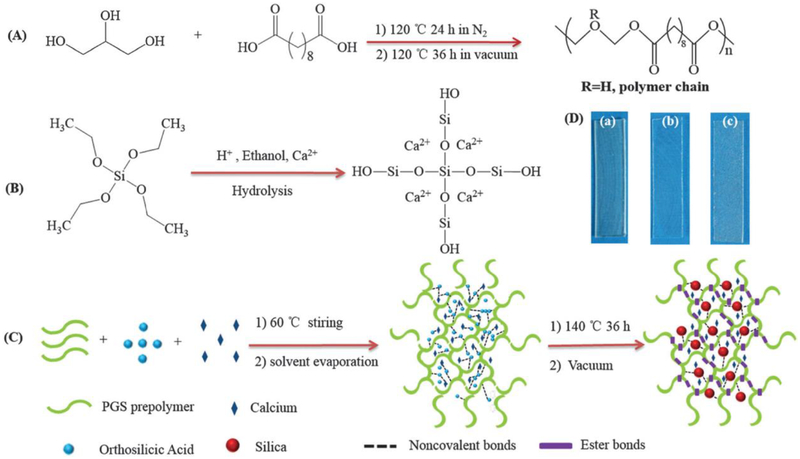
To overcome the limitations of polymer-based elastomers, inorganic phase reinforced hybrid polymer elastomers have been developed in recent years [73–75]. As osteoconductive biomaterials, hydroxyapatite nanoparticles were incorporated into PCD-based elastomers to fabricate composites for orthopedic implants [76]. Uniform distribution of HA in the polymer matrix significantly enhanced the mechanical properties and osteoconductive biocompatibility of PCD-HA hybrid elastomers. Melt-derived bioglass particles were also introduced into PGS elastomers to improve their range of biomedical applications [77]. Bioglass particles efficiently enhanced the elastomeric strain and cellular biocompatibility of PGS. These hybrid elastomers still have the intrinsic problem of poor interface intensity between the inorganic phase and polymers. Therefore, our group introduced bioactive silica into PCD elastomers through a one-step thermal polymerization method [30,31,78,79]. The inorganic silica phase was bonded with the PCD polymer chain through covalent bonds. The resulting hybrid polycitrate-silicon (PCS) elastomers demonstrated significantly improved elastomeric behavior, mechanical strength and cellular biocompatibility (Figure 7) [30]. SBGS-based PGS hybrid elastomers were also fabricated successfully through the direct hybridization of SBGS and PGS solution [29]. SBGS-PGS hybrid elastomers exhibited significantly enhanced mechanical properties, biominerialization and cellular biocompatibility (Figure 8). The inorganic phase-grafted PGS and PCD hybrid elastomers have shown promise for applications in bone tissue regeneration.
Figure 7.
Synthesis of multifunctional silica-poly(citrate)-based hybrid prepolymers and elastomers. a,b) Fabrication of Multifunctional silica-poly(citrate) (MSPC) and crosslinked MSPC (CMSPC) elastomers by polycondensation of citric acid (CA), 1,8-octylene glycol (OD), aminosilane (AS), as well as the chemical crosslinking with hexamethylene diisocyanate (HDI) and c) schematic diagram showing the formation of CMSPC hybrid elastomers matrix. Reproduced from Ref [30].
Figure 8.
Schematic illustration for preparing poly(glycerol sebacate)-silica-calcium (PGSSC) hybrid elastomers. (A) Synthesis of PGS pre-polymers; (B) formation of silica-based bioactive glass sols; (C) fabrication of PGSSC hybrid elastomers; (D) optical images of PGS and PGSSC hybrid elastomers ((a) PGS; (b) PGS15mol%Si (PGS15Si); and (c) PGS-15mol%Si-20mol%Ca (PGS15Si20Ca).
Reproduced from Ref [29] with permission from the Royal Society of Chemistry.
2.3. Electroactive hybrid polymers
Conducting polymers are organic polymers that possess electrical, magnetic and optical properties that are similar to metal, while maintaining desirable mechanical properties as well as ease of processing of polymers [80,81]. Recently, it was found that conductive polymers could tune the properties of cells in electrically sensitive tissues under electrical stimulation, including neural, muscle, cardiac, and bone [82–84]. Regenerative biomaterials for the treatment of bone diseases that need surgical intervention have attracted more attention, particularly with extended life expectancies. Scaffolds that regulate cellular behavior are particularly interesting for such applications [85–87]. A 3-D conductive scaffold that can locally deliver an electrical signal is needed. 3D conductive scaffolds were prepared using poly(3,4-ethylenedioxythiophene) poly(4-styrene sulfonate) (PEDOT:PSS), gelatin, and bioactive glass [88]. Introduction of PEDOT:PSS enhanced the physiochemical stability and improved mechanical properties of the composite. Increasing the content of PEDOT:PSS in the scaffolds improved cell viability. Together, these results indicated that these conductive scaffolds exhibited more favorable structural properties for bone repair. The use of conducting polymers loaded with a bioactive molecule has been an emerging approach to functional biomaterial use in tissue regeneration. Chondroitin sulfate (CS)-doped polypyrrole (PPy) was coated via an in situ chemical oxidative polymerization onto the non-conductive polylactide to fabricate novel osteogenic scaffolds [89]. Electrical conductivity of PPy-coated polylactide (PPy-PLA) scaffolds was obvious, but it decreased with time due to de-doping.
3. Applications in bone tissue regeneration
Biomaterials-based bone regeneration aims to develop bioactive bone-substitutes that repair damaged issue and restore tissue functionality. Native bone ECM is a hybrid structure that consists of a polymer and inorganic phase. Therefore, biodegradable hybrid polymer biomaterials with representative nanostructures are desirable for bone tissue regeneration [90]. Inorganic phase reinforced hybrid biomaterials with multifunctional properties have demonstrated enhanced bone-binding ability, osteoblast activity, and bone regeneration potential as compared to pure biodegradable polymers [91]. Here, we review the recent development of biodegradable hybrid polymer biomaterials for osteoblastic proliferation, differentiation, and in vivo bone regeneration.
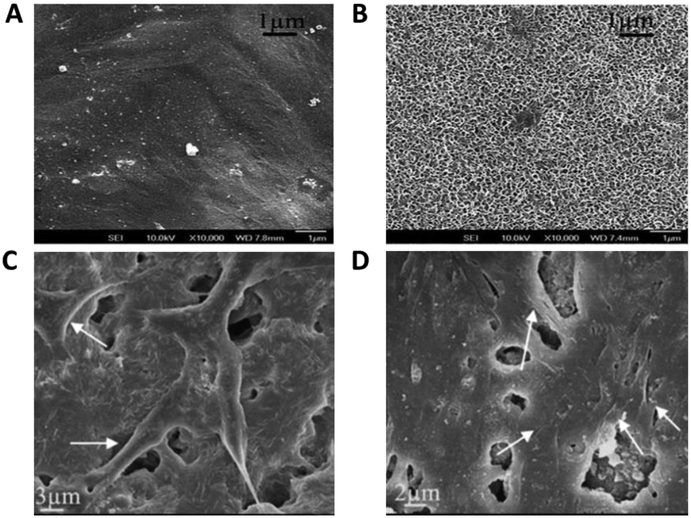
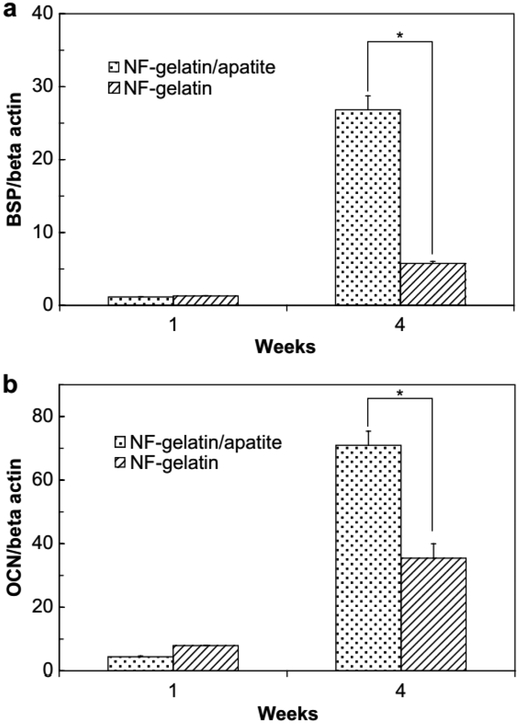
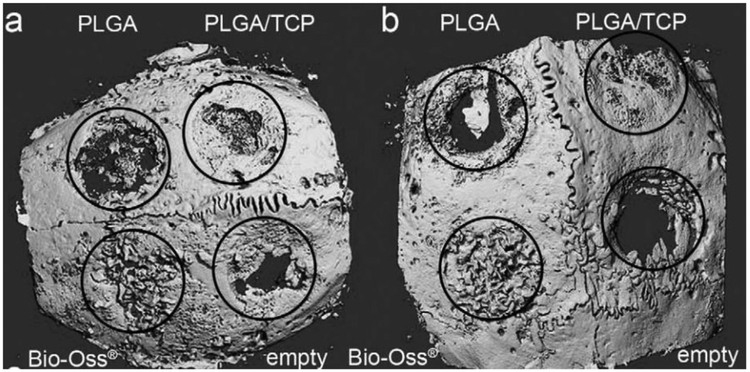
Silicon-based BG particles with microscale and nanoscale sizes have been employed to enhance biominerialization and biocompatibility [41,43]. As shown in Figure 6, hybrid polymers reinforced with BG microparticles or nanoparticles could efficiently induce deposition of biological apatite after soaking in SBF [41]. Osteoblast attachment was also improved on the hybrid BGN-PCL, as compared to PCL scaffold alone [43]. In addition to bioactive glass-based hybrid polymer, the apatite-based polymer also showed enhanced osteoblastic activity (Figure 9). After 1 and 4 week culture of MC3T3-E1 cells on nanofibrous gelatin-apatite hybrid scaffolds (NF-gelatin/apatite) and pure NF-gelatin scaffolds, cells grown on the hybrid scaffolds showed significantly increased expression of genes for bone sialoprotein (BSP) and osteocalcin (OCN) (Figure 10) [49]. In vivo experimentation demonstrated that the incorporation of osteoconductive components can significantly improve bone formation. For example, compared with pure poly(lactide-co-glycolide) (PLGA), amorphous tricalcium phosphate nanoparticles reinforced PLGA (PLGA-TCP) nanocomposites enhanced the rapid regeneration of bone defects in a New Zealand white rabbit model (Figure 11) [92].
Figure 9.
In vitro biomineralization activity and osteoblast biocompatibility (MC3T3-E1) of BG micro-nanoscale particles-PCL hybrid polymers. (A, B) Apatite formation on surface of PCL (A) and BG-PCL (B) after soaking in SBF for 7 days; (C, D) Cell attachment morphology on the surface of PCL (C) and BG-PCL (D) after culture for 3 days. Reproduced from Ref. [41] and Ref.[43].
Figure 10.
Quantitative RT-PCR results of bone sialoprotein (BSP) and osteocalcin (OCN) gene expression. MC3T3-E1 cells were cultured on NF-gelatin and NF-gelatin/apatite scaffolds for 1 and 4 weeks. The Y-axis of the figure is the gene expression results normalized by beta actin. (*) represents statistically significant differences (p < 0.05). Reproduced from Ref.[49]
Figure 11.
Micro-computed tomography of the cranial defects (diameter=6 mm) in New Zealand White rabbits after 4-week implantation using PLGA, PLGA/TCP composites. (a, b) Two examples of the CT of the entire cranial bone are shown. Defect margins and treatment modalities are indicated. Adapted from Ref [92].
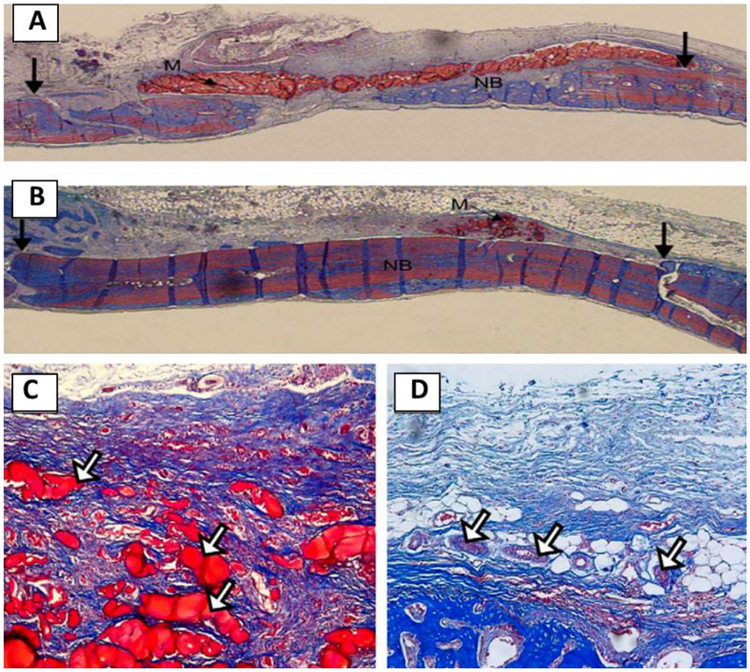
As compared to osteoconductive particle-based hybrid polymers, silica- based sol-polymers possessed uniform nanostructure distribution and inorganic-organic interface, which could mimic the structure of native bone ECM. Silica-based chitosan hybrid polymer has been used to guide bone tissue regeneration successfully [93]. As compared to pure chitosan, new bone formation was significantly enhanced by the hybrid polymer while the hybrid membrane was degraded after 3 week implantation at bone defect sites (Figure 12). A significantly higher rate of bone formation was observed for the hybrid (93%) but not the chitosan membrane (60%) [93]. Due to the inherent elastomeric behavior of native bone, elastomeric hybrid biomaterials are appealing for applications in bone regeneration [94]. Our group developed silicon-based polymer elastomers with controlled biodegradation for applications in bone regeneration [29–31]. The results demonstrated that poly(citrate-siloxane) (PCS) hybrid elastomers significantly enhanced attachment and proliferation of various cells, including cells derived from both hard and soft tissue [30,31]. PCS-based hybrid polymer could also significantly enhance osteoblastic differentiation, cellular biominerialization of MC3T3-E1 cells [78,79]. PCS-based hybrid biomaterials have shown promising potential for in vivo bone tissue regeneration. Additional in vivo experiments should be carried out to evaluate the potential value of PCS-based hybrid biomaterials.
Figure 12.
Optical micrographs of the rat bone tissue regeneration responses after the 3 weeks implantation of the membranes: (A, C) pure chitosan and (B, D) the chitosan–silica xerogel hybrid. The fresh-formed bone tissue was revealed in blue, the calcified bones and materials were stained in red. Reproduced from Ref.[93]
Proliferation and osteogenic differentiation of human adipose stem cells (hASCs) on the coated and conductive scaffolds was compared to non-coated polylactide scaffolds under electrical stimulation. The conductive hybrid scaffolds greatly enhanced hASC proliferation compared to pure PLA scaffolds [89]. Alkaline phosphatase (ALP) activity of hASCs seeded on PLA-PPy scaffolds was generally higher; however, electrical stimulation did not show a significant effect on hASCs. These results highlighted the potential application of PPy-coated PLA scaffolds for bone regeneration. Mesenchymal stem cells (MSCs) have great potential and are commonly used progenitor cells in bone tissue engineering. Osteogenic differentiation of MSCs can be guided by various types of biomaterials. Our group found that the electroactive biodegradable copolymers can enhance osteogenic differentiation of bone marrow derived MSCs (BMSCs) [95]. These copolymers were composed of polylactide and tunable contents of conductive aniline tetramer. Culture of BMSCs on the electroactive copolymer films indicated that these copolymers were not cytotoxic, in fact proliferation of BMSCs was significantly enhanced. Osteogenic differentiation of BMSCs showed that the electroactive copolymers greatly promoted osteogenic differentiation compared to pure PLA with respect to expression of ALP, OPN, and Runx2 and deposition of calcium measured by von Kossa staining. The electroactive copolymer surface can adsorb more protein than pure PLA, which may be a factor that enhanced proliferation and differentiation of MSCs. These results indicated that the electroactive degradable polymers based on polylactide and aniline tetramer have great potential as scaffolding materials for bone regeneration.
3. Summary and Perspective
Degradable hybrid polymer biomaterials with osteoconductivity, biomimetic elastomeric behavior and electroactivity have shown promise in applications in bone tissue repair and regeneration. However, to meet the requirements of efficient bone regeneration, there are still many areas in need of improvement for these polymer hybrid biomaterials. First, high osteoinductive activity should be incorporated into these hybrid polymers. Second, maintaining high mechanical strength of hybrid polymers while preserving their elastomeric behavior should be prioritized. Third, other functions including antibacterial activity and bioimaging ability should be also considered in the design of next generation hybrid polymer biomaterials.
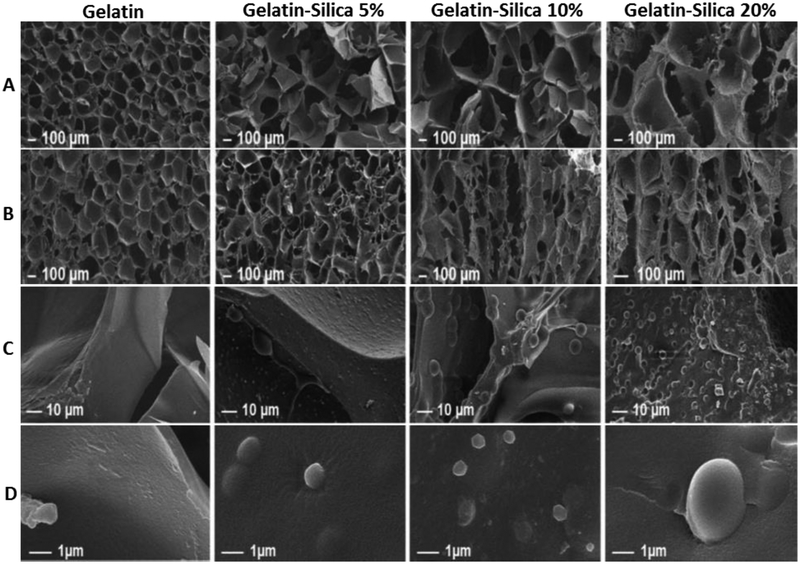
Figure 5.
Porous morphology of gelatin-silica hybrid polymer scaffolds. (A, C) Transverse direction; (B, D) Axial direction. Reproduced from Ref.[68]
Acknowledgements
This work was financially supported by US DOD (W81XWH-12-2-0008), the National Institutes of Health (NIDCR DE022327), Natural Science Foundation of China (grant number: 51502237, 21304073 and 51673155), and Xi’an Jiaotong University.
References
- [1].Watt FM, Huck WTS, Role of the extracellular matrix in regulating stem cell fate, Nat. Rev. Mol. Cell Biol 14 (2013) 467–473. doi: 10.1038/nrm3620. [DOI] [PubMed] [Google Scholar]
- [2].Erickson IE, Kestle SR, Zellars KH, Farrell MJ, Kim M, Burdick JA, et al. , High mesenchyrnal stem cell seeding densities in hyaluronic acid hydrogels produce engineered cartilage with native tissue properties, Acta Biomater 8 (2012) 3027–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lee SS, Huang BJ, Kaltz SR, Sur S, Newcomb CJ, Stock SR, et al. , Bone regeneration with low dose BMP-2 amplified by biomimetic supramolecular nanofibers within collagen scaffolds, Biomaterials 34 (2013) 452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].V Dorozhkin S, Calcium Orthophosphate-Containing Biocomposites and Hybrid Biomaterials for Biomedical Applications, J Funct Biomater 6 (2015) 708–832. doi: 10.3390/jfb6030708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wu W, Wang WG, Li JS, Star polymers: Advances in biomedical applications, Prog. Polym. Sci 46 (2015) 55–85. [Google Scholar]
- [6].Nicolas J, Mura S, Brambilla D, Mackiewicz N, Couvreur P, Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery, Chem. Soc. Rev 42 (2013) 1147–1235. [DOI] [PubMed] [Google Scholar]
- [7].Tian HY, Tang ZH, Zhuang XL, Chen XS, Jing XB, Biodegradable synthetic polymers: Preparation, functionalization and biomedical application, Prog. Polym. Sci 37 (2012) 237–280. [Google Scholar]
- [8].Pan Z, Ding J, Poly(lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicine, Interface Focus 2 (2012) 366–377. doi: 10.1098/rsfs.2011.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Igwe JC, Mikael PE, Nukavarapu SP, Design, fabrication and in vitro evaluation of a novel polymer-hydrogel hybrid scaffold for bone tissue engineering, J. Tissue Eng. Regen. Med 8 (2014) 131–142. doi: 10.1002/term.1506. [DOI] [PubMed] [Google Scholar]
- [10].Venkatesan J, Bhatnagar I, Manivasagan P, Kang K-H, Kim S-K, Alginate composites for bone tissue engineering: A review, Int. J. Biol. Macromol 72 (2015) 269–281. doi: 10.1016/j.ijbiomac.2014.07.008. [DOI] [PubMed] [Google Scholar]
- [11].Venkatesan J, Vinodhini PA, Sudha PN, Kim S-K, Chitin and Chitosan Composites for Bone Tissue Regeneration, in: Adv. Food Nutr. Res, 2014: pp. 59–81. doi: 10.1016/B978-0-12-800268-1.00005-6. [DOI] [PubMed] [Google Scholar]
- [12].Yunus Basha R, S.K. TS, M. Doble, Design of biocomposite materials for bone tissue regeneration, Mater. Sci. Eng. C 57 (2015) 452–463. doi: 10.1016/J.MSEC.2015.07.016. [DOI] [PubMed] [Google Scholar]
- [13].Sun F, Zhou H, Lee J, Various preparation methods of highly porous hydroxyapatite/polymer nanoscale biocomposites for bone regeneration, Acta Biomater 7 (2011) 3813–3828. doi: 10.1016/j.actbio.2011.07.002. [DOI] [PubMed] [Google Scholar]
- [14].Gkioni K, Leeuwenburgh SCG, Douglas TEL, Mikos AG, Jansen JA, Mineralization of hydrogels for bone regeneration., Tissue Eng. Part B. Rev 16 (2010) 577–85. doi: 10.1089/ten.TEB.2010.0462. [DOI] [PubMed] [Google Scholar]
- [15].Wei Q, Lu J, Wang Q, Fan H, Zhang X, Novel synthesis strategy for composite hydrogel of collagen/hydroxyapatite-microsphere originating from conversion of CaCO 3 templates, Nanotechnology 26 (2015) 115605. doi: 10.1088/0957-4484/26/11/115605. [DOI] [PubMed] [Google Scholar]
- [16].Vo TN, Shah SR, Lu S, Tatara AM, Lee EJ, Roh TT, et al. , Injectable dual-gelling cell-laden composite hydrogels for bone tissue engineering, Biomaterials 83 (2016) 1–11. doi: 10.1016/j.biomaterials.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nejadnik MR, Mikos AG, Jansen JA, Leeuwenburgh SCG, Facilitating the mineralization of oligo(poly(ethylene glycol) fumarate) hydrogel by incorporation of hydroxyapatite nanoparticles, J. Biomed. Mater. Res. Part A 100A (2012) 1316–1323. doi: 10.1002/jbm.a.34071. [DOI] [PubMed] [Google Scholar]
- [18].Samavedi S, Whittington AR, Goldstein AS, Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior, Acta Biomater 9 (2013) 8037–8045. [DOI] [PubMed] [Google Scholar]
- [19].Wei G, Jin Q, Giannobile WV, Ma PX, The enhancement of osteogenesis by nano-fibrous scaffolds incorporating rhBMP-7 nanospheres, Biomaterials 28 (2007) 2087–2096. doi: 10.1016/j.biomaterials.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang R, Ma PX, Poly(alpha-hydroxyl acids)/hydroxyapatite porous composites for bone-tissue engineering. I. Preparation and morphology., J. Biomed. Mater. Res 44 (1999) 446–455. [DOI] [PubMed] [Google Scholar]
- [21].Kango S, Kalia S, Celli A, Njuguna J, Habibi Y, Kumar R, Surface modification of inorganic nanoparticles for development of organic-inorganic nanocomposites-A review, Prog. Polym. Sci 38 (2013) 1232–1261. [Google Scholar]
- [22].Pina S, Oliveira JM, Reis RL, Natural-Based Nanocomposites for Bone Tissue Engineering and Regenerative Medicine: A Review, Adv. Mater 27 (2015) 1143–1169. [DOI] [PubMed] [Google Scholar]
- [23].Sahoo NG, Pan YZ, Li L, He CB, Nanocomposites for bone tissue regeneration, Nanomedicine 8 (2013) 639–653. [DOI] [PubMed] [Google Scholar]
- [24].Ma PX, Zhang R, Xiao G, Franceschi R, Engineering new bone tissue in vitro on highly porous poly(alpha-hydroxyl acids)/hydroxyapatite composite scaffolds., J. Biomed. Mater. Res 54 (2001) 284–293. [DOI] [PubMed] [Google Scholar]
- [25].Shinzato S, Nakamura T, Ando K, Kokubo T, Kitamura Y, Mechanical properties and osteoconductivity of new bioactive composites consisting of partially crystallized glass beads and poly(methyl methacrylate)., J. Biomed. Mater. Res 60 (2002) 556–563. [DOI] [PubMed] [Google Scholar]
- [26].Koleganova VA, Bernier SM, Dixon SJ, Rizkalla AS, Bioactive glass/polymer composite materials with mechanical properties matching those of cortical bone., J. Biomed. Mater. Res. A 77 (2006) 572–579. doi: 10.1002/jbm.a.30561. [DOI] [PubMed] [Google Scholar]
- [27].Marcolongo M, Ducheyne P, Garino J, Schepers E, Bioactive glass fiber/polymeric composites bond to bone tissue., J. Biomed. Mater. Res 39 (1998) 161–170. [DOI] [PubMed] [Google Scholar]
- [28].Kerativitayanan P, Gaharwar AK, Elastomeric and mechanically stiff nanocomposites from poly(glycerol sebacate) and bioactive nanosilicates, Acta Biomater 26 (2015) 34–44. [DOI] [PubMed] [Google Scholar]
- [29].Zhao X, Wu Y, Du Y, Chen X, Lei B, Xue Y, et al. , A highly bioactive and biodegradable poly(glycerol sebacate)–silica glass hybrid elastomer with tailored mechanical properties for bone tissue regeneration, J. Mater. Chem. B 3 (2015) 3222–3233. doi: 10.1039/C4TB01693A. [DOI] [PubMed] [Google Scholar]
- [30].Du YZ, Yu M, Ge J, Ma PX, Chen XF, Lei B, Development of a Multifunctional Platform Based on Strong, Intrinsically Photoluminescent and Antimicrobial Silica-Poly(citrates)-Based Hybrid Biodegradable Elastomers for Bone Regeneration, Adv. Funct. Mater 25 (2015) 5016–5029. [Google Scholar]
- [31].Du YZ, Ge J, Shao YP, Ma PX, Chen XF, Lei B, Development of silica grafted poly(1,8-octanediol-co-citrates) hybrid elastomers with highly tunable mechanical properties and biocompatibility, J. Mater. Chem. B 3 (2015) 2986–3000. [DOI] [PubMed] [Google Scholar]
- [32].Balint R, Cassidy NJ, Cartmell SH, Conductive polymers: Towards a smart biomaterial for tissue engineering, Acta Biomater 10 (2014) 2341–2353. [DOI] [PubMed] [Google Scholar]
- [33].Hopley EL, Salmasi S, Kalaskar DM, Seifalian AM, Carbon nanotubes leading the way forward in new generation 3D tissue engineering, Biotechnol. Adv 32 (2014) 1000–1014. [DOI] [PubMed] [Google Scholar]
- [34].Liu XH, Holzwarth JM, Ma PX, Functionalized Synthetic Biodegradable Polymer Scaffolds for Tissue Engineering, Macromol. Biosci 12 (2012) 911–919. [DOI] [PubMed] [Google Scholar]
- [35].Jones JR, Review of bioactive glass: From Hench to hybrids, Acta Biomater 9 (2013) 4457–4486. [DOI] [PubMed] [Google Scholar]
- [36].Lei B, Chen XF, Wang YJ, Zhao N, Synthesis and in vitro bioactivity of novel mesoporous hollow bioactive glass microspheres, Mater. Lett 63 (2009) 1719–1721. [Google Scholar]
- [37].Lei B, Chen XF, Wang YJ, Zhao NR, Du C, Fang LM, Surface nanoscale patterning of bioactive glass to support cellular growth and differentiation, J. Biomed. Mater. Res. Part A 94a (2010) 1091–1099. [DOI] [PubMed] [Google Scholar]
- [38].Chen XF, Lei B, Wang YJ, Zhao N, Morphological control and in vitro bioactivity of nanoscale bioactive glasses, J. Non. Cryst. Solids 355 (2009) 791–796. [Google Scholar]
- [39].Zakaria SM, Sharif SH, Othman MR, Yang F, Jansen JA, Nanophase Hydroxyapatite as a Biomaterial in Advanced Hard Tissue Engineering: A Review, Tissue Eng. Part B-Reviews 19 (2013) 431–441. [DOI] [PubMed] [Google Scholar]
- [40].Boccaccini AR, Erol M, Stark WJ, Mohn D, Hong ZK, Mano JF, Polymer/bioactive glass nanocomposites for biomedical applications: A review, Compos. Sci. Technol 70 (2010) 1764–1776. [Google Scholar]
- [41].Lei B, Shin KH, Noh DY, Koh YH, Choi WY, Kim HE, Bioactive glass microspheres as reinforcement for improving the mechanical properties and biological performance of poly(e-caprolactone) polymer for bone tissue regeneration, J. Biomed. Mater. Res. Part B-Applied Biomater 100b (2012) 967–975. [DOI] [PubMed] [Google Scholar]
- [42].Lei B, Chen XF, Han X, Zhou JA, Versatile fabrication of nanoscale sol-gel bioactive glass particles for efficient bone tissue regeneration, J. Mater. Chem 22 (2012) 16906–16913. [Google Scholar]
- [43].Lei B, Shin KH, Noh DY, Jo IH, Koh YH, Kim HE, et al. , Sol-gel derived nanoscale bioactive glass (NBG) particles reinforced poly(epsilon-caprolactone) composites for bone tissue engineering, Mater. Sci. Eng. C-Materials Biol. Appl 33 (2013) 1102–1108. [DOI] [PubMed] [Google Scholar]
- [44].Roohani-Esfahani SI, Nouri-Khorasani S, Lu ZF, Appleyard R, Zreiqat H, The influence hydroxyapatite nanoparticle shape and size on the properties of biphasic calcium phosphate scaffolds coated with hydroxyapatite-PCL composites, Biomaterials 31 (2010) 5498–5509. [DOI] [PubMed] [Google Scholar]
- [45].Rahaman MN, Day DE, Bal BS, Fu Q, Jung SB, Bonewald LF, et al. , Bioactive glass in tissue engineering, Acta Biomater 7 (2011) 2355–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Peter M, Binulal NS, V Nair S, Selvamurugan N, Tamura H, Jayakumar R, Novel biodegradable chitosan-gelatin/nano-bioactive glass ceramic composite scaffolds for alveolar bone tissue engineering, Chem. Eng. J 158 (2010) 353–361. [Google Scholar]
- [47].Mozafari M, Moztarzadeh F, Rabiee M, Azami M, Maleknia S, Tahriri M, et al. , Development of macroporous nanocomposite scaffolds of gelatin/bioactive glass prepared through layer solvent casting combined with lamination technique for bone tissue engineering, Ceram. Int 36 (2010) 2431–2439. [Google Scholar]
- [48].Hong ZK, Reis RL, Mano JF, Preparation and in vitro characterization of scaffolds of poly(L-lactic acid) containing bioactive glass ceramic nanoparticles, Acta Biomater 4 (2008) 1297–1306. [DOI] [PubMed] [Google Scholar]
- [49].Liu X, Smith LA, Hu J, Ma PX, Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering, Biomaterials 30 (2009) 2252–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].He CL, Xiao GY, Jin XB, Sun CH, Ma PX, Electrodeposition on Nanofibrous Polymer Scaffolds: Rapid Mineralization, Tunable Calcium Phosphate Composition and Topography, Adv. Funct. Mater 20 (2010) 3568–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lei B, Wang L, Chen XF, Chae SK, Biomimetic and molecular level-based silicate bioactive glass-gelatin hybrid implants for loading-bearing bone fixation and repair, J. Mater. Chem. B 1 (2013) 5153–5162. [DOI] [PubMed] [Google Scholar]
- [52].Chen J, Que W, Xing Y, Lei B, Molecular level-based bioactive glass-poly (caprolactone) hybrids monoliths with porous structure for bone tissue repair, Ceram. Int 41 (2015) 3330–3334. doi: 10.1016/j.ceramint.2014.10.147. [DOI] [Google Scholar]
- [53].Xie MH, Ge J, Lei B, Zhang Q, Chen XF, Ma PX, Star-Shaped, Biodegradable, and Elastomeric PLLA-PEG-POSS Hybrid Membrane With Biomineralization Activity for Guiding Bone Tissue Regeneration, Macromol. Biosci 15 (2015) 1656–1662. [DOI] [PubMed] [Google Scholar]
- [54].Chen J, Du YZ, Que WX, Xing YL, Chen XF, Lei B, Crack-free polydimethylsiloxane-bioactive glass-poly(ethylene glycol) hybrid monoliths with controlled biomineralization activity and mechanical property for bone tissue regeneration, Colloids and Surfaces B-Biointerfaces 136 (2015) 126–133. [DOI] [PubMed] [Google Scholar]
- [55].Chen J, Du YZ, Que WX, Xing YL, Lei B, Content-dependent biomineralization activity and mechanical properties based on polydimethylsiloxane-bioactive glass-poly(caprolactone) hybrids monoliths for bone tissue regeneration, Rsc Adv 5 (2015) 61309–61317. [DOI] [PubMed] [Google Scholar]
- [56].Lei B, Shin KH, Moon YW, Noh DY, Koh YH, Jin Y, et al. , Synthesis and Bioactivity of Sol-Gel Derived Porous, Bioactive Glass Microspheres Using Chitosan as Novel Biomolecular Template, J. Am. Ceram. Soc 95 (2012) 30–33. [Google Scholar]
- [57].Mahony O, Tsigkou O, Ionescu C, Minelli C, Ling L, Hanly R, et al. , Silica-Gelatin Hybrids with Tailorable Degradation and Mechanical Properties for Tissue Regeneration, Adv. Funct. Mater 20 (2010) 3835–3845. [Google Scholar]
- [58].Lei B, Shin KH, Jo IH, Koh YH, Kim HE, Highly porous gelatin-silica hybrid scaffolds with textured surfaces using new direct foaming/freezing technique, Mater. Chem. Phys 145 (2014) 397–402. [Google Scholar]
- [59].Lei B, Shin KH, Noh DY, Jo IH, Koh YH, Choi WY, et al. , Nanofibrous gelatin-silica hybrid scaffolds mimicking the native extracellular matrix (ECM) using thermally induced phase separation, J. Mater. Chem 22 (2012) 14133–14140. [Google Scholar]
- [60].Xue YM, Wang L, Shao YP, Yan J, Chen XF, Lei B, Facile and green fabrication of biomimetic gelatin-siloxane hybrid hydrogel with highly elastic properties for biomedical applications, Chem. Eng. J 251 (2014) 158–164. [Google Scholar]
- [61].Duan S, Yang X, Mei F, Tang Y, Li X, Shi Y, et al. , Enhanced osteogenic differentiation of mesenchymal stem cells on poly(l -lactide) nanofibrous scaffolds containing carbon nanomaterials, J. Biomed. Mater. Res. Part A 103 (2015) 1424–1435. doi: 10.1002/jbm.a.35283. [DOI] [PubMed] [Google Scholar]
- [62].Sitharaman B, Shi X, Walboomers XF, Liao H, Cuijpers V, Wilson LJ, et al. , In vivo biocompatibility of ultra-short single-walled carbon nanotube/biodegradable polymer nanocomposites for bone tissue engineering., Bone 43 (2008) 362–70. doi: 10.1016/j.bone.2008.04.013. [DOI] [PubMed] [Google Scholar]
- [63].Park S, Park J, Jo I, Cho S-P, Sung D, Ryu S, et al. , In situ hybridization of carbon nanotubes with bacterial cellulose for three-dimensional hybrid bioscaffolds, Biomaterials 58 (2015) 93–102. doi: 10.1016/J.BIOMATERIALS.2015.04.027. [DOI] [PubMed] [Google Scholar]
- [64].Siqueira IAWB, Corat MAF, Cavalcanti B. das N., Neto WAR, Martin AA, Bretas RES, et al. , In Vitro and in Vivo Studies of Novel Poly(d, l -lactic acid), Superhydrophilic Carbon Nanotubes, and Nanohydroxyapatite Scaffolds for Bone Regeneration, ACS Appl. Mater. Interfaces 7 (2015) 9385–9398. doi: 10.1021/acsami.5b01066. [DOI] [PubMed] [Google Scholar]
- [65].Mikael PE, Amini AR, Basu J, Josefina Arellano-Jimenez M, Laurencin CT, Sanders MM, et al. , Functionalized carbon nanotube reinforced scaffolds for bone regenerative engineering: fabrication, in vitro and in vivo evaluation, Biomed. Mater 9 (2014) 35001. doi: 10.1088/1748-6041/9/3/035001. [DOI] [PubMed] [Google Scholar]
- [66].Hirata E, Ménard-Moyon C, Venturelli E, Takita H, Watari F, Bianco A, et al. , Carbon nanotubes functionalized with fibroblast growth factor accelerate proliferation of bone marrow-derived stromal cells and bone formation, Nanotechnology 24 (2013) 435101. doi: 10.1088/0957-4484/24/43/435101. [DOI] [PubMed] [Google Scholar]
- [67].Das B, Chattopadhyay P, Maji S, Upadhyay A, Das Purkayastha M, lata Mohanta C, et al. , Bio-functionalized MWCNT/hyperbranched polyurethane bionanocomposite for bone regeneration, Biomed. Mater 10 (2015) 25011. doi: 10.1088/1748-6041/10/2/025011. [DOI] [PubMed] [Google Scholar]
- [68].Lei B, Shin K-H, Koh Y-H, Kim H-E, Porous gelatin-siloxane hybrid scaffolds with biomimetic structure and properties for bone tissue regeneration, J. Biomed. Mater. Res. Part B Appl. Biomater 102 (2014) 1528–1536. doi: 10.1002/jbm.b.33133. [DOI] [PubMed] [Google Scholar]
- [69].Nettles DL, Chilkoti A, Setton LA, Applications of elastin-like polypeptides in tissue engineering, Adv. Drug Deliv. Rev 62 (2010) 1479–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Chen QZ, Liang SL, Thouas GA, Elastomeric biomaterials for tissue engineering, Prog. Polym. Sci 38 (2013) 584–671. [Google Scholar]
- [71].Sant S, Hwang CM, Lee SH, Khademhosseini A, Hybrid PGS-PCL microfibrous scaffolds with improved mechanical and biological properties, J. Tissue Eng. Regen. Med 5 (2011) 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kharaziha M, Nikkhah M, Shin SR, Annabi N, Masoumi N, Gaharwar AK, et al. , PGS:Gelatin nanofibrous scaffolds with tunable mechanical and structural properties for engineering cardiac tissues, Biomaterials 34 (2013) 6355–6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Bokobza L, Mechanical, electrical and spectroscopic investigations of carbon nanotube-reinforced elastomers, Vib. Spectrosc 51 (2009) 52–59. [Google Scholar]
- [74].Pei AH, Malho JM, Ruokolainen J, Zhou Q, Berglund LA, Strong Nanocomposite Reinforcement Effects in Polyurethane Elastomer with Low Volume Fraction of Cellulose Nanocrystals, Macromolecules 44 (2011) 4422–4427. [Google Scholar]
- [75].Paul DR, Mark JE, Fillers for polysiloxane (“silicone”) elastomers, Prog. Polym. Sci 35 (2010) 893–901. [Google Scholar]
- [76].Moradi A, Dalilottojari A, Pingguan-Murphy B, Djordjevic I, Fabrication and characterization of elastomeric scaffolds comprised of a citric acid-based polyester/hydroxyapatite microcomposite, Mater. Des 50 (2013) 446–450. [Google Scholar]
- [77].Liang SL, Cook WD, Thouas GA, Chen QZ, The mechanical characteristics and in vitro biocompatibility of poly(glycerol sebacate)-Bioglass (R) elastomeric composites, Biomaterials 31 (2010) 8516–8529. [DOI] [PubMed] [Google Scholar]
- [78].Du YZ, Yu M, Chen XF, Ma PX, Lei B, Development of Biodegradable Poly(citrate)-Polyhedral Oligomeric Silsesquioxanes Hybrid Elastomers with High Mechanical Properties and Osteogenic Differentiation Activity, ACS Appl. Mater. Interfaces 8 (2016) 3079–3091. [DOI] [PubMed] [Google Scholar]
- [79].Du YZ, Xue YM, Ma PX, Chen XF, Lei B, Biodegradable, Elastomeric, and Intrinsically Photoluminescent Poly(Silicon-Citrates) with high Photostability and Biocompatibility for Tissue Regeneration and Bioimaging, Adv. Healthc. Mater 5 (2016) 382–392. [DOI] [PubMed] [Google Scholar]
- [80].Guimard NK, Gomez N, Schmidt CE, Conducting polymers in biomedical engineering, Prog. Polym. Sci 32 (2007) 876–921. [Google Scholar]
- [81].Patil AO, Heeger AJ, Wudl F, Optical-Properties of Conducting Polymers, Chem. Rev 88 (1988) 183–200. [Google Scholar]
- [82].Guo BL, Glavas L, Albertsson AC, Biodegradable and electrically conducting polymers for biomedical applications, Prog. Polym. Sci 38 (2013) 1263–1286. [Google Scholar]
- [83].Xie M, Wang L, Ge J, Guo B, Ma PX, Strong Electroactive Biodegradable Shape Memory Polymer Networks Based on Star-Shaped Polylactide and Aniline Trimer for Bone Tissue Engineering, ACS Appl. Mater. Interfaces 7 (2015) 6772–6781. doi: 10.1021/acsami.5b00191. [DOI] [PubMed] [Google Scholar]
- [84].Xie MH, Wang L, Guo BL, Wang Z, Chen YE, Ma PX, Ductile electroactive biodegradable hyperbranched polylactide copolymers enhancing myoblast differentiation, Biomaterials 71 (2015) 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Hardy JG, Geissler SA, Aguilar D, Villancio-Wolter MK, Mouser DJ, Sukhavasi RC, et al. , Instructive Conductive 3D Silk Foam-Based Bone Tissue Scaffolds Enable Electrical Stimulation of Stem Cells for Enhanced Osteogenic Differentiation, Macromol. Biosci 15 (2015) 1490–1496. [DOI] [PubMed] [Google Scholar]
- [86].Meng SY, Zhang Z, Rouabhia M, Accelerated osteoblast mineralization on a conductive substrate by multiple electrical stimulation, J. Bone Miner. Metab 29 (2011) 535–544. [DOI] [PubMed] [Google Scholar]
- [87].Meng SY, Rouabhia M, Zhang Z, Electrical stimulation modulates osteoblast proliferation and bone protein production through heparin-bioactivated conductive scaffolds, Bioelectromagnetics 34 (2013) 189–199. [DOI] [PubMed] [Google Scholar]
- [88].Yazdimamaghani M, Razavi M, Mozafari M, Vashaee D, Kotturi H, Tayebi L, Biomineralization and biocompatibility studies of bone conductive scaffolds containing poly(3,4-ethylenedioxythiophene): poly(4-styrene sulfonate) (PEDOT:PSS), J. Mater. Sci. Med 26 (2015). [DOI] [PubMed] [Google Scholar]
- [89].Pelto J, Bjorninen M, Palli A, Talvitie E, Hyttinen J, Mannerstrom B, et al. , Novel Polypyrrole-Coated Polylactide Scaffolds Enhance Adipose Stem Cell Proliferation and Early Osteogenic Differentiation, Tissue Eng. Part A 19 (2013) 882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Guo B, Lei B, Li P, Ma PX, Functionalized scaffolds to enhance tissue regeneration, Regen Biomater 2 (2015) 47–57. doi: 10.1093/rb/rbu016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Jiang T, Carbone EJ, Lo KWH, Laurencin CT, Electrospinning of polymer nanofibers for tissue regeneration, Prog. Polym. Sci 46 (2015) 1–24. [Google Scholar]
- [92].Schneider OD, Weber F, Brunner TJ, Loher S, Ehrbar M, Schmidlin PR, et al. , In vivo and in vitro evaluation of flexible, cottonwool-like nanocomposites as bone substitute material for complex defects, Acta Biomater 5 (2009) 1775–1784. [DOI] [PubMed] [Google Scholar]
- [93].Lee EJ, Shin DS, Kim HE, Kim HW, Koh YH, Jang JH, Membrane of hybrid chitosan-silica xerogel for guided bone regeneration, Biomaterials 30 (2009) 743–750. [DOI] [PubMed] [Google Scholar]
- [94].Xie MH, Ge J, Xue YM, Du YZ, Lei B, Ma PX, Photo-crosslinked fabrication of novel biocompatible and elastomeric star-shaped inositol-based polymer with highly tunable mechanical behavior and degradation, J. Mech. Behav. Biomed. Mater 51 (2015) 163–168. [DOI] [PubMed] [Google Scholar]
- [95].Li LC, Yu M, Ma PX, Guo BL, Electroactive degradable copolymers enhancing osteogenic differentiation from bone marrow derived mesenchymal stem cells, J. Mater. Chem B. 4 (2016) 471–481. [DOI] [PubMed] [Google Scholar]