Abstract
C/EBPβ is a key mediator of cancer-induced skeletal muscle wasting. However, the signaling mechanisms that activate C/EBPβ in the cancer milieu are poorly defined. Here we report cancer-induced muscle wasting requires the transcriptional co-factor p300 which is critical for the activation of C/EBPβ. Conditioned media from diverse types of tumor cells as well as recombinant HSP70 and HSP90 provoked rapid acetylation of C/EBPβ in myotubes, particularly at its Lys39 residue. Overexpression of C/EBPβ with mutated Lys39 impaired Lewis lung carcinoma (LLC)-induced activation of the C/EBPβ-dependent catabolic response, which included upregulation of E3 ligases UBR2 and atrogin1/MAFbx, increased LC3-II, and loss of muscle proteins both in myotubes and mouse muscle. Silencing p300 in myotubes or overexpressing a dominant negative p300 mutant lacking acetyltransferase activity in mouse muscle attenuated LLC tumor-induced muscle catabolism. Administration of pharmacological p300 inhibitor C646, but not PCAF/GCN5 inhibitor CPTH6, spared LLC tumor-bearing mice from muscle wasting. Furthermore, mice with muscle-specific p300 knockout were resistant to LLC tumor-induced muscle wasting. These data suggest that p300 is a key mediator of LLC tumor-induced muscle wasting whose acetyltransferase activity may be targeted for therapeutic benefit in this disease.
Keywords: Cancer Cachexia, HSP70, HSP90, Lys39 of C/EBPβ, C646
Precis:
Findings demonstrate that tumor-induced muscle wasting in mice is abrogated by knockout, mutation of Lys39 or Asp1399, and pharmacological inhibition of p300.
INTRODUCTION
Cancer has been increasingly recognized as a systemic disease that causes metabolic disorders in multiple organs that are not resided by cancer per se. Cachexia is a wasting syndrome frequently seen in cancer patients and accounts for one third of cancer-associated deaths, which involves predominantly loss of skeletal muscle mass and function known as muscle wasting. The etiology of cancer-induced cachexia remains poorly defined and consequently there has been no FDA-approved medication for this lethal metabolic disorder1, 2.
Cancer-induced muscle wasting is associated with accelerated muscle catabolism due to hyper-activation of the ubiquitin-proteasome pathway (UPP) leading to the degradation of myofibrillar proteins, as well as the autophagy-lysosomal pathway (ALP) leading to the degradation of cytoplasmic protein aggregates and organelles3. Although these proteolytic pathways are also activated in non-cancer-induced muscle atrophy including fasting, disuse and denervation, the signaling mechanism that mediates their activation in the cancer milieu appears distinct. For example, the Akt/FoxO pathway inversely regulates both UPP and ALP activation by fasting, disuse, or denervation4–6, however, it appears not essential for muscle catabolism in animal cancer models7, 8 or cancer patients9, 10 due to activation of Akt that inhibits FoxOs11. In contrast, inflammation-activated signaling plays a key role in mediating the activation of UPP and ALP in response to cancer. In mouse models of cancer, TLR4 mediates muscle catabolism through a direct effect in muscle cells as well as an indirect effect by increasing circulating inflammatory cytokines, all of which activate such intracellular signaling molecules as p38 MAPK and NF-κB to promote muscle catabolism12, 13. Further, we recently found that diverse types of cachexia-inducing cancer cells release high levels of HSP70 and HSP90 via extracellular vesicles that activate TLR4-mediated muscle wasting14. We also observed that downstream of TLR4 and cytokine receptors, activation of transcription factor C/EBPβ by p38β MAPK is required for cancer-induced muscle catabolism through upregulation of E3 ligases atrogin1/MAFbx8, 15 and UBR216, as well as key autophagy-related genes LC3b and Gabarapl117. Thus, C/EBPβ plays a key role in mediating cancer-induced activation of UPP and ALP in cachectic muscle, which makes it a potential therapeutic target of cancer cachexia.
As a transcription factor involved in numerous cellular processes, the signaling mechanisms that mediate C/EBPβ activation is highly complex18, 19. The mechanisms by which C/EBPβ is activated in muscle cells in the cancer milieu have only been deciphered partially. C/EBPβ activity is regulated primarily by posttranscriptional modifications. The transactivation activity of C/EBPβ is normally auto-repressed in the absence of an activating signal, which is yet to be identified in cancer cachexia. Although we showed previously that p38β MAPK-mediated phosphorylation of C/EBPβ at Thr188 activates its DNA-binding activity in muscle cells15, the signaling mechanism that removes the auto-repression is undefined. The regulation of C/EBPβ activity involves acetylation in addition to phosphorylation at various sites by transcriptional co-activators/acetyltransferases p300, GCN5 or PCAF18, 19. In cultured myotubes, dexamethasone induces p300-dependent acetylation of C/EBPβ, although the acetylation sites are unknown20. Intriguingly, different C/EBPβ-responsive promoters require different patterns of acetylated lysine in C/EBPβ for transcriptional activation21. In HEK293T cells, binding of p300 to and acetylation of the N-terminal transactivation domain (aa 22–104) of C/EBPβ removes the auto-repression, although the specific acetylation site(s) involved in this critical process remains unknown22. These data suggest that acetylation of the N-terminal transactivation domain of C/EBPβ by p300 plays a primary role in the regulation of C/EBPβ activity. However, it is not clear whether and how p300-mediated acetylation of C/EBPβ releases it from auto-repressed state in muscle cells in response to a tumor burden.
In the present study, we tested the hypothesis that cancer activates C/EBPβ-mediated muscle catabolism through specific acetylation of its N-terminal transactivation domain. We demonstrate that multiple types of cachexia-inducing cancer as well as exogenous HSP70 and HSP90 induce C/EBPβ acetylation at its Lys39 residue within the N-terminal transactivation domain in myotubes. In addition, activation of muscle catabolism by Lewis lung carcinoma (LLC) in mice requires p300-mediated acetylation of Lys39 in C/EBPβ. Critically, we show that the acetyltransferase activity of p300 is essential for the development of muscle wasting in LLC tumor-bearing mice by genetic as well as pharmacological manipulations of p300. These data suggest that p300 is a key mediator and a promising therapeutic target of cancer-induced muscle wasting.
MATERIALS AND METHODS
Muscle and cancer cell cultures
Murine C2C12 myoblasts (American Type Culture Collection, ATCC) were grown in growth medium (10% fetal bovine serum in DMEM) at 37°C under 5% CO2. Myoblast differentiation was induced at 85–90% confluence by changing to differentiation medium (4% heat-inactivated horse serum in DMEM) for 96 h with medium replacement in every 24 h interval to form myotubes. Conditioned medium from 48-hour cultures of cancer cells including Lewis lung carcinoma, C26 adenocarcinoma cells (both from National Cancer Institute, Frederick, MD in 2007), BxPC-3 and AGS (both from ATCC in 2011 and 2009, respectively) were collected and centrifuged (1000 × g, 5 min). The supernatant was used to treat myotubes (25% final volume in fresh medium) when indicated, and replaced every 24 h. Baculovirus expression system-derived human recombinant protein HSP70 (Sigma-Aldrich) and human HSP90α generated as previously described14 were added to myotube culture medium (100 ng/ml each) when indicated. Pre-treatment with 10 μM C646 (Sigma, St. Louis, MO) or 10 μM CPTH6 (Glixx Laboratories, Hopkinton, MA) was performed 30 mins prior to the conditioned medium challenge when indicated. All cell lines were aliquoted upon receipt and kept in liquid nitrogen. Each frozen aliquot of cells were passaged for < 10 times. All cell lines were verified to be mycoplasma free using the Mycoplasma Detection Kit (Lonza Bioscience; in March 2017 and October 2018). Cell culture-based experiments were replicated independently for three times.
Animal use
Experimental protocols were pre-approved by the institutional Animal Welfare Committee at the University of Texas Health Science Center at Houston. For LLC-induced cancer cachexia model, 100 μl LLC cells (1 × 106), or an equal volume of vehicle (PBS) was injected subcutaneously into the flanks of 8-week-old male C57BL/6 mice (#000664, The Jackson Laboratories, Bar Harbor, ME). When indicated, C646 dissolved in DMSO and then diluted with equal volume of PBS, or the vehicle (50% DMSO in PBS), were administered 10 mg/kg/day from day 7 after cancer cell injection, when the tumor became palpable, through a subcutaneously implanted Alzet® Osmotic Pump (Alzet, Cupertino, CA). Plasmids encoding dominant negative p300 mutant or C/EBPβ Lys39 mutants were transfected into TA muscle on day 7 and repeated on day 14 following cancer cell injection using a protocol adopted from Witczak et al.23 and McMahon et al.24. Briefly, mice were anaesthetized prior to intramuscular injection of 0.4 U/μl of bovine hyaluronidase (H-4272; Sigma-Aldrich Products, Poole, UK) in 30 μl saline. After 2 h, the right TA muscle was injected with 30 μg K39A-encoding plasmids whereas the left TA was injected with the empty vector as a contralateral control. Ten square-wave electrical pulses (100 V/cm) were applied with an electrical pulse generator (Model 830, BTX) at a rate of 1 pulse/s to both muscles immediately after plasmid injection. Each pulse (20 ms in duration) was delivered through a pair of stainless steel needles that were 5 mm apart. Development of cachexia was monitored by body weight and grip strength test, and usually took place within 21 days after cancer cell injection. On day 21, mice were euthanized and muscle samples were collected immediately for analyses.
Generation of mice with muscle specific knockout of p300
Muscle-specific p300 knockout mice (p300 mKO) were created by crossbreeding p300-floxed mice25 (#025168, The Jackson Laboratory, Bar Harbor, ME) in C57BL/6 background with muscle creatine kinase-Cre mice in the same background (#006475, The Jackson Laboratory, Bar Harbor, ME). Expected PCR genotyping band sizes for the p300-floxed transgene (400 bp) and MCK-Cre (450 bp) were assigned to the p300 mKO mice (Figure S1). Primer sets used in genotyping have the following sequences: for p300-floxed transgene-Forward 5’GTGAGTTGATGTCCCTGTCG3’ and Reverse 5’CAGACACCCTCTTGCACTCA; for MCK-Cre: Forward 5’TAAGTCTGAACCCGGTCTGC3’ and Reverse 5’GTGAAACAGCATTGCTGTCACTT.
Transfection of plasmids and siRNA in C2C12 myotubes
Plasmids encoding various proteins and siRNAs specific for p300 (SASI_Mm01_00159721), PCAF (SASI_Mm01_00043888 & SASI_Mm01_00043889, Sigma-Aldrich) or scrambled control siRNA (Ambion, Austin, TX) were transfected into C2C12 myoblasts using the jetPRIME reagent (Polyplus-transfection Inc., Illkirch, France) according to the manufacturer’s protocol. In 24 h, myoblasts were changed into differentiating medium and experiments were started in another 96 h when myotubes were formed. Transfection of p300 siRNA caused an initial delay in differentiation during the first 24 h, however, at 96 h there was no difference in the degree of differentiation between control and p300 siRNA-transfected myotubes. Transfection of PCAF did not alter differentiation.
Western blotting
Cell lysate was prepared and Western blotting was performed as described previously8. The primary antibodies used for western blotting were as follows: anti-acetylated lysine (1:1000, 9814, Cell Signaling Technology), anti-p300 (1:500, sc584, Santa Cruz Biotechnology), anti-PCAF (1:1000, p3378, Cell Signaling Technology), anti-C/EBPβ (1:1000, MA1–827, ThermoFisher), anti-atrogin1/MAFbx (1:1000, AP2041, ECM Bioscience), anti-UBR2 (1:500, NBP1–45243, Novus Biologicals), anti-LC3 (1:2000, NB100–2220, Novus Biologicals), anti-p62 (1:1000, H00008878-M01, Novus Biologicals), anti-p38 MAPK (1:1000, 9212, Cell Signaling), anti-pp38 MAPK (1:1000, 4511, Cell Signaling), anti-p65 (1:250, 8008, Santa Cruz), anti-pp65 (1:500, 166748, Santa Cruz) and anti-MHC (1:1000, MAB4470, R&D Systems); whereas the custom-antibody targeting acetylated Lys39 of C/EBPβ (1:2000) were generated by Pocono Rabbit Farm & Laboratory from rabbits using a peptide (CLAYGAK(Ac)AARAAPRA) synthesized by Novoprotein Scientific Inc (Summit, NJ 07901). Data were normalized to α-Tubulin (antibody was from Development Studies Hybridoma Bank at the University of Iowa, Iowa City, IA).
Fluorescence microscopy and histology study
C2C12 myotubes were stained with anti-MHC antibody (1:1000, MAB4470, R&D Systems) and anti-mouse Alexa Fluor® Plus 488 secondary antibody (1:200, A32723, ThermoFisher), and examined using a Zeiss Axioskop 40 microscope and a Zeiss Axiocam MRM camera system controlled by Axiovision Release 4.6 imaging software. Acquired images were edited using the Photoshop software. Myotube diameter was measured in MHC-stained myotubes as previously described26. Cross-sectional area of H&E stained muscle sections was quantified by using the ImageJ software (NIH). Five view-fields with ~100 myofibers per field in each section were measured.
Immunoprecipitation
Immunoprecipitation was carried out as previously described8 using an anti-C/EBPβ antibody (2 μg per 1 mg total protein, MA1–827, ThermoFisher).
Statistical analyses
Statistical analyses were conducted using the SPSS 22.0 software package (IBM, Chicago, IL). A normality test was performed to examine data distributions. All data were expressed as means ± standard deviation (SD). Comparisons were made by one-way ANOVA followed by Tukey post-hoc test, Paired Student t-test or Chi-Square test as appropriate. Statistical significance was considered at p < 0.05.
RESULTS
Cancer cell-released cachexins cause C/EBPβ acetylation at Lys39
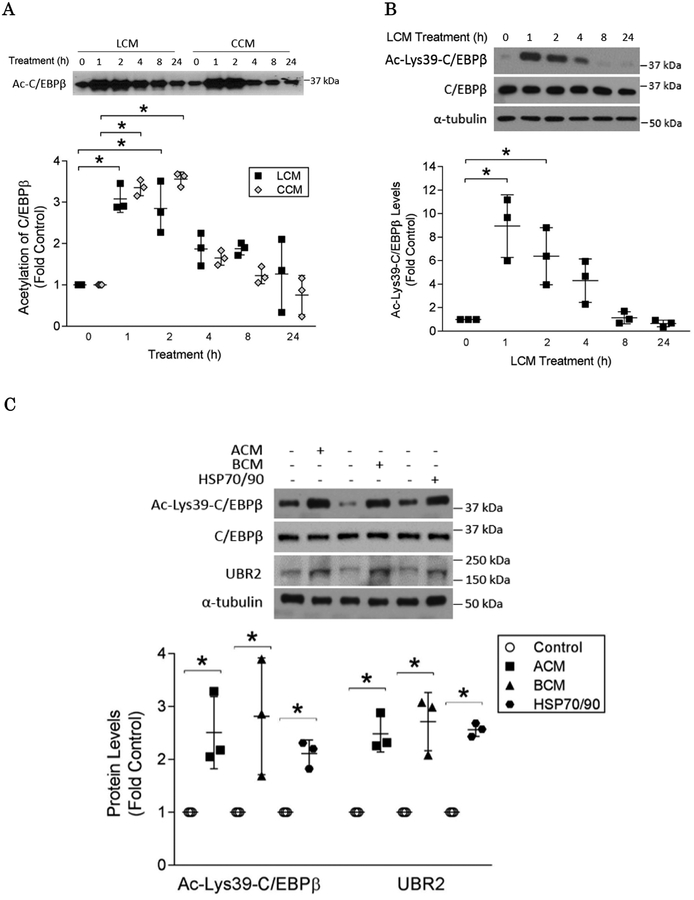
Given that murine Lewis lung carcinoma (LLC) and C26 adenocarcinoma trigger muscle catabolism through releasing HSP70 and HSP90 that activate Toll-like receptor 4 (TLR4) on muscle cells14, we first examined if their conditioned media (denoted as LCM and CCM respectively) induce C/EBPβ acetylation in muscle cells. C/EBPβ was immunoprecipitated from the lysates of LCM/CCM-treated C2C12 myotubes followed by Western blotting using an antibody against acetylated lysine residues. As shown in Figure 1A, the total acetylation status of C/EBPβ was rapidly increased within 1 h and then gradually returned to basal levels around 8 h. To identify acetylated lysine residues that are relevant to C/EBPβ activity, we examined C/EBPβ acetylation on Lys39, which is within the N-terminal transactivation domain of C/EBPβ (aa 22–104) that is acetylated by p300 in HEK293T cells to remove auto-repression22, by using a custom-made polyclonal antibody specific for C/EBPβ acetylated on Lys39. We observed that LCM increased Lys39 acetylation robustly with a time course similar to the total acetylation of C/EBPβ (Figure 1B). In addition, treatment with conditioned media of human gastric adenocarcinoma AGS (ACM) and pancreatic adenocarcinoma BxPC-3 (BCM) resulted in similar increases in Lys39 acetylation and up-regulation of C/EBPβ-controlled E3 ligase UBR216; thus indicating that C/EBPβ acetylation at Lys39 is induced by diverse types of cachexia-inducing cancer cells (Figure 1C). Given that the four types of cancer cells tested above all release high levels of HSP70 and HSP90 that are responsible for the activation of muscle catabolism in diverse tumor models14, we treated myotubes with recombinant HSP70 and HSP90, which resulted in a similar increase in Lys39 acetylation and upregulation of the E3 ligase UBR2 (Figure 1C). Collectively, these data indicate that acetylation of C/EBPβ at Lys39 is a catabolic response induced by diverse types of cachexia-inducing cancer cells through releasing cachexins, primarily HSP70 and HSP90.
Figure 1. Cancer cell-released cachexins stimulate acetylation of C/EBPβ at Lys39 in myotubes.
C2C12 myotubes were incubated with diverse cancer cell-conditioned media or HSP70 and HSP90 as indicated in durations ranging from 0 to 24 hrs. (A) LLC and CCM induces acetylation of C/EBPβ. Cell lysates were subjected to immunoprecipitation with an anti-C/EBPβ antibody. The immunocomplexes were analyzed by Western blotting using an anti-acetyl lysine antibody. (B) LCM induces acetylation of C/EBPβ at Lys39. Western blotting was conducted to analyze lysate of LCM-treated myotubes using a custom-made antibody targeting acetylated Lys39 of C/EBPβ. (C) ACM and BCM as well as HSP70 and HSP90 induce acetylation of C/EBPβ at Lys39 (2 h) and up-regulation of UBR2 (8 h) in myotubes as analyzed by Western blotting. * p<0.05 signifies a statistically significant difference after One-way ANOVA analyses.
C/EBPβ acetylation at Lys39 is required for LLC-induced skeletal muscle catabolism
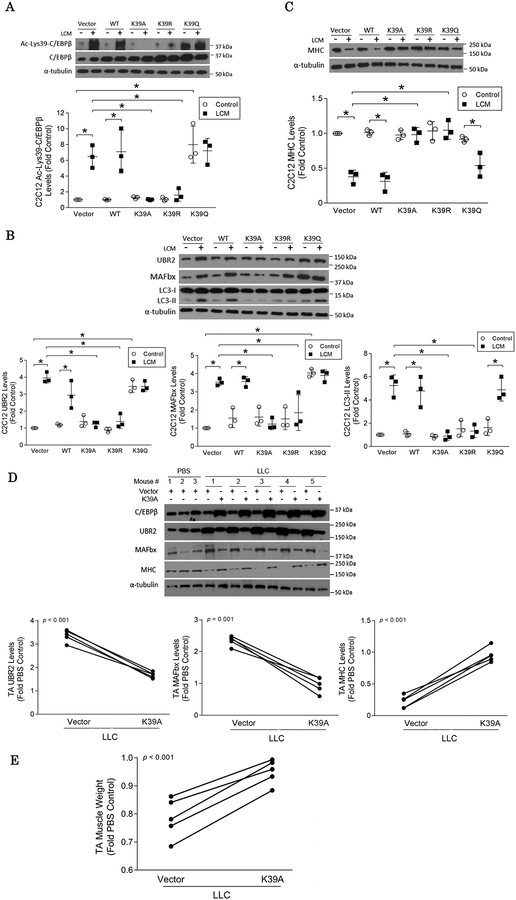
We then attempted to determine the significance of acetylation of C/EBPβ at Lys39 in the induction of atrophic signaling. Two Lys39 acetylation-defective mutants of C/EBPβ in which Lys39 was replaced with alanine (K39A) or arginine (K39R) and an acetylation-mimicking mutant in which Lys39 was replaced with glutamine (K39Q)27 were over-expressed in C2C12 myotubes. Western blot analysis of the cell lysate using the custom-made antibody specific for C/EBPβ acetylated on Lys39 revealed that overexpressed K39A and K39R mutants abrogated the LCM-provoked Lys39 acetylation whereas the K39Q mutant mimicked Lys39 acetylation (Figure 2A). This result also demonstrates that the custom-made antibody is specific for Lys39-acetylated C/EBPβ. Consequently, C/EBPβ-dependent catabolic events including the up-regulations of UBR216 and atrogin1/MAFbx8, increase in LC3-II and resultant loss of myofibrillar protein myosin heavy chain (MHC) in LCM-challenged myotubes were halted by K39A and K39R, but not by K39Q (Figure 2B & 2C). On the other hand, LCM-induced elevation of C/EBPβ phosphorylated at Thr188 (which regulates its DNA-binding activity15) was not altered by any of the mutants studied (Figure S2). This suggests that Thr188 phosphorylation is independent of Lys39 acetylation. Thus, Lys39 acetylation, not Thr188 phosphorylation, is likely the primary regulatory mechanism of C/EBPβ activity.
Figure 2. LLC-induced muscle wasting is dependent on Lys39 acetylation of C/EBPβ.
(A) Over-expressed mutants of C/EBPβ alters acetylation of C/EBPβ at Lys39. C2C12 myotubes over-expressing Lys39 acetylation-defective C/EBPβ (K39A or K39R) or Lys39 acetylation-mimicking C/EBPβ (K39Q) were treated with LCM for 2 h. Cell lysates were analyzed by Western blotting using our custom-made antibody against C/EBPβ acetylated at Lys39. (B) LCM-induced catabolism in C2C12 myotubes requires C/EBPβ that is acetylated at Lys39. C2C12 myotubes over-expressing the indicated C/EBPβ mutants were treated with LCM for 8 h. Levels of catabolic markers UBR2, atrogin1/MAFbx and LC3-II were analyzed by Western blotting. (C) LCM-induced MHC loss requires C/EBPβ acetylated at Lys39. C2C12 myotubes over-expressing the indicated C/EBPβ mutants were treated with LCM for 72 h. Levels of MHC in cell lysates were analyzed by Western blotting. (D) C/EBPβ acetylation at Lys39 is critical to the activation of muscle catabolism in LLC tumor-bearing mice. The K39A mutant was over-expressed in TA of LLC tumor-bearing mice with vector overexpressed in contralateral TA as control. After the mice had developed cachexia (day 21), markers of muscle catabolism and MHC in muscle lysate were analyzed by Western blotting. (E) C/EBPβ acetylation at Lys39 is critical to muscle mass loss in LLC tumor-bearing mice. TA was excised from the mice and weighed. * p<0.05 signifies a statistically significant difference after One-way ANOVA analyses or Paired t-test (A to E).
We then validated these findings in vivo by over-expressing K39A in the tibialis anterior (TA) muscle of LLC-bearing mice. Compared with the vector-transfected contralateral TA, forced expression of K39A attenuated the upregulations of atrogin1/MAFbx and UBR2 and the loss of MHC (Figure 2D). Furthermore, K39A ameliorated the loss of TA mass (Figure 2E) and the decrease in myofiber cross-sectional area under the tumor burden (Figure S3). These data suggest that C/EBPβ acetylation at Lys39 is required for cancer-induced skeletal muscle catabolism.
p300 mediates LLC-induced C/EBPβ acetylation at Lys39 and muscle catabolism
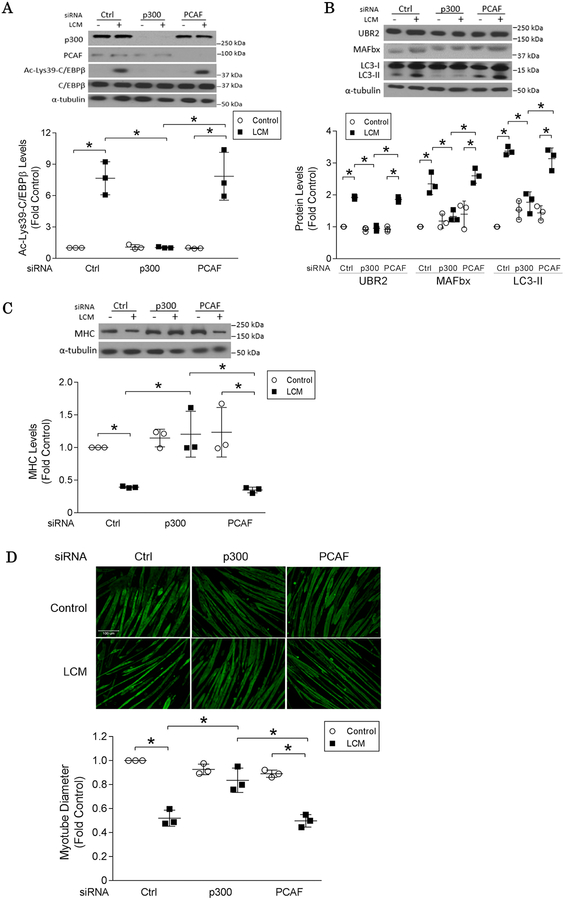
C/EBPβ can be acetylated by multiple acetyltransferases including p30028, PCAF and GCN529. To identify the acetyltransferase responsible for C/EBPβ acetylation at Lys39 in muscle cells in cancer milieu, p300 or PCAF were knocked down in C2C12 myotubes with specific siRNAs. LCM-induced acetylation of C/EBPβ at Lys39 was abrogated in p300-deficient myotubes, but not in that deficient in PCAF (Figure 3A). Consequently, p300 silencing abolished LCM-induced catabolic responses including upregulations of UBR2 and atrogin1/MAFbx, increase in LC3-II (Figure 3B) and loss in MHC (Figure 3C) and myotube mass (Figure 3D). It is noteworthy that the protein level of p300 was not altered in LCM-treated myotubes (Figure 3A), suggesting the regulation of p300 activity by cancer primarily involves post-transcriptional mechanisms.
Figure 3. p300 mediates LLC-induced C/EBPβ acetylation at Lys39.
(A) Cancer cell-induced Lys39 acetylation of C/EBPβ requires p300 specifically.
Transcriptional repression of p300 or PCAF in myotubes was achieved by specific siRNA. After 2 h of LCM treatment, the expression of p300 and PCAF, as well as C/EBPβ acetylation at Lys39 in C2C12 myotubes were analyzed by Western blotting. (B) Cancer cell-induced activation of muscle catabolic pathways requires p300 specifically. p300 or PCAF in myotubes was knocked-down by specific siRNA. After 8 h of LCM treatment, the levels of UBR2, MAFbx and LC3-II were analyzed by Western blotting. (C) Cancer cell-induced MHC loss is dependent on p300. Myotubes in which p300 or PCAF was knocked-down by siRNA were treated with LCM for 72 h, and subjected to Western blot analysis of MHC. (D) Cancer cell-induced loss of myotube mass is dependent on p300. Myotubes treated as described in (C) were subjected to immunofluorescence staining using an anti-MHC antibody and myotube diameter was measured. * p<0.05 signifies a statistically significant difference determined by One-way ANOVA analyses.
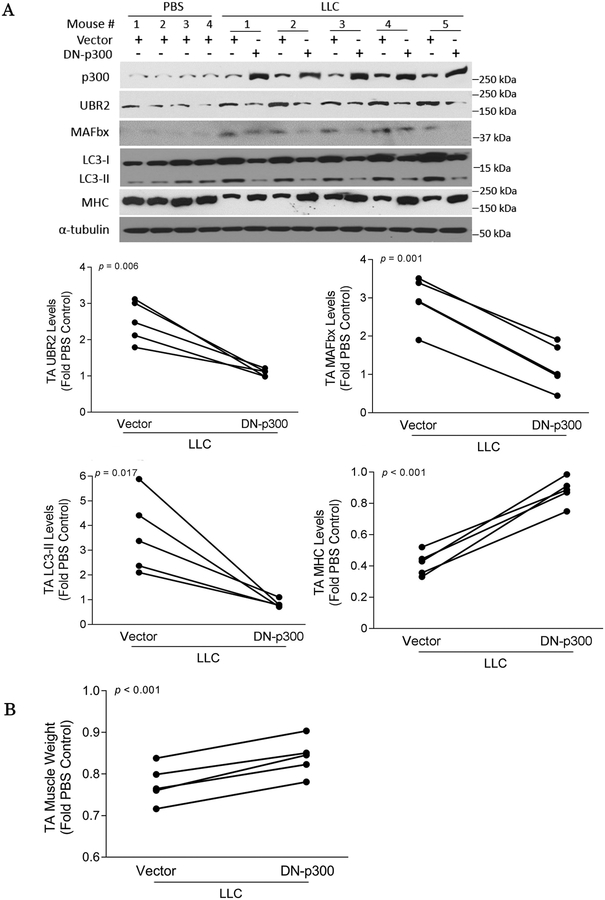
To verify whether the acetyltransferase activity of p300 is required for cancer-induced muscle catabolism in vivo, a dominant negative p300 mutant (DN-p300) that lacks acetyltransferase activity due to the conversion of aspartic acid 1399 to tyrosine30 was transfected into the TA of LLC tumor-bearing mice. Expression of this mutant mitigated the elevations of UBR2, atrogin1/MAFbx and LC3-II, loss of MHC (Figure 4A) and TA mass (Figure 4B). In addition, DN-300 abrogated the loss of myofiber cross-sectional area (Figure S4).
Figure 4. Acetyltransferase activity of p300 is essential to LLC-induced muscle wasting.
(A) Cancer-induced activation of catabolic pathways in muscle requires p300 with intact acetyltransferase activity. A dominant negative p300 mutant (DN-p300) with defective acetyltransferase activity was over-expressed in the TA muscle of LLC tumor-bearing mice through plasmid transfection. Empty Vector was transfected into the contralateral TA as control. Muscle catabolism measured as UBR2, atrogin1/MAFbx, LC3-II and MHC, was analyzed by Western blotting. (B) Cancer-induced muscle loss is dependent on p300 with intact acetyltransferase activity. TA of mice described in (A) was excised and weighed. * p<0.05 signifies a statistically significant difference after One-way ANOVA analyses or Paired t-test (A and B).
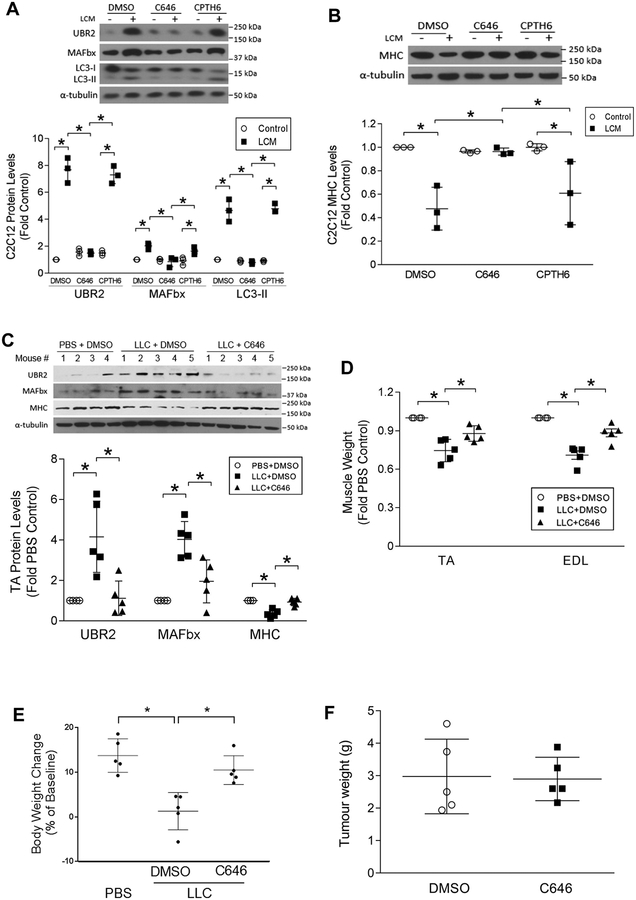
Next, we investigated whether cancer-induced muscle wasting can be intervened by pharmacological inhibition of p300. C2C12 myotubes were pretreated with the specific p300 inhibitor C64631 or a dual inhibitor of PCAF and GCN5, CPTH632. Myotube catabolism provoked by LCM was impeded significantly by C646, but not by CPTH6 (Figure 5A & 5B). Furthermore, administration of C646 to LLC tumor-bearing mice hampered the catabolic responses (Figure 5C) and attenuated the loss of muscle mass (Figure 5D) as well as body weight (Figure 5E), without affecting tumor growth (Figure 5F). These data support that the acetyltransferase activity of p300 specifically mediates C/EBPβ acetylation at Lys39 and the ensuing muscle wasting in the tumor milieu. Therefore, pharmacological inhibition of p300 appears to be a promising strategy for combating cancer cachexia.
Figure 5. Pharmacological inhibition of p300 ameliorates LLC-induced muscle wasting.
(A) C646, a specific pharmacological inhibitor of p300, abrogates the LCM-induced myotube catabolism. C2C12 myotubes were pre-treated (30 min) with pharmacological inhibitor of p300 (C646, 10 μM) or PCAF/GCN5 (CPTH6, 10 μM) prior to LCM exposure for 8 h. Markers of catabolic pathways were analyzed by Western blotting. (B) C646 abrogates the LCM-induced MHC loss. Myotubes were pre-treated with pharmacological inhibitor of p300 (C646) or PCAF/GCN5 (CPTH6) prior to LCM treatment for 72 h. The expression of MHC was analyzed by Western blotting. (C) C646 abrogates muscle catabolism in LLC tumor-bearing mice. Subcutaneous administration of C646 was initiated 7 days after the implantation of LLC. On day 21, mice were euthanized and analyzed for muscle catabolism markers by Western blotting. (D) C646 attenuates muscle mass loss in LLC tumor-bearing mice. (E) C646 attenuates body weight loss in LLC tumor-bearing mice. (F) C646 does not alter tumor growth. * p<0.05 signifies a statistically significant difference after One-way ANOVA analyses.
Mice with muscle-specific deletion of p300 are resistant to LLC-induced muscle wasting
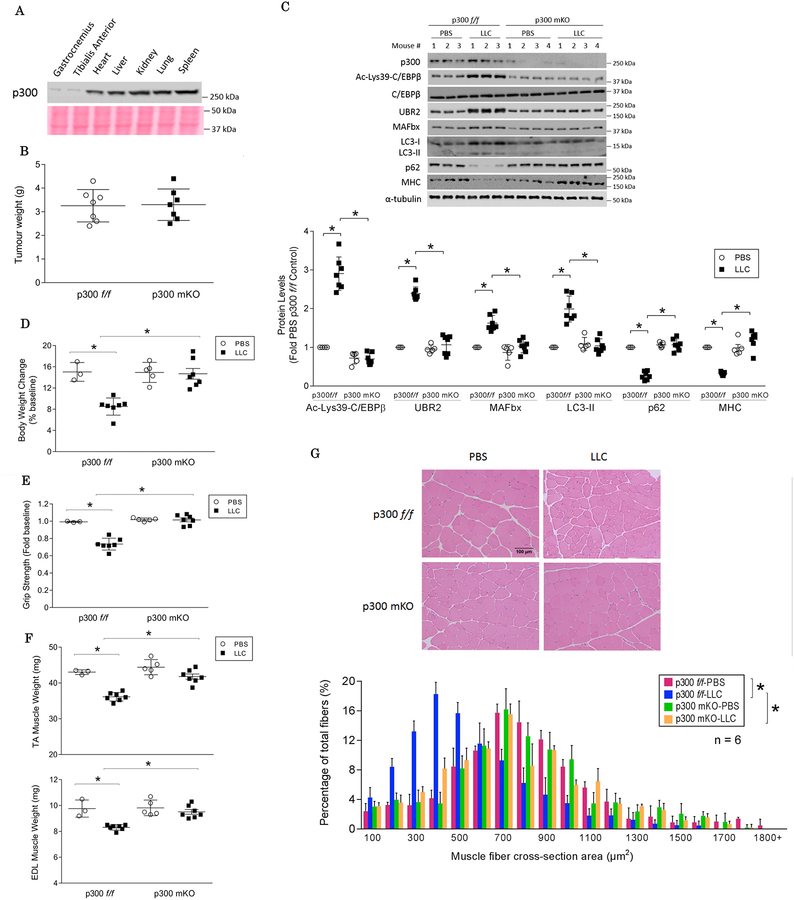
Finally, to further verify the key role of p300 in mediating cancer-induced muscle wasting, we generated mice with skeletal muscle-specific knockout of p300 (p300 mKO) by crossing p300-floxed mice (p300f/f)25 to MCK-Cre mice (Figure S4), both were in the C57BL/6 background. Muscle-specific knockout of p300 was confirmed by Western blot analysis of p300 in various tissues (Figure 6A). There was no difference in tumor growth in p300 mKO mice compared to p300f/f mice (Figure 6B). LLC tumor-provoked increase in C/EBPβ acetylation on Lys39 was absent in the TA of p300 mKO mice, so as the C/EBPβ-dependent activation of the catabolic pathways (Figure 6C). Given that LLC activates TLR4 and provokes muscle catabolism through its release of HSP70 and HSP9012, 14, the activities of TLR4 downstream effectors p38 MAPK and NF-κB were monitored. The increase in the active forms of p38 MAPK and NF-κB were not altered in LLC tumor-bearing p300 mKO mice (Figure S5). Consequently, p300 mKO mice were spared from muscle wasting as measured by body weight (Figure 6D), muscle strength (Figure 6E), muscle weight (Figure 6F), and muscle fiber cross sectional area (Figure 6G). In agreement with a previous report33, p300 mKO mice did not manifest significant phenotypic differences as compared to p300f/f mice in terms of body weight (Figure 6D), muscle strength (Figure 6E), muscle weight (Figure 6F), and histology (Figure 6G). These data allow us to conclude that p300 is a critical mediator of LLC tumor-induced muscle wasting.
Figure 6. Mice with muscle-specific knockout of p300 are resistant to LLC-induced muscle wasting.
(A) Muscle-specific knock out of p300 in mice (p300 mKO). p300 mKO mice were generated by crossing p300-floxed mice (p300 f/f) to MCK-Cre mice. The expression of p300 in multiple tissues of mKO mice were analyzed by Western blotting with Ponceau S staining of the membrane as loading control. (B) Muscle-specific knock out of p300 did not alter tumor growth (day 21). (C) Muscle catabolic response is abrogated in LLC tumor-bearing p300 mKO mice. p300 mKO and p300f/f mice were implanted with LLC cells or received PBS as control and euthanized on day 21. Lys39 acetylation of C/EBPβ, activation of muscle catabolic markers and the level of MHC in the TA lysates were analyzed by Western blotting. (D) Body weight loss is attenuated in LLC tumor-bearing p300 mKO mice. (E) Muscle strength was preserved in LLC tumor-bearing p300 mKO mice. (F) Muscle weight (TA and EDL) was preserved in LLC tumor-bearing p300 mKO mice. (G) Reduction of muscle fiber cross-sectional area was prevented in LLC tumor-bearing p300 mKO mice. * p<0.05 signifies a statistically significant difference after One-way ANOVA or Chi-square test.
DISCUSSION
The present study demonstrates for the first time a key role of p300 in mediating cancer-induced muscle wasting by activating C/EBPβ through specific acetylation of its Lys39 residue. This study not only extends our scarce understanding of the etiology of cancer-induced muscle wasting through detailed analyses of the underlying signaling mechanism that activates muscle catabolic pathways, but also identifies a critical therapeutic target p300 for this metabolic disorder. Remarkably, from the therapeutic point of view, our findings that genetic or pharmacological inhibition of p300 is sufficient to abrogate muscle wasting in a widely studied mouse cancer model (LLC) may have significant translational values. Given the unsatisfactory results in intervening cancer cachexia with various therapeutic strategies in previous clinical trials34,35, our success in abrogating cancer-induced muscle wasting both in vitro and in vivo by targeting p300 suggests that improved understandings in pathogenic mechanisms can lead to the design of more specific and effective therapeutic strategies for the intervention of cancer cachexia.
Our data demonstrate that in response to a tumor burden, p300 activates C/EBPβ-mediated catabolic pathways in muscle cells through acetylating its Lys39 residue. In HEK293T cells, the auto-repressed state of C/EBPβ is released upon the binding of p300 to acetylate its N-terminal transactivation domain (aa 22–104)22. Our data suggest that p300-mediated acetylation of Lys39, which is within the N-terminal transactivation domain, serves the same purpose in muscle cells. In addition, our data indicate that in absence of Lys39 acetylation, LCM-induced C/EBPβ phosphorylation of Thr188 remains intact (Figure S1), which enables C/EBPβ to bind DNA15. Thus, Lys39 acetylation, but not Thr188 phosphorylation appears to play a primary role in the regulation of C/EBPβ activity by removing auto-repression. According to previous structural analyses by Lee et al., the N-terminal transactivation domain is mostly unstructured with pockets of hydrophobicity. Lys39 resides in a subdomain within this region (aa 22–40) that is populated by short hydrophobic helices22. It is likely that the Lys39 acetylation of C/EBPβ alters conformation of this domain to allow access of regulatory proteins or interaction with the target genes.
Exerting important roles in development, physiology, and disease via its network with many proteins, p300 serves not only as a histone acetyltransferase (HAT), but also as a factor acetyltransferase (FAT) for certain transcription factors36, 37. The effect of p300-mediated acetylation on muscle mass is highly complex. Previous studies reported that p300-mediated acetylation modulates FoxO1 and FoxO3 activity differentially in response to denervation, disuse, starvation, insulin or dexamethasone38–41; and HDAC1 promotes disuse-induced muscle atrophy by activating FoxO through deacetylation42. In contrast, the current study identifies p300 as a key mediator of cancer-induced muscle wasting by acetylating C/EBPβ at Lys39. Thus, the regulation of muscle mass by acetylation of transcription factors appears to be stimulus and substrate-dependent.
Our observations that conditioned media of diverse types of cancer cells as well as recombinant HSP70 and HSP90 similarly activate p300-mediated C/EBPβ acetylation at Lys39 in myotubes (Figure 1) suggest that this effect is mediated by HSP70 and HSP90 that are released by various cachectic cancer cells and cause muscle wasting by activating TLR4 as previously demonstrated14. Thus, p300-mediated C/EBPβ acetylation at Lys39 is likely important for muscle wasting induced by diverse types of cancer that release HSP70 and HSP90. We also observed that the abundance of p300 was not altered in response to cancer (Figure 3A), which implies that the regulation of p300 activity by cancer is primarily at the post-transcriptional level through an unknown mechanism downstream of TLR4. NF-κB and p38 MAPK are downstream effectors of TLR4 that mediate muscle wasting12. However, we observed that neither the activation of NF-κB nor p38 MAPK was attenuated in the muscle of tumor-bearing p300 mKO mice. This data suggests that p300 activation is downstream of or unrelated to NF-κB and p38 MAPK. Given that p300-mediated C/EBPβ acetylation on Lys39 takes place within 1 h of exposure to cancer cell-released cachexins (Figure 1), transcription factor NF-κB is unlikely to be involved in this event.
Acetylation of Gcn5-related N-acetyltransferases (GNATs) has been shown to promote gene transcription that governs diverse biological processes such as lipid metabolism and oxidative homeostasis43. In skeletal muscle, the mRNA levels of p300 and PCAF were elevated concomitantly during atrophy associated with denervation and nutrient deprivation44 whereas forced expression of enzymatic-inactive GCN5 down-regulated a panel of atrophic genes in fasted animals45. However, our data that ablation of PCAF alone or in combination with GCN5 by either genetic or pharmacological approach failed to avert C/EBPβ-dependent catabolism suggest that these acetyltransferases are not essential for cancer-induced muscle wasting. In contrast, we demonstrated that mice with muscle-specific deletion of p300 are resistant to cancer-induced muscle wasting. Mice with muscle-specific knockout of p300 (p300 mKO) was generated by crossbreeding p300f/f mice to mice with the Cre transgene controlled by the muscle creatine kinase promoter. This approach allows p300 mKO mice to bypass the myogenic differentiation stage for which p300 is required46, resulting in normal development of skeletal muscle. Concordantly, we observed a remarkable effectiveness of the specific p300 inhibitor C646 in blocking C/EBPβ acetylation at Lys39 and C/EBPβ-dependent muscle catabolism. These data support specific p300 inhibitors as potential therapeutic agents for cancer-induced muscle wasting.
Taken together, the current study reveals p300 as an indispensable mediator of LLC tumor-induced muscle wasting due to its activation of C/EBPβ by acetylating Lys39. Thus, specific inhibitors of p300 could be further tested for the intervention of cancer-associated muscle wasting.
Supplementary Material
ACKNOWLEDGEMENT
This study was supported by a grant from National Institute of Arthritis and Musculoskeletal and Skin Diseases to Y.-P. Li (R01 AR067319). The authors would like to acknowledge the technical assistance of Amy HoangAnh Doan and Zhelong Liu, and thank Alemayehu Gorfe (UTHealth) for consulting on the structural aspect of C/EBPβ. The authors also thank Jessica Schwartz (University of Michigan) for sharing C/EBPβ mutant plasmids, and Tso-Pang Yao (Duke University) for sharing DN-p300 plasmid.
Footnotes
CONFLICT OF INTEREST
The authors declare there is no conflict of interest.
REFERENCES
- 1.Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Primers. 2018;4: 17105. [DOI] [PubMed] [Google Scholar]
- 2.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12: 489–495. [DOI] [PubMed] [Google Scholar]
- 3.Ventadour S, Attaix D. Mechanisms of skeletal muscle atrophy. Curr Opin Rheumatol. 2006;18: 631–635. [DOI] [PubMed] [Google Scholar]
- 4.Zhao J, Brault JJ, Schild A, et al. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007;6: 472–483. [DOI] [PubMed] [Google Scholar]
- 5.Milan G, Romanello V, Pescatore F, et al. Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat Commun. 2015;6: 6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugiura T, Abe N, Nagano M, et al. Changes in PKB/Akt and calcineurin signaling during recovery in atrophied soleus muscle induced by unloading. Am J Physiol Regul Integr Comp Physiol. 2005;288: R1273–1278. [DOI] [PubMed] [Google Scholar]
- 7.Penna F, Bonetto A, Muscaritoli M, et al. Muscle atrophy in experimental cancer cachexia: is the IGF-1 signaling pathway involved? International journal of cancer. Journal international du cancer. 2010;127: 1706–1717. [DOI] [PubMed] [Google Scholar]
- 8.Zhang G, Jin B, Li YP. C/EBPbeta mediates tumour-induced ubiquitin ligase atrogin1/MAFbx upregulation and muscle wasting. The EMBO journal. 2011;30: 4323–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Op den Kamp CM, Langen RC, Snepvangers FJ, et al. Nuclear transcription factor kappa B activation and protein turnover adaptations in skeletal muscle of patients with progressive stages of lung cancer cachexia. The American journal of clinical nutrition. 2013;98: 738–748. [DOI] [PubMed] [Google Scholar]
- 10.Stephens NA, Skipworth RJ, Gallagher IJ, et al. Evaluating potential biomarkers of cachexia and survival in skeletal muscle of upper gastrointestinal cancer patients. Journal of cachexia, sarcopenia and muscle. 2015;6: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandri M, Sandri C, Gilbert A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117: 399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang G, Liu Z, Ding H, Miao H, Garcia JM, Li YP. Toll-like receptor 4 mediates Lewis lung carcinoma-induced muscle wasting via coordinate activation of protein degradation pathways. Sci Rep. 2017;7: 2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cannon TY, Guttridge D, Dahlman J, et al. The effect of altered Toll-like receptor 4 signaling on cancer cachexia. Arch Otolaryngol Head Neck Surg. 2007;133: 1263–1269. [DOI] [PubMed] [Google Scholar]
- 14.Zhang G, Liu Z, Ding H, et al. Tumor induces muscle wasting in mice through releasing extracellular Hsp70 and Hsp90. Nat Commun. 2017;8: 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang G, Li YP. p38beta MAPK upregulates atrogin1/MAFbx by specific phosphorylation of C/EBPbeta. Skeletal muscle. 2012;2: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang G, Lin RK, Kwon YT, Li YP. Signaling mechanism of tumor cell-induced up-regulation of E3 ubiquitin ligase UBR2. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27: 2893–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Z, Sin KWT, Ding H, Doan HA, Gao S, Miao H, Wei Y, Wang Y, Zhang G, Li Y-P p38beta MAPK mediates ULK1-dependent induction of autophagy in skeletal muscle of tumor-bearing mice. Cell stress. 2018;2: 311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J. 2002;365: 561–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nerlov C C/EBPs: recipients of extracellular signals through proteome modulation. Current opinion in cell biology. 2008;20: 180–185. [DOI] [PubMed] [Google Scholar]
- 20.Chamberlain W, Gonnella P, Alamdari N, Aversa Z, Hasselgren PO. Multiple muscle wasting-related transcription factors are acetylated in dexamethasone-treated muscle cells. Biochemistry and cell biology = Biochimie et biologie cellulaire. 2012;90: 200–208. [DOI] [PubMed] [Google Scholar]
- 21.Cesena TI, Cardinaux JR, Kwok R, Schwartz J. CCAAT/enhancer-binding protein (C/EBP) beta is acetylated at multiple lysines: acetylation of C/EBPbeta at lysine 39 modulates its ability to activate transcription. The Journal of biological chemistry. 2007;282: 956–967. [DOI] [PubMed] [Google Scholar]
- 22.Lee S, Miller M, Shuman JD, Johnson PF. CCAAT/Enhancer-binding protein beta DNA binding is auto-inhibited by multiple elements that also mediate association with p300/CREB-binding protein (CBP). The Journal of biological chemistry. 2010;285: 21399–21410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witczak CA, Fujii N, Hirshman MF, Goodyear LJ. Ca2+/calmodulin-dependent protein kinase kinase-alpha regulates skeletal muscle glucose uptake independent of AMP-activated protein kinase and Akt activation. Diabetes. 2007;56: 1403–1409. [DOI] [PubMed] [Google Scholar]
- 24.McMahon JM, Signori E, Wells KE, Fazio VM, Wells DJ. Optimisation of electrotransfer of plasmid into skeletal muscle by pretreatment with hyaluronidase -- increased expression with reduced muscle damage. Gene Ther. 2001;8: 1264–1270. [DOI] [PubMed] [Google Scholar]
- 25.Kasper LH, Fukuyama T, Biesen MA, et al. Conditional knockout mice reveal distinct functions for the global transcriptional coactivators CBP and p300 in T-cell development. Molecular and cellular biology. 2006;26: 789–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doyle A, Zhang G, Abdel Fattah EA, Eissa NT, Li YP. Toll-like receptor 4 mediates lipopolysaccharide-induced muscle catabolism via coordinate activation of ubiquitin-proteasome and autophagy-lysosome pathways. FASEB J. 2011;25: 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cesena TI, Cui TX, Subramanian L, et al. Acetylation and deacetylation regulate CCAAT/enhancer binding protein beta at K39 in mediating gene transcription. Molecular and cellular endocrinology. 2008;289: 94–101. [DOI] [PubMed] [Google Scholar]
- 28.Mink S, Haenig B, Klempnauer KH. Interaction and functional collaboration of p300 and C/EBPbeta. Molecular and cellular biology. 1997;17: 6609–6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiper-Bergeron N, Salem HA, Tomlinson JJ, Wu D, Hache RJ. Glucocorticoid-stimulated preadipocyte differentiation is mediated through acetylation of C/EBPbeta by GCN5. Proc Natl Acad Sci U S A. 2007;104: 2703–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ito A, Lai CH, Zhao X, et al. p300/CBP-mediated p53 acetylation is commonly induced by p53-activating agents and inhibited by MDM2. The EMBO journal. 2001;20: 1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowers EM, Yan G, Mukherjee C, et al. Virtual ligand screening of the p300/CBP histone acetyltransferase: identification of a selective small molecule inhibitor. Chemistry & biology. 2010;17: 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trisciuoglio D, Ragazzoni Y, Pelosi A, et al. CPTH6, a thiazole derivative, induces histone hypoacetylation and apoptosis in human leukemia cells. Clin Cancer Res. 2012;18: 475–486. [DOI] [PubMed] [Google Scholar]
- 33.LaBarge SA, Migdal CW, Buckner EH, et al. p300 is not required for metabolic adaptation to endurance exercise training. FASEB J. 2016;30: 1623–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dingemans AM, de Vos-Geelen J, Langen R, Schols AM. Phase II drugs that are currently in development for the treatment of cachexia. Expert Opin Investig Drugs. 2014;23: 1655–1669. [DOI] [PubMed] [Google Scholar]
- 35.Solheim TS, Laird BJA, Balstad TR, et al. Cancer cachexia: rationale for the MENAC (Multimodal-Exercise, Nutrition and Anti-inflammatory medication for Cachexia) trial. BMJ Support Palliat Care. 2018. [DOI] [PubMed] [Google Scholar]
- 36.Bedford DC, Brindle PK. Is histone acetylation the most important physiological function for CBP and p300? Aging. 2012;4: 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dancy BM, Cole PA. Protein lysine acetylation by p300/CBP. Chem Rev. 2015;115: 2419–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Heide LP, Smidt MP. Regulation of FoxO activity by CBP/p300-mediated acetylation. Trends in biochemical sciences. 2005;30: 81–86. [DOI] [PubMed] [Google Scholar]
- 39.Perrot V, Rechler MM. The coactivator p300 directly acetylates the forkhead transcription factor Foxo1 and stimulates Foxo1-induced transcription. Molecular endocrinology. 2005;19: 2283–2298. [DOI] [PubMed] [Google Scholar]
- 40.Senf SM, Sandesara PB, Reed SA, Judge AR. p300 Acetyltransferase activity differentially regulates the localization and activity of the FOXO homologues in skeletal muscle. American journal of physiology. Cell physiology. 2011;300: C1490–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bertaggia E, Coletto L, Sandri M. Posttranslational modifications control FoxO3 activity during denervation. American journal of physiology. Cell physiology. 2012;302: C587–596. [DOI] [PubMed] [Google Scholar]
- 42.Beharry AW, Sandesara PB, Roberts BM, Ferreira LF, Senf SM, Judge AR. HDAC1 activates FoxO and is both sufficient and required for skeletal muscle atrophy. Journal of cell science. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelly TJ, Lerin C, Haas W, Gygi SP, Puigserver P. GCN5-mediated transcriptional control of the metabolic coactivator PGC-1beta through lysine acetylation. J Biol Chem. 2009;284: 19945–19952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beharry AW, Judge AR. Differential expression of HDAC and HAT genes in atrophying skeletal muscle. Muscle Nerve. 2015;52: 1098–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee D, Goldberg AL. Muscle Wasting in Fasting Requires Activation of NF-kappaB and Inhibition of AKT/Mechanistic Target of Rapamycin (mTOR) by the Protein Acetylase, GCN5. J Biol Chem. 2015;290: 30269–30279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puri PL, Avantaggiati ML, Balsano C, et al. p300 is required for MyoD-dependent cell cycle arrest and muscle-specific gene transcription. EMBO J. 1997;16: 369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.