Abstract
Graft-versus-host disease (GVHD) remains the most common treatment-related complication after allogeneic hematopoietic cell transplantation (allo-HCT). Lymphocyte migration plays a critical role in the pathogenesis of GVHD. A previous phase I/II trial demonstrated that CCR5 blockade with maraviroc in the first 30 days after transplant resulted in a low incidence of early acute GVHD, primarily in visceral organs, but without impact on late acute and chronic GVHD.
We conducted a phase II trial to examine the efficacy of an extended course of maraviroc, administered through post-transplant day +90 in addition to standard prophylaxis in 37 reduced-intensity conditioned unrelated donor allo-HCT recipients with hematologic malignancies.
Extended maraviroc treatment was safe and feasible. The primary endpoint, day-180 rate (±s.e.) of grade 2 to 4 acute GVHD was 22% ±7%, liver GVHD was not observed and gut GVHD was uncommon. The day-180 rate of grade 3 to 4 acute GVHD was 5% ±4%. The 1-year rate of moderate-severe chronic GVHD was 8% ±5% and disease relapse 30% ±8%. Overall survival at 1 year was 70% ±8%. In comparison to the previously studied short course of maraviroc, the extended course resulted in a significantly higher GVHD-free, relapse-free survival (adjusted HR 0.45, 95% CI [0.25 to 0.82], p=.009) and overall survival (adjusted HR 0.48, 95% CI [0.24 to 0.96], p=.037). A combined analysis of both trials showed that high maraviroc trough concentrations on the day of hematopoietic cell infusion were associated with lower rates of acute GVHD.
An extended course of maraviroc after reduced intensity unrelated donor allo-HCT is safe and effective in preventing acute and chronic GVHD and is associated with favorable survival.
Introduction
Graft-versus-host disease (GVHD) remains a significant barrier to successful allogeneic hematopoietic cell transplantation (allo-HCT) and represents the most common cause for treatment-related mortality in patients with hematologic malignancies, especially when the gastrointestinal (GI) tract is involved.1 Acute GVHD complicates approximately 30%-50% of HLA-matched transplants from related donors and 50%-70% of transplants from unrelated donors with standard prophylaxis regimens.2 Chronic GVHD further complicates allo-HCT and leads to long-term debilitating symptoms and opportunistic infections in many patients.3 Therefore, additional strategies to prevent GVHD are desperately needed.
Blocking lymphocyte migration may prevent GvHD without interfering with graft-versus-tumor activity. Donor T cells must home to secondary lymphoid organs and then into target organs in order to recognize alloantigens presented by antigen-presenting cells and to cause tissue injury.4 This migration is carefully regulated by adhesion molecules and chemokine receptors expressed by lymphocytes, such as CCR5. In animal models, murine GVHD can be prevented by blocking alloreactive CCR5+ T cell homing.5-7 We previously reported that brief (up to day +30) CCR5 blockade using maraviroc in patients with hematologic malignancies resulted in a low incidence of acute GVHD and absence of early liver and gut GVHD, although some cases of delayed acute GVHD still occurred and the incidence of chronic GVHD was unaffected.8,9 In addition, the majority of the treatment effect was seen in unrelated donor transplants and not in matched related donor transplants. We therefore conducted a phase II study to test extended administration of maraviroc in unrelated donor allo-HCT.
Methods
Trial Design
We conducted a phase II clinical trial to study the role of a 3-month course of maraviroc when added to conventional GVHD prophylaxis after reduced intensity allo-HCT from well-matched or single-antigen mismatched unrelated donors in patients with hematologic malignancies. A 3-month treatment course parallels the course of calcineurin inhibitors at therapeutic levels on our standard protocols and was not extended further due to an unknown impact on disease relapse. Our primary objective was to determine the efficacy of an extended course of maraviroc in the prevention of acute GVHD. Study participants were considered eligible if they were at least 18 years old, had an available unrelated donor with at least 7/8 HLA-A, -B, -C and -DRB1 matching by high resolution typing and met institutional criteria of performance status and organ function for reduced-intensity allo-HCT. Eligible diseases included acute leukemia in complete remission, chronic myelogenous leukemia or myelodysplastic syndrome with <5% marrow blasts, lymphomas, chronic lymphocytic leukemia and myeloproliferative neoplasms other than primary myelofibrosis.
All participants were admitted to the Hospital of the University of Pennsylvania, and received a uniform conditioning regimen of fludarabine i.v. 120 mg/m2 and busulfan i.v. 6.4 mg/kg (Flu/Bu), followed by the infusion of a granulocyte colony-stimulating factor-mobilized peripheral blood stem cell graft from an unrelated donor on day 0.
All participants received a uniform GVHD prophylaxis regimen of oral tacrolimus 0.06 mg/kg/d in 2 divided doses starting on day −3 and intravenous methotrexate 15 mg/m2 on day +1 and 10 mg/m2 on days +3, +6 and +11. Trough tacrolimus concentrations were checked at least twice weekly and doses were adjusted to target levels between 5–15 ng/mL.
Maraviroc was administered orally twice daily between day −3 and day +90. An oral suspension was used as a substitute for tablets in patients who developed severe mucositis. Dose adjustments were performed with interacting medications according to the maraviroc package insert, however no dose adjustments were made for concomitant use of voriconazole or posaconazole, based on pharmacokinetic data from our previous study. Dose reduction was allowed for symptomatic orthostatic hypotension, which is dose-dependent.
Clinical Endpoints
The primary endpoint was the cumulative incidence of grade 2 to 4 acute GVHD by day 180 according to the consensus conference criteria.10 Safety was monitored and recorded using the NCI CTCAE v4.0 criteria. Neutrophil and/or platelet engraftment were defined as absolute neutrophil count >0.5×109/L on the first of 3 consecutive days and platelet count >20×109/L on the first of 7 consecutive days without transfusion support, respectively. Time to disease relapse, acute GVHD, nonrelapse mortality (NRM), overall survival (OS) and chronic GVHD were defined as the time from transplantation (day 0) to the event. GVHD-free, relapse-free survival (GRFS) was defined as the time from transplantation to grade 3 to 4 acute GVHD, moderate to severe chronic GVHD, disease relapse or death, whichever occurred first. Patients were censored at the time of last contact alive and event-free or a second transplantation for all outcomes, and at the time of donor lymphocyte infusion when applicable for GVHD outcomes. Disease relapse was defined as morphologic, cytogenetic or radiologic evidence of disease demonstrating pre-transplantation characteristics. Restaging evaluation, including bone marrow biopsies and appropriate imaging, was routinely performed at day +100 or earlier in patients with signs indicating early relapse. The Consensus Conference criteria and National Institutes of Health criteria were used for acute and chronic GVHD grading, respectively.10,11
Lab Assessment
Donor chimerism levels were measured in whole blood and after immunomagnetic positive selection of CD3+ cells from peripheral blood and bone marrow samples (STEMCELL Technologies, Vancouver, BC, Canada). IgG levels and CD4+ T-cell counts were measured by clinical labs using standard assays. Maraviroc trough concentrations were measured in plasma on day 0 following 6 doses of maraviroc and then again on day +14. The method of maraviroc concentration measurement is provided in the Supplementary Information.
Statistical Analysis
We hypothesized that maraviroc treatment through day +90 would decrease the day-180 rate of grade 2 to 4 acute GVHD to less than 30% from a historical rate of 52%. The historical rate was established in patients who underwent unrelated donor transplant with Flu/Bu2 conditioning and tacrolimus/methotrexate prophylaxis at Penn between 2009 and 2014. A sample size of 31 patients provided 80% power for a one sample chi-square test of the null hypothesis that π0 >0.52 vs. πA <0.30 at a one-sided 5% significance level. The sample size was increased to 37 patients to account for possible mortality in the first 6 months. The planned follow up was 1 year. The cumulative incidence function was used to analyze time to GVHD and disease relapse, taking into account death as a competing risk. Relapse was considered a competing risk for NRM and GVHD. OS and GRFS were assessed using the Kaplan-Meier method. An exploratory analysis comparing the outcomes of the current study with the previous study (1 month of maraviroc) were conducted using the Gray’s test12 or Cox regression followed by multivariable Cox regression modeling with backward elimination to adjust for significant covariates using P<0.05 as a criterion for inclusion in the models. For this analysis, long-term follow-up data were used and are presented up to 48 months. OS and time to disease relapse were adjusted for the Disease Risk Index,13 GRFS was adjusted for donor age, GVHD outcomes were adjusted for graft CD3+ T-cell doses and NRM had no significant covariates. A similar method was used to compare patients with high vs. low day 0 maraviroc concentrations, with the planned treatment duration (1 month vs. 3 months) as a fixed covariate. The proportional hazards assumption was tested for each covariate to ensure that this assumption was not violated. Analyses were conducted in R (The R Project for Statistical Computing, http://www.r-project.org) or in STATA v13.1 (STATA, College Station, TX).
Study Approval
The study was approved by the Institutional Review Board of the University of Pennsylvania, and written informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
Results
Patient Characteristics
We enrolled 37 patients who received allo-HCT from unrelated donors using Flu/Bu conditioning and peripheral blood stem cells between May 2013 and August 2015. Patient characteristics are presented in Table 1. The median and mean ages were 64, 84% had a matched unrelated donor, 16% had 7/8 mismatched unrelated donor, and 49% had a comorbidity index >2. Underlying diseases were predominantly acute leukemia (78%) and MDS (16%) and the Disease Risk Index was high or very high in 49% of patients. At the time of analysis, median follow up was 36.1 months. All patients were included in the efficacy and safety analysis.
Table 1.
Patient Characteristics
| Variable | Value |
|---|---|
| Recipient age: mean (range) | 64 (49-72) |
| Recipient sex: M/F (%) | 62/38 |
| Comorbidity Index: N (%) | |
| Low (0) | 7 (19) |
| Intermediate (1-2) | 12 (32) |
| High (>2) | 18 (49) |
| Diagnosis: N (%) | |
| AML | 27 (73) |
| MDS | 6 (16) |
| ALL | 2 (5) |
| MPN | 1 (3) |
| NHL | 1 (3) |
| Disease Risk Index: N (%) | |
| Low | 3 (8) |
| Intermediate | 16 (43) |
| High / Very High | 18 (49) |
| Donor: N (%) | |
| Matched unrelated | 31 (84) |
| Single-antigen mismatched unrelated | 6 (16) |
| Donor age: mean (range) | 32 (19-53) |
| Donor sex: M/F (%) | 65/35 |
| Cytomegalovirus status: N (%) | |
| Recipient positive | 18 (49) |
| Donor positive | 9 (24) |
| Cell doses: mean (range) | |
| CD34+ ×106 cells/kg | 6.2 (1.5-20.2) |
| CD3+ ×108 cells/kg | 2.2 (0.6-8.1) |
| CD4+ ×108 T cells/kg | 1.5 (0.3-5.4) |
| CD8+ ×108 T cells/kg | 0.7 (0.1-2.2) |
Safety and Feasibility
The 3-month course of maraviroc was well tolerated; 8 patients did not complete treatment for the following reasons: disease relapse and early withdrawal of all GvHD prophylactic agents (5 patients), skin reaction that was thought to be related to sulfa but maraviroc was discontinued as well (1 patient), early infection-related death (1 patient) and poor oral tolerance (1 patient). One patient underwent dose reduction to 150 mg twice daily due to orthostatic hypotension and completed the treatment course at the modified dose.
Neutrophil engraftment occurred in all patients (median 12 days, range 10-24) and platelets engrafted at a median of 17 days (range 10-43) in all patients. Donor chimerism levels in whole blood, CD3+ T-cells and bone marrow are presented in Supplemental Figure 1 and were not different from historical controls (not shown).
Clinical Outcomes
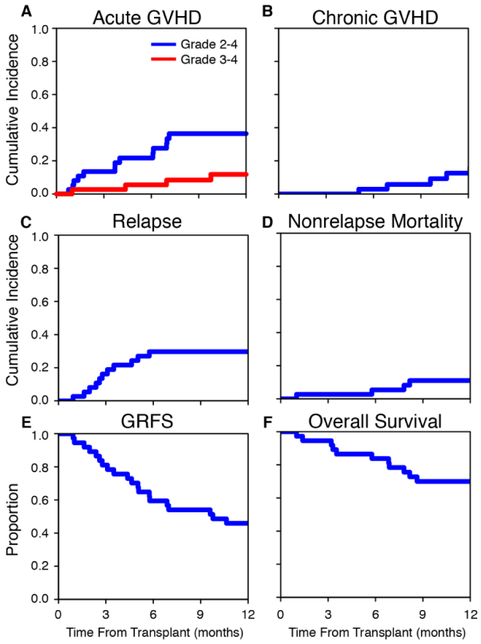
The study met its primary end-point; the day-180 rate (±s.e.) of grade 2 to 4 acute GVHD was 22 ±7% (95% confidence interval (CI) [8 to 36]; Fig. 1A), rejecting the null hypothesis of a 52% rate. In addition, the day-180 rate (±s.e.) of grade 3 to 4 acute GVHD was 5 ±4% (Fig. 1A). In the first 100 days there were no cases of liver GVHD, 2 patients developed stage 1 upper GI GVHD and 1 patient developed stage 3 lower GI GVHD together with aggressive relapse with documented leukemic infiltrates in the GI mucosa. At 1 year, the incidence of moderate to severe chronic GVHD was 8 ±5%, NRM was 11 ±5% and disease relapse was 30 ±8% (Fig. 1B-D). The GRFS rate at 1 year was 46 ±8% and OS rate at 1 year was 70 ±8% (Fig. 1E-F). With a median follow-up of 36.1 months, the median survival has not been reached; 20 of 37 patients are alive and 19 are in complete remission. Six patients received donor lymphocyte infusions due to disease relapse (5) or incomplete donor engraftment (1). Two of these patients are alive. One patient underwent a second transplant due to disease relapse. Causes of death are listed in Table 2. Two patients died from infectious complications related to delayed acute GVHD on day 234 and day 245. To date, there have been no deaths due to chronic GVHD.
Figure 1. Clinical outcomes with extended course of maraviroc.
Cumulative incidence plots of grade 2 to 4 acute graft-versus-host disease (GVHD) (A), grade 3 to 4 acute GVHD (A), moderate to severe chronic GVHD (B), disease relapse (C), nonrelapse mortality (D) and Kaplan-Meier plots of GVHD-free, relapse-free survival (GRFS) (E) and overall survival (F).
Table 2.
Causes of Death
| Cause of Death | Number (%) |
|---|---|
| Disease Relapse | 12 (32) |
| Acute GVHD | 2 (5) |
| Infection | 2 (5) |
| Second Malignancy | 1 (3) |
Immune Reconstitution and Infections
CD4+ and CD8+ T-cell counts and IgG levels on days +30, +60, +100 and +180 are displayed in Supplementary Figure 2. CD4 counts <200/μL were observed in the minority of patients at each time point, ranging from 8% to 23%. Only 2 patients (6%) on day +100 and 5 patients (16%) on day +180 had IgG levels <400 mg/dL. Of 18 CMV-positive recipients, 6 (33%) required treatment for CMV reactivation. Four patients (11%) had a Clostridium difficile infection during the first year after transplant. Other infections occurring during the treatment course included 8 bacteremias, 2 urinary tract infections, 1 pneumonia and 1 cholecystitis. Of note, antibacterial prophylaxis was not used in any of the patients and our standard prophylaxis included only antifungal, antiviral and anti-PCP prophylaxis. These results show that extended maraviroc treatment does not have an adverse effect on immune reconstitution or infectious complications.
Maraviroc Extended Course versus Short Course
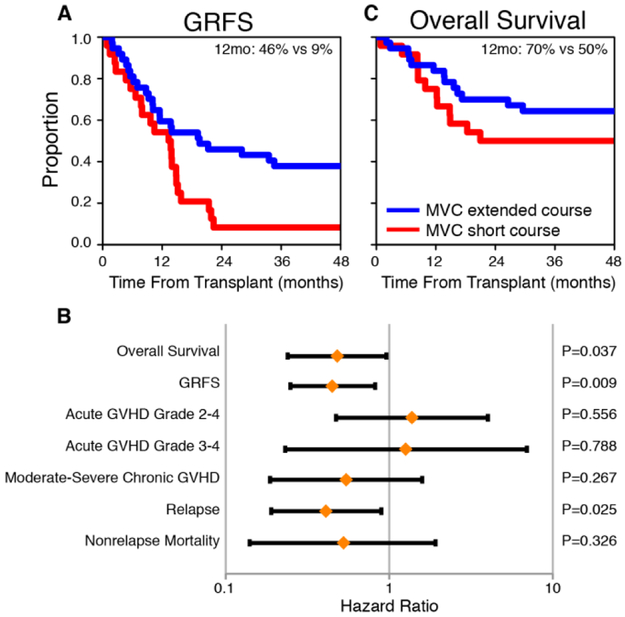
We conducted an exploratory analysis comparing the GRFS in this trial with the previously studied short (1-month) course of maraviroc. We excluded HLA-identical related donor transplants from the previous study to allow for comparison of similar patients. In this analysis we examined the duration of treatment (i.e., 3 months vs. 1 month) as the primary variable of interest with adjustment for significant covariates. The GRFS was significantly better for 3 months of maraviroc vs. 1 month (adjusted HR, 0.45, 95% CI [0.25 to 0.82], p=.009; Fig. 2A) and the GRFS rates at 1 year were 46 ±8% vs. 8 ±6%. In a detailed analysis of the components of the GRFS, there were no significant differences in the incidence rates of acute GVHD, but the incidence of disease relapse was significantly lower for the extended treatment course with adjustment for the Disease Risk Index (adjusted HR, 0.41, 95% CI [0.19 to 0.89], p=.025; Fig. 2B). The incidence rates of chronic GVHD and NRM were also lower for the extended course but the differences did not reach statistical significance (Fig. 2B). Importantly, the extended course of maraviroc was associated with an overall survival advantage (adjusted HR, 0.48, 95% CI [0.24 to 0.96], p=0.037, Fig. 2C) and the survival rates at 1 year were 70% ±8% for the extended course and 50% ±10% for the short course.
Figure 2. Comparison of outcomes of short course (1 month) and extended course (3 months) of maraviroc.
Kaplan-Meier plots of GVHD-free, relapse-free survival (GRFS) (A) and overall survival (C) with a comparison of 1-year estimates. A forest plot (B) shows adjusted hazard ratios, standard errors and p-values from multivariable analyses of transplant outcomes.
Maraviroc Blood Concentrations Correlate with Efficacy
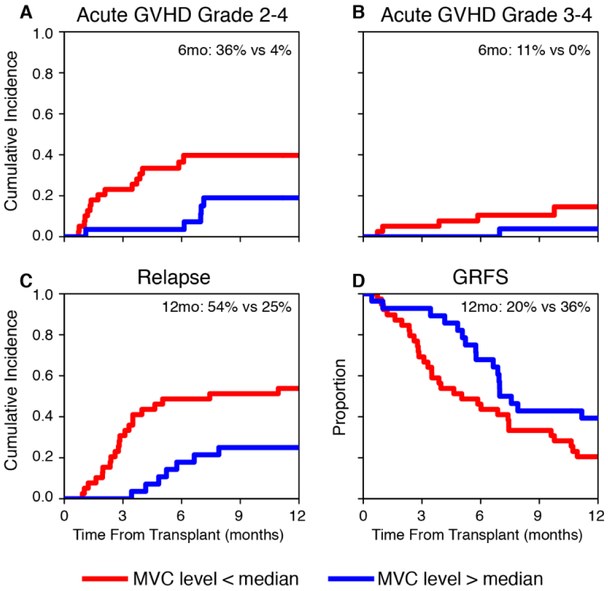
We combined data from both trials to examine the impact of day 0 trough maraviroc concentration on outcomes. Data were evaluable for 67 patients. There was notable variability in trough concentrations between patients (median 65 ng/mL, range 12-316) and a similarly high variability was also demonstrated on day +7 and day +14 (Supplemental Fig. 3). Maraviroc concentrations were not associated with age or sex and were not significantly different between the two trials (not shown). To analyze the independent impact of day 0 trough drug concentration on outcomes, we included the treatment duration (short vs. extended) as a fixed covariate in all multivariable models. We found that maraviroc concentrations above the median were associated with lower incidence of grade 2 to 4 acute GVHD (4% vs. 36% at 6 months; adjusted HR, 0.33, 95% CI [0.12 to 0.87], p=.025; Fig. 3A). The rate of grade 3 to 4 acute GVHD was also lower for higher maraviroc concentrations (0% vs. 11% at 6 months; Fig. 3B) but did not reach statistical significance in multivariable analysis. Similarly, lower relapse rate at 1 year (25% vs. 54%, Fig. 3C) and higher GRFS rate at 1 year (36% vs. 20%, Fig. 3D) were achieved with higher maraviroc concentrations but lacked statistical significance. Maraviroc day 0 concentrations had no associations with chronic GVHD, NRM or OS. Interestingly, of 21 patients who had day 0 trough concentrations >100 ng/mL, only one patient developed delayed acute GVHD 7 months after transplant and no other acute GVHD cases were observed.
Figure 3. Clinical outcomes in patients with high (>median) vs. low (<median) day-0 trough maraviroc concentrations.
Cumulative incidence plots of acute graft-versus-host disease (GVHD) grade 2 to 4 (A), grade 3 to 4 (B), relapse (C) and a Kaplan-Meier plot of GVHD-free, relapse-free survival (GRFS) (D).
Discussion
Our group previously conducted the first proof-of-concept study with the CCR5 antagonist maraviroc, demonstrating biologic activity in blocking lymphocyte chemotaxis and clinical benefit that manifested as low rates of early severe acute GvHD, primarily in visceral organs.8,9 However, while 1 month of maraviroc is biologically active, long-term outcomes may not be significantly improved with this brief treatment, as was also suggested in a recent multicenter phase II study that compared maraviroc-treated patients with a contemporary control cohort.14 Our current study examined whether longer treatment, specifically in unrelated donor transplants, would benefit patients further by decreasing the rates of delayed acute or chronic GVHD. We found that the longer treatment duration was well tolerated and that the composite endpoint of GRFS was improved compared to our previous study, leading to a 1-year GRFS rate of 46%. This rate compares favorably with a recent CIBMTR analysis that showed a GRFS of 23% in over 5,000 patients treated with standard tacrolimus and methotrexate.15 It is also higher than a recent University of Minnesota analysis, where 1-year GRFS rate in a heterogeneous group of adult patients who received calcineurin-inhibitor-based GVHD prophylaxis was 24% overall and 26% after reduced intensity conditioning.16 Although our cross-trial comparison of 3-month vs. historical 1-month treatment is exploratory, these data strongly suggest that the extended course of CCR5 blockade is associated with improved transplantation outcomes.
Surprisingly, the high GRFS rate in our study was driven by low rates of disease relapse and chronic GVHD, while rates of acute GvHD were not significantly different from the shorter course of maraviroc. Importantly, this study also demonstrated favorable long-term survival outcomes with the median survival not reached after a median follow up of 3 years. Considering the patients’ older age, high-risk disease mix and high comorbidity index, these survival outcomes compare favorably to the published data in reduced intensity transplants.17 While a historical comparison of relapse risk across studies is limited and highly exploratory, the relapse risk was not only lower for the extended course of treatment, but also independently improved with high maraviroc concentrations when analyzing both trials together. These findings support the possibility of a direct antitumor mechanism of maraviroc, either by potentiating the graft-versus-tumor response or through a direct cytotoxic effect. It has been previously shown that absence of CCR5 delayed growth of murine melanoma and enhanced dendritic cell-based vaccination.18 CCR5 antagonists have demonstrated a direct anti-metastatic effect in models of CCR5-expressing prostate cancer and breast cancer and synergism with DNA-damaging cyotoxic agents has been demonstrated.19-21 CCR5 blockade also modified the tumor microenvironment in colorectal and pancreatic cancer models, an effect that was confirmed in early phase clinical trials.22-24 The potential therapeutic effect of maraviroc in cancer is actively explored in clinical trials (NCT03274804, NCT01736813).
This study confirmed the previous finding that the impact of CCR5 blockade is primarily in visceral organs, demonstrated by absence of liver GVHD in the first 100 days and low rates and severity of gut GVHD. We have previously also shown that low levels of the gut biomarker Reg3α accompany the clinical protective effect.9 The gut-protective effect of maraviroc is particularly important considering the high morbidity, mortality and cost associated with gut GVHD. This study also confirms the safety profile of maraviroc, which in both studies was not associated with graft failure, an increase in infectious complications or poor immune reconstitution.
We observed a strong and independent association between maraviroc blood concentrations on day 0 and the incidence of subsequent acute GVHD. In vitro, chemotaxis blockade by maraviroc is dose-dependent.8 The effect of chemotherapy on maraviroc bioavailability has not been formally explored. Additionally, maraviroc metabolism involves cytochrome P450-3A4 and is potentially affected by common genomic polymorphisms.25 Finally, we have previously described pharmacodynamic variability by conducting CCR5 phosphoflow in real-time, which also correlated with outcomes.26 Together, these factors may explain the heterogeneity in responses to maraviroc, resulting in inferior efficacy in some patients. The importance of adequate levels of GVHD prophylaxis agents early after the hematopoietic cell infusion has been previously demonstrated for tacrolimus and cyclosporine27-29 and potentially mirrors the findings in murine models that T-cell activation and migration into tissues is an early event that occurs within the first 24-72 hours after transplantation.30 Further studies are needed to characterize the impact of conditioning on maraviroc absorption and to define the optimal maraviroc concentration and a dosing strategy that targets this concentration.
While this study adds critical information about lymphocyte chemotaxis blockade as a strategy in preventing GVHD, we acknowledge certain limitations. This was a single-arm study conducted in a single center. Although we limited the study to a single conditioning regimen and to unrelated donors, it was still a heterogeneous population in terms of disease type and HLA matching. While a comparison to our initial trial using short course maraviroc provides important insights, these were sequential studies and not a randomized controlled trial. In particular, the comparison of relapse rates is potentially confounded by variables that are not captured by the DRI (e.g., somatic mutations). In addition, we and others have focused on maraviroc and CCR5 as a proof of concept,8,31 inspired by work in mouse models that lends support to this approach,5-7,32 but other chemokine receptors and adhesion molecules were implicated and some are being explored in clinical trials.33-42 Finally, the results of this study should be examined in the context of the recent BMT-CTN 1203 phase 2 study, which did not identify a benefit for a short course of maraviroc compared to non-randomized contemporary controls.14 Our current results suggest that the short treatment course of maraviroc in the 1203 study was suboptimal, but additional differences between these studies should be noted. The 1203 study allowed both unrelated and HLA-identical related donors, while the benefit in our phase 1/2 study was exclusive to unrelated donor transplants. The 1203 study also allowed several conditioning regimens and mouse models have previously shown that the role of CCR5 in GVHD strongly depends on conditioning intensity and may become redundant with more intensive conditioning regimens than Flu/Bu2.7
In summary, an extended course of maraviroc is safe and effective and results in a high rate of GVHD-free and relapse-free survival. Pharmacologic variables may impact maraviroc concentrations and therefore its efficacy; these should be studied prospectively.
Supplementary Material
Highlights:
A 3-months course of maraviroc prevented acute and chronic graft-versus-host disease.
Survival and GRFS were improved compared historically to 1 month of maraviroc.
High day-0 maraviroc concentrations were associated with lower rates of acute GVHD.
Acknowledgements
We thank the patients, their family members and the team of research coordinators and nurses at the Abramson Cancer Center. This work was supported by National Institutes of Health grants U01-HL069286 (D.L.P.), K23-CA178202 (R.R.) and P30-CA016520 (R.M, R.H.V.) Additional support included a Career Development Award from the Conquer Cancer Foundation (R.R.) and the Amy Strelzer Manasevit Award from the National Marrow Donor Program (R.R.). We thank Jayvant Heera and Manoli Vourvahis (both at Pfizer) for helpful discussions. Maraviroc was supplied for the study by Pfizer Inc.
Support: National Institutes of Health grants U01-HL069286, K23-CA178202 and P30-CA016520, Conquer Cancer Foundation and the National Marrow Donor Program.
Footnotes
Disclaimers: Maraviroc supplied for the trial by Pfizer Inc. The authors indicated no other potential conflicts of interest that are relevant to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferrara JL, Smith CM, Sheets J, et al. : Altered homeostatic regulation of innate and adaptive immunity in lower gastrointestinal tract GVHD pathogenesis. J Clin Invest 127:2441–2451, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jagasia M, Arora M, Flowers ME, et al. : Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood 119:296–307, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeiser R, Blazar BR: Pathophysiology of Chronic Graft-versus-Host Disease and Therapeutic Targets. N Engl J Med 377:2565–2579, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, et al. : Leukocyte migration and graft-versus-host disease. Blood 105:4191–9, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murai M, Yoneyama H, Harada A, et al. : Active participation of CCR5(+)CD8(+) T lymphocytes in the pathogenesis of liver injury in graft-versus-host disease. J Clin Invest 104:49–57, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murai M, Yoneyama H, Ezaki T, et al. : Peyer’s patch is the essential site in initiating murine acute and lethal graft-versus-host reaction. Nat Immunol 4:154–60, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Wysocki CA, Burkett SB, Panoskaltsis-Mortari A, et al. : Differential roles for CCR5 expression on donor T cells during graft-versus-host disease based on pretransplant conditioning. J Immunol 173:845–54, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Reshef R, Luger SM, Hexner EO, et al. : Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. N Engl J Med 367:135–45, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moy RH, Huffman AP, Richman LP, et al. : Clinical and immunologic impact of CCR5 blockade in graft-versus-host disease prophylaxis. Blood 129:906–916, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Przepiorka D, Weisdorf D, Martin P, et al. : 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 15:825–8, 1995 [PubMed] [Google Scholar]
- 11.Jagasia MH, Greinix HT, Arora M, et al. : National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant 21:389–401 e1, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruan PK, Gray RJ: A method for analyzing disease-specific mortality with missing cause of death information. Lifetime Data Anal 12:35–51, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Armand P, Kim HT, Logan BR, et al. : Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood 123:3664–71, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolaños-Meade J, Reshef R, Fraser R, et al. : Novel Approaches for Graft-Versus-Host Disease (GvHD) Prophylaxis: Primary Results of Progress I Multicenter Trial of Matched Allogeneic Hematopoietic Cell Transplantation (alloHCT) Using Reduced Intensity Conditioning (RIC) BMT CTN 1203. Presented at the BMT Tandem Meetings, Salt Lake City, UT , 2/25/2018, 2018 [Google Scholar]
- 15.Pasquini MC, Logan B, Jones RJ, et al. : Blood and Marrow Transplant Clinical Trials Network Report on Development of Novel Endpoints and Selection of Promising Approaches for Graft-Versus-Host Disease Prevention Trials. Biol Blood Marrow Transplant, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holtan SG, DeFor TE, Lazaryan A, et al. : Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood 125:1333–8, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reshef R, Porter DL: Reduced-intensity conditioned allogeneic SCT in adults with AML. Bone Marrow Transplant 50:759–69, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Ng-Cashin J, Kuhns JJ, Burkett SE, et al. : Host absence of CCR5 potentiates dendritic cell vaccination. J Immunol 170:4201–8, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Velasco-Velazquez M, Jiao X, De La Fuente M, et al. : CCR5 antagonist blocks metastasis of basal breast cancer cells. Cancer Res 72:3839–50, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Jiao X, Velasco-Velazquez MA, Wang M, et al. : CCR5 Governs DNA Damage Repair and Breast Cancer Stem Cell Expansion. Cancer Res 78:1657–1671, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sicoli D, Jiao X, Ju X, et al. : CCR5 receptor antagonists block metastasis to bone of v-Src oncogene-transformed metastatic prostate cancer cell lines. Cancer Res 74:7103–14, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halama N, Zoernig I, Berthel A, et al. : Tumoral Immune Cell Exploitation in Colorectal Cancer Metastases Can Be Targeted Effectively by Anti-CCR5 Therapy in Cancer Patients. Cancer Cell 29:587–601, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Ward ST, Li KK, Hepburn E, et al. : The effects of CCR5 inhibition on regulatory T-cell recruitment to colorectal cancer. Br J Cancer 112:319–28, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan MC, Goedegebuure PS, Belt BA, et al. : Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J Immunol 182:1746–55, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abel S, Russell D, Whitlock LA, et al. : Assessment of the absorption, metabolism and absolute bioavailability of maraviroc in healthy male subjects. Br J Clin Pharmacol 65 Suppl 1:60–7, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huffman AP, Richman LP, Crisalli L, et al. : Pharmacodynamic Monitoring Predicts Outcomes of CCR5 Blockade as Graft-versus-Host Disease Prophylaxis. Biol Blood Marrow Transplant, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganetsky A, Shah A, Miano TA, et al. : Higher tacrolimus concentrations early after transplant reduce the risk of acute GvHD in reduced-intensity allogeneic stem cell transplantation. Bone Marrow Transplant 51:568–72, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malard F, Szydlo RM, Brissot E, et al. : Impact of cyclosporine-A concentration on the incidence of severe acute graft-versus-host disease after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 16:28–34, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Martin P, Bleyzac N, Souillet G, et al. : Relationship between CsA trough blood concentration and severity of acute graft-versus-host disease after paediatric stem cell transplantation from matched-sibling or unrelated donors. Bone Marrow Transplant 32:777–84, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Beilhack A, Schulz S, Baker J, et al. : In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood 106:1113–22, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khandelwal P, Fukuda T, Mizuno K, et al. : A Pharmacokinetic and Pharmacodynamic Study of Maraviroc as Acute Graft-versus-Host Disease Prophylaxis in Pediatric Allogeneic Stem Cell Transplant Recipients with Nonmalignant Diagnoses. Biol Blood Marrow Transplant 22:1829–1835, 2016 [DOI] [PubMed] [Google Scholar]
- 32.Burger DR, Parker Y, Guinta K, et al. : PRO 140 Monoclonal Antibody to CCR5 Prevents Acute Xenogeneic Graft-versus-Host Disease in NOD-scid IL-2Ry(null) Mice. Biol Blood Marrow Transplant 24:260–266, 2018 [DOI] [PubMed] [Google Scholar]
- 33.Terwey TH, Kim TD, Kochman AA, et al. : CCR2 is required for CD8-induced graft-versus-host disease. Blood 106:3322–30, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He S, Cao Q, Qiu Y, et al. : A new approach to the blocking of alloreactive T cell-mediated graft-versus-host disease by in vivo administration of anti-CXCR3 neutralizing antibody. J Immunol 181:7581–92, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Duffner U, Lu B, Hildebrandt GC, et al. : Role of CXCR3-induced donor T-cell migration in acute GVHD. Exp Hematol 31:897–902, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Schreder A, Moschovakis GL, Halle S, et al. : Differential Effects of Gut-Homing Molecules CC Chemokine Receptor 9 and Integrin-beta7 during Acute Graft-versus-Host Disease of the Liver. Biol Blood Marrow Transplant 21:2069–2078, 2015 [DOI] [PubMed] [Google Scholar]
- 37.Leonhardt F, Grundmann S, Behe M, et al. : Inflammatory neovascularization during graft-versus-host disease is regulated by alphav integrin and miR-100. Blood 121:3307–18, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Chen YB, McDonough S, Chen H, et al. : Expression of alpha4beta7 integrin on memory CD8(+) T cells at the presentation of acute intestinal GVHD. Bone Marrow Transplant 48:598–603, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Waldman E, Lu SX, Hubbard VM, et al. : Absence of beta7 integrin results in less graft-versus-host disease because of decreased homing of alloreactive T cells to intestine. Blood 107:1703–11, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dutt S, Ermann J, Tseng D, et al. : L-selectin and beta7 integrin on donor CD4 T cells are required for the early migration to host mesenteric lymph nodes and acute colitis of graft-versus-host disease. Blood 106:4009–15, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petrovic A, Alpdogan O, Willis LM, et al. : LPAM (alpha 4 beta 7 integrin) is an important homing integrin on alloreactive T cells in the development of intestinal graft-versus-host disease. Blood 103:1542–7, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Lu SX, Holland AM, Na IK, et al. : Absence of P-selectin in recipients of allogeneic bone marrow transplantation ameliorates experimental graft-versus-host disease. J Immunol 185:1912–9, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.