Abstract
Human cytomegalovirus (CMV) infection and disease remains a significant cause of morbidity and mortality for hematopoietic cell transplantation (HCT) recipients. Disruption of or weak reconstitution of virus-specific cellular immune function, such as with certain HCT approaches, poses significant risk for CMV-related complications. The incidence of and risk factors for CMV infection and the nature of CMV disease were evaluated retrospectively among 356 consecutive HCT recipients transplanted at the National Institutes of Health using all graft sources, including bone marrow, peripheral blood stem cell (PBSC), and umbilical cord blood (UCB), and a range of in vivo and ex vivo approaches for graft-versus-host disease (GVHD) prophylaxis. The cumulative incidence of CMV infection was higher for CMV-seropositive recipients at 33%, regardless of donor CMV serostatus. Patients transplanted with CMV-seropositive donors had a significantly shorter duration of antiviral therapy. Among graft sources, UCB was associated with the highest cumulative incidence of CMV infection at 65%, as well as significantly longer treatment duration with median of 36 days, while PBSC HCT was associated with the lowest incidence at 26% and the shortest CMV treatment duration with median of 21 days. There were significant differences in the cumulative incidence of CMV infection by T-cell manipulation strategy when systemic steroids were included as a risk-modifying event. Over a third of CMV infections occurred in the setting of systemic steroid administration. CMV disease occurred in 5% of HCT recipeints, with 70% of cases in the setting of treatment for GVHD. Although factors related to serostatus, graft source, and GVHD prophylaxis were associated with varied CMV infection incidence, unplanned post-HCT corticosteroid therapy contributed greatly to the incidence of both CMV infection and disease across HCT approaches, highlighting this post-HCT intervention as a key time to potentially tailor the approach to monitoring, pre-emptive therapy, and even prophylaxis.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a potentially curative therapy for a variety of benign and malignant hematologic and immunologic conditions. Despite diagnostic and therapeutic advances in the management of post-HCT opportunistic infections, human cytomegalovirus (CMV) remains associated with significant morbidity, organ toxicity, and transplant-related mortality.1–3 Uncontrolled CMV infection can lead to pneumonitis or enterocolitis, and more rarely hepatitis, retinitis, and encephalitis. CMV infection increases the risk of secondary bacterial and fungal infections,4,5 while antiviral therapies incur morbidity from myelotoxicity (ganciclovir) or nephrotoxicity (foscarnet). In addition CMV infection increases the risks of graft failure and graft-versus-host disease (GVHD), while treating CMV infection in the presence of poor graft function or in conjunction with immunosuppressive agents (especially steroids) for GVHD increases the risk from infections.4,6,7 Finally the treatment of CMV-associated complications post-HCT is associated with longer hospital length-of-stay, and CMV therapy carries a substantial financial burden.8,9
Although letermovir is now approved for CMV prophylaxis in all CMV-seropositive recipients in the first three months post-HCT, the benefit is seen mainly in recipients at high risk for CMV disease. Administering letermovir to all CMV-seropositive recipients is expensive, increases pill burden, and the chance of drug interactions. Therefore, determining which HCT recipients are most appropriate for letermovir is an important question.10 Furthermore, identifying recipients who need monitoring for CMV infection beyond day +100 post-HCT and require extended prophylaxis requires a better understanding of pre- and post-HCT factors that affect the risk from CMV.
Post-HCT CMV infection risk increases at times when cellular immunity is hindered or compromised, such as with GVHD or post-HCT relapse and their treatments, or in the setting of graft failure or poor immune reconstitution.11 This risk period varies with the HCT regimen and with the pace of immune reconstitution and post-HCT events such as GVHD and its treatment. Thus while grafts depleted of donor T cells may carry an increased risk of viral infection they incur less risk of GVHD and treatment with systemic steroids which particularly impair immune reconstitution.
At the National Institutes of Health (NIH), the HCT platforms employed within the Clinical Center vary widely, including umbilical cord blood (UCB) HCT, ex vivo T-cell depletion (TCD), in vivo T-cell depletion with proximal serotherapy, calcineurin-inhibitor (CNI) or mammalian target of rapamycin inhibitor (mTORi)-based pharmacologic GVHD prophylaxis, and in vivo immunomodulation with high-dose, post-transplantation cyclophosphamide (PTCy). Despite this diversity, all NIH Clinical Center HCT recipients are uniformly monitored for CMV in the blood using quantitative polymerase chain reaction (qPCR) performed in a central clinical laboratory and patients with CMV infection or disease are treated under NIH-wide HCT guidelines. This unique combination of diverse HCT approaches within a single center that applies a uniform approach to CMV monitoring and treatment offers an opportunity to comprehensively evaluate the cumulative incidence (CInc) of and risk factors for post-HCT CMV infection and disease across different HCT regimens and across all graft sources. Additionally, this framework allowed the evaluation of the impact of systemic steroids after HCT on the CI of CMV infection, accounting for differences in the incidence of this risk-modifying event within individual HCT platforms.
Patients and Methods
Clinical Record Review
After Institutional Review Board approval, we retrospectively evaluated the incidence and risk factors for CMV infection among patients undergoing allogeneic HCT at NIH between August 10, 2011 and February 11, 2017. All patients transplanted at the NIH were enrolled on prospective clinical trials and enrollment lists from all HCT trials actively accruing during the study interval were used to generate a list of all HCT recipients. The Biomedical Translational Research Information System and the Clinical Research Information System were queried for HCT and CMV data. Of 390 consecutive HCT recipients transplanted during the study period, recipients were included if the following criteria were met: first allogeneic HCT, clinical follow-up of survivors through 1 year post-HCT, and at least 64% of weekly CMV qPCRs, equating to at least 9 of 14 weekly specimens for survivors through day +100, or fewer specimens but meeting at least the 64% threshold for those with death before day +100. Recipients on single-patient HCT protocols, an HCT protocol that included prophylactic post-HCT virus-specific cytotoxic T-cell therapy, and those who died before receipt of donor cells on day 0 were excluded.
All HCT recipients at NIH remained under direct local NIH care for at least 100 days post-HCT. The approaches to monitoring, pre-emptive therapy, and treatment of CMV infection and disease are uniform across HCT protocols, as formalized by NIH Bone Marrow Transplant Consortium Infectious Disease guidelines. All recipients were monitored at least weekly with blood CMV qPCR through day +100 post-HCT, with twice weekly monitoring for UCB HCT recipients, ex vivo TCD HCT, and haplo recipients with hemoglobinopathy/thalassemia. Standard blood components for HCT recipients were leukoreduced to reduce the risk of CMV transmission but CMV-seronegative recipients with CMV-seronegative HCT donors were not exclusively given blood components from CMV-seronegative donors.
Recipients were considered to be at-risk for CMV infection if either donor and/or recipient were CMV-seropositive. For recipients of dual HLA-haploidentical (haplo) CD34+-selected peripheral blood stem cell (PBSC) and UCB (haplo-UCB) grafts, recipients were considered at-risk for CMV infection only if the recipient was CMV-seropositive.
UCB HCT were classified to include all HCTs using UCB as a graft source and included haplo-UCB since the engrafting component of the haplo-UCB almost always comes from the UCB component.
T-cell manipulation strategies to prevent GVHD were grouped into four strategies. The approaches were PTCy, proximal serotherapy (alemtuzumab starting day −12 or more proximal to day 0 or antithymocyte globulin days −5 through −2), ex vivo TCD, and CNI/mTORi-based. HCTs using both PTCy and proximal serotherapy were designated as serotherapy transplants, as serotherapy so proximal to the graft infusion was considered to have the dominant effect on graft immunomodulation. Within the serotherapy group, the approach to HCT conditioning was distinct for recipients with hemoglobinopathy and thalassemia (minimal intensity conditioning with serotherapy and radiation) as compared to recipients with primary immunodeficiency disease (PID) or malignancy who received a mostly myeloablative chemotherapy-based conditioning. Thus, the serotherapy group was further subdivided by recipient diagnosis (hemoglobinopathy/thalassemia versus other) for some exploratory analyses.
Definitions of Endpoints
CMV infection (synonymous with CMV reactivation) was defined as any of the following: 1) two qPCR values between 3.08–4.11 log10 IU/mL separated by one week, 2) one qPCR value > 4.11 log10 IU/mL, or 3) sufficient clinical suspicion for CMV disease to prompt therapy, regardless of CMV qPCR level. Before October 10, 2014, CMV qPCR was reported as log10 copies/mL (minimum level of detection <2.40 log10 copies/mL, equivalent to <250 copies/mL); as of October 10, 2014, CMV qPCR was reported as log10 IU/mL (same minimum level of detection, reported as <3.08 log10 IU/mL). For results prior to this change, values were converted to log10 IU/mL by adding 1.1 to the log10 copies/mL value. The CMV qPCR is a laboratory developed assay designed and validated by the Molecular Lab of the NIH Department of Laboratory Medicine. Recurrent infection was defined according to a published definition as a subsequent episode of CMV infection meeting the above criteria, after prior post-HCT CMV infection followed by at least a 4-week period of no virus detection on weekly monitoring.6 Recipients were designated as having CMV disease if the treating HCT team at the time documented such a diagnosis in the chart, or based on autopsy findings. CMV infection data were captured through day +100 post-HCT and disease data were captured through 1 year post-HCT, as it was presumed that the occurrence of CMV disease in the first year post-HCT would be known to the HCT team, even if diagnosed and treated at another hospital. Treatment of CMV was defined as the administration of ganciclovir, valganciclovir, foscarnet, or cidofovir for CMV infection or disease.
Competing risks (CRs) for CMV infection included death from any cause, as well as the risk-modifying events of graft failure, malignancy relapse requiring systemic therapy, second HCT, donor lymphocyte infusion, and systemic steroid use. Systemic steroid use was chosen as a CR given that it is an objective event that would be expected to modulate CMV infection risk and was an event that captured all patients with GVHD requiring first-line therapy, as well as the use of steroids for other post-HCT complications. Graft failure was defined as the absence of hematopoietic recovery post-HCT or, in the setting of autologous recovery or secondary graft failure, myeloid chimerism <5% donor cells. Malignancy relapse was defined as post-HCT recurrence or progression of a hematologic malignancy requiring systemic therapy. Systemic steroid use was defined as the post-HCT administration of ≥0.5 mg/kg/day of prednisone or prednisone equivalent, excluding hydrocortisone, based on patient weight upon starting steroid therapy.
Data were locked for analysis on March 1, 2018.
Statistical Analyses
Descriptive statistics were used for HCT characteristics and the timing and duration of CMV therapy. Comparisons of HCT characteristics across sub-groups were performed using the Chi-squared test for categorical variables and the Kruskall-Wallis test for continuous variables.
100-day post-HCT CMV-free survival was defined as survival of recipients who did not have CMV infection or death occur within 100 days post-HCT, with CMV-infection-free survivors censored at 100-days post-HCT. CMV infection-free, steroid-free, graft failure-free survival was defined as survival of recipients who did not have CMV infection, graft failure, or systemic steroid use within the 100-days post-HCT, with event-free survivors censored at 100-days post-HCT. The corresponding survival curves were constructed using the Kaplan-Meier method and compared using the log-rank p-value.
CInc curves of CMV infection were constructed using the method of Fine and Gray and compared using K-sample tests.12,13 Death, malignancy relapse requiring systemic therapy, graft failure, second HCT, and donor lymphocyte infusion were considered CRs in every analysis. A second set of CInc curves were constructed including systemic steroid use as an additional CR to account for the risk modification of increased systemic immunosuppression, most often in the setting of acute GVHD.
Survival curves were generated using GraphPad Prism, version 7.01 (GraphPad Software, La Jolla, California, USA, www.graphpad.com). CInc curves were generated using R program, version 3.3.3 (R Core Development Team, Vienna, Austria).
Multiple logistic regression analyses to evaluate risk factors for CMV infection were performed. Univariate associations between a set of parameters and CMV infection were initially determined prior to performing multiple logistic regression analyses. Following the initial univariate screening analyses, multiple logistic regression analyses were used to determine the association between HCT characteristics and CMV infection. These analyses were performed using SAS 9.3 (SAS Institute, Cary NC).
For all the above, non-CInc analyses, results were considered statistically significant if two-tailed p-value was <0.05. For the CInc analyses, because of the exploratory nature of some of the analyses and the varying degrees of independence of the tests performed, no formal adjustment for multiple tests was performed and p-values are presented without any correction. However, in view of the number of tests performed, in order to provide proper interpretation, the p-values are interpreted such that p<0.01 would be considered statistically significant while if the p-value is between 0.01 and 0.05, then the result would be considered to be associated with a trend toward statistical significance.
Results
Recipient, Donor, and HCT Characteristics
Of 390 HCT recipients transplanted during the study period, 356 (91%) met inclusion criteria. Reasons for exclusion included <64% of CMV qPCRs despite survival through 100-days post-HCT (n=14), second HCT (n=8), survivor with less than 1 year follow-up (n=8), death prior to day 0 (n=2), single-patient protocol (n=1), and receipt of prophylactic virus-specific cytotoxic T cells (n=1). Of the 356, 263 (74%) were at-risk for post-HCT CMV infection, with no significant difference among T-cell manipulation strategies.
The recipient, donor, and HCT characteristics for the 356 HCTs are shown in Table 1, grouped by T-cell manipulation strategy. There were significant differences among groups with respect to these characteristics, as expected given that different T-cell manipulation strategies at NIH were employed in the setting of clinical trials, which were largely disease- and HCT approach-specific.
Table 1.
Recipient, donor, and transplant characteristics.
| Patients (n=356) | CNI/mTORi-based (n=145) | Serotherapy (n=82) | PTCy-based (n=58) | TCD (n=46) | UCB (n=25) | p-value | |
|---|---|---|---|---|---|---|---|
| Male, n (%) | 215 (60%) | 82 (56%) | 61 (74%) | 28 (49%) | 29 (63%) | 15 (60%) | p=0.02 |
| Age at HCT, median (range) | 31 (3–71) | 38.5 (8–71) | 28 (4–68) | 22 (6–66) | 30 (6–70) | 20 (3–52) | p<0.0001 |
| Diagnosis, n (%) | p<0.0001 | ||||||
| Malignancy | 178 (50%) | 112 (77%) | 9 (11%) | 10 (17%) | 44 (96%) | 2 (8%) | |
| Primary Immunodeficiency | 97 (27%) | 20 (14%) | 29 (35%) | 48 (83%) | 0 | 1 (4%) | |
| Hemoglobinopathy/Thalassemia | 44 (12%) | 0 | 44 (54%) | 0 | 0 | 0 | |
| Aplastic Anemia | 37 (10%) | 13 (9%) | 0 | 0 | 2 (4%) | 22 (88%) | |
| Intensity of conditioning, n (%) | p<0.0001 | ||||||
| NMA/RIC | 196 (55%) | 92 (63%) | 53 (65%) | 15 (26%) | 12 (26%) | 24 (96%) | |
| Myeloablative | 160 (45%) | 53 (37%) | 29 (35%) | 43 (74%) | 34 (74%) | 1 (4%) | |
| Graft source, n (%) | p<0.001 | ||||||
| PBSC | 264 (74%) | 118 (81%) | 80 (98%) | 20 (35%) | 46 (100%) | 0 | |
| Bone Marrow | 67 (19%) | 27 (19%) | 2 (2%) | 38 (65%) | 0 | 0 | |
| UCB or CD34+ selected haplo+UCB | 25 (7%) | 0 | 0 | 0 | 0 | 25 (100%) | |
| Donor type, n (%) | p<0.0001 | ||||||
| HLA-matched related | 183 (51%) | 107 (74%) | 33 (40%) | 6 (10%) | 38 (83%) | 0 | |
| HLA-matched unrelated | 84 (24%) | 33 (23%) | 32 (39%) | 12 (21%) | 7 (15%) | 0 | |
| HLA-haploidentical | 56 (16%) | 0 | 15 (18%) | 40 (69%) | 0 | 0 | |
| UCB or CD34+ selected haplo+UCB | 25 (7%) | 0 | 0 | 0 | 0 | 25 (100%) | |
| HLA-mismatched unrelated | 8 (2%) | 5 (3%) | 2 (2%) | 0 | 1 (2%) | 0 | |
| Donor age, median (range)a | 32 (4–71) | 38 (5–71) | 30 (4–67) | 34 (5–65) | 30 (12–69) | n/a | p=0.02 |
| Female --> male HCT, n (%) | 89 (25%) | 32 (22%) | 29 (35%) | 8 (14%) | 11 (24%) | 9 (36%) | p=0.03 |
| At-risk for post-HCT CMV infectionb | 263 (74%) | 118 (81%) | 55 (67%) | 43 (74%) | 30 (65%) | 17 (68%) | NS |
| D/R CMV serostatus, n (%)c | p<0.001 | ||||||
| +/+ | 161 (45%) | 78 (54%) | 33 (40%) | 27 (47%) | 22 (48%) | 0 | |
| −/− | 88 (24%) | 27 (18%) | 26 (32%) | 12 (21%) | 15 (33%) | 8 (32%) | |
| −/+ | 67 (19%) | 23 (16%) | 14 (17%) | 7 (12%) | 6 (13%) | 17 (68%) | |
| +/− | 31 (9%) | 17 (12%) | 8 (10%) | 5 (9%) | 1 (2%) | 0 | |
| T-cell dose x 107/kg, median (IQR)d | 10.5 (2.0–22.7) | 12.1 (3.9–19.3) | 31.5 (21.9–38.8) | 6.17 (3.8–20) | 0.09 (0.06–0.13) | 0.74 (0.5–1.1) | p<0.0001 |
Abbreviations: CNI, calcineurin inhibitor; mTORi, mammalian target of rapamycin inhibitor; PTCy, post-transplantation cyclophosphamide; HCT, hematopoietic cell transplantation; NMA, non-myeloablative; RIC, reduced intensity conditioning; PBSC, peripheral blood stem cells; UCB, umbilical cord blood; HLA, human leukocyte antigen; D/R, donor/recipient; CMV, cytomegalovirus; IQR, interquartile range; TCD, ex vivo T-cell depletion
Excluding UCB and haplo-UCB since engrafting cells are UCB
Recipients were considered at-risk for CMV infection if either donor or recipient were CMV-seropositive, including the haplo donor of haplo-UCB HCTs.
D/R unknown for 10 (3%) recipients; for HCT using haplo+UCB donors, UCB was presumed CMV-seronegative and haplo donor CMV serostatus was used to determine at-risk designations for recipients, as it was presumed that a CMV-seropositive CD34+ selected haplo product could transmit CMV to the recipient. However, haplo donor serostatus was not used to designate donor CMV serostatus for use as a variable for CMV infection risk analyses, as the T-cell immunity was provided by the UCB donor.
T-cell dose unknown in 18 (5%) recipients
Survival and Freedom from CMV Infection
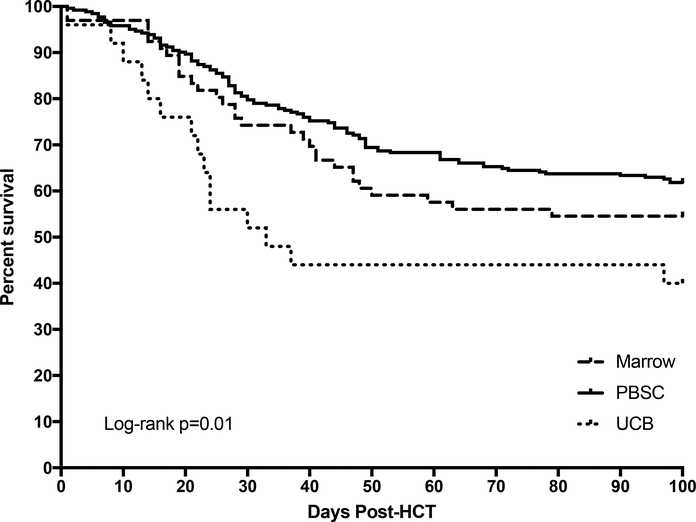
Survival point estimates are shown in Table 2. There was no difference in 1-year OS by D/R CMV serostatus, graft source, or T-cell manipulation strategy. Among graft sources, UCB was associated with lower CMV-infection-free survival as compared with marrow and PBSC grafts, p=0.01, Figure 1. There were no differences in CMV infection-free, steroid-free, graft failure-free survival estimates by graft source or T-cell manipulation strategy.
Table 2.
Kaplan-Meier survival curve point estimates.
| 1 year Overall Survival | p-value | Day +100 CMV-free survival | p-value | Day +100 CMV-free, steroid-free, GF-free survival | p-value | |
|---|---|---|---|---|---|---|
| D/R Serostatus | NS | <0.0001 | <0.0001 | |||
| −/− | 86% (77–92%) | 93% (85–97%) | 51% (40–61%) | |||
| −/+ | 76% (64–85%) | 39% (28–51%) | 27% (17–38%) | |||
| +/− | 84% (65–93%) | 84% (66–93%) | 55% (36–70%) | |||
| +/+ | 78% (71–84%) | 41% (33–49%) | 25% (19–32%) | |||
| Graft Source | NS | 0.01 | 0.08 | |||
| Marrow | 83% (72–90%) | 55% (42–66%) | 35% (24–46%) | |||
| PBSC | 79% (74–84%) | 62% (56–67%) | 37% (31–43%) | |||
| UCB | 84% (63–94%) | 40% (21–58%) | 20% (7–37%) | |||
| T-cell Manipulation | NS | 0.08 | NS | |||
| PTCy-based | 84% (72–91%) | 57% (43–69%) | 39% (27–52%) | |||
| Serotherapy | 91% (83–96%) | 67% (56–76%) | 41% (31–52%) | |||
| TCD | 87% (73–94%) | 60% (44–73%) | 29% (17–42%) | |||
| CNI/mTORi-based | 78% (70–84%) | 58% (44–65%) | 35% (28–43%) |
Abbreviations: CMV, cytomegalovirus; GF, graft failure; D/R, donor/recipient; PBSC, peripheral blood stem cell; UCB, umbilical cord blood; PTCy, post-transplantation cyclophosphamide; TCD, ex vivo T-cell depletion; CNI, calcineurin inhibitor; mTORi, mammalian target of rapamycin inhibitor
Figure 1.
Kaplan-Meier curve of CMV infection-free survival through day +100 post-HCT by graft source.
Impact of CMV Serostatus and Graft Dose on CMV Infection
The 100-day CInc of CMV infection differed by donor and recipient CMV serostatus, p<0.001, with CMV infection CInc the highest for CMV-seropositive recipients, regardless of donor serostatus, Table 3. When evaluating the impact of T-cell graft dose, not accounting for the effect of the various in vivo T-cell manipulation strategies, there was no difference in the 100-day CInc of CMV infection among at-risk recipients (n=263) who received 1–9.9 × 108 CD3 cells/kg compared with those who received one, two, or > two log fewer T cells/kg, although the 100-day CInc of CMV infection trended upward with each grouped decrease in T-cell graft dose, Table 3.
Table 3.
Cumulative incidence of CMV infection and competing-risk events.
| 100-day CInc of CMV infection, including steroids as CR | p-value | 100-day CInc of CMV infection, excluding steroids as CR | p-value | |
|---|---|---|---|---|
| Entire At-risk Cohort | 30% (24–35%) | 47% (40–52%) | ||
| D/R Serostatus | <0.0001 | <0.0001 | ||
| −/− | 2% (0–7%) | 4% (0–9%) | ||
| −/+ | 33% (22–44%) | 52% (40–63%) | ||
| +/− | 9% (2–21%) | 12% (4–25%) | ||
| +/+ | 33% (26–40%) | 53% (45–60%) | ||
| Graft Source | p=0.001 | p=0.0001 | ||
| Marrow | 33% (20–46%) | 54% (39–67%) | ||
| PBSC | 26% (20–33%) | 43% (36–49%) | ||
| UCB | 65% (34–84%) | 82% (51–95%) | ||
| T-cell Manipulation Strategy | 0.007 | NS | ||
| PTCy-based | 26% (14–40%) | 49% (32–63%) | ||
| Serotherapy | 36% (24–49%) | 44% (30–56%) | ||
| TCD | 47% (28–64%) | 60% (40–75%) | ||
| CNI/mTORi-based | 19% (13–27%) | 40% (31–49%) | ||
| Graft T-cell Dose | NS | NS | ||
| <1 × 106 CD3/kg | 41% (23–57%) | 56% (37–72%) | ||
| 1–9.9 × 106 CD3/kg | 33% (11–58%) | 53% (25–75%) | ||
| 1–9.9 × 107 CD3/kg | 33% (23–43%) | 52% (40–62%) | ||
| 1–9.9 × 108 CD3/kg | 24% (17–31%) | 40% (32–49%) |
Abbreviations: CMV, cytomegalovirus; D/R, donor/recipient; PBSC, peripheral blood stem cell; UCB, umbilical cord blood; PTCy, post-transplantation cyclophosphamide; TCD, ex vivo T-cell depletion; CNI, calcineurin inhibitor; mTORi, mammalian target of rapamycin inhibitor; CInc, cumulative incidence; CR, competing risk
Impact of Systemic Steroids and HCT Approach on CMV Infection
The CInc of CMV infection, with and without steroids as a CR, are shown in Table 3. For the at-risk cohort, the estimated 100-day CInc of CMV infection was 47% (95% CI 40–52%) without steroids as a CR and 30% (95% CI 24–35%) with steroids as a competing risk, demonstrating that many CMV infections occurred after the initiation of systemic steroid therapy. Of the 124 recipients with CMV infection in the first 100-day post-HCT, 45 (36%) recipients developed CMV infection after initiation of steroids. UCB grafts were associated with the highest CInc of CMV infection among graft sources, Figure 2. By T-cell manipulation strategy, there were significant differences between groups with regard to the estimated 100-day CInc of CMV infection, but only when including steroids as a CR, highlighting the significant impact of steroids in changing the CMV risk associated with specific HCT approaches, Figure 2.
Figure 2. Cumulative incidence of CMV infection, not including (A,C,E) and including (B,D,F) systemic steroids as a competing risk.
A and B: 100-day cumulative incidence of CMV infection by donor/recipient serostatus. C and D: 100-day cumulative incidence of CMV infection by graft source. E and F: 100-day cumulative incidence of CMV infection by T-cell manipulation strategy.
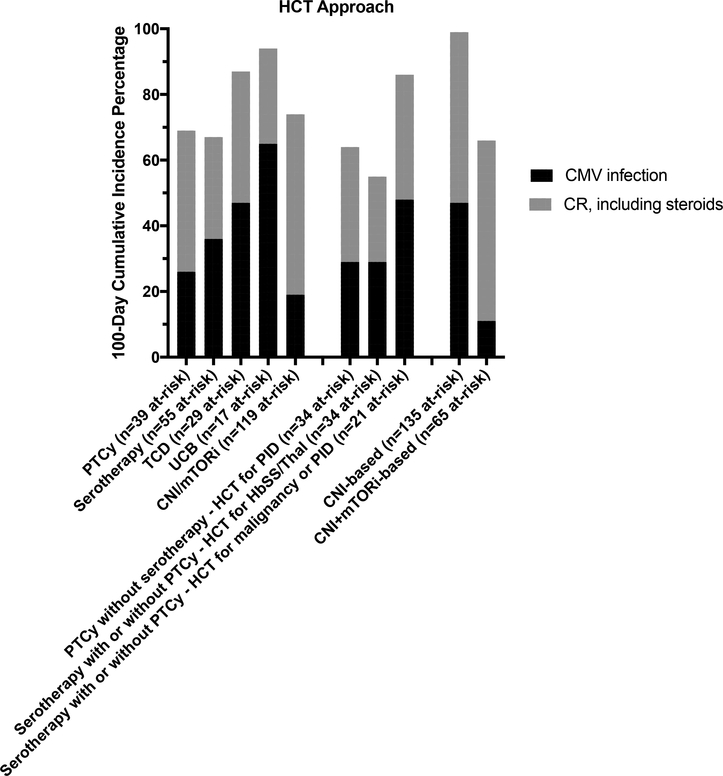
Figure 3 shows the CInc estimates of CMV infection and of CRs, including steroids, by T-cell manipulation strategy to demonstrate the overall rates of experiencing CMV infection or a CR event across HCT approaches. As PTCy-based and serotherapy-based approaches were associated with some of the more moderate rates of both CMV infection and CRs, but the HCT approaches varied by HCT indication/disease, these sub-groups are also shown in Figure 3. The CInc of CMV infection was further explored comparing HCT using PTCy without serotherapy for recipients with PID (PTCy-alone, n=34 at-risk), HCT using serotherapy with or without PTCy for recipients with hemoglobinopathy/thalassemia (serotherapy-based, n=34 at-risk), and HCT using serotherapy with or without PTCy for PID or malignancy (n=21 at-risk). Including steroids as a CR, there were no significant difference in 100-day CInc of CMV infection, estimated at 29% (95% CI 15–45%) for recipients of serotherapy-based HCT for hemoglobinopathy/thalassemia, 29% (95% CI 15–45%) for recipients of PTCy-alone HCT for PID, and 48% (95% CI 25–67%) for recipients of serotherapy with or without PTCy for PID or malignancy. Among pharmacologic, non-PTCy-based and non-serotherapy-based approaches to GVHD prophylaxis, the CInc of CMV infection between CNI-based (n=57) versus CNI+mTORi-based (n=61) approaches were compared among at-risk recipients. The estimated 100-day CInc of CMV infection was significantly higher in the CNI-based group as compared with the CNI+mTORi-based group, both with (32% vs. 8%, p = 0.001) and without (60% vs 21%, p<0.001) steroids as a CR. The incidence of CMV infection and CRs for CNI-based approaches and CNI+mTORi-based approaches are also shown in Figure 3.
Figure 3. 100-day cumulative incidence of CMV infection and competing risks, including steroids, by HCT approach, shown as a percentage where the remainder to reach 100% are event-free through day +100.
Sub-groups of the serotherapy-based and PTCy-based approaches, by recipient diagnosis, and sub-groups of CNI-based and CNI+mTORi-based pharmacologic approaches to GVHD prophylaxis are also shown.
Risk Factors for CMV Infection and Impact of HLA-Mismatch
Based on univariate analyses of factors predicting CMV infection, recipient CMV serostatus, donor/recipient CMV serostatus, T-cell dose of the graft as a continuous variable, graft source, and transplant indication (recipient disease) were included in the multiple logistic regression model, while conditioning intensity, T-cell manipulation strategy, and recipient age were parameters not carried forward to the multiple logistric regression model. Across all approaches, this resulted in a classification rule that ultimately included recipient CMV serostatus alone to successfully predict 94.1% of recipients who developed CMV infection by day +100. However, this rule only predicted 52.1% of recipients who did not develop CMV infection by day +100.
Given that HLA-mismatching has been associated with increased risk of CMV infection and that a recipient may often have multiple haplo donor options of different CMV serostatus,14,15 we specifically evaluated the CInc of CMV infection among PTCy-treated HCT recipients, as recipients of haplo grafts (excluding haplo-UCB) all received PTCy. Among PTCy-treated recipients, an identical multiple logistic regression analysis was run. The only parameters carried forward to the multiple logistic regression model were recipient CMV serostatus and donor/recipient CMV serostatus. The model reduced down to just using donor/recipient CMV serostatus to result in classification rule where, in contrast to the findings across all approaches, both donor and recipient CMV serostatus were necessary to include to optimally predict 85.7% of recipients who developed CMV infection by day +100 and 68.3% of those who did not develop CMV infection by day +100. For exploratory purposes given the multiple logistic regression model findings, we evaluated the impact of donor/recipient CMV serostatus on the CInc of CMV infection among at-risk recipients of haplo grafts. Including steroids as a CR, CMV infection only occurred among CMV seropositive recipients with CMV seropositive donors, with a 100-day CInc of CMV infection of 45%, compared to 0% in both donor/recipient +/− and −/+ groups, p=0.03. We also evaluated the impact of HLA-match on the CInc of CMV infection for PTCy-treated recipients, comparing at-risk recipients of HLA-matched related or unrelated grafts (n=13) to at-risk recipients of haplo grafts (n=46). There was no difference in the CInc of CMV infection or the CInc of CRs, with and without steroids as a CR, for haplo graft recipients as compared to matched graft recipients, with 100-day CInc of CMV infection of 38% versus 47%, respectively, without steroids as a CR and 15% versus 30%, respectively, with steroids as a CR.
Timing and Duration of CMV Infection
By T-cell manipulation strategy, there was no difference among groups in the timing of CMV infection post-HCT or the duration of therapy for infection, where the median day post-HCT of CMV infection ranged from day +21 for PTCy, day +21 for serotherapy, day +23 for UCB, day +25 for TCD, and day +35 for CNI/mTORi-based approaches.
Treatment duration was longer for recipients of UCB grafts at a median of 36 days, as compared with 27 days for marrow and 21 days for PBSCs, p=0.03. Treatment duration was longer for recipients of grafts from CMV-seronegative donors with a median duration of 30 treatment days, as compared with recipients of grafts from CMV-seropositive donors with a median duration of 21 treatment days, p=0.02. When non-UCB, CMV-seronegative grafts were considered as a separate group and compared with UCB grafts and CMV-seropositive grafts, the treatment duration was different between groups with median of 21 days with CMV-seropositive grafts, 29 days for non-UCB, CMV-seronegative grafts, and 36 days for UCB grafts, p=0.04. The timing of CMV infection requiring treatment post-HCT did not differ by donor serostatus or whether the seronegative donor was UCB.
At-risk recipients of HCT for hemoglobinopathy/thalassemia received CMV treatment significantly less frequently as compared to recipients of HCT for malignancy, PIDs, or aplastic anemia, χ2 p=0.0005. Among recipients of serotherapy-based HCT, those transplanted for a diagnosis fo hemoglobinopathy/thalassemia had a shorter treatment duration (median 16 days) as compared to those transplanted for malignancy or PID (median 31 days), p=0.008.
Antiviral resistance mutation testing was performed in 9 HCT recipients. In 4 recipients with CMV infection, no resistance mutations were found. In 5 recipients, all with CMV disease, resistance mutations were found (Table 4).
Table 4.
Recipient, transplant, and clinical characteristics of cases of CMV disease
| Days Post HCT of Diagnosis | Graft | HCT Indication (Disease) | HCT Approach | Organ | D/R CMV Serostatus | Setting of CMV Disease | CMV Infection in 1st 100 days | Day of Post-HCT Death | Antiviral Resistance | Other Infectious Issues | Cause of Death |
|---|---|---|---|---|---|---|---|---|---|---|---|
| +34 | PBSC | Malignancy | CNI/mTORi | Colon | +/+ | Relapse | Day +31 | +38 | None | Relapse | |
| +84 | PBSC | Malignancy | CNI/mTORi | Stomach | +/+ | GVHD, relapse | Day +61 | +258 | Giardia | Relapse | |
| +29 | BM | Malignancy | CNI/mTORi | Lung | +/− | No events | Day +29 | Alive | None | N/A | |
| +212 | PBSC | Malignancy | CNI/mTORi | Colon | +/+ | GVHD | Day +49 | +372 | Ganciclovir (F412L,V812L in UL54 gene), foscarnet (V812L), cidofovir (F412L,V812L) | Paecilomyces sinusitis, VRE bacteremia, C. difficile colitis, BK-associated cystitis | GVHD with infectious complications |
| +254 | PBSC | Malignancy | TCD | Retina | +/+ | After 2nd HCT for graft failure | +897 | HHV6 reactivation, EBV elevation in blood, Klebsiella and E. coli UTIs, S. pneumoniae pneumonia | CNS relapse after 2nd HCT | ||
| +237 | PBSC | Malignancy | CNI/mTORi | Lung | +/− | Relapse, GVHD | Day +42 | +255 | Foscarnet (S585A in UL54 gene) | Aspergillus pneumonia | Secondary malignancy |
| +88 | PBSC | PID | Serotherapy | Lung | −/− | GVHD | Day +8 | +93 | Ganciclovir and foscarnet (A809V in UL54 gene) | Pyrenochaeta liver abscess, P. romeroi pneumonia, Klebsiella pneumonia and bacteremia | GVHD with infectious complications |
| #1: +22 #2: +85 | PBSC | Malignancy | CNI/mTORi | #1: Stomach #2: Colon | +/+ | #1: CMV just prior to GVHD #2: GVHD | Day +6 | +710 | #1: H. pylori gastritis, BK viremia, E. faecalis bacteremia, S. epidermidis bacteremia #2: Cholecystitis | Relapse | |
| +30 | PBSC | Malignancy | CNI/mTORi | Esophagus | +/+ | Relapse – 2nd HCT, then relapse again | Day +30 | +261 | HHV6 reactivation | Relapse | |
| +42 | PBSC | PID | PTCy | CNS | +/+ | GVHD | Day +43 | +83 | HHV6 reactivation, EBV elevation in blood, BKassociated cystitis, Cryptosporidium, S. aureus bacteremia, Pseudomonas bacteremia, disseminated Basidiomycetes infection | GVHD with infectious complications | |
| +237 | UCB | Aplastic Anemia | UCB | Lung | −/+ | GVHD | Day +22 | +416 | Ganciclovir (T503I,A809V in UL54 gene), foscarnet (A809V), cidofovir (T503I); CMV-CTLs | HHV6 reactivation, RSV LRTI, Cryptococcal sinusitis, MAC pneumonia, Aspergillus pneumonia | CMV pneumonitis |
| +15 | PBSC | Malignancy | Serotherapy | Colon | +/+ | GVHD | Day +21 | +83 | HSV viremia, EBV elevation in blood | Relapse | |
| +269 | PBSC | Malignancy | CNI/mTORi | Lung | +/+ | GVHD | Day +51 | Alive | Adenoviremia | N/A | |
| +108 | PBSC | PID | Serotherapy | Colon | +/+ | GVHD | Day +91 | +277 | Adenoviremia, EBV elevation in blood, BK-associated cystitis, Legionella peritonitis, Candida glabrata fungemia, VRE bacteremia, C. difficile colitis | GVHD with infectious complications | |
| +346 | PBSC | Malignancy | TCD | Colon | +/+ | Relapse – 2nd HCT, then GVHD | +595 | Disseminated Fusarium, S. mitis bacteremia, Klebsiella pneumoniae bacteremia | Relapse with infectious complications | ||
| +145 | PBSC | Malignancy | CNI/mTORi | Colon | +/+ | GVHD | Alive | BK-associated cystitis | |||
| +340 | PBSC | Malignancy | Serotherapy | Lung | −/+ | GVHD | Day +12 | +398 | Ganciclovir (C603W in UL97 gene), foscarnet (V715M in UL54 gene) | HHV6 reactivation, BK-associated cystitis | GVHD, uncontrolled CMV, TMA |
| #1: +48 #2: +385 | PBSC | PID | PTCy | #1: Stomach #2: Lungs | +/+ | #1 and 2: GVHD | Day +49 | +414 | BK-associated cystitis, primary EBV infection | GVHD with uncontrolled CMV | |
| +25 | PBSC | Hemoglobinopathy | Serotherapy | Colon | +/+ | No events | Day +19 | Alive | None | N/A |
Abbreviations: CMV, cytomegalovirus; HCT, hematopoietic cell transplantation; D/R, donor/recipient; PBSC, peripheral blood stem cell; BM, bone marrow; UCB, umbilical cord blood; PTCy, post-transplantation cyclophosphamide; TCD, ex vivo T-cell depletion; CNI/mTORi, calcineurin inhibitor or mammalian target of rapamycin inhibitor-based; GVHD, graft-versus-host disease; CMV-CTLs, CMV-specific cytotoxic T-lymphocytes; VRE, vancomycin resistant enterococcus; BK, BK virus; HHV6, human herpesvirus 6; EBV, Epstein-Barr virus; UTI, urinary tract infection; RSV, respiratory syncytial virus; LRTI, lower respiratory tract infection; MAC, M avium complex; HSV, herpes simplex virus; TMA, thrombotic microangiopathy; PID, primary immunodeficiency disease
CMV Disease
In the year post-HCT, 19 recipients (5%) had 21 CMV disease events (colon=7; lungs=7, upper gastrointestinal tract=5; retinitis=1; central nervous system=1) at a median of 88 days post-HCT, although 10 recipients were diagnosed beyond day +100, Table 4. Two cases were resistant to ganciclovir, foscarnet, and cidofovir, two cases were resistant to ganciclovir and foscarnet, and one case was resistant to foscarnet. Three cases of CMV disease developed without prior CR, while 12 cases were in the setting of GVHD, three in the setting of relapse and GVHD, two in the setting of relapsed hematologic malignancy requiring systemic therapy, and one in the setting of second HCT for graft failure. One case was a D/R −/− recipient who had received granulocytes post-HCT, while two were D/R −/+ and two were D/R +/−. All but two cases occurred in recipients of PBSC grafts. One patient, after developing resistance to antiviral therapy, received multiple infusions of CMV-specific cytotoxic T-cells without response.
Discussion
In the modern era of improved methods to monitor, treat, and even prevent CMV infection after HCT, understanding the circumstances in which HCT recipients are or become at risk of CMV infection post-HCT remains an important focus.16 Our results are consistent with those from a Center for International Blood and Marrow Transplant Research (CIBMTR) study, which demonstrated that recipient CMV serostatus is the main determinant of CMV infection incidence post-HCT across HCT approaches. Our data also indicate that among PTCy-treated recipients, donor CMV serostatus also may inform CMV infection risk.17 The mechanism behind donor CMV seropositivity contributing to the prediction of CMV infection risk among PTCy-treated recipients is unclear but might relate to differences in immunomodulation of donor-derived antiviral immunity by PTCy that depend on donor/graft serostatus. Furthermore, our finding that UCB was associated with high rates of CMV infection and prolonged treatment duration are consistent with a prior study.18 Unique to our study is how post-HCT events, particularly steroid use, modify the known pre-HCT predictive factors. Furthermore, given that CMV diagnosis and treatment as well as other supportive care elements were standardized across patients, this study eliminates confounding influences present in the CIBMTR registry study. While the level of detail available to investigate CMV infection and risk modifiers exceeds a registry based approach, our study is limited by the fact that the variables evaluated across the HCT approaches investigated herein such as disease type, graft source, and T-cell manipulation strategy, were determined in aggregate by protocol design and not as independent variables.
The degree of HLA matching and donor relatedness are known determinants of CMV infection among at-risk recipients,14 although it is difficult to separate the GVHD prophylaxis approach and the risk for developing GVHD determined by HLA match, from differences in post-HCT CMV-specific immunity related directly to the degree of HLA-matching. Among recipients of haplo grafts, others have shown that in vivo TCD with anti-thymocyte globulin carries higher rates of CMV infection and viral infection-related mortality when compared to PTCy-based approaches to haplo HCT.15 In an exploratory analysis, we found no difference in CMV infection incidence between PTCy-treated recipients by degree of HLA-match, even when accounting for post-HCT steroid use as a CR. These findings likely are related at least in part to overall low incidences of severe acute and chronic GVHD and thus low immunosuppressive burdens in patients treated with PTCy. This is further supported by our overall data illustrating the impact of post-HCT interventions, such as steroid therapy for GVHD, on CMV infection incidence, regardless of other specifics of the approach.19,20
In comparing CNI-based GVHD prophylaxis to CNI+mTORi-based approaches, the latter was associated with less CMV infection. There are data to suggest that mTORi may protect against CMV infection,21 although two randomized trials of CNI/methotrexate versus CNI/mTORi reported no differences in CMV infection incidence between treatment arms, with similar rates of GVHD between the arms.22,23 However, the relationship between GVHD onset/treatment and CMV infection was not examined for differences between arms in either trial.22,23 As mTORi have been shown to not directly inhibit CMV replication,24 any protective effect of mTORi, if real, would seem to be indirect and related to reconstitution of CMV-specific cellular immunity. Additionally, recipients transplanted for hemoglobinopathy/thalassemia received alemtuzumab during conditioning and post-HCT mTORi without CNI, with a relatively low incidence of CMV infection despite the proximal serotherapy they received. Our finding of lower CMV incidence in recipients of mTORi is interesting in that it suggests mTORi may indirectly mitigate CMV infection risk, perhaps related to the early mixed chimerism often observed in these recipients of mTORi-based GVHD prophylaxis.25–27
Our data support that unplanned, post-HCT events, particularly steroid use, contribute greatly to the incidence of CMV infection. Analyses with and without steroids as a CR quantify the added risk posed by this very common post-HCT intervention. Of the recipients in this study who had CMV disease, post-HCT risk-modifying events were a co-factor in the majority of cases. As reported by others28 and also illustrated here, CMV disease resulting in death was often in the seen with multiple concurrent infectious complications arising in the setting of steroid-refractory GVHD.
These data illustrate the utility in tailoring the monitoring and preemptive treatment, strategies in a dynamic manner, based on the nature and severity of post-HCT risk-modifying events and on an individual level. Our analysis from the pre-letermovir era should serve as a historical comparison as new prophylactic approaches are incorporated into practice. Durable restoration of CMV-specific cellular immune function should mark the point post-HCT when risk for clinically significant CMV infection and disease ends for a given individual. However, post-HCT factors may weaken or incapacitate CMV-specific cellular immunity and make patients again at-risk for CMV infection or disease. While assays to quantify CMV-specific cytotoxic T cells are now clinically available, what represents a protective, functional level of cellular immunity is still largely investigational, and the clinical application of these tests post-HCT remains very much uncharted.29,30 Further work may provide insight into whether these assays are more informative when applied to recipients recovering from a risk-modifying event, such as tapering off unplanned immunosuppression or recovering from chemotherapy plus donor lymphocyte infusion for relapse, to provide additional insight at that time into CMV-specific immunity and guide CMV monitoring approach moving foward. Letermovir is now approved for CMV prophylaxis in CMV seropositive recipients through day +100,10 however the recipients most vulnerable to significant CMV infection and disease may be best identified not just by their serostatus pre-HCT nor the HCT platform utilized, but by post-HCT events that impair immune reconstitution, even after day +100.
Highlights.
Systemic steroid use after HCT increases the risk of CMV infection and disease
Risk for CMV infection post-HCT is dynamic and depends on post-HCT events
Approaches to GVHD prophylaxis that include mTOR inhibitors may protect against CMV
Acknowledgements
The authors thank Seth Steinberg for his input and guidance regarding the statistical design and analyses. The authors thank the research teams and patients for their contributions and participation in the research. This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crocchiolo R, Bramanti S, Vai A, et al. Infections after T-replete haploidentical transplantation and high-dose cyclophosphamide as graft-versus-host disease prophylaxis. Transpl Infect Dis. 2015;17(2):242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Illiaquer M, Imbert-Marcille BM, Guillaume T, et al. Impact of stem cell graft on early viral infections and immune reconstitution after allogeneic transplantation in adults. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2017;93:30–36. [DOI] [PubMed] [Google Scholar]
- 3.Styczynski J Who Is the Patient at Risk of CMV Recurrence: A Review of the Current Scientific Evidence with a Focus on Hematopoietic Cell Transplantation. Infect Dis Ther. 2018;7(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camargo JF, Komanduri KV. Emerging concepts in cytomegalovirus infection following hematopoietic stem cell transplantation. Hematol Oncol Stem Cell Ther. 2017;10(4):233238. [DOI] [PubMed] [Google Scholar]
- 5.Boeckh M, Ljungman P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood. 2009;113(23):5711–5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ljungman P, Boeckh M, Hirsch HH, et al. Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials. Clin Infect Dis. 2017;64(1):87–91. [DOI] [PubMed] [Google Scholar]
- 7.Miller HK, Braun TM, Stillwell T, et al. Infectious Risk after Allogeneic Hematopoietic Cell Transplantation Complicated by Acute Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2017;23(3):522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain NA, Lu K, Ito S, et al. The clinical and financial burden of pre-emptive management of cytomegalovirus disease after allogeneic stem cell transplantation-implications for preventative treatment approaches. Cytotherapy. 2014;16(7):927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robin C, Hemery F, Dindorf C, et al. Economic burden of preemptive treatment of CMV infection after allogeneic stem cell transplantation: a retrospective study of 208 consecutive patients. BMC Infect Dis. 2017;17(1):747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marty FM, Ljungman P, Chemaly RF, et al. Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic-Cell Transplantation. N Engl J Med. 2017;377(25):2433–2444. [DOI] [PubMed] [Google Scholar]
- 11.Hill JA, Mayer BT, Xie H, et al. Kinetics of Double-Stranded DNA Viremia After Allogeneic Hematopoietic Cell Transplantation. Clin Infect Dis. 2018;66(3):368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.R: A language and environment for statistical computing [computer program]. 2017.
- 13.cmprsk: Subdistribution Analysis of Competing Risks [computer program]. 2014.
- 14.Atay D, Akcay A, Erbey F, Ozturk G. The impact of alternative donor types on viral infections in pediatric hematopoietic stem cell transplantation. Pediatr Transplant.2018;22(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tischer J, Engel N, Fritsch S, et al. Virus infection in HLA-haploidentical hematopoietic stem cell transplantation: incidence in the context of immune recovery in two different transplantation settings. Ann Hematol. 2015;94(10):1677–1688. [DOI] [PubMed] [Google Scholar]
- 16.Styczynski J Who Is the Patient at Risk of CMV Recurrence: A Review of the Current Scientific Evidence with a Focus on Hematopoietic Cell Transplantation. Infect Dis Ther. 2018;7(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teira P, Battiwalla M, Ramanathan M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood. 2016;127(20):2427–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramanathan M, Teira P, Battiwalla M, et al. Impact of early CMV reactivation in cord blood stem cell recipients in the current era. Bone Marrow Transplant. 2016;51(8):1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanakry CG, Bolanos-Meade J, Kasamon YL, et al. Low immunosuppressive burden after HLA-matched related or unrelated BMT using posttransplantation cyclophosphamide. Blood. 2017;129(10):1389–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCurdy SR, Kanakry CG, Tsai HL, et al. Grade II Acute Graft-versus-Host Disease and Higher Nucleated Cell Graft Dose Improve Progression-Free Survival after HLA-Haploidentical Transplant with Post-Transplant Cyclophosphamide. Biol Blood Marrow Transplant. 2018;24(2):343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinana JL, Perez-Pitarch A, Guglieri-Lopez B, et al. Sirolimus exposure and the occurrence of cytomegalovirus DNAemia after allogeneic hematopoietic stem cell transplantation. Am J Transplant. 2018:1–10. [DOI] [PubMed] [Google Scholar]
- 22.Torlen J, Ringden O, Garming-Legert K, et al. A prospective randomized trial comparing cyclosporine/methotrexate and tacrolimus/sirolimus as graft-versus-host disease prophylaxis after allogeneic hematopoietic stem cell transplantation. Haematologica. 2016;101(11):1417–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cutler C, Logan B, Nakamura R, et al. Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood. 2014;124(8):1372–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glover TE, Kew VG, Reeves MB. Rapamycin does not inhibit human cytomegalovirus reactivation from dendritic cells in vitro. J Gen Virol. 2014;95(Pt 10):2260–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fowler DH, Mossoba ME, Steinberg SM, et al. Phase 2 clinical trial of rapamycin-resistant donor CD4+ Th2/Th1 (T-Rapa) cells after low-intensity allogeneic hematopoietic cell transplantation. Blood. 2013;121(15):2864–2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mossoba ME, Halverson DC, Kurlander R, et al. High-Dose Sirolimus and Immune-Selective Pentostatin plus Cyclophosphamide Conditioning Yields Stable Mixed Chimerism and Insufficient Graft-versus-Tumor Responses. Clin Cancer Res. 2015;21(19):4312–4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsieh MM, Fitzhugh CD, Weitzel RP, et al. Nonmyeloablative HLA-matched sibling allogeneic hematopoietic stem cell transplantation for severe sickle cell phenotype. JAMA. 2014;312(1):48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erard V, Guthrie KA, Seo S, et al. Reduced Mortality of Cytomegalovirus Pneumonia After Hematopoietic Cell Transplantation Due to Antiviral Therapy and Changes in Transplantation Practices. Clin Infect Dis. 2015;61(1):31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gratama JW, Boeckh M, Nakamura R, et al. Immune monitoring with iTAg MHC Tetramers for prediction of recurrent or persistent cytomegalovirus infection or disease in allogeneic hematopoietic stem cell transplant recipients: a prospective multicenter study. Blood. 2010;116(10):1655–1662. [DOI] [PubMed] [Google Scholar]
- 30.Borchers S, Bremm M, Lehrnbecher T, et al. Sequential anti-cytomegalovirus response monitoring may allow prediction of cytomegalovirus reactivation after allogeneic stem cell transplantation. PLoS One. 2012;7(12):e50248. [DOI] [PMC free article] [PubMed] [Google Scholar]