Abstract
Although African-American (AA) prostate cancer (PCa) patients tend to develop greater therapeutic resistance and faster PCa recurrence compared to Caucasian-American men, the molecular mechanisms of this racial PCa disparity remain undefined. In this study, we provide the first comprehensive evidence that cytochrome c (CC) deficiency in AA primary tumors and cancer cells abrogates apoptosome-mediated caspase activation and contributes to mitochondrial dysfunction, thereby promoting therapeutic resistance and PCa aggressiveness in AA men. In AA PCa cells, decreased nuclear accumulation of Nrf1 and its subsequent loss of binding to the CC promoter mediated CC deficiency. Activation of c-Myc and NF-κB or inhibition of AKT prevented nuclear translocation of Nrf1. Genetic and pharmacological inhibition of c-Myc and NF-κB or activation of AKT promoted Nrf1 binding to CC promoter, CC expression, caspase activation, and cell death. The lack of p-Drp1S616 in AA PCa cells contributed to defective CC release and increased resistance to apoptosis, indicating that restoration of CC alone may be insufficient to induce effective apoptosis. CC-deficiency promoted acquisition of glycolytic phenotypes and mitochondrial dysfunction, whereas CC restoration via inhibition of c-Myc and NF-κB or activation of AKT attenuated glycolysis in AA PCa cells. Inhibition of c-Myc and NF-κB enhanced the efficacy of docetaxel in tumor xenografts. Therefore, restoring CC may overcome therapeutic resistance and PCa aggressiveness in AA men. Overall, this study provides the first comprehensive experimental, mechanistic, and clinical evidence for apoptosome and mitochondrial dysfunction in PCa racial disparity.
Keywords: Prostate Cancer, Health Disparity, Cytochrome c, Apoptosome Dysfunction, Mitochondrial Dysfunction
Introduction
African-American (AA) men are more often diagnosed with prostate cancer (PCa) and suffer higher mortality rates than Caucasian-American (CA) men (1,2). These poor outcomes are due to the fact that AA PCa patients respond more poorly than their CA counterparts to current therapeutic approaches (3). AA PCa is more aggressive, takes less time to relapse, shows molecular differences, and has greater likelihood of metastasis than CA PCa (2–8). While the molecular mechanisms driving acquisition of these characteristics in AA PCa remain largely unknown, we and others have demonstrated that mitochondrial dysfunction is a key contributing factor to therapeutic resistance (9–12). One of the reasons for greater PCa aggressiveness in AA men is the existence of defective oxidative phosphorylation (OXPHOS) system in AA PCa cells and tumors (9,12,13). We reported that mitochondrial DNA (mtDNA) copy number is reduced in non-tumor prostatic tissues in AA men with PCa compared to CA men with PCa (11). MtDNA encodes proteins critical for OXPHOS Complexes I, III, IV, and V (14). Therefore, the reduced level of mtDNA may compromise OXPHOS function leading to aberrant activity/expression of other components of the OXPHOS system, such as cytochrome c (CC). CC transfers electrons from Complex III to Complex IV during electron transport for ATP production. Thus OXPHOS defects due to reduced mtDNA and aberrant CC expression may promote aerobic glycolysis in AA PCa compared to CA PCa. This hypothesis is supported by the fact that AA PCa, compared to CA PCa, exhibits higher levels of key proteins, such as c-Myc and NF-κB (6,15,16), that foster a glycolytic phenotype (17,18). Whether and how upregulation of c-Myc and other regulators of aerobic glycolysis compromise OXPHOS function in AA men with PCa remain unclear.
We reported that AA PCa cells resist apoptosis due to lack of caspase activation (9). However, the underlying causes of resistance to apoptosis in response to various anticancer agents remain undefined. In solid epithelial cancers, such as PCa, mitochondria are required for efficient apoptosis triggered by release of CC (19), a key component of the OXPHOS system (14). CC release from mitochondria interacts with and activates an adapter protein, apoptotic protease-activating factor-1 (Apaf-1), which undergoes oligomerization to form the apoptosome that recruits and activates caspase-9 at the apoptosome complex. Caspase-9 then activates effector caspases, such as caspase-3, to execute apoptosis (20–22). Apoptosome dysfunction has been reported in some cancer types (23–25), but whether higher therapeutic resistance in AA PCa patients is due to apoptosome dysfunction remains unknown. Apoptosome dysfunction may occur via protein deficiency of apoptosomal components or due to defects in CC release from mitochondria. Here we provide the first comprehensive evidence that CC-deficiency in AA PCa cells contributes to development of aggressive PCa and therapeutic resistance. Defining underlying mechanisms causing CC deficiency have revealed novel therapeutic approaches to restore CC, inhibit aerobic glycolysis, and sensitize PCa cells to first line chemotherapeutic agents, such as docetaxel (DOC).
Materials and Methods
Patient samples:
Primary prostate tumors (PT), matching non-tumor (MN) prostate tissues, and total RNA from CA and AA PCa patients were collected at Roswell Park Comprehensive Cancer Center (Roswell Park) by the Pathology Network Shared Resource (PNSR) under approved IRB protocol. The patient’s samples were de-identified by PNSR and patient informations were not provided to researchers.
Mice:
Animal experiments were approved by and performed in compliance with the guidelines and regulations by the Roswell Park Institutional Animal Care and Use Committee (IACUC, protocol # 1306M). 6–8 weeks old SCID male mice were purchased from the Roswell Park Division of Laboratory Animal Resources (DLAR). All mice were kept under standard conditions and diet.
Cell lines:
LNCaP, DU145, and PC-3 cells were maintained in RPMI 1640 media (Life Technologies, Carlsbad, CA) supplemented with 7% FBS and 100U ml−1 penicillin/streptomycin. E006AA and E006AA-hT cells were maintained in high glucose DMEM (Life Technologies, Carlsbad, CA) supplemented with 7% FBS and 100U ml−1 penicillin/streptomycin. RWPE-1, RC-77 T/E and RC-77 N/E cells were maintained in keratinocytes-SFM (Life Technologies, Carlsbad, CA) supplemented with EGF and BPE. Human cell lines acquired from ATCC or collaborators are profiled by short tandem repeat (STR) analysis every 6 months. Early passage cells are cryopreserved for subsequent use in all experiments to reduce possible genetic drift. Cultures are passaged for no more than 3 months at which time they are replaced from cryopreserved stocks. Cell lines are screened routinely for mycoplasma contamination using Hoechst staining or a more sensitive PCR assay. Details about all cells studied are provided in supplemental methods.
Compounds:
Docetaxel (DOC) was purchased from Cayman chemicals, Ann Arbor, MI (Cat # 11637). Pharmacological inhibitors of c-Myc (10058-F4; Cat # 15929) and NF-κB (JSH-23; Cat # 15036) transcription factors were purchased from Cayman chemicals, Ann Arbor, MI. bpV(pic) (AKT activator) was purchased from Cayman chemicals, Ann Arbor, MI (Cat # 14434). All compounds were reconstituted in 100% DMSO and diluted in cell culture media before use.
Gene specific silencing using shRNA lentiviral particles:
Cells were seeded in 6 well plates and after 24 hrs, polybrene (8 µg/ml) was added to the media. After 1 hr, mock shRNA or gene specific shRNA (CYCS, Drp1 and Nrf1) lentiviral particles were added at MOI of 2. After 48 hrs of transduction, media was replaced with fresh media containing 1 µg/ml puromycin for selection of transduced cells. Knock down of targeted gene was confirmed using immunoblotting.
Gene specific silencing using siRNA:
Cells were transfected with siRNA for c-Myc or p65 subunit of NF-κB or PTEN using lipofectamine 3000 system as per manufacturer’s instructions. Cells were treated with DOC (10 nM for 24 hrs) alone or in combination with either c-Myc inhibitor (c-Myc I, 75 µM) or NF-κB inhibitor (NF-κB I, 50 µM) or AKT activator (AKT Act, 5 µM). Whole cell lysates were prepared and used for DEVDase activity and immunoblotting for CC. Knock down of the targeted gene was confirmed using immunoblotting.
Statistical analysis.
Significant differences between means were assessed using analysis of variance (ANOVA) and GraphPad Prism Version 6.0. A *p < 0.05 value was accepted as significant. Significance was denoted as compared to control, unless otherwise indicated.
Supplemental Materials and Methods:
Experimental details for CC overexpression using CRISPR-SAM, MitoROS and MitoMass quantification, Annexin/PI staining, mtDNA determination, subcellular fractionation, ChiP and CC promoter assay, real time PCR, immunoblotting, immunofluorescence, immunohistochemistry (IHC), cell viability and caspase-3 (DEVDase) assay, bioenergetics and clonogenic assays, PCa cell xenograft, and cell cycle analysis as well as a list of antibodies (Supplemental Table 1), shRNA sequences (Supplemental Table 2), and siRNA sources (Supplemental Table 3) are included in the Supplemental Materials and Methods.
Results
CC, a key component of apoptosome and OXPHOS system, is reduced in PCa cell lines and tumor specimens derived from AA men with PCa
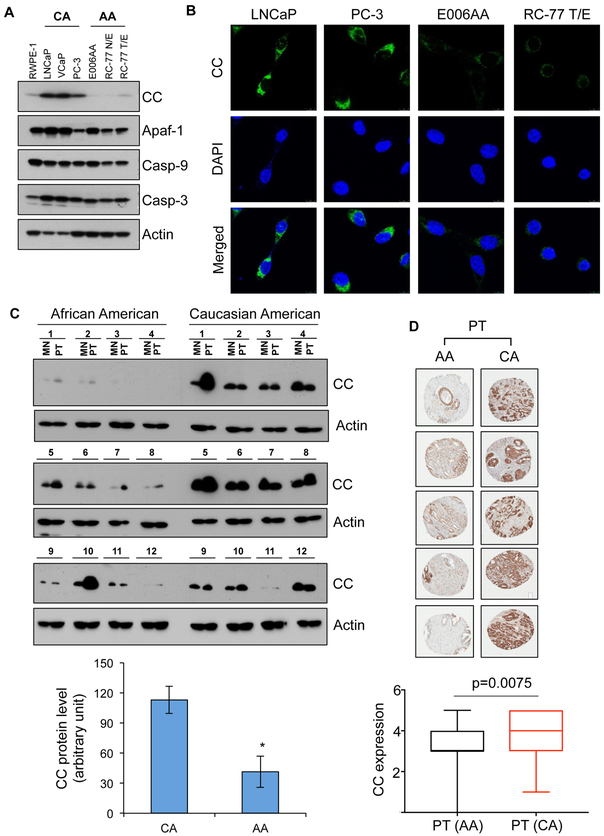
We have previously demonstrated that PCa cell lines derived from AA PCa patients are more resistant to anticancer agents than to PCa cell lines derived from CA PCa patients (9). One possible explanation for greater therapeutic resistance in AA men with PCa is apoptosome dysfunction in AA PCa cells compared to CA PCa cells. We first measured expression of the apoptosome components in AA PCa cells. Analysis of mRNA in E006AA and E006AA hT (AA PCa), and PC-3 and LNCaP (CA PCa) cells demonstrated reduced levels of CC mRNA in AA PCa cells compared to CA PCa cells (Figure S1A). The reduced level of CC protein via immunoblotting in AA PCa cells validated reduced expression of CC mRNA (Figure 1A). Immunolabeling of CC supported CC-deficiency in AA PCa cells compared to CA PCa cells (Figure 1B and S1B). In contrast, the levels of other components of the apoptosome, such as Apaf-1, caspase-9, and caspase-3, were not altered significantly in AA and CA PCa cells (Figure 1A). The clinical relevance of apoptosome dysfunction in AA men with PCa was evaluated by measuring the levels of CC in primary tumor (PT) and matched non-tumor (MN) prostate tissues using immunoblotting. CC protein expression was reduced in PT and MN tissues of AA men compared to CA men (Figure 1C). Sections of a PCa tissue microarray (TMA) constructed from PT and MN from AA (n=92) and CA (n=89) patients (Figure 1D) were immunostained with CC antibody. Analysis of MN and PT from AA and CA men with PCa provides two important outcomes. First, PT from AA PCa patients showed reduced CC level compared to CA counterparts that suggests apoptosome dysfunction is due to the lack of CC in AA PCa. Second, MN in AA PCa patients show reduced expression of CC compared to CA men with PCa (Figure 1C and D). CC is critical for the assembly of apoptosome, so lack of CC suggests the existence of apoptosome dysfunction in PCa cells, and PT and MN from AA men with PCa.
Figure 1. CC, a key component of apoptosome and OXPHOS system, is reduced in PCa cell lines and tumor specimens derived from AA men with PCa.
A, Expression of the components of apoptosome complex, which include CC, Apaf-1, caspase-9 (Casp-9), and caspase-3 (Casp-3) were examined using immunoblotting in RWPE-1 (normal prostate epithelial cells), LNCaP, VCaP, PC-3, E006AA, RC-77 N/E and RC-77 T/E cells. Actin serves as a loading control. B, Expression of CC in LNCaP, PC-3, E006AA and RC-77 T/E cells using immunofluorescence. C, Immunoblot analysis of CC in primary tumor (PT) and matched non-tumor (MN) prostatic tissues from AA and CA men with PCa. Actin serves as a loading control. Densitometry analysis of immunoblots of CC in PT tissues from AA and CA men with PCa (n=12 for each race). D, Immunohistochemistry (IHC) analysis of CC expression in PT in AA and CA men with PCa using tissue microarray (TMA) sections. Scoring analysis of IHC of CC in PT tissues from AA (n=92) and CA (n=89) men with PCa. Data in C represent mean ± SD of n=12. Significant differences between means were assessed using analysis of variance (ANOVA) and GraphPad Prism Version 6.0. *p < 0.05 vs CA primary tumor.
Lack of CC causes apoptosome dysfunction and apoptosis resistance in AA PCa cells
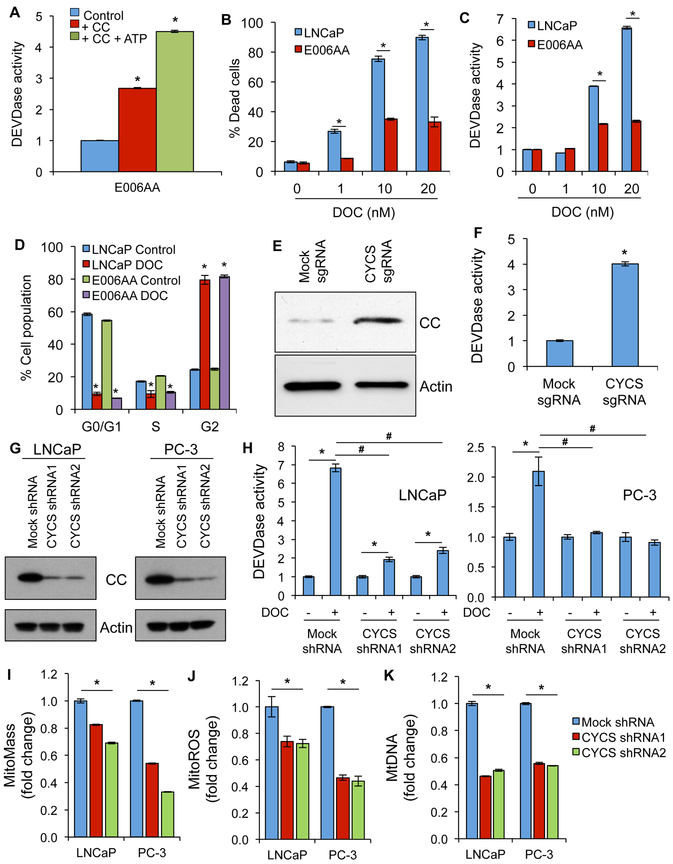
Reconstitution experiments using purified cytosol and CC demonstrated that lack of CC is a key reason for inhibition of apoptosome-mediated caspase activation in AA PCa cells (Figure 2A). DOC-induced caspase activation and cell death were reduced in various AA PCa cells compared to CA PCa cells (Figure 2B and C, and Figure S2A-C). To rule out the possibility that lack of apoptosis in AA PCa cells is due to expression of multidrug transporters (26), the effect of DOC on the cell cycle were evaluated. Both AA and CA PCa cells showed similar cell cycle arrest at G2/M phase after DOC treatment (Figure 2D). To test whether CC deficiency in AA PCa confers therapeutic resistance, we increased expression of endogenous CC in E006AA cells using the CRISPR-SAM technique. Increased expression of endogenous CC induced robust caspase-3 activation in E006AA cells (Figure 2E and F). Thus, CC is a limiting factor for DOC-induced apoptosis in AA PCa cells
Figure 2. Lack of CC causes apoptosome dysfunction and apoptosis resistance in AA PCa cells whereas CC-silencing induces mitochondrial/apoptosome dysfunction in CA PCa cells.
A, Purified cytosol isolated from E006AA cells was reconstituted with CC with or without ATP to quantitate apoptosome-mediated caspase-3 activity using a substrate cleavage (DEVDase) assay. B, Cell death in LNCaP and E006AA cells upon docetaxel (DOC) treatment (for 24 hrs) was quantified using a Trypan blue assay. C, Caspase-3 activity (i.e., DEVDase activity) was determined in LNCaP and E006AA cells after treatment with DOC for 24 hrs. D, Cell cycle phase analysis was quantified in LNCaP and E006AA cells after treatment with DOC for 24 hrs. E, Endogenous CC was overexpressed in E006AA cells using a CRISPR-SAM approach and expression of CC was determined using immunoblotting. Actin serves as a loading control. F, Endogenous CC was overexpressed in E006AA cells using a CRISPR-SAM approach and caspase-3 activity was measured using a DEVDase assay. G, CC was knocked down in LNCaP and PC-3 cells using shRNAs and expression of CC was determined using immunoblotting. Actin serves as a loading control. H, Caspase-3 activity (DEVDase activity) was measured in mock- and CC-silenced LNCaP and PC-3 cells treated with DOC (10 nM) for 24 hrs. I, Mitochondrial mass (mitoMass) was measured in mock- and CC-silenced LNCaP and PC-3 cells using flow cytometry. J, Mitochondrial ROS (mitoROS) was measured in mock- and CC-silenced LNCaP and PC-3 cells using flow cytometry. K, MtDNA copy number was analyzed in mock- and CC-silenced LNCaP and PC-3 cells. The bar color for panels I, J, and K represents mock and CYCS shRNAs. Data represent mean ± SD of 3 independent experiments. Significant differences between means were assessed using analysis of variance (ANOVA) and GraphPad Prism Version 6.0. *p < 0.05 vs respective controls or groups; #p<0.05 vs mock shRNA.
CC-silencing in CA PCa cells induces mitochondrial and apoptosome dysfunction leading to inhibition of caspase activation and apoptosis resistance
CC is an important component of apoptosome formation, but it also plays a critical role in energy metabolism by participating in the electron transport chain (ETC) of the oxidative phosphorylation (OXPHOS) system (25). To test whether lack of CC contributes to mitochondrial and apoptosome dysfunction, we adopted a reverse approach by generating CC-silenced CA PCa (LNCaP and PC-3) cells using shRNA lentiviral particles (Figure 2G). CC-silenced CA PCa cells were resistant to DOC treatment as evidenced by inhibition of caspase-3 activity, apoptotic cell death, and levels of cleaved PARP and caspase 3 (Figure 2H; Figure S3A and B). CC-silencing significantly decreased mitochondrial mass (mitoMass), mitochondrial reactive oxygen species (mitoROS), and mitochondrial DNA (mtDNA) in CA PCa cells (Figure 2I, J and K). MtDNA content was reduced in AA PCa cells compared to CA PCa cells (Figure S3C). These findings suggest that lack of CC contributes to mitochondrial dysfunction and apoptosis resistance.
Abrogated nuclear respiration factor-1 (Nrf1) translocation to nucleus contributes to CC loss in AA PCa cells
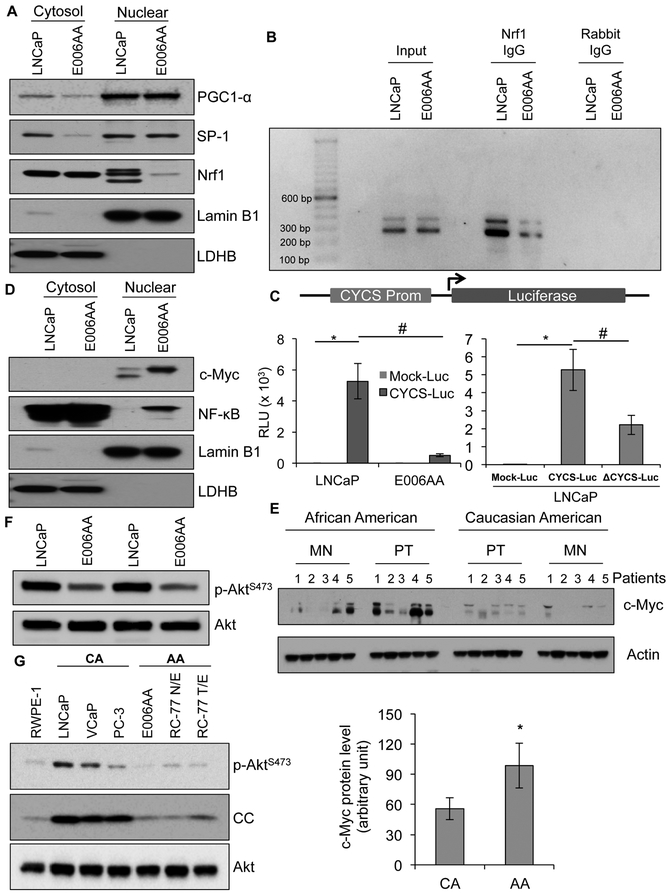
Expression of CC in mammalian cells is regulated by peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1-α), specificity protein 1 (SP-1), and nuclear respiratory factor 1 (Nrf1) transcription factors (27,28). To define the mechanism of reduced expression of CC, we first analyzed the level of these transcription factors using nuclear fractions of E006AA (low CC expression) and LNCaP (high CC expression) cells. Similar levels of PGC1-α and SP-1 were observed in the nuclear fractions of both high and low CC expressing cells, but Nrf1 was reduced in the nuclear fraction of E006AA cells compared to LNCaP cells (Figure 3A), suggesting that reduced Nrf1 nuclear translocation contributes to CC-deficiency in E006AA cells. Chromatin immunoprecipitation (ChIP) analysis of the CC promoter demonstrated reduced binding of Nrf1 in AA PCa cells compared to CA PCa cells (Figure 3B). To confirm that abrogation of Nrf1 binding to the CC promoter reduced expression of CC, we cloned the CC promoter region containing PGC1-α, SP-1 and Nrf1 binding sites in pLightSwitch-Luc vector with luciferase as the reporter gene (CYCS-Luc). The promoter-reporter assay analysis confirmed that the CC gene (CYCS) promoter activity was reduced in E006AA cells compared to LNCaP cells. Deletion of the Nrf1 binding site from p-CYCS-LightSwitch-Luc vector (ΔCYCS-Luc) abolished its promoter activity as evidenced by decreased luciferase activity upon its transfection in LNCaP cells (Figure 3C). Taken together, these data demonstrated that nuclear Nrf1 is the rate-limiting factor for the expression of CC in AA PCa and tumor cells.
Figure 3. Inhibition of nuclear respiration factor-1 (Nrf1) translocation to nucleus contributes to reduced CC expression in AA PCa cells.
A, Cytosolic and nuclear levels of PGC1-α, SP-1, and Nrf1 in LNCaP and E006AA cells were determined using immunoblot analysis. Lamin B1 and LDHB serve as marker proteins as well as loading controls for nuclear and cytosolic fractions, respectively. B, Nrf1 binding efficiency with CC promoter in LNCaP and E006AA cells was determined using a chromatin immunoprecipitation (ChIP) assay. C, LNCaP and E006AA cells were transfected with CC promoter (CYCS Prom) constructs. Luciferase assay detected CC promoter activity. Deletion of Nrf1 binding site on CC promoter inhibited luciferase activity in LNCaP cells. D, Cytosolic and nuclear level of c-Myc and NF-kB in LNCaP and E006AA cells were determined using immunoblot analysis. Lamin B1 and LDHB serve as marker proteins as well as loading controls for nuclear and cytosolic fractions, respectively. E, Representative immunoblot analysis of c-Myc in MN and PT tissue AA and CA men with PCa. Actin serves as a loading control. Densitometry analysis of immunoblots of c-Myc in PT tissues from AA and CA men with PCa (n=12 for each race). F, Expression level of phosphorylated form of Akt (p-AktS473) in LNCaP and E006AA cells. Total Akt serves as a loading control. G, Expression of p-AktS473 and CC using immunoblotting in RWPE-1 (normal prostate epithelial cells), LNCaP, VCaP, PC-3, E006AA, RC-77 N/E and RC-77 T/E cells. Total Akt serves as a loading control. Data represent mean ± SD of 3 independent experiments. Significant differences between means were assessed using analysis of variance (ANOVA) and GraphPad Prism Version 6.0. *p < 0.05 vs respective controls, and #p < 0.05 vs respective groups.
The cytosolic level of Nrf1 was similar in LNCaP and E006AA cells, that prompted an experiment to test if nuclear translocation of Nrf1 was inhibited in E006AA cells (Figure 3A). Cellular Myc (c-Myc) and NF-κB transcription factors regulate Nrf1 translocation and its target genes (29,30). Both c-Myc and NF-κB are hyperactivated in AA PCa patients (6,15,16,31,32), indicating their involvement in abrogating Nrf1 nuclear translocation. Nuclear accumulation of both c-Myc and NF-κB transcription factors was elevated in AA PCa cells (E006AA and RC-77 T/E cells), which may lead to suppression of nuclear translocation of Nrf1 (Figure 3D, and Figure S4A). Expression of c-Myc was upregulated in both MN and PT from AA patients compared to CA patients (Figure 3E). We next determined the involvement of AKT signaling, which phosphorylates and activates Nrf1 and its target genes (33,34). We observed reduced levels of active AKT (p-AKTS473) in E006AA compared to LNCaP cells (Figure 3F). A larger panel of CA and AA PCa cells further establish that reduced Akt phosphorylation correlates with reduced CC expression (Figure 3G). These findings demonstrate that activation of c-Myc or NF-κB and suppression of p-AKT signaling may contribute to the abrogation of Nrf1 nuclear translocation that leads to reduced expression of CC in AA PCa cells compared to CA PCa cells.
Genetic and pharmacological inhibition of c-Myc and/or NF-κB, and activation of AKT enhance Nrf1 nuclear translocation to promote CC expression
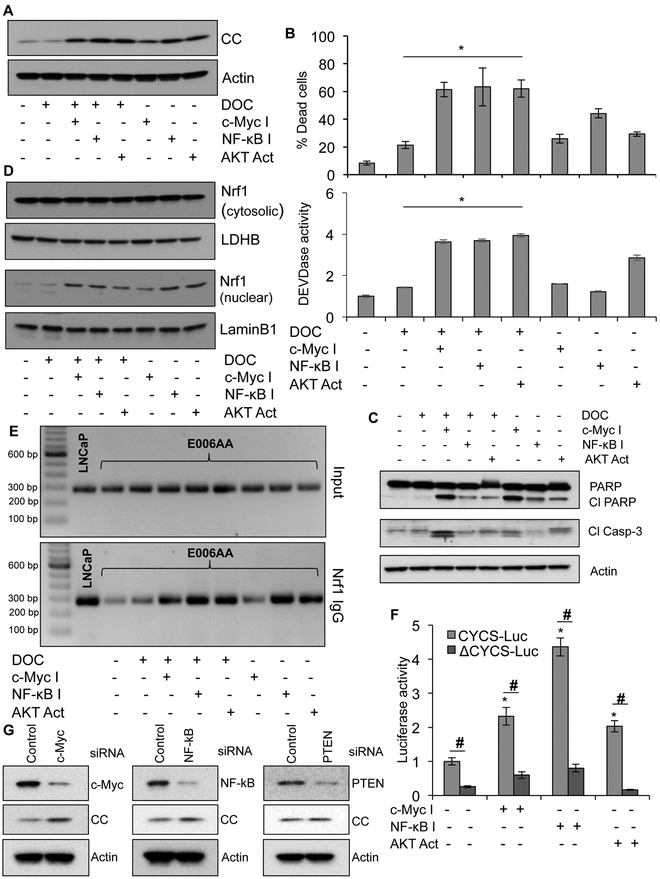
To establish the biological significance of c-Myc and/or NF-κB activation and AKT inhibition in abrogating CC expression and apoptosis, we measured CC expression and cell death in response to specific pharmacological inhibitors of c-Myc or NF-κB alone, AKT activator alone and their combinations with DOC. We observed increased CC expression, increased caspase-3 activation, PARP cleavage, and enhanced cell death in response to combined treatment with DOC and either c-Myc inhibitor, NF-κB inhibitor, or AKT activator in E006AA and other AA PCa cells (Figure 4A-C, Figure S4B and C). Enhanced translocation of Nrf1 to nucleus in AA PCa cells in response to c-Myc or NF-κB inhibitors or AKT activator alone, and when combined with DOC demonstrated that increased expression of CC was due to nuclear translocation of Nrf1 (Figure 4D). Re-activation of Nrf1 transcriptional activity was validated using ChIP analysis, which demonstrated enhanced binding of Nrf1 to the CC promoter in response to c-Myc or NF-κB inhibitors or AKT activator alone and when combined with DOC (Figure 4E). Nrf1-silencing inhibited drug sensitivity to c-Myc inhibitor, NF-κB inhibitor, and AKT activator alone and when combined with DOC (Figure S5). We confirmed the involvement of Nrf1 in CC expression by treating CYCS-Luc and ΔCYCS-Luc transfected E006AA cells with either c-Myc inhibitor or NF-κB inhibitor or AKT activator for 24 hrs followed by luciferase activity measurement. Treatment with c-Myc inhibitor, NF-κB inhibitor, or AKT activator increased the CYCS promoter activity in CYCS-Luc transfected cells but not in ΔCYCS-Luc transfected cells (Figure 4F). Silencing of c-Myc, NF-κB and PTEN in AA PCa cells enhanced CC expression and caspase-3 activity with or without DOC treatment (Figure 4G and S6A and B). Taken together, these data provide evidence that induced expression of CC upon Nrf1 activation sensitized AA PCa cells to DOC albeit not to the same degree as in CA PCa cells.
Figure 4. Inhibition of c-Myc and/or NF-κB and activation of AKT enhance Nrf1 nuclear translocation, restores CC expression, and induces cell death.
A, Immunoblot analysis of CC in E006AA cells after treatment with DOC (10 nM for 24 hrs) alone or in combination with either c-Myc inhibitor (c-Myc I, 75 µM) or NF-κB inhibitor (NF-κB I, 50 µM) or AKT activator (AKT Act, 5 µM). Actin serves as a loading control. B, Quantification of cell death using Trypan blue assay and caspase-3 activity determination in E006AA cells upon treatment with DOC (for 24 hrs) alone or in combination with either c-Myc inhibitor or NF-κB inhibitor or AKT activator. *p < 0.05 vs DOC treated cells. C, Immunoblot analysis of cleaved PARP (Cl PARP) and cleaved caspase-3 (Cl Casp-3) in E006AA cells treated with DOC alone, c-Myc inhibitor alone, NF-κB inhibitor alone, AKT activator alone or DOC in combination with Myc inhibitor or NF-κB inhibitor or AKT activator. Actin serves as a loading control. D, Immunoblot analysis of Nrf1 in cytosolic and nuclear fractions isolated from E006AA cell treated with DOC (for 24 hrs) alone or in combination with either c-Myc inhibitor or NF-κB inhibitor or AKT activator. LDHB and TBP serve as marker proteins and loading controls for cytosolic and nuclear fractions, respectively. E, Nrf1 binding efficiency with CC promoter in E006AA cells upon treatment with DOC (for 24 hrs) alone or in combination with either c-Myc inhibitor or NF-κB inhibitor or AKT activator using ChIP analysis. LNCaP cells were used as positive controls. F, E006AA cells were transfected with CC promoter constructs (CYCS-Luc or ΔCYCS-Luc) and treated with either c-Myc inhibitor or NF-κB inhibitor or AKT activator, followed by luciferase assay after 24 hrs to detect CC promoter activity. *p < 0.05 vs untreated control, #p< 0.05 vs respective groups. G, c-Myc, p65 subunit of NF-κB and PTEN was knock down in E006AA cells using siRNA. Expression of these proteins and CC was determined using immunoblotting. Actin serves as a loading control. Data represent mean ± SD of 3 independent experiments.
CC release machinery at the mitochondrial outer membrane in AA PCa cells is defective compared to CA PCa cells
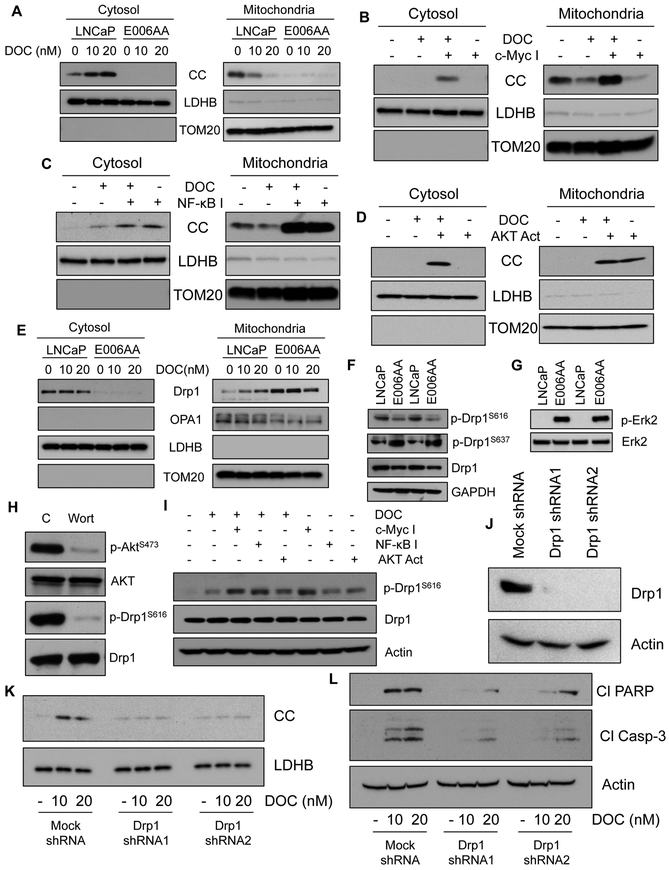
DOC induced CC expression and release from mitochondria to the cytosolic compartment in CA PCa cells, but not in AA PCa cells (Figure 5A). These findings suggest that CC expression was not altered upon DOC treatment, and the CC release machinery is defective in AA PCa cells, which causes apoptosis resistance in response to DOC. Analysis of cytosolic and mitochondrial fractions for CC levels upon inhibition of either c-Myc or AKT activation in combination with DOC treatment demonstrated CC release only in combination treatment but not with single agent exposure, whereas inhibition of NF-κB alone caused CC release (Figure 5B, C, and D). Another AA cell line, RC-77 T/E cells, showed reduced nuclear Nrf1, high c-Myc, and NF-κB compared to LNCaP (Figure S4A). Inhibition of either c-Myc or NF-κB induced CC expression and activated caspase-3 activity, which lead to enhanced cell death in both AA PCa cell lines albeit not as effective as in CA PCa cells (Figure S4B and C).
Figure 5. CC release machinery at the mitochondrial outer membrane is defective and deficiency of Drp1 phosphorylation at serine 616 contributes to defective CC release in AA cells.
A, Immunoblot analysis of CC in LNCaP and E006AA cells after treatment with DOC for 24 hrs. LDHB and TOM20 serve as markers and loading controls for cytosolic and mitochondrial fractions, respectively. B-D, Immunoblot analysis of CC in cytosolic and mitochondrial fractions isolated from E006AA cells upon treatment with DOC (for 24 hrs) alone or c-Myc inhibitor alone or c-Myc inhibitor and DOC (B); DOC alone or NF-κB inhibitor alone or NF-κB inhibitor and DOC (C); DOC alone or AKT activator alone; or AKT activator and DOC (D). LDHB and TOM20 serve as markers and loading controls for cytosolic and mitochondrial fractions, respectively. E, Immunoblot analysis of Drp1 and Opa1 in cytosolic and mitochondrial fractions isolated from LNCaP and E006AA cells treated with DOC alone. LDHB and TOMM20 serve as markers and loading controls for cytosolic and mitochondrial fractions, respectively. F, Immunoblot analysis of total Drp1, p-Drp1S616, and p-Drp1S637 in LNCaP and E006AA cells. GAPDH serves as a loading control. G, Immunoblot analysis of total Erk2 and its phosphorylated form in LNCaP and E006AA cells. Total Erk2 serves as a loading control. H, Expression level of total Akt, p-AktS473, total Drp1, and p-Drp1S616 in LNCaP cells treated with AKT inhibitor wortmanin (Wort, 1 µM). Total Akt and Drp1 serve as loading controls. I, Immunoblot analysis of p-Drp1S616 and Drp1 in E006AA cells after treatment with DOC (10 nM for 24 hrs) alone or in combination with either c-Myc inhibitor (c-Myc I, 75 µM) or NF-κB inhibitor (NF-κB I, 50 µM) or AKT activator (AKT Act, 5 µM). Actin serves as a loading control. J, Drp1 was knocked down in LNCaP cells using shRNAs and expression of Drp1 was determined using immunoblotting. Actin serves as a loading control. K, Immunoblot analysis of CC expression in cytosolic fractions isolated from mock and Drp1-silenced LNCaP cells treated with DOC for 24 hrs. LDHB serves as a loading control. L, Immunoblot analysis of PARP cleavage (Cl PARP) and caspase-3 cleavage (Cl Casp-3) in mock and Drp1-silenced LNCaP cells treated with DOC for 24 hrs. Actin serves as a loading control.
Deficiency of dynamin-related protein (Drp1) phosphorylation at serine 616 (p-Drp1S616) contributes to defective CC release in AA PCa cells
Phosphorylation of Drp1 protein plays a crucial role in mitochondrial dynamics and CC release (35,36). Drp1 primarily localizes in the cytosolic compartment, translocates to the outer mitochondrial membrane, and modulates mitochondrial cristae structure to cause CC release into the cytosol in response to cellular stress (37). Drp1 was translocated to mitochondria in response to DOC in CA PCa cells (Figure 5E). Surprisingly, AA PCa cells expressed higher Drp1 protein that was detected mostly in the mitochondrial fraction (Figure 5E). Phosphorylation of Drp1 at serine 616 (activating phosphorylation, p-Drp1S616) promotes mitochondrial fission and cell death, whereas phosphorylation site serine 637 (inhibitory phosphorylation; p-Drp1S637) inhibits mitochondrial fission (38–40). DOC-responsive LNCaP cells expressed higher levels of activating pDrp1S616 than DOC-unresponsive E006AA, while E006AA expressed higher levels of inhibitory pDrp1S637 than LNCaP cells (Figure 5F). We further observed that p-Erk2, which phosphorylates Drp1 at S616 (41), was upregulated in AA PCa, but the levels of p-Drp1S616 phosphorylation in AA PCa cells was reduced. In contrast to AA PCa cells, the expression level of p-Drp1S616 was higher, but p-Erk2 was not detected, in LNCaP cells (Figure 5F and G). These findings preclude the involvement of p-Erk2 in Drp1 phosphorylation at S616 in these cells. Reduced expression of p-AKT (Figure 3F) with concomitant decrease of p-DrpS616 (Figure 5F) in AA PCa cells suggests that p-AKT may play a critical role in Drp1 phosphorylation at S616 in PCa cells. Inhibition of Drp1 phosphorylation at S616 by the AKT inhibitor, wortmanin, provided evidence that p-AKT contributes to the phosphorylation of Drp1 at S616 in PCa cells (Figure 5H). We also confirmed the levels of pDrp1S616 and pDrp1S637 in MN and PT from AA and CA PCa patients, and observed that pDrp1S616 was lower and pDrp1S637 was higher in AA compared to CA patients (Figure S7A and B). Reduced accumulation of p-AKT in AA PCa cells suggests that either activation of AKT or inhibition of c-Myc and NF-κB may contribute to Drp1 phosphorylation at S616, which promotes CC release and sensitizes AA PCa cells to DOC. We observed that c-Myc or NF-κB inhibitor or AKT activator increased the levels of p-Drp1S616 in AA PCa cells (Figure 5I) that resulted in CC release in response to DOC treatment (Figure 5B-D).
We silenced Drp1 in DOC-responsive LNCaP cells and treated with DOC (Figure 5J). Drp1-silencing inhibited CC release, caspase-3 activation, PARP cleavage, and apoptosis in LNCaP cells (Figure 5K and L, and S8A and B). To confirm that increased caspase-3 activation is mediated by Drp1 and CC, Drp1-silenced or CC-silenced AA PCa cells were treated with c-Myc or NF-κB inhibitor or AKT activator with or without DOC. Our findings demonstrated that Drp1 and CC knock down greatly attenuated caspase-3 activation induced by either c-Myc/NF-κB inhibition or AKT activation with or without DOC treatment (Figure S9A and B). Taken together, these data showed that deficiency of Drp1 phosphorylation at S616 abrogated CC release and inhibited apoptosis in response to DOC in AA PCa cells.
CC-deficiency confers metabolic reprogramming in AA primary tumor and PCa cell lines
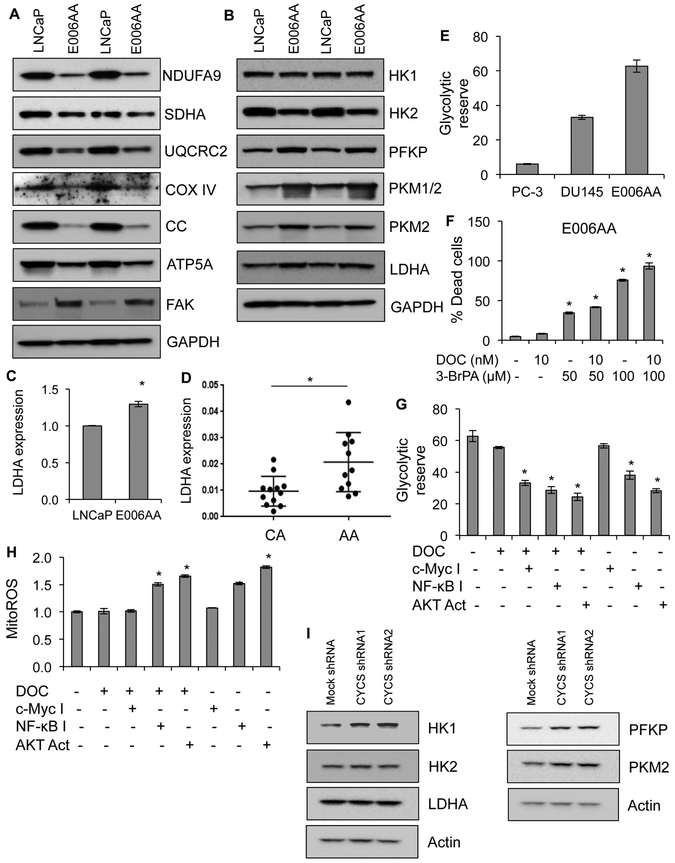
The physiological function of CC is to transport electrons from Complex III to Complex IV of OXPHOS system (14,25). Therefore, loss of CC may lead to metabolic reprogramming in AA PCa cells and AA PT tissues. OXPHOS subunits of Complexes I-V were reduced (Figure 6A), whereas glycolytic enzymes and other glycolysis modulators were upregulated in AA PCa compared to CA PCa cells (Figure 6B). Immunoblot analysis of MN and PT tissue samples from AA and CA men with PCa demonstrated that OXPHOS subunits of Complexes III, IV, and V were downregulated (Figure S10A and B), whereas lactate dehydrogenase A (LDHA) was upregulated, in AA PT compared to CA PT tissues (Figure S11A and B). Increased expression of LDHA mRNA in AA PCa cells and PT established that lack of CC caused the acquisition of a glycolytic phenotype in AA PCa cells and AA PT (Figure 6C and D). Higher glycolytic reserve capacity in AA PCa cells than CA PCa cells (Figure 6E) suggested that AA PCa cells depend more on glycolysis than CA PCa cells for survival, resistance, and proliferation. Disruption of glycolysis via 3-bromopyruvate (3-BrPA) induced dose-dependent cell death in response to DOC in AA PCa cells (Figure 6F).
Figure 6. CC-deficiency causes metabolic reprogramming in AA primary tumor and AA PCa cells.
A, Expression levels of OXPHOS complex subunits and FAK in LNCaP and E006AA cells by immunoblot analysis. NDUFA9 for Complex I, succinate dehydrogenase A (SDHA) for Complex II, UQCRC2 for Complex III, cytochrome c oxidase subunit IV (COX IV) for Complex IV; ATP5A for Complex V. GAPDH serves as a loading control. B, Expression level of glycolytic enzymes including focal adhesion kinase (FAK), hexokinase 1 (HK1), hexokinase 2 (HK2), phosphofructokinase platelet isoform (PFKP), pyruvate kinase M ½ (PKM ½), and lactate dehydrogenase A (LDHA) in LNCaP and E006AA cells using immunoblot analysis. GAPDH serves as a loading control. C, Expression of LDHA at mRNA level using RT-PCR in LNCaP and E006AA cells. D, Expression of LDHA at mRNA level using RT-PCR in primary tumor isolated from AA and CA men with PCa. E, Measurement of glycolytic reserve capacity in PC-3, DU145, and E006AA cells using Seahorse XF analyzer. F, Cell death quantification in E006AA cells treated with DOC alone or DOC in combination with glycolytic disruptor 3-BrPA (3-Bromopyruvate) for 24 hrs. G, Measurement of glycolytic reserve capacity using Seahorse XF analyzer in E006AA cells treated with DOC or DOC in combination with either c-Myc inhibitor or NF-κB inhibitor or AKT activator. H, Measurement of mitochondrial ROS production using MitoSOX dye in E006AA cells treated with DOC or DOC in combination with either c-Myc inhibitor or NF-κB inhibitor or AKT activator using flow cytometry. I, Expression level of glycolytic enzymes including hexokinase 1 (HK1), hexokinase 2 (HK2), phosphofructokinase platelet isoform (PFKP), pyruvate kinase M 2 (PKM2), and lactate dehydrogenase A (LDHA) in mock- and CC- silenced LNCaP cells using immunoblot analysis. Actin serves as a loading control. Data represent mean ± SD of 3 independent experiments. Significant differences between means were assessed using analysis of variance (ANOVA) and GraphPad Prism Version 6.0. *p < 0.05 vs respective controls.
Treatment of AA PCa cells with c-Myc/NF-κB inhibitors or the AKT activator in the presence of DOC inhibited glycolytic reserve capacity in AA PCa cells compared to control or DOC alone (Figure 6G). AKT activation alone inhibited glycolytic reserve in AA PCa cells, suggesting that AKT activation is sufficient to block aerobic glycolysis in AA PCa cells. MitoROS production in response to NF-κB inhibition or AKT activation alone or in combination with DOC further provides evidence that enhanced mitochondrial activity and sensitivity to DOC in AA PCa cells (Figure 6H). To analyze the effect of CC expression on metabolic reprogramming, we knocked down CC in LNCaP cells and observed elevated expression of glycolytic proteins in these CA PCa cells (Figure 6I). Taken together, these data suggest that CC-deficiency and the glycolytic phenotype contribute to higher therapy resistance and aggressiveness in AA men with PCa.
Inhibition of c-Myc or NF-κB enhances therapeutic efficacy of DOC via CC upregulation, caspase-3 activation, and PARP cleavage in AA PCa xenograft tumors
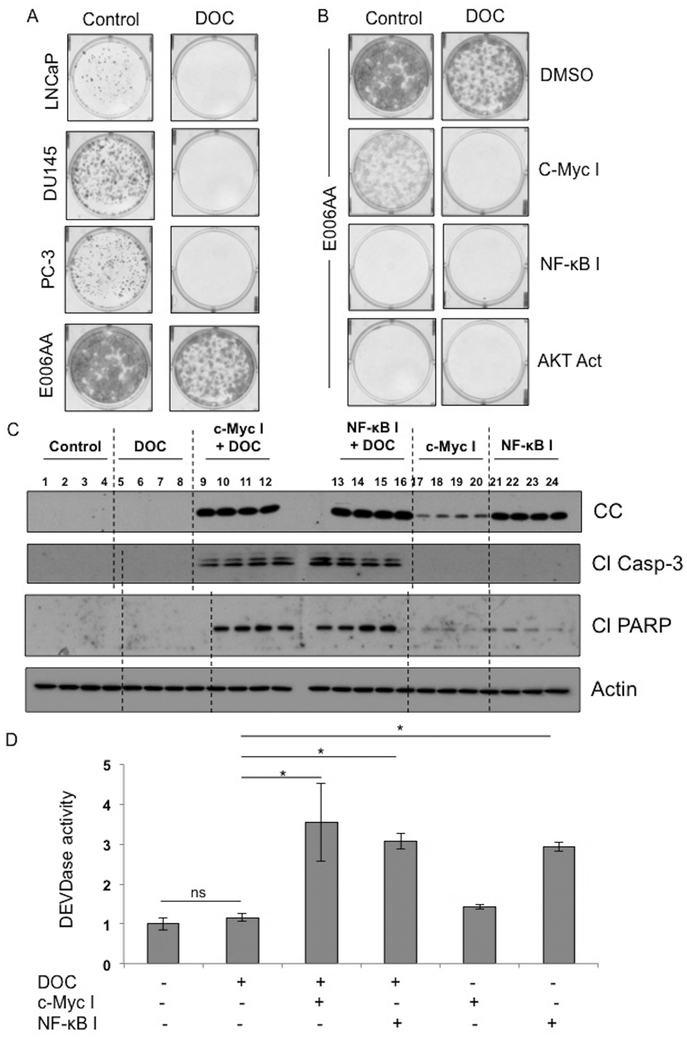
To determine therapeutic efficacy of DOC upon inhibition of c-Myc/NF-κB, we first analyzed clonogenicity or colony forming ability (CFA) of AA and CA PCa cells. We observed higher CFA in AA PCa cells than CA PCa cells (Figure 7A). Exposure of DOC abolished CFA of CA PCa cells but not AA PCa (Figure 7A). Either c-Myc/NF-κB inhibition or AKT activation with or without DOC reduced the CFA of AA PCa cells (Figure 7B). These findings prompted the evaluation of the effect of c-Myc or NF-κB inhibition on efficacy of DOC in vivo using PCa xenografts. AA PCa E006AA hT xenografts in SCID mice were treated with c-Myc or NF-κB inhibitors with or without DOC twice weekly. Inhibition of either c-Myc or NF-κB alone induced CC expression in E006AA hT xenografts (Figure 7C). In combination with DOC, the expression of CC was further upregulated leading to caspase-3 activation, and PARP cleavage in E006AA hT xenografts (Figure 7C and D). Taken together, these data clearly suggest that inhibition of c-Myc or NF-κB and DOC may be an effective therapeutic approach for the management of PCa in AA patients.
Figure 7. Inhibition of c-Myc or NF-κB enhances therapeutic efficacy of DOC in AA PCa xenografts.
A, Clonogenic analysis of LNCaP, DU145, PC-3 and E006AA cells in response to DOC treatment. B, Clonogenic analysis of E006AA cells treated with DOC or DOC in combination with either c-Myc inhibitor or NF-κB inhibitor or AKT activator. C, Immunoblot analysis of CC, caspase-3 cleavage, or PARP cleavage in E006AA hT xenografts treated with DOC or DOC in combination with c-Myc inhibitor or NF-κB inhibitor. D, Caspase-3 activity in E006AA hT xenografts treated with DOC or DOC in combination with c-Myc inhibitor or NF-κB inhibitor. Data represent mean ± SD of 4 independent experiments. Significant differences between means were assessed using analysis of variance (ANOVA) and GraphPad Prism Version 6.0. *p < 0.05 vs respective controls.
Discussion
This study provides the first comprehensive evidence that lack of CC plays a critical role in therapeutic resistance and development of aggressive disease among AA men with PCa. Patients with relapsed PCa after androgen deprivation therapy are treated often with taxane-based therapy, such as DOC. Lack of CC or reduced CC release is the driving force for apoptosome dysfunction leading to inhibition of apoptotic cell death (42), which may contribute to therapeutic resistance and recurrence upon treatment with chemotherapeutic agents, such as DOC. Our findings using a variety of AA and CA PCa cell lines, and PT specimens suggest that CC-deficiency is a key reason for abrogated apoptosome formation/function in AA men with PCa. This notion is supported by the demonstration that exogenous addition of CC in purified cytosol activates caspases, suggesting that all required components except CC are active for apoptosome formation and function. Expression of endogenous CC using CRISPR-SAM technique induces caspase activation and cell death in AA PCa cells. Knockdown of CC in CA PCa cells inhibits caspase activation and cell death. Taken together, our findings provide evidence that lack of CC in PCa cells in AA men is a key reason for higher therapeutic resistance and faster relapse of advanced PCa. Apoptosis also can be executed by a caspase-independent mechanism (43), defects in permeabilization of the mitochondrial membrane preclude this possibility.
Apoptosome dysfunction could result from defects in permeabilization of the outer mitochondrial membrane because pharmacological restoration of CC in AA PCa is not sufficient to induce apoptosis. Our findings establish that outer mitochondrial membrane permeabilization machinery is faulty in AA PCa cells due to increased accumulation of inactivating phosphorylation of Drp1 at serine637 residue (p-Drp1S637) at mitochondria. Compelling evidence suggests that p-Drp1S637 inhibits mitochondrial fragmentation and CC release (38,44), but other studies reveal that p-Drp1S637 may also promote permeabilization of mitochondrial membrane in some types of cells (39). Our data indicate that accumulation of Drp1S637 inhibits outer mitochondrial membrane permeabilization in AA PCa cells. In contrast to AA PCa cells, robust accumulation of activating phosphorylation of Drp1 at serine616 (p-Drp1S616) was observed in CA PCa cells, which promotes outer mitochondrial permeabilization leading to CC release and caspase activation (20,37,40). Although phosphorylation of Drp1 at S616 is mediated by AMPK and Erk2 in other cell types (39,41), our study identifies that AKT, and not Erk2, is the key kinase responsible for Drp1 phosphorylation at S616 in PCa cells.
Aerobic glycolysis confers selective advantage to cancer cells, such as AA PCa cells, and leads to inhibition of apoptotic cell death, increased proliferation, and the aggressive tumor phenotype (45). Our findings provide evidence that lack of CC concomitantly associates with higher expression of various glycolytic proteins including LDHA, c-Myc, and NF-κB. These proteins are critical for possible reprogramming of mitochondrial metabolism and bioenergetics in AA PCa cells and AA tumor tissues, which promote survival, proliferation, and aggressiveness of AA PCa. Knockdown of CC expression in CA PCa cells leads to acquisition of glycolytic characteristics and mitochondrial dysfunction that causes CA PCa cells to adopt the AA PCa cell phenotype. These observations further support our conclusion that CC-deficiency is the cause of mitochondrial dysfunction in AA PCa.
PGC1-α and Nrf1, two major transcription factors, monitor mitochondrial mass and function by regulating the expression of mitochondrial proteins critical for mitochondrial biogenesis, such as mitochondrial transcription factor A (TFAM), OXPHOS complexes, CC, and other metabolism pathways like glutaminolysis (28,46). Thus, expression of PGC1-α and Nrf1 in PCa has been reported to correlate with favorable clinical outcome (47,48). Our findings suggest that acquisition of mitochondrial dysfunction and therapeutic resistance is due to the abolished nuclear accumulation of Nrf1 that causes loss of CC in AA PCa tissues and AA PCa cells.
How is Nrf1 nuclear translocation inhibited in AA PCa cells? Proto-oncogenes c-Myc and NF-κB were upregulated in the nuclear compartment, whereas p-AKT was reduced in AA PCa cells compared to CA PCa cells. c-Myc, NF-κB, and AKT are key players that promote mitochondrial dysfunction and aerobic glycolysis in malignant cells (49), and our observations confirm that c-Myc expression is increased in AA PT compared to CA PT tissues. If c-Myc and NF-κB contribute to acquisition of a glycolytic phenotype in AA PCa, inhibition of c-Myc and NF-κB should block Nrf1 nuclear translocation or transcriptional activity in AA PCa cells. Genetic and pharmacological inhibition of these two proteins induces Nrf1 nuclear translocation and it’s binding to the CC promoter, which ultimately leads to increased expression of CC. On the contrary, AKT signaling was suppressed in AA PCa cells compared to CA PCa cells and activation of AKT in AA PCa cells by inhibiting PTEN enhances Nrf1 activity and CC expression in AA PCa.
Although further experiments are required for making firm conclusions, increased expression of c-Myc in matched non-tumor prostate tissues in AA PCa patients may contribute to increased incidence of clinical PCa in AA compared to CA men. This notion is based on the understanding that c-Myc is a known promoter of prostate carcinogenesis and overexpression of human c-Myc in murine prostate leads to PCa development (50). Thus c-Myc overexpression may be an early alteration during prostate tumorigenesis among AA men. Overexpression of c-Myc induces oncogenic transformation in organoids generated from AA non-tumor prostate epithelial tissue, which further establishes the importance of c-Myc upregulation in PCa health disparity (15). Overall NF-κB expression was similar between AA and CA PCa cells, but increased NF-κB nuclear translocation was observed in AA PCa cells. Previous reports showed increased expression of NF-κB in AA PCa compared to CA PCa (16). NF-κB, a key promoter of inflammation, is a pre-requisite for PCa development and progression by regulating pro-growth cytokines and chemokines. Overexpression of c-Myc and NF-κB may serve as initiating events in prostate tumorigenesis, so loss-of-function mutation in tumor suppressor p53 and Rb1 or gain-of-function mutations in tumor promoters, such as Ras may contribute to higher incidence, greater acquisition of the aggressive phenotype, and enhanced resistance to therapy in AA compared to CA men.
This study provides the first comprehensive and mechanistic analysis of apoptosome and mitochondrial dysfunction, which contribute to therapeutic resistance and higher aggressiveness in AA compared to CA PCa patients. The key reason for apoptosome and mitochondrial dysfunction in AA PCa patients is the loss of CC in PT tissues. c-Myc and NF-κB-mediated suppression of Nrf1 pinpoint the loss of CC, and inhibition of c-Myc/NF-κB sensitizes AA PCa cells to DOC both in vitro and in vivo. Taken together, our findings conclude that loss of CC in AA PCa men is a hallmark event that is succeeded by mitochondrial and apoptosome dysfunction, which drives the development of therapeutic resistance an aggressive phenotype in AA men. This study also supports future research and clinical trials testing the combination of DOC and either c-Myc or NF-κB inhibition for AA PCa patients.
Supplementary Material
Significance:Mechanistic insights on prostate cancer health disparity among American men provide novel approaches to restore mitochondrial function, which can address therapeutic resistance and aggressiveness in African American men with prostate cancer
Acknowledgement.
Drs. Dean G. Tang and David W. Goodrich critically read the manuscript. This work was supported by the National Cancer Institute of the National Institutes of Health under Award Number RO1-CA160685 and the American Cancer Society Research Scholar Grant RSG-12–214-01 – CCG to DC, and in part by the National Cancer Institute Center Support Grant P30-CA016056 to the Roswell Park Comprehensive Cancer Center that supports the Pathology Network, Flow and Image Cytometry, Bioinformatics, Biostatistics, Immune Analysis Shared Resources, Genomic Shared Resources and the Onsite Supply Center.
Financial support: This work was supported by the National Cancer Institute (Award Number RO1-CA160685 and the American Cancer Society Research Scholar Grant RSG-12–214-01 – CCG to D. Chandra. This work is also supported in part by the National Cancer Institute Center Support Grant P30-CA016056 to the Roswell Park Comprehensive Cancer Center.
Footnotes
Conflict of interest statement:
The authors declare no potential conflicts of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Williams VL, Awasthi S, Fink AK, Pow-Sang JM, Park JY, Gerke T, et al. African-American men and prostate cancer-specific mortality: a competing risk analysis of a large institutional cohort, 1989–2015. Cancer Med 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang BD, Ceniccola K, Hwang S, Andrawis R, Horvath A, Freedman JA, et al. Alternative splicing promotes tumour aggressiveness and drug resistance in African American prostate cancer. Nat Commun 2017;8:15921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khani F, Mosquera JM, Park K, Blattner M, O’Reilly C, MacDonald TY, et al. Evidence for molecular differences in prostate cancer between African American and Caucasian men. Clinical cancer research : an official journal of the American Association for Cancer Research 2014;20(18):4925–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuletra JG, Kamenko A, Ramsey F, Eun DD, Reese AC. African-American men with prostate cancer have larger tumor volume than Caucasian men despite no difference in serum prostate specific antigen. Can J Urol 2018;25(1):9193–98. [PubMed] [Google Scholar]
- 6.Huang FW, Mosquera JM, Garofalo A, Oh C, Baco M, Amin-Mansour A, et al. Exome Sequencing of African-American Prostate Cancer Reveals Loss-of-Function ERF Mutations. Cancer Discov 2017;7(9):973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGinley KF, Tay KJ, Moul JW. Prostate cancer in men of African origin. Nat Rev Urol 2016;13(2):99–107. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Wang J, Zhang L, Karatas OF, Shao L, Zhang Y, et al. RGS12 Is a Novel Tumor-Suppressor Gene in African American Prostate Cancer That Represses AKT and MNX1 Expression. Cancer Res 2017;77(16):4247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhary AK, Bhat TA, Kumar S, Kumar A, Kumar R, Underwood W, et al. Mitochondrial dysfunction-mediated apoptosis resistance associates with defective heat shock protein response in African-American men with prostate cancer. Br J Cancer 2016;114(10):1090–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ray AM, Zuhlke KA, Levin AM, Douglas JA, Cooney KA, Petros JA. Sequence variation in the mitochondrial gene cytochrome c oxidase subunit I and prostate cancer in African American men. Prostate 2009;69(9):956–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koochekpour S, Marlowe T, Singh KK, Attwood K, Chandra D. Reduced mitochondrial DNA content associates with poor prognosis of prostate cancer in African American men. PLoS One 2013;8(9):e74688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalsbeek AMF, Chan EKF, Corcoran NM, Hovens CM, Hayes VM. Mitochondrial genome variation and prostate cancer: a review of the mutational landscape and application to clinical management. Oncotarget 2017;8(41):71342–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCrow JP, Petersen DC, Louw M, Chan EK, Harmeyer K, Vecchiarelli S, et al. Spectrum of mitochondrial genomic variation and associated clinical presentation of prostate cancer in South African men. Prostate 2016;76(4):349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yadav N, Chandra D. Mitochondrial DNA mutations and breast tumorigenesis. Biochimica et biophysica acta 2013;1836(2):336–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Unno K, Roh M, Yoo YA, Al-Shraideh Y, Wang L, Nonn L, et al. Modeling African American prostate adenocarcinoma by inducing defined genetic alterations in organoids. Oncotarget 2017;8(31):51264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powell IJ, Dyson G, Land S, Ruterbusch J, Bock CH, Lenk S, et al. Genes associated with prostate cancer are differentially expressed in African American and European American men. Cancer Epidemiol Biomarkers Prev 2013;22(5):891–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson RF, Perkins ND. Nuclear factor-kappaB, p53, and mitochondria: regulation of cellular metabolism and the Warburg effect. Trends Biochem Sci 2012;37(8):317–24. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh AL, Walton ZE, Altman BJ, Stine ZE, Dang CV. MYC and metabolism on the path to cancer. Semin Cell Dev Biol 2015;43:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Algeciras-Schimnich A, Pietras EM, Barnhart BC, Legembre P, Vijayan S, Holbeck SL, et al. Two CD95 tumor classes with different sensitivities to antitumor drugs. Proceedings of the National Academy of Sciences of the United States of America 2003;100(20):11445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997;91(4):479–89. [DOI] [PubMed] [Google Scholar]
- 21.Malladi S, Challa-Malladi M, Fearnhead HO, Bratton SB. The Apaf-1*procaspase-9 apoptosome complex functions as a proteolytic-based molecular timer. The EMBO journal 2009;28(13):1916–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng TC, Hong C, Akey IV, Yuan S, Akey CW. A near atomic structure of the active human apoptosome. eLife 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu JR, Opipari AW, Tan L, Jiang Y, Zhang Y, Tang H, et al. Dysfunctional apoptosome activation in ovarian cancer: implications for chemoresistance. Cancer Res 2002;62(3):924–31. [PubMed] [Google Scholar]
- 24.Schafer ZT, Kornbluth S. The apoptosome: physiological, developmental, and pathological modes of regulation. Developmental cell 2006;10(5):549–61. [DOI] [PubMed] [Google Scholar]
- 25.Yadav N, Chandra D. Mitochondrial and postmitochondrial survival signaling in cancer. Mitochondrion 2014;16:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer 2013;13(10):714–26. [DOI] [PubMed] [Google Scholar]
- 27.Evans MJ, Scarpulla RC. Interaction of nuclear factors with multiple sites in the somatic cytochrome c promoter. Characterization of upstream NRF-1, ATF, and intron Sp1 recognition sequences. J Biol Chem 1989;264(24):14361–8. [PubMed] [Google Scholar]
- 28.Vercauteren K, Pasko RA, Gleyzer N, Marino VM, Scarpulla RC. PGC-1-related coactivator: immediate early expression and characterization of a CREB/NRF-1 binding domain associated with cytochrome c promoter occupancy and respiratory growth. Mol Cell Biol 2006;26(20):7409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang H, Magilnick N, Lee C, Kalmaz D, Ou X, Chan JY, et al. Nrf1 and Nrf2 regulate rat glutamate-cysteine ligase catalytic subunit transcription indirectly via NF-kappaB and AP-1. Mol Cell Biol 2005;25(14):5933–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrish F, Giedt C, Hockenbery D. c-MYC apoptotic function is mediated by NRF-1 target genes. Genes Dev 2003;17(2):240–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith CJ, Dorsey TH, Tang W, Jordan SV, Loffredo CA, Ambs S. Aspirin Use Reduces the Risk of Aggressive Prostate Cancer and Disease Recurrence in African-American Men. Cancer Epidemiol Biomarkers Prev 2017;26(6):845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang BD, Yang Q, Ceniccola K, Bianco F, Andrawis R, Jarrett T, et al. Androgen receptor-target genes in african american prostate cancer disparities. Prostate Cancer 2013;2013:763569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hock MB, Kralli A. Transcriptional control of mitochondrial biogenesis and function. Annu Rev Physiol 2009;71:177–203. [DOI] [PubMed] [Google Scholar]
- 34.Piantadosi CA, Suliman HB. Mitochondrial transcription factor A induction by redox activation of nuclear respiratory factor 1. J Biol Chem 2006;281(1):324–33. [DOI] [PubMed] [Google Scholar]
- 35.Estaquier J, Arnoult D. Inhibiting Drp1-mediated mitochondrial fission selectively prevents the release of cytochrome c during apoptosis. Cell Death Differ 2007;14(6):1086–94. [DOI] [PubMed] [Google Scholar]
- 36.Landes T, Martinou JC. Mitochondrial outer membrane permeabilization during apoptosis: the role of mitochondrial fission. Biochimica et biophysica acta 2011;1813(4):540–5. [DOI] [PubMed] [Google Scholar]
- 37.Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Developmental cell 2008;14(2):193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han XJ, Lu YF, Li SA, Kaitsuka T, Sato Y, Tomizawa K, et al. CaM kinase I alpha-induced phosphorylation of Drp1 regulates mitochondrial morphology. The Journal of cell biology 2008;182(3):573–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO reports 2007;8(10):939–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wieder SY, Serasinghe MN, Sung JC, Choi DC, Birge MB, Yao JL, et al. Activation of the Mitochondrial Fragmentation Protein DRP1 Correlates with BRAF(V600E) Melanoma. J Invest Dermatol 2015;135(10):2544–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kashatus JA, Nascimento A, Myers LJ, Sher A, Byrne FL, Hoehn KL, et al. Erk2 phosphorylation of Drp1 promotes mitochondrial fission and MAPK-driven tumor growth. Mol Cell 2015;57(3):537–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ichim G, Lopez J, Ahmed SU, Muthalagu N, Giampazolias E, Delgado ME, et al. Limited mitochondrial permeabilization causes DNA damage and genomic instability in the absence of cell death. Mol Cell 2015;57(5):860–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tait SW, Green DR. Caspase-independent cell death: leaving the set without the final cut. Oncogene 2008;27(50):6452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang CR, Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem 2007;282(30):21583–7. [DOI] [PubMed] [Google Scholar]
- 45.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009;324(5930):1029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev 2004;18(4):357–68. [DOI] [PubMed] [Google Scholar]
- 47.Piao Y, Kim HG, Oh MS, Pak YK. Overexpression of TFAM, NRF-1 and myr-AKT protects the MPP(+)-induced mitochondrial dysfunctions in neuronal cells. Biochimica et biophysica acta 2012;1820(5):577–85. [DOI] [PubMed] [Google Scholar]
- 48.Torrano V, Valcarcel-Jimenez L, Cortazar AR, Liu X, Urosevic J, Castillo-Martin M, et al. The metabolic co-regulator PGC1alpha suppresses prostate cancer metastasis. Nat Cell Biol 2016;18(6):645–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okoh VO, Felty Q, Parkash J, Poppiti R, Roy D. Reactive oxygen species via redox signaling to PI3K/AKT pathway contribute to the malignant growth of 4-hydroxy estradiol-transformed mammary epithelial cells. PLoS One 2013;8(2):e54206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R, et al. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell 2003;4(3):223–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.