Abstract
Introduction:
Treatments for colorectal and anal cancers can have a detrimental impact on sexual function. Type of treatment, which may include surgery, radiation and/or chemotherapy, varies by disease site and severity. Treatment and long-term side effects can impact sexual function and intimacy for patients and their partners.
Aim:
To review the literature regarding treatment for colorectal/anal cancer and its impact on female sexual function, and to provide an assessment of medical outcomes and patient-reported outcomes (PROs) of women with a history of colon, rectal, or anal cancer seeking sexual health treatment.
Methods:
We performed a PubMed search to identify peer-reviewed, English-language articles, published from 2008 to 2018, using the following search terms: “colorectal cancer,” or “rectal cancer,” or “anal cancer” and “sexual function,” or “sexual dysfunction.” We also assessed the medical outcomes and PROs from our recent cross-sectional cohort study of 99 women with a history of colon, rectal, or anal cancer seeking sexual health treatment.
Main Outcome Measures:
Sexual function, quality of life, and PROs after colorectal/anal cancer
Results:
Twenty-three studies were identified. Study designs included 15 cross-sectional survey studies, 5 longitudinal studies, 2 psychoeducational interventions, and 1 pilot study. Ten studies included women and 13 included both men and women. The literature and our cohort confirmed that women with colorectal/anal cancer experience changes in sexual function after diagnosis and through the continuum of care. The scarcity of data in this area, however, indicates a need for additional intervention trials and longitudinal studies.
Conclusions:
Research studies with large sample sizes and long study durations are needed to help us better understand the needs of female survivors of colorectal/anal cancer. Women with colorectal/anal cancer need simple strategies and resources to address concerns of sexual function after cancer treatment. Such interventions have been shown to enhance survivorship and quality of life.
Keywords: colorectal cancer, anal cancer, sexual health, female, survivorship
Introduction
The U.S. population of cancer survivors is expected to grow to 20 million by 2026 [1]. Despite early screening/detection and cancer prevention strategies, colorectal cancer is a common malignancy among both men and women [2]. Anal cancer is less common; however, the number of newly diagnosed anal cancer cases is rising every year [3]. The incidence and mortality rates of colorectal cancer are higher in men than in women, and rates of anal cancer are slightly higher in women [3]. In 2018, there will be an estimated 47,530 newly diagnosed cases of colon cancer, 17,100 new cases of rectal cancer, and 5,620 new cases of anal cancer among women [4].
Treatment for colorectal/anal cancers varies by disease site and severity. Standard treatment typically includes a combination of surgery, chemotherapy, and/or radiation therapy. Management of colorectal cancer relies primarily on surgical resection of the bowel with the adjacent draining lymph nodes. The use of neoadjuvant or adjuvant chemotherapy (with or without radiation) to treat colorectal cancer depends on tumor location and disease stage. Treatment for colorectal/anal cancer may require a temporary or permanent stoma (an artificial opening on the abdomen through which the bowel or bladder diverts). The stoma opening allows for the attachment of a changeable bag through which feces can be eliminated [5]. For anal cancer, primary radical radiotherapy and concomitant chemotherapy improves survival while also preserving the anal sphincter [6]. Five-year survival rates for colorectal/anal cancer have improved in recent years with screening advancements, an increase in the removal of colonic polyps (precursors to cancer), novel surgical techniques and therapies (adjuvant therapy), better preoperative staging, and targeted therapies [7]. The use of adjuvant chemotherapy for colon cancer and neoadjuvant chemoradiation for rectal cancer has also improved survival rates [8].
Female survivors of colorectal/anal cancer are typically older adults, with an average age of 72 for colon, 63 for rectal, and early 60s for anal cancer [1]. Common issues of aging, such as comorbid illnesses and vulvovaginal tissue quality, can be exacerbated by cancer therapy [7]. As these women live longer, understanding the long-term side effects of treatment are a priority.
Common physical side effects of treatment include autonomic nerve injury, bowel function issues (incontinence, increased stool frequency, flatulence), buttock pain, and vulvovaginal health issues (dryness, fibrosis, adhesions, shortening) [9–11], all of which can adversely impact quality of life (QOL). Colorectal/anal cancer can have adverse and persisting effects on sexual function and psychological wellbeing. It should be noted that urological issues such as erectile dysfunction are common among male cancer survivors and can result in significant difficulties in survivorship [12]; however in this review, we will explore the limited but recent literature on the sexual health of women diagnosed and treated for colorectal/anal cancer, as well as offer insights from our recent cohort of women who sought treatment to address vulvovaginal sexual health concerns.
Methods
Literature Review
We searched PubMed for peer-reviewed, English-language articles published from 2008–2018 using the following search terms: “colorectal cancer,” or “rectal cancer,” or “anal cancer” and “female,” and “sexual function,” or “sexual dysfunction.” Since most of the studies we identified were not exclusively female focused, we included studies that had both male and female patients. The criteria for inclusion consisted of peer-reviewed articles (cross-sectional, longitudinal, interventional, or pilot studies) addressing sexual function in women with a history of colon, rectal, or anal cancer. The study had to incorporate the use of measures assessing sexual function. Review papers were excluded. Within the literature, the prevalence rates for specific sexual dysfunction are difficult to define, in part because of different methods for data collection and lack of baseline data on sexual activity prior to cancer treatment. All studies presented in this review used at least one validated measure.
Colorectal/Anal Cancer Cohort
An IRB-approved limited waiver of authorization was obtained for our recent cross-sectional cohort study. Ninety-nine women with a history of colon, rectal, or anal cancer were seen for an initial consult at the Female Sexual Medicine and Women’s Health Program at Memorial Sloan Kettering Cancer Center from 01/12–07/17. A female sexual medicine clinical assessment form was used to collect medical and patient characteristics and patient-reported outcomes (PROs). The form consists of PRO measures, including the Vaginal Assessment Scale (VAS), Vulvar Assessment Scale (VuAS), Female Sexual Function Index (FSFI), Sexual Activity Questionnaire (SAQ). In addition, exploratory items addressing self-efficacy and sexual/vaginal health regarding confidence about sexual activity, using vaginal health promotion strategies, and managing sexual health/vaginal health issues in the future were included, as well as the degree of concern about sexual function and vaginal health. Medical data were obtained using a pelvic exam checklist and documentation of use of vulvovaginal/sexual health promotion strategies. Descriptive statistics were calculated using SPSS.
Results
Overview of the Literature
Of 65 initially identified studies, 23 met our inclusion criteria (Table 1) [13–35]. Of the 23 studies, 10 included women only [13–18, 23, 25, 30, 34] and 13 included both men and women [19–22, 24, 26–29, 31–33, 35]. There were 15 cross-sectional survey studies [13–15, 17, 19–21, 24–26, 30–34], 2 psychoeducational interventions [23, 27], 5 longitudinal studies [16, 18, 22, 29, 345], and 1 pilot study [28].
Table 1.
Literature Review
| Study | Sample | Study Design/Aim | Measures | Key Sexuality Results | Strengths | Limitations |
|---|---|---|---|---|---|---|
| Traa et al. 2014 [32] |
n=439; nonadvanced disease (80), locally advanced rectal cancer (292), locally recurrent rectal cancer (67) (men and women) | Cross-sectional study to compare the HR-QOL of 3 patient populations with a focus on sexual functioning, and to compare to a normative population | Participants completed the following questionnaires: EORTC general HR-QOL questionnaire (QLQ-C30), disease specific EORTC-colorectal 38 (QLQ-CR38) | Patients with more extensive disease reported lower HR-QOL. Greater than 50% of women were sexually active before treatment and <35% after treatment. All groups reported lower sexual functioning scores compared to a normative population. | High response rate (85%); only study of HR-QOL with 3 types of rectal cancer populations; comparison to a normative population; validated/reliable questionnaires | Cross-sectional design; no information on patients’ functioning before treatment; QLQ-CR38 only asks 5 questions on sexuality |
| Benedict et al. 2016 [13] |
n=70 anorectal cancer (women only) | Cross-sectional survey study seeking to describe body image problems, including sociodemographic and disease/treatment correlates and examine relations between body image and sexual function | Participants completed the following questionnaires as part of a baseline assessment from sexual health intervention study: EORTC-QLQ-CR38 (Body Image subscale), FSFI | Body image is a potentially modifiable target to address sexual difficulties. Poor body image is inversely related to all aspects of sexual function, except pain. Strongest Association between FSFI sexual/relationship satisfaction. | Importance of addressing sexual concerns in women who are not sexually active; near complete response rate for FSFI | Women agreeing to participate in a sexual health intervention study; cross-sectional design |
| Welzel et al. 2010 [33] |
n=88 anal cancer (men and women) | Cross-sectional survey study to assess self-reported long-term QOL experienced by anal cancer patients after radiochemotherapy and identify patient and disease-related factors associated with QOL | Participants completed the following questionnaires: Cancer-specific QOL: EORTC QLQ-C30; Colorectal cancer module QLQ-CR38 | Female sexual dysfunction is among the most frequently reported symptoms and was affected by marital status and radiotherapy dose (>50.4 Gy); 27% of females had engaged in sexual activity in past 4 weeks. | Rare cancer and limited research on anal cancer | Small sample size, which limited power size; retrospective nature |
| Segelman et al. 2013 [30] |
n=82 rectal (women only) | Cross-sectional survey study to describe preoperative sexual functioning in a large group of women with rectal cancer | Participants completed the following questionnaire during a preoperative outpatient visit: FSFI | Sexual dysfunction and sexual inactivity are common in women with rectal cancer demonstrated by low FSFI scores. | Use of validated questionnaire; evaluation of preoperative functioning; high response rate (70%) | Recall bias may have influenced FSFI results; selection bias (non-Swedish-speaking women not included) |
| Traa et al. 2012 [31] |
n=242; colorectal patients (136); partners of the patients (106) (men and women) | Cross-sectional survey study to describe preoperative sexual functioning, QOL, and relationship functioning of colorectal patients and their partners. | Participants completed the following questionnaires at home before their scheduled surgery for colorectal cancer: IIEF, FSFI, Golombok-Rust Inventory of Sexual Satisfaction, Maudsley Marital Questionnaire | Female patients had lower QOL than male patients. All patients and partners reported lower sexual functioning and lower QOL than a normative population but similar relationship functioning. The study highlights that colorectal patients may already experience low sexual functioning and quality of sex life before surgical treatment, showing surgical intervention may not be solely responsible for high sexual dysfunction. | Use of validated and comprehensive measures; in-depth view of preoperative sexual functioning, QOL and relationship functioning in patients and partners of both sexes | Patients completed assessments after diagnosis, so no statements can be made about functioning or sex life prior to diagnosis; time between diagnosis and completing questionnaires varied; one important reason for declining participation was high levels of stress and the intimate questions, perhaps underestimating the concerns of this population |
| Bohm et al. 2008 [15] |
n=26; colon (11) and rectal (15); all had transabdominal rectal resection (women only) | Cross-sectional survey study to establish the incidence of potential postoperative anorectal, bladder and sexual dysfunction in women after total mesorectal excision compared to colonic resection (control group) | Participants completed the following questionnaires: “general questionnaire,” Wexner questionnaire, King’s Health Questionnaire, FSFI | Worse sexual functioning in the total mesorectal excision group compared to the control group | Comparative group of patients undergoing surgery during the same time | Unable to compare pre- and postoperative results |
| Das et al. 2010 [19] |
n=80 anal cancer (men and women) | Cross-sectional survey study to evaluate long-term QOL in patients treated with definitive radiotherapy or chemoradiation for squamous cell cancer of the anal canal | Participants completed the following questionnaires at home: FACT-C, MOS Sexual Problems Scale | Mean MOS Sexual Problems Scale score was 51 (out of 100) in a relatively young sample (median, 51) | Squamous cell cancer of the anal canal is a rare malignancy; supported the need for more modern radiation techniques to reduce toxicity from radiotherapy | Small sample; 40% response rate; unable to account for baseline sexual function; cross-sectional design |
| Corte et al. 2011 [17] |
n= 47 anal cancer (women only) | Cross-sectional survey study to evaluate sexual life of women after surgery for squamous cel carcinoma of anus (abdominoperineal resection) | Participants completed the following questionnaire: FSFI | 76% of women who had an active sex life before abdominoperineal resection still had intercourse after surgery. Colpectomy reduces sex life. | Homogenous group regarding surgical technique, which facilitates comparison | Small sample; low response rate; use of one questionnaire |
| Benedict et al. 2016 [14] |
n=70 rectal and anal (women only) | Cross-sectional survey study to evaluate the degree to which bowel and Gastrointestinal dysfunction related to psychological distress in women treated for rectal or anal cancer. Additionally, to examine whether body image mediated the effect of bowel and gastrointestinal dysfunction on psychological distress | Participants completed the following questionnaires as part of a baseline assessment from sexual health intervention study: EORTC-QLQ-CR38 (Diarrhea, Gastrointestinal Symptoms and Body Image subscales), Brief Symptom Index (depression and anxiety subscales) | Long-term bowel and gastrointestinal dysfunction are distressing and affect how women perceive and relate to their body. | Insight into how body image perception can exacerbate other treatment side effects | Cross-sectional design; small sample; primary focus prevented inclusion of covariates (history of psychopathology); participants were in an intervention study for sexual health and may not represent other rectal/anal cancer survivors |
| Philip et al. 2013 [25] |
n=70 anorectal (women only) | Cross-sectional survey study to examine the characteristics of women enrolled in an intervention trial to treat sexual dysfunction and explore the relationship between sexual functioning and psychological wellbeing | Participants completed the following questionnaires: EORTC-QLQ-C30, QLQ-CR38, FSFI, BSI-18, IES-R, CR-38 Body image | Of those in an intervention trial, sexual dysfunction was significantly and consistently associated with measure of psychological wellbeing. | Explored an understudied clinical issue within cancer care | Homogenous sample of ethnicity and all received care at a large cancer center; participants were at varying stages of posttreatment survivorship; no data on age-matched controls; no information on the sexual functioning of patients’ partners |
| Di Fabio et al. 2008 [21] |
n=62 colorectal patients (men and women) | Cross-sectional survey study to explore aspects of QOL in long-term survivors (including sexual dysfunction) and surgeons’ awareness of patients’ needs to determine how to improve follow-up care programs | Participants completed the following questionnaires: EORTC QLQ-C30, QLQ-C38, postoperative sexual problems evaluated with 6 items | 61% colon patients and 24% rectal patients reported no sexual problems. Patients who reported no sexual problems had better QOL. | QOL measures can help detect problems that may go unnoticed in routine followup care; novel study of perception mismatch between doctors and patients; studies like this help to reduce long-term postoperative dissatisfaction | Small sample; single-center setting; cross-sectional design |
| Den Oudsten et al. 2012 [20] |
n=1759; colon and rectal survivors (1359) and normative population (400) (men and women) | Cross-sectional survey study to explore the prevalence of erectile dysfunction, ejaculation problems, dyspareunia, and dry vagina in colon and recall cancer survivors and a normative population. Additionally, to compare dysfunction between groups and describe the sociodemographic, clinical and psychological correlates of sexual dysfunction in survivors | Participants completed the following questionnaires: SCQ, EORTC, QLQ-CR38, FAS, HADS | Vaginal dryness was common in colon (28%) and rectal (35%) cancer survivors. Colon and rectal cancer survivors experienced more pain with intercourse than a normative population | Inclusion of a normative population contributed to debate on whether sexual dysfunction in a higher age is normal or pathological; high response rate | Cross-sectional; no information on sexual functioning before cancer treatment/diagnosis; EORTC QLQ-CR-38 provides limited information on sexual functioning |
| Milbury et al. 2013 [24] |
n=261 colorectal (144 male and 117 female) | Cross-sectional survey study to identify demographic, medical, and psychosocial risk factors to explain a proportion of the variance in male and female sexual function in colorectal cancer survivors | Participants completed the following questionnaires in clinic or at home: IIEF, FSFI, EORTC-QLQ-CR38, CES-D, BSI-18, MOS-SSS, A-DAS | Patients’ age and more destructive procedures are related to poor sexual function | Helped to identify patients at high risk for sexual dysfunction after cancer treatment; inclusive of sexually inactive survivors; high response rate for clinic follow-up (90%) | Cross-sectional; no pre-treatment assessment; low power (small sample because the rarity of abdominoperineal resection); homogenous sample; potential sampling bias from different recruitment methods |
| Yu-Hua et al. 2014 [34] |
n=64 rectal (32 with low anterior resection and 32 healthy women) (women only) | Cross-sectional survey study to investigate the prevalence of sexual functioning problems among women who have had a low anterior resection | Participants completed the following questionnaires: FSFI | Women who have had a low anterior resection are at higher risk of sexual function problems; FSFI domain scores lower for the low anterior resection group compared to healthy women | Insight into the sexual dysfunction experienced by Taiwanese women following low anterior resection | Cross-sectional design (no causality inference); selection bias (healthy women recruited from women known to researcher); did not expand to other relevant parameters (hormonal status, previous sexual experience or spousal attitude) |
| Reese et al. 2014 [26] |
n=141 colorectal (18 post ostomy; 25 current ostomies; and 98 no ostomy) (men and women) | Cross-sectional survey study where data were collected from a larger prospective study examining physical and emotional predictors of sexual QOL in colorectal patients. The aim was to compare colorectal cancer patients by ostomy classification group with respect to sexual function, perceived impact of disease, and treatment on sexual function and body image distress | Participants completed the following questionnaires: Medical Impact on Sexual Function, FSFI, IIEF, Body Image Scale, CES-D | Current and past ostomy groups had worse sexual functioning and body image than the no ostomy group | Use of validated and comprehensive measures; prospective study design | Cross-sectional design (limits generalizability and no causal interpretations); uneven groups; low response rate for women answering sexual function questions |
| Reese et al. 2016 [27] |
n=141 colorectal cancer (men and women) | Intervention study to examine the importance of sexuality within the self-view and cross-sectional correlations. To determine if the importance of sexuality changes after participating in an intimacy enhancement intervention | Participants completed the following questionnaires as part of a larger prospective study examining physical and emotional predictors of sexual QOL in colorectal patients: 2 importance of sexuality items, FSFI, IIEF; participants were randomized to an intimacy enhancement condition (intervention) | Perceived importance of sexuality decreased for individuals treated for colorectal cancer and can increase through participating in an intimacy-focused intervention. | Theory-driven approach;, use of validated measures; inference of causality (secondary analyses of outcomes from an intervention study) | Use of two single items (non-validated) to assess importance of sexuality; some comparisons on small subsamples not allowing for testing of longitudinal hypotheses |
| DuHamel et al. 2016 [23] |
n=70 rectal and anal cancer survivors (women only) | Intervention trial to assess the efficacy of a telephone-based, four-session cancer survivorship intervention for rectal and anal cancer survivors | Participants randomized to assessment only or CSI-SH and completed the telephone-based Intervention and/or assessments at baseline, 4 mo. and 8 mo. FSFI, IES-R, BSI, EORTC-QLQ-C30 | Improved sexual and psychological functioning and QOL in the CSI-SH group. The intervention was more efficacious for sexually active women. | This study provided preliminary data to inform and develop a larger intervention trial for sexual dysfunction and QOL | Pilot study and represents small sample of survivors; inclusion criteria were limited to women who reported low to moderate degrees of satisfaction; challenging recruitment; heterogenous sample |
| Carter et al. 2017 [16] |
n=175; colorectal/anal (15), breast (90), gynecologic (54), other (16) (women only) | Longitudinal intervention study (baseline and 1 follow-up) to evaluate patient adherence and response to vaginal and sexual health treatment strategies in a female sexual medicine and women’s health program. | Participants completed the following questionnaires: VAS, VuAS, FSFI, SAQ, SSS; all patients had two gynecologic exams (pelvic exam checklist) | After two visits to the program, participants showed improvement in vulvar/vaginal symptoms, enhanced sexual function, increased sexual activity and confidence in sexual activity, decline in elevated vaginal pH, and decreased pain with gynecologic exam. | Simple strategies can be helpful to women treated for cancer; intervention that has substantial clinical relevance (evaluation of emotion and physical implications of changes to vulvovaginal health and sexual functioning in women with cancer) | Only included women with follow-up; potential subject bias to self-report measures; absence of a control group |
| da Silva et al. 2008 [18] |
n=93; colon (57) and rectal (36) (women only) | Longitudinal survey study to evaluate women’s sexual function, self-esteem, body image and health-related QOL after colorectal surgery | Participants completed the following questionnaires preoperatively, 5 and 12 months postoperatively: FSFI, Rosenburg Sel-Esteem Scale, Body Image scale, SF-36 | Significant deterioration of sexual function 6 Months postoperatively with partial recovery at 12 months. 81% stated that it was somewhat-extremely important to discuss sexual issues. | Validated questionnaires; longitudinal design | Low response rate to FSFI; use of one questionnaire |
| Doeksen et al. 2011 [22] |
n=136; underwent rectal resection (83) or colonic resection leaving rectum in situ (53) (men and women) | Longitudinal survey study (preoperatively, 3 mo., 12 mo., 8 years) to prospectively compare long-term sexual and urinary function between patients who underwent rectal resection and a control group (hemicolectomy, subtotal colectomy or ileocolic resection). Bowel function and QOL were secondary endpoints. | The following questionnaires were sent to participants to complete at home: items assessing sexual, urinary and bowel functioning with a QOL questionnaire (SF-36) | Patients who underwent rectal resection experienced more sexual and bowel function issues than rectum in situ patients up to 1 year postoperatively. | Longitudinal study from preop to 8.5 years postop | Small sample; heterogenous population |
| Reese et al. 2010 [29] |
n=113; colon or rectal (56) and anal (1) (men and women) | Longitudinal survey study to assess sexual concerns in gastrointestinal cancer patients compared with breast cancer patients. Also examined if sexual concerns change over time and evaluated whether sexual concerns are significantly associated with functioning in other domains (QOL, symptom severity, disease interference, disease-related distress) | Participants completed the following questionnaires during 4 outpatient clinic visits over 6 months: Sexual concerns (reduced sexual enjoyment, interest or performance), quality of life (FACT-G), symptom severity, disease interference (MD Anderson Symptom Inventory), disease-related distress (NCCN Distress Scale) | Sexual concerns were common in 57% of the gastrointestinal patients. Concerns were stable over time and significantly associated with lower levels of functioning in QOL, symptom severity, disease interference, and disease-related distress. | Prospective design with multiple timepoints; validated instruments to measure perceived disease impact and QOL; large sample | Single item to assess sexual concerns (not validated); subjective nature of sexual concern item; brief timespan (6 months); lack of information on type of treatment received |
| Zutshi et al. 2012 [35] |
n=260 rectal cancer (male and female) | Longitudinal survey study to assess the effect of rectal cancer treatment on bowel function, QOL, and sexual activity | Participants completed the following questionnaires over 5 years (3 mo., 6 mo., 1 year, 3 years, 5 years): SF-36, CGQOL, QOL questions, Kirwan score, Sexual function questions (validated) | Sexual function declined sharply within the first year after surgery and gradually over 5 years. | Prospective observation over 5 years | Only 4 items to assess sexual function in women |
| Reese et al. 2012 [28] |
n=14 couples (men and women) | Pilot study to collect preliminary data on the feasibility and efficacy of an intervention for physical intimacy and sexual concerns in colorectal cancer | Participants completed four 50-minute phone-based intimacy enhancement intervention sessions. Additionally, participants completed the following questionnaires: use of skills questions, program evaluation questions, Index of Sexual Satisfaction, FSFI, IIEF, Dyadic Sexual Communication Scale, Miller Social Intimacy Scale, Dyadic Adjustment Scale | The majority rated the intervention as at least “quite a bit” helpful overall, easy to participate in, and as an important program for people with colorectal cancer. | Comprehensive set of outcome and feasibility measures; grounding in empirically supportive cognitive and behavioral studies; test of a novel intervention; implemented strategy to overcome recruitment challenges, such as telephone-based format that eliminated participant travel burden | Limited generalizability because of small sample size and lack of control group, so unable to draw definitive conclusions on efficacy of the intervention; no long-term followup |
| Bentzen et al., 2013 [41] |
n=128 anal cancer (male and female) | Cross-sectional cohort study with Norwegian national registry | EORTC QLQ-CR29 assesses sexual interest and dyspareunia | Statistically significant difference between anal cancer survivors and matched volunteers sexual function. Anal cancer patients had decreased sexual interest (p<0.0001) and greater dyspareunia (p<0.0001). | Large sample of female anal cancer survivors, with 64% response rate; comparison with Dutch and Norwegian normative data | Single items to assess sexual interest and dyspareunia and only at one time point |
| Reese et al. 2018 [29] |
n=141 colon and rectal cancer patients (men and women) | Longitudinal mail-based survey with 2 assessment points – study enrollment (baseline) and 6 months follow-up. | Sexual Distress Scale (ISS), SFQ, (FSFI | 73.8% of women experienced sexual dysfunction (as per FSFI). Sexual QOL and body image were compromised after colorectal cancer and remained over time. | Assessment of multiple domains of sexual QOL; 2 assessment points over time | Mixed sample of patients actively on treatment and post-treatment; mean time since treatment was 31.5 months; homogenous sample (race/ethnicity); predominantly male participants |
EORTC, European Organization for Research and Treatment of Cancer; HR-QOL, health-related quality of life; FSFI, Female Sexual Function Index; IIEF, International Index of Erectile Functioning; FACT-C, Functional Assessment of Cancer Therapy-Colorectal; MOS, Medical Outcomes Study; BSI, Brief Syndrome Inventory; IES-R, Impact of Event Scale-Revised; SCQ, Self-administered Comorbidity Questionnaire; FAS, Fatigue Assessment Scale; HADS, Hospital Anxiety and Depression Scale; CES-D, Center for Epidemiological Studies-Depression; MOS-SSS, Medical Outcomes Study Social Support Survey; A-DAS, Abbreviated Dyadic Adjustment Scale; CSI-SH, Cancer Survivor Intervention--Sexual Health; SF-36, Short Form Health Survey-36; SFQ, Sexual Function Questionnaire
The most common instruments used were the EORTC-QLQ-C30 [21, 23, 25, 33, 36], the EORTC-QLQ-CR38 [13, 14, 20, 21, 24, 25, 32, 33, 37], and the FSFI [13, 15–18, 23, 24, 26–28, 30, 31, 33, 34, 38, 39]. These measures have strong reliability and validity. The FSFI asks participants to recall their sexual activity within the past 4 weeks, making it difficult to assess function when no sexual activity occurred within that time frame.
Patients who do not report any sexual function concerns before their cancer diagnosis may experience changes during or after cancer treatment [40]. An estimated 75% of patients with colorectal cancer experience sexual difficulties after treatment, and up to one-third refrain from sexual activity post-treatment regardless of physical dysfunction [28]. Sexual dysfunction is the impairment of one’s typical pattern of intimate sexual response and can include changes in sexual desire, arousal, orgasm ability, pain, vaginal dryness or atrophy, as well as feeling a lack of femininity, sexual attractiveness, and confidence [24, 41–43]. Contributing factors include use of neoadjuvant or adjuvant radiation, age, tumor stage, and surgical experience and technique. Older patients tend to have pre-existing sexual changes [5, 44]. Patients undergoing nerve-sparing surgical techniques to help mitigate symptoms can still experience sexual dysfunction [45].
Though sexual and vulvovaginal health concerns after treatment for colorectal/anal cancer are prevalent, they are rarely studied. Patients with a history of colorectal/anal cancer should undergo regular follow-up and evaluation for the management of treatment-related side effects; however, the data regarding associated changes in sexual function to guide such management are limited. The existing literature is mainly retrospective, consisting of small sample sizes and mostly male patients. Furthermore, patient engagement with healthcare providers to address these issues is low [21]. Many female cancer patients and survivors have indicated that sexual matters were never discussed before or after their treatment, and many did not initiate such conversations with their healthcare providers [10]. In a study by Da Silva et al., 81% of women stated that it was somewhat-to-extremely important to discuss sexual issues with their provider [18]. Studies with cancer survivors suggest they would like to have conversations on sexuality with their medical team [46]. Additionally, many couples report a desire for candid conversations about sexual health challenges [5, 47].
Targeted interventions and resources addressing sexual and vulvovaginal health can aid women and their partners in adjusting to changes during and after treatment. Sexual health interventions have shown promising results in addressing these concerns and emphasize the need for targeted therapies for sexual/relationship satisfaction. The implications of these limited intervention trials are significant. For example, Barsky-Reese et al. and DuHammel et al. provided evidence that it is essential to not only evaluate patient concerns but efficiently address worry/distress to enhance intimacy [23, 27]. Increased attention and considerable effort to identify and manage symptoms of colorectal/anal patients in survivorship can promote adjustment and foster realistic expectations. Findings by Park et al. suggest that the information patients receive, as well as personal attitudes, play a role in expectations for post-treatment function (e.g., bowel function) [48]. Research into the psychological, informational, and experiential components that underlie patient expectations of surgical recovery can be useful for healthcare professionals looking to develop strategies for proactive patient education [48]. Recording quantitative outcomes and qualitative findings post treatment may reveal symptomatic trends that can be discussed by healthcare professionals in early clinical conversations and monitored throughout survivorship [48]. Additionally, setting realistic expectations on post-treatment function can help healthcare professionals and patients work together to manage symptoms throughout the continuum of cancer care.
Some patients may require either a temporary or permanent stoma after colorectal surgery. For these patients, having sufficient information and support around stoma care (how to prevent skin breakdown, manage waste and leaks, and reduce embarrassment from sounds and smells) can be beneficial in helping them adapt and minimize challenges. Healthcare providers can help mitigate patient anxiety about stomas by acknowledging that sexual health issues are common and educating their patients [49]. Women adjusting to living with an ostomy may experience issues of body image, concerns about interpersonal/intimate relationships, and possibly anxiety related to participating in physical/social activities, all of which can impact QOL [7, 26]. These women should be instructed to empty their pouches and check the seal of the appliance before engaging in sexual activity [7]. While an ostomy surgery does not directly affect sexual desire or function, women may worry about their partner’s response or leakage of their pouch during intimacy [5]. Rehabilitation and support of ostomy patients are critical, and patients can benefit from appropriate counseling from healthcare providers.
Changes in body function after cancer treatment have been shown to impact the way survivors view themselves and interact with others [13, 14, 26, 31, 33]. Reintegrating into social relationships, including intimate relationships and friendships, can be challenging. The unpredictability of incontinence/bowel clustering can interfere with social activities, as bowel dysfunction is associated with social stigma and fear [50]. The ability to participate in life activities (such as sexual or intimate contact) is strongly associated with QOL among rectal cancer survivors and should be evaluated as a causal factor in reduced QOL [25]. The ability to participate in life activities (such as sexual or intimate contact) is strongly associated with QOL among rectal cancer survivors and should be evaluated as a causal factor in reduced QOL [25]. It was found that stoma-related gastrointestinal toxicity (as measured by the LENT/SOMA scale) is associated with poorer social functioning [33]. Also, since perceived social support has been shown to decline following a colorectal cancer diagnosis and treatment, assessing social support from initial diagnosis throughout survivorship can enable targeted interventions to improve health-related QOL [51]. Social support is an essential aspect of recovery, as high levels of social support show better health outcomes and health-related QOL after cancer treatment. Women of low socioeconomic status who present with co-morbidities typically experience limited social support due to caregiver burden and decreased contact with providers in survivorship [51]. This is a crucial dichotomy for health providers to acknowledge when caring for this patient population. Benedict C. et al elaborates on the social/intimacy challenges post treatment for survivors and emphasizes dialogue on these topics as a crucial tool in managing survivorship [13]. Healthcare professionals can address this concern by initiating conversations about options for social support and intimacy from the time of initial diagnosis throughout survivorship follow-ups.
Study Weaknesses
Many studies examined the QOL of patients with colorectal/anal cancer; however, few explored the sexual health concerns specific to women with a history of such cancers. The studies we identified had small numbers of female patients. Small sample sizes can reduce statistical power and impact the generalizability of findings to all female patients/survivors of colorectal/anal cancer. For instance, Philip et al. surveyed a homogenous sample (without ethnic diversity) of patients receiving care at a large urban cancer center [25]. This cross-sectional survey study examined the characteristics of women enrolled in an intervention trial to treat sexual dysfunction and explore the relationship between sexual function and psychological wellbeing. Of those in the intervention trial, sexual dysfunction was significantly and consistently associated with psychological wellbeing. Even though the small homogenous sample limits the generalizability of the findings, this study explored an understudied clinical issue within cancer care. Small samples can be viewed as a weakness, but can also facilitate group comparison and provide important insights into rare malignancies.
Including patients at varying stages of post-treatment survivorship can serve as another limitation, as their needs may differ across the continuum of care. It can be misleading to compare patients at different stages of survivorship. For example, Philip et al. conducted a study with 70 women with anorectal cancer; however, all participants were at varying stages of post-treatment survivorship [25].
While retrospective cross-sectional survey studies are feasible and convenient methods to examine areas of interest [13–15, 17, 19–21, 24–26, 30–34], this study design is also subject to recall bias. The studies reported in this review used various PRO measures to assess aspects of QOL, including the EORTC-QLQ-C30 [21, 23, 25, 33], EORTC-QLQ-CR38 [13, 14, 20, 21, 24, 25, 32, 33], and the FSFI [13, 15–18, 23–28, 30, 31, 33, 34], but additional validated measures specific to sexual function are needed to understand the complexity of intimacy during and after colorectal/anal cancer treatment.
Although cross-sectional survey studies can give a snapshot of the patient experience, they are limited by their single timepoint assessment. Longitudinal measurement is needed to explain chronicity or transitive nature of changes. Cross-sectional studies are useful for providing descriptive analyses and generating hypotheses, yet this method is susceptible to potential sampling bias. To address the risk of self-report bias and poor retrospective recall, some researchers asked their patients about their pre-cancer levels of sexual function to compare them to current levels [31, 32]. Overall, however, cross-sectional studies lack strength without the ability to understand conditions over time [28].
To ensure a more systematic approach to understanding the needs of patients across the continuum of care, more long-term follow-up designs with pre-treatment baselines are needed. Also, a lack of control groups [28] does not allow researchers to draw definitive conclusions. Patients may have had sexual health concerns before cancer, so without a pre-treatment assessment, it may be difficult to pinpoint the specific treatment-related contributing factors for the sexual health issues. Many women experience sexual dysfunction with age, including a lack of sexual desire and arousal, as well as poor vulvovaginal tissue quality, emphasizing the need for pre-treatment assessment and comparisons with general population norms.
Within this review, many studies had high response rates [13, 24, 30, 32], while others had low response rates [19, 31]. Of note, high response rates were seen when participants were asked to complete a questionnaire during a preoperative visit, ensuring completion of study materials. Traa et al. noted high levels of stress and discomfort with intimate questions as reasons for declining participation in their cross-sectional survey study [31]. Participants’ willingness or unwillingness to discuss their sexual life may underestimate the concerns of this population. Data on why patients decline participation are needed. Future research studies should incorporate methods to elicit this information in ways that make the patient feel respected and comfortable.
Study Strengths
Recent intervention studies have provided important insights into the impact of treatment on the sexual function of patients with colorectal/anal cancer. Reese and colleagues used a flexible coping model in an intervention study focused on the sexual concerns of patients with colorectal cancer and their partners (n=18) [28]. The goal of this phone-based intervention was to aid couples in making cognitive and behavioral shifts in their relationship. The participants had four 50-minute sessions focused on couples coping skills, sex therapy, communication strategies and intimacy-building activities. The results suggested that telephone-based couple’s interventions for this population are feasible and may enhance intimacy. The strengths of this study included the use of validated measures and access to a novel intervention.
Reese and Haythornthwaite looked at the importance of sexuality in terms of predictors, changes, and response to an intimacy enhancement intervention in patients with colorectal cancer [27]. This study noted several factors contributing to how an individual perceives sexuality, such as the presence of an intimate partner, being on active treatment, or having metastatic disease. Patients classified as sexually dysfunctional rated sexuality as less important and indicated sex was less important to them after cancer. The strengths of this intervention included its theory-driven approach and use of a validated sexual health measure, the FSFI.
Duhamel et al. conducted a randomized controlled trial to assess the efficacy of a telephone-based, four-session Cancer Survivorship Intervention-Sexual Health (CSI-SH) in women with a history of rectal/anal cancer [23]. Participants (n=70) were randomized to CSI-SH or assessment-only and received four 1-hour individual sessions that included the following: (1) an overview of sexual health, (2) discussion of strategies to improve sexual functioning, (3) education on effective communication methods, and (4) provision of additional resources (i.e., educational booklets or relevant referrals). The results demonstrated improved sexual and psychological functioning and QOL in the women on the CSI-SH arm. This study highlights the importance of providing patients with sexual health education, support, and resources during survivorship care.
Recent Clinical Findings of a Cohort of Survivors of Colorectal/Anal Cancer Who Sought Sexual Health Treatment
Patients consisted of women with a history of colon (n=9), rectal (n=49), or anal cancer (n=41) who sought sexual/vulvovaginal health care at the Female Sexual Medicine and Women’s Health Program at Memorial Sloan Kettering Cancer Center [16]. Participants were diagnosed with cancer between 1989 and 2015. Mean age was 48 years (range, 18–77 years). On average, women were seen approximately 3 years from diagnosis (range, 9 months to 9.5 years). Demographic and medical characteristics are presented in Table 2.
Table 2.
Demographic and Medical Characteristics
| Basic Demographics (N=99) | ||
|---|---|---|
| n | % | |
| Disease Site | ||
| Colon | 9 | 9% |
| Rectal | 49 | 50% |
| Anal | 41 | 41% |
| Age at diagnosis | ||
| Colon | 40 (range, 24–55) | |
| Rectal | 46 (range, 18–77) | |
| Anal | 53 (range, 38–69) | |
| Race | ||
| White | 88 | 89% |
| Black | 5 | 5% |
| Asian | 3 | 3% |
| Other/Unknown | 3 | 3% |
| Marital Status | ||
| Married | 64 | 65% |
| Single | 19 | 19% |
| Divorced/Widowed/Separated | 17 | 16% |
| Engage in Sexual Activity (n= 88) | ||
| Yes | 29 | 33% |
| No | 59 | 67% |
| Date of Diagnosis | ||
| 1989–2000 | 3 | 3% |
| 2001–2009 | 26 | 26% |
| 2010–2015 | 70 | 71% |
| Time from diagnosis to initial consult | ||
| 3 months – 1 year | 54 | 55% |
| 2 years – 9 years | 37 | 37% |
| 10 years – 25 years | 8 | 8% |
| Age at initial consult (mean, 48 yrs; range, 20–78 yrs) | ||
| 20–39 | 6 | 6% |
| 40–50 | 42 | 43% |
| 51–60 | 38 | 38% |
| 61–78 | 13 | 13% |
| Treated with radiation | ||
| Yes | 94 | 95% |
| No | 6 | 5% |
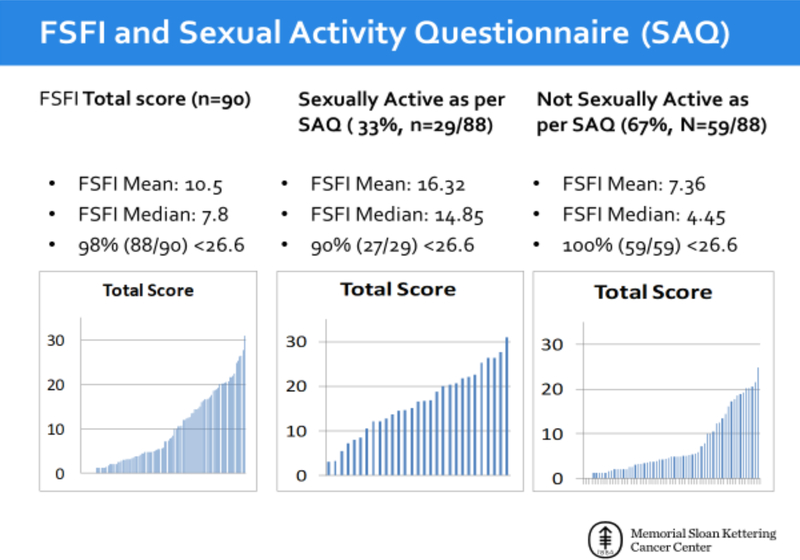
Sixty-seven percent (n=56/83) reported symptoms of mild-severe vaginal dryness, 36% (n=30/84) mild-severe dyspareunia (VAS), and 60% (n=44/73) mild-severe vulvar dryness (VuAS) (Table 3). Upon gynecologic exam, 54% (n=30/56) had mild-severe vaginal atrophy, and 67% (n=44/66) experienced pain with an exam. Forty percent (n=21/54) had issues of fecal incontinence, 47% (n=27/58) had urinary incontinence, and 56% (n=32/57) were currently wearing a pad/panty liner. Thirty-three percent (n=29/88) were currently sexually active per the SAQ. The main reason for not engaging in sexual activity (as cited on the SAQ) was a physical issue making sexual activity difficult or uncomfortable (64%, n=38/59).
Table 3.
Vaginal Assessment Scale (VAS) and Vulvar Assessment Scale (VuAS)
| VAS (N=99) | None | Mild | Moderate | Severe |
|---|---|---|---|---|
| Dryness | 27% (27) | 20% (20) | 11% (11) | 25% (25) |
| Soreness | 66% (65) | 7% (7) | 8% (8) | 2% (2) |
| Irritation | 68% (67) | 10% (10) | 2% (2) | 2% (2) |
| VuAS (N=99) | None | Mild | Moderate | Severe |
| Dryness | 29% (29) | 16% (16) | 15% (15) | 13% (13) |
| Soreness | 46% (46) | 13% (13) | 9% (9) | 4% (4) |
| Irritation | 46% (46) | 10% (10) | 11% (11) | 5% (5) |
When queried about “How concerned are you about sexual/vaginal health?” (n=82), 91% (n=75) reported being somewhat to very concerned about this issue. Forty-eight percent (n=39/82) indicated a lack of confidence about future sexual activity. In all, 98% (n=88/90) scored <26.6 on the FSFI (mean, 10.5; median, 7.8), indicating female sexual dysfunction. The FSFI examined those who were and were not sexually active per the SAQ. Of those who were sexually active, 90% (n=27/29) scored <26.6 (mean, 16.32; median, 14.85). Of those who were not sexually active, 100% (n=59/59) scored <26.6 (mean, 7.36; median, 4.45). The mean FSFI score of those who were sexually active compared to those who were not sexually active varied greatly, highlighting the caution with this instrument (Figure 1).
Figure 1.
Female Sexual Function Index (FSFI) and Sexual Activity Questionnaire (SAQ) Results
Overall, women who sought treatment for sexual and vaginal health concerns after treatment for colorectal/anal cancer showed symptoms on a gynecologic exam that were in line with results on the PRO measures. Within this cohort, many had vulvovaginal tissue quality concerns and few were sexually active or confident about future sexual activity in survivorship. Throughout colorectal/anal cancer survivorship, many women experience persistent vaginal/sexual health concerns. Simple vulvovaginal health promotion strategies have been shown to provide symptom relief and promote intimacy [16] (Table 4).
Table 4.
Vulvovaginal Health Promotion Strategies
| Strategy | Purpose | Recommendation |
|---|---|---|
| Vaginal moisturizers Ex. Hydrating Moisturizers Replens ®HyaloGYN ® |
Manage vaginal and vulvar dryness and improve tissue quality by increasing moisture and elasticity of the tissues | Use 3–5 times per week Apply before bedtime Can be used in the vagina and on external (vulvar) tissues |
| Vaginal lubricants Ex. Water-based – Astroglide, KY, Yes, Luvena, Good Clean Love Silicone – Pjur, Astroglide silicone, Pink Natural Oils Coconut or almond oil |
Used to add to natural lubrication and minimize discomfort with sexual touch and/or vaginal insertion | Use silicone- or water-based lubricants Apply with all sexual activity Avoid petroleum jelly, colored, flavored or warming lubricants that may cause irritation |
| Skin/Tissue Protectants Ex. Aquaphor®, Balmex® or Desitin® |
Pads/panty liners can be very drying to the vulvar tissues Apply a skin/tissue protectant to protect the skin and help seal in moisture |
Before using a pad/pantyliner apply the protectant cream to the external (vulvar) tissues |
| Pelvic floor muscle exercises | To strengthen pelvic floor muscles and prevent physical problems (incontinence) and improve sexual health and pleasure Kegels can relax vaginal muscles, improve arousal/blood circulation, and increase vaginal tone and lubrication |
Perform pelvic floor exercises daily During each session, pull up and contract pelvic floor muscles for 3–6 seconds; and equal time to fully relax the pelvic floor for 3–6 seconds Repeat till muscle fatigue |
| Vaginal dilators | Vaginal dilators can help with comfort with exam, prevent the vagina from becoming too narrow, maintain vaginal elasticity, and reduce discomfort with sexual activity | Practice dilator therapy several times per week (every other day) Gradually increase the size of dilators without discomfort |
Our study had limitations. The data were from women who sought treatment for sexual/vulvovaginal health issues. There are no data on survivors of colorectal/anal cancer who did not seek treatment. It is possible that our sample may be more symptomatic or motivated to address vulvovaginal health and intimacy concerns. Of note, our patients were much younger than the average age of patients with colorectal/anal cancer. Perhaps younger women are more motivated to seek treatment for these issues and may be an ideal risk group for interventions. Nevertheless, women of all ages need support, and providers should be encouraged to have discussions with their patients regarding vulvovaginal health and intimacy concerns, providing information and making referrals, as needed.
Future Directions of Research and Clinical Care
Studies that have examined the sexual function of women with colorectal/anal cancer have shown that sexual dysfunction is a prevalent issue after cancer treatment. Although the rates of sexual dysfunction vary by study design and treatment modality, research shows that the management of sexual function in patients with colorectal/anal cancer should be part of standard care. Furthermore, sexual function concerns should be re-assessed throughout treatment and into survivorship.
Future research should look to further validate measures, using larger sample sizes, and should focus specifically on female patients. Unlike with male sexual dysfunction, studies on female sexual dysfunction after colorectal/anal cancer are limited. This may reflect that female sexual dysfunction is multifaceted, complex [47–52], and not well understood. Female sexual function is challenging to measure due to dynamic and non-linear changes that take place over time when compared to male sexual function (e.g., erectile dysfunction). Until recently, few validated scoring items regarding female sexual dysfunction were available. Furthermore, male healthcare providers may be apprehensive or reluctant to speak with female patients regarding sexual function, as it is a sensitive topic [52].
Recent psychoeducational interventions have shown promising results [25, 27]. The intervention trial for couples conducted by Barsky-Reese and colleagues showed how these types of interventions may benefit this population by addressing symptoms and concerns [28]. One innovative approach is pre-rehabilitation, or “pre-hab.” The essence of pre-hab is to reduce potential side effects of treatment before the treatment begins. Most research on pre-hab focuses on proper nutrition and exercise before treatment; however, in the realm of female sexual medicine, it could be beneficial to teach pelvic floor exercises, initiate moisturizer regimens for tissue quality, and advocate lubricant use with sexual activity before the initiation of treatment. Additionally, encouraging patients to seek support from medical professionals to reinforce realistic expectations may help reduce psychological distress post-treatment. Patients need access to high-quality information about the short- and long-term effects of cancer treatment. It is vital that as treatment modalities change, research follows suit.
Early intervention can consist of a comprehensive assessment of current concerns, education, and symptom management. Often in the cancer setting, oncologists are focused on controlling and curing the cancer, while side effects of treatment, including sexuality and intimacy issues, are relegated to other health professionals (e.g., social workers, primary care providers) [53]. In evaluating sexual dysfunction, clinicians should be candid and direct in asking patients if they have any issues or changes with sex and intimacy. Normalizing concerns can be helpful and can be accomplished by reassuring patients that sexual issues after cancer treatment are common experiences. Standardized questionnaires can be used to screen for sexual function and can be given to patients before clinical appointments. The (VAS and VuAS are effective screens for tissue quality changes. The PROMIS measure has a validated 1-item sexual function screener that can be easily administered within a busy clinical setting [54]. The FSFI can help evaluate the specific sexual function concerns of desire, arousal, orgasm, vaginal dryness, and pain. Methods to assess sexual distress are also critical, such as with the Female Sexual Distress Scale (FSDS).
Simple vulvovaginal health promotion strategies and education can help mitigate patient concerns (Table 4); however, complex problems may require intervention with medical/psychological therapies. Available treatments for sexual dysfunction in females are more limited than options for males. Common treatment suggestions include water or silicone-based lubricants during sexual activity, and vaginal moisturizers to alleviate vaginal and vulvar dryness. These products can make sexual intercourse and gynecologic exams more comfortable. Low-dose estrogens (vaginal) can also be used to relieve symptoms. Issues of pelvic floor dysfunction (e.g., painful sex, incontinence) after colorectal/anal cancer treatment are common. Pelvic floor muscle exercises can be helpful to enhance pelvic floor function and control. It is essential for women to learn how to relax the pelvic floor, especially in the setting of a history of pain, in addition to contraction [16, 55]. Pelvic floor dysfunction is common after pelvic surgery, contributing to pain (with exams and sex) and incontinence issues, also seen in our cohort. Pelvic floor physical therapy is an excellent resource to address these issues. It is also important to screen for chronic pad/pantyliner usage, as these may affect vulvar health by increasing vulvar dryness or trapping moisture. The types of pads/pantyliners that can provide optimal vulvovaginal support should be recommended, given that certain products, such as perfumed pads, can aggravate symptoms. Skin/tissue protectant creams, such as Aquaphor®, Balmex® or Desitin®, applied to the vulva can enhance comfort and protect the tissues from the drying and/or trapping effects of pads and/or external influences (urine or feces). Vaginal dilators can help prevent vaginal shortening and maintain vaginal elasticity [7], which can increase confidence and decrease anxiety over vaginal insertion discomfort.
Conclusions
A review of the existing literature shows that more research is needed to explore the sexual health concerns of women treated for colorectal/anal cancer with regard to long-term follow-up, pre-treatment assessments, and interventions. Future trials need to include more women and younger patients. Addressing the sexual health concerns of women with cancer is imperative for maximizing QOL into long-term survivorship. During the shared decision-making process, healthcare providers should prepare their patients to have clear, reasonable expectations from treatment and to be aware of the potential side effects of treatment, including sexual health side effects that may persist into survivorship.
Sexual health is often not addressed in survivorship care. As female survivors of colorectal/anal cancer live longer, it is crucial to address post-treatment function for psychological wellbeing. A better understanding of the concerns of this patient population is pivotal in developing and implementing effective interventions. As healthcare providers, we should be proactive in identifying vulnerable patients and fostering symptom management, educational support, counseling, and referrals.
Acknowledgements
We would like to thank Sally Saban and George Monemvasitis for their careful review and feedback.
Funding: This study was funded in part through the NIH/NCI Memorial Sloan Kettering Cancer Center Support Grant P30 CA008748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors report no conflicts.
References
- [1].American Society of Cancer. Cancer Treatment and Survivorship: Facts and Figures (2016–2017). Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-treatment-and-survivorship-facts-and-figures/cancer-treatment-and-survivorship-facts-and-figures-2016-2017.pdf. Accessed September 21, 2018.
- [2].Simon K Colorectal cancer development and advances in screening. Clin Interv Aging 2016;11:967–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Moscicki AB, Darragh TM, Berry-Lawhorn JM, Roberts JM, Khan MJ, Boardman LA, et al. Screening for Anal Cancer in Women. J Low Genit Tract Dis 2015;19:S27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- [5].Albaugh JA, Tenfelde S, Hayden DM. Sexual Dysfunction and Intimacy for Ostomates. Clin Colon Rectal Surg 2017;30:201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Incrocci L, Jensen PT. Pelvic radiotherapy and sexual function in men and women. J Sex Med 2013;10 Suppl 1:53–64. [DOI] [PubMed] [Google Scholar]
- [7].Haggstrom DA, Cheung WY. Approach to the long-term survivor of colorectal cancer Available from: https://www.uptodate.com/contents/approach-to-the-long-term-survivor-of-colorectal-cancer/print 2018. Accessed September 14, 2018.
- [8].Alberts SR, Citrin D, Schwartz D, Rodriguez-Bigas M. Colon, Rectal, and Anal Cancers Available from: http://www.cancernetwork.com/cancer-management/colon-rectal-and-anal-cancers 2016. Accessed July 14, 2018.
- [9].Bruheim K, Guren MG, Skovlund E, Hjermstad MJ, Dahl O, Frykholm G, et al. Late side effects and quality of life after radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys 2010;76:1005–11. [DOI] [PubMed] [Google Scholar]
- [10].Hendren SK, O’Connor BI, Liu M, Asano T, Cohen Z, Swallow CJ, et al. Prevalence of male and female sexual dysfunction is high following surgery for rectal cancer. Ann Surg 2005;242:212–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Panjari M, Bell RJ, Burney S, Bell S, McMurrick PJ, Davis SR. Sexual function, incontinence, and wellbeing in women after rectal cancer--a review of the evidence. J Sex Med 2012;9:2749–58. [DOI] [PubMed] [Google Scholar]
- [12].Sukhu T, Ross S, Coward RM. Urological survivorship issues among adolescent boys and young men who are cancer survivors. Sex Med Rev 2018;6:396–409. [DOI] [PubMed] [Google Scholar]
- [13].Benedict C, Philip EJ, Baser RE, Carter J, Schuler TA, Jandorf L, et al. Body image and sexual function in women after treatment for anal and rectal cancer. Psychooncology 2016;25:316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Benedict C, Rodriguez VM, Carter J, Temple L, Nelson C, DuHamel K. Investigation of body image as a mediator of the effects of bowel and GI symptoms on psychological distress in female survivors of rectal and anal cancer. Support Care Cancer 2016;24:1795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bohm G, Kirschner-Hermanns R, Decius A, Heussen N, Schumpelick V, Willis S. Anorectal, bladder, and sexual function in females following colorectal surgery for carcinoma. Int J Colorectal Dis 2008;23:893–900. [DOI] [PubMed] [Google Scholar]
- [16].Carter J, Stabile C, Seidel B, Baser RE, Goldfarb S, Goldfrank DJ. Vaginal and sexual health treatment strategies within a female sexual medicine program for cancer patients and survivors. J Cancer Surviv 2017;11:274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Corte H, Lefevre JH, Dehnis N, Shields C, Chaouat M, Tiret E, et al. Female sexual function after abdominoperineal resection for squamous cell carcinoma of the anus and the specific influence of colpectomy and vertical rectus abdominis myocutaneous flap. Colorectal Dis 2011;13:774–8. [DOI] [PubMed] [Google Scholar]
- [18].da Silva GM, Hull T, Roberts PL, Ruiz DE, Wexner SD, Weiss EG, et al. The effect of colorectal surgery in female sexual function, body image, self-esteem and general health: a prospective study. Ann Surg 2008;248:266–72. [DOI] [PubMed] [Google Scholar]
- [19].Das P, Cantor SB, Parker CL, Zampieri JB, Baschnagel A, Eng C, et al. Long-term quality of life after radiotherapy for the treatment of anal cancer. Cancer 2010;116:822–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Den Oudsten BL, Traa MJ, Thong MS, Martijn H, De Hingh IH, Bosscha K, et al. Higher prevalence of sexual dysfunction in colon and rectal cancer survivors compared with the normative population: a population-based study. Eur J Cancer (Oxford, England : 1990) 2012;48:3161–70. [DOI] [PubMed] [Google Scholar]
- [21].Di Fabio F, Koller M, Nascimbeni R, Talarico C, Salerni B. Long-term outcome after colorectal cancer resection. Patients’ self-reported quality of life, sexual dysfunction and surgeons’ awareness of patients’ needs. Tumori 2008;94:30–5. [DOI] [PubMed] [Google Scholar]
- [22].Doeksen A, Gooszen JA, van Duijvendijk P, Tanis PJ, Bakx R, Slors JF, et al. Sexual and urinary functioning after rectal surgery: a prospective comparative study with a median follow-up of 8.5 years. Int J Colorectal Dis 2011;26:1549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].DuHamel K, Schuler T, Nelson C, Philip E, Temple L, Schover L, et al. The sexual health of female rectal and anal cancer survivors: results of a pilot randomized psycho-educational intervention trial. J Cancer Surviv 2016;10:553–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Milbury K, Cohen L, Jenkins R, Skibber JM, Schover LR. The association between psychosocial and medical factors with long-term sexual dysfunction after treatment for colorectal cancer. Support Care Cancer 2013;21:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Philip EJ, Nelson C, Temple L, Carter J, Schover L, Jennings S, et al. Psychological correlates of sexual dysfunction in female rectal and anal cancer survivors: analysis of baseline intervention data. J Sex Med 2013;10:2539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Reese JB, Finan PH, Haythornthwaite JA, Kadan M, Regan KR, Herman JM, et al. Gastrointestinal ostomies and sexual outcomes: a comparison of colorectal cancer patients by ostomy status. Support Care Cancer 2014;22:461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Reese JB, Haythornthwaite JA. Importance of sexuality in colorectal cancer: predictors, changes, and response to an intimacy enhancement intervention. Support Care Cancer 2016;24:4309–17. [DOI] [PubMed] [Google Scholar]
- [28].Reese JB, Porter LS, Somers TJ, Keefe FJ. Pilot feasibility study of a telephone-based couples intervention for physical intimacy and sexual concerns in colorectal cancer. J Sex Marital Ther 2012;38:402–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Reese JB, Shelby RA, Keefe FJ, Porter LS, Abernethy AP. Sexual concerns in cancer patients: a comparison of GI and breast cancer patients. Support Care Cancer 2010;18:1179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Segelman J, Martling A, Machado M, Holm T, Bergmark K, Floter Radestad A. Preoperative sexual function in women with rectal cancer. Eur J Surg Oncol 2013;39:1079–86. [DOI] [PubMed] [Google Scholar]
- [31].Traa MJ, De Vries J, Roukema JA, Den Oudsten BL. The preoperative sexual functioning and quality of sexual life in colorectal cancer: a study among patients and their partners. J Sex Med 2012;9:3247–54. [DOI] [PubMed] [Google Scholar]
- [32].Traa MJ, Orsini RG, Den Oudsten BL, De Vries J, Roukema JA, Bosman SJ, et al. Measuring the health-related quality of life and sexual functioning of patients with rectal cancer: does type of treatment matter? Int J Cancer 2014;134:979–87. [DOI] [PubMed] [Google Scholar]
- [33].Welzel G, Hagele V, Wenz F, Mai SK. Quality of life outcomes in patients with anal cancer after combined radiochemotherapy. Strahlenther Onkol 2011;187:175–82. [DOI] [PubMed] [Google Scholar]
- [34].Yu-Hua L Sexual dysfunction in women after low anterior resection. Clin Nurs Res 2014;23:216–26. [DOI] [PubMed] [Google Scholar]
- [35].Zutshi M, Hull T, Shedda S, Lavery I, Hammel J. Gender differences in mortality, quality of life and function after restorative procedures for rectal cancer. Colorectal Dis 2013;15:66–73. [DOI] [PubMed] [Google Scholar]
- [36].Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76. [DOI] [PubMed] [Google Scholar]
- [37].Sprangers MA, te Velde A, Aaronson NK. The construction and testing of the EORTC colorectal cancer-specific quality of life questionnaire module (QLQ-CR38). European Organization for Research and Treatment of Cancer Study Group on Quality of Life. Eur J Cancer (Oxford, England : 1990) 1999;35:238–47. [DOI] [PubMed] [Google Scholar]
- [38].Baser RE, Li Y, Carter J. Psychometric validation of the Female Sexual Function Index (FSFI) in cancer survivors. Cancer 2012;118:4606–18. [DOI] [PubMed] [Google Scholar]
- [39].Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther 2000;26:191–208. [DOI] [PubMed] [Google Scholar]
- [40].Hawkins Y, Ussher J, Gilbert E, Perz J, Sandoval M, Sundquist K. Changes in sexuality and intimacy after the diagnosis and treatment of cancer: the experience of partners in a sexual relationship with a person with cancer. Cancer Nurs 2009;32:271–80. [DOI] [PubMed] [Google Scholar]
- [41].Bentzen AG, Balteskard L, Wanderas EH, Frykholm G, Wilsgaard T, Dahl O, et al. Impaired health-related quality of life after chemoradiotherapy for anal cancer: late effects in a national cohort of 128 survivors. Acta Oncol (Stockholm, Sweden) 2013;52:736–44. [DOI] [PubMed] [Google Scholar]
- [42].Donovan KA, Thompson LM, Hoffe SE. Sexual function in colorectal cancer survivors. Cancer Control 2010;17:44–51. [DOI] [PubMed] [Google Scholar]
- [43].Reese JB, Handorf E, Haythornthwaite JA. Sexual quality of life, body image distress, and psychosocial outcomes in colorectal cancer: a longitudinal study. Support Care Cancer 2018. [DOI] [PMC free article] [PubMed]
- [44].Temple LK, Wong WD, Minsky B. The impact of radiation on functional outcomes in patients with rectal cancer and sphincter preservation. Semin Radiat Oncol 2003;13:469–77. [DOI] [PubMed] [Google Scholar]
- [45].Brotto LA, Yule M, Breckon E. Psychological interventions for the sexual sequelae of cancer: a review of the literature. J Cancer Surviv 2010;4:346–60. [DOI] [PubMed] [Google Scholar]
- [46].Stabile C, Goldfarb S, Baser RE, Goldfrank DJ, Abu-Rustum NR, Dickler MN, et al. Sexual needs and educational intervention preferences for women with cancer. Breast Cancer Res Treat 2017;165:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Manderson L Boundary breaches: the body, sex and sexuality after stoma surgery. Soc Sci Med (1982) 2005;61:405–15. [DOI] [PubMed] [Google Scholar]
- [48].Park J, Neuman HB, Bennett AV, Polskin L, Phang PT, Wong WD, et al. Patient expectations of functional outcomes after rectal cancer surgery: a qualitative study. Diseases of the colon and rectum 2014;57:151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Silva NM, Santos MAD, Rosado SR, Galvao CM, Sonobe HM. Psychological aspects of patients with intestinal stoma: integrative review. Rev Lat Am Enfermagem 2017;25:e2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].McMullen C, Liu L, Bulkley JE, Hornbrook MC, Wendel C, Grant M, et al. Participation in Activities Associated With Quality of Life for Long-Term Survivors of Rectal Cancer. Perm J 2017;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Haviland J, Sodergren S, Calman L, Corner J, Din A, Fenlon D, et al. Social support following diagnosis and treatment for colorectal cancer and associations with health-related quality of life: Results from the UK ColoREctal Wellbeing (CREW) cohort study. Psychooncology 2017;26:2276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Breukink SO, Wouda JC, Van Der Werf-Eldering MJ, Van De Wiel HBM, Bouma EMC, Pierie JP, et al. Psychophysiological assessment of sexual function in women after radiotherapy and total mesorectal excision for rectal cancer: a pilot study on four patients. J Sex Med 2009;6:1045–53. [DOI] [PubMed] [Google Scholar]
- [53].Falk SJ, Dizon DS. Sexual dysfunction in women with cancer. Fertil Steril 2013;100:916–21. [DOI] [PubMed] [Google Scholar]
- [54].Flynn KE, Lindau ST, Lin L, Reese JB, Jeffery DD, Carter J, Baron SR, Abramsohn E, Weinfurt KP. Development and Validation of a Single-Item Screener for Self-Reporting Sexual Problems in US Adults. J Gen Intern Med 2015. April 18 [DOI] [PMC free article] [PubMed]
- [55].Faubion SS, Shuster LT, Bharucha AE. Recognition and management of nonrelaxing pelvic floor dysfunction. Mayo Clin Proc 2012;87:187–93. [DOI] [PMC free article] [PubMed] [Google Scholar]