Abstract
Objective:
Challenges with efficient patient recruitment including socio-technical barriers for clinical trials are major barriers to the timely and efficacious conduct of translational studies. We conducted a time-and-motion study to investigate the workflow of clinical trial enrollment in a pediatric emergency department.
Methods:
We observed clinical research coordinators during three clinically-staffed shifts. One clinical research coordinator was shadowed at a time. Tasks were marked in 30 second intervals and annotated to include: patient screening, patient contact, performing procedures, and physician contact. Statistical analysis was conducted on the patient enrollment activities.
Results:
We conducted fifteen 120-minute observations from 12/12/2013 to 1/3/2014 and shadowed 8 clinical research coordinators. Patient screening took 31.62% of their time, patient contact took 18.67%, performing procedures took 17.6%, physician contact was 1%, and other activities took 31.0%.
Conclusions:
Screening patients for eligibility constituted the most time. Automated screening methods could help reduce this time. The findings suggest improvement areas in recruitment planning to increase the efficiency of clinical trial enrollment.
Keywords: Medical Informatics, Emergency Services, Hospital, Pediatrics, Clinical Trials as Topic
1. INTRODUCTION
Clinical trials are experiments in biomedical research involving human participants. These trials are critical to the progress of medical science. However, challenges with patient recruitment for clinical trials represent major barriers to the timely and efficacious conduct of translational research.1–3 In current practice clinical trial staff (e.g., clinical research coordinators (CRCs)) and physicians conduct the eligibility screening manually, including reviewing patients’ electronic health records (EHRs) for demographic and clinical information, then collating and matching this information to trial requirements, and finally identifying eligible patients for the trial. After screening, the staff then approaches the eligible patients for enrollment this involves walking the patient through the informed consent process, study procedures, any study-specific enrollment tasks, and finally baseline data collection and study enrollment and start. The workflow is difficult and time-consuming with coordinators screening electronic data manually, posing a significant financial burden for an institution undertaking clinical research.3
The pediatric emergency department (ED) is an appropriate place for many types of research activities due to the variety and complexity of presenting complaints and varied demographics of patients.4–7 In addition, prior literature studies have shown, patients are amenable to participation in research studies in the ED.8 Successful trials have included those facilitated by large research networks,9 site-initiated studies,10, 11 and external division-based studies.
Despite the advantages of ED research, due to the unplanned nature of ED visits, the CRCs must screen and enroll patients during their visit. Frequently, eligibility screening (ES) is performed manually by the CRCs during a clinic visit, but in this setting, there is time prior to the visit to review records and improve the efficiency of who to approach apriori.12–15 Manual ES of patients in the ED is often time consuming as it may require repetitive review of medical records as well as identifying and locating clinical staff to answer questions regarding a patient’s condition and/or treatment during the active care of the patient. Manual ES is inefficient, but is standard practice in conducting clinical trials. Time saved not performing manual ES could be redirected to conducting enrollment procedures that research staff are most equipped to do, maximizing efficient use of CRC skill set. Patient recruitment in the ED has many other challenges including fluctuating patient volumes and that active medical treatment takes precedence over trial recruitment. For these reasons, we wanted to identify the key areas of clinical trial enrollment performed by CRCs could be improved or streamlined to help facilitate research in the ED.
Time-and-motion methodology has been used to evaluate efficiency of clinical activities,16, 17 and to study how to increase the efficiency in care and can result in changes to documentation and unit organization.16, 18 A time-and-motion study is a continuous, observational study where an observer watches the subject performing a task and uses a timekeeping device (e.g., stopwatch) to record the time taken to accomplish the task. The methodology is mainly used in documentation analysis19 and when there is a variety of dissimilar tasks assigned to one individual, as it is able to pin-point time-wasting steps in busy, fragmented clinical care; although it can be used to study other common clinical tasks as well.20 Results of time and motion analyses can suggest ways to improve efficiency, reduce redundant work, and improve workflow.21 They can also identify the negative effects of technology implementations and improved patient care through safer systems.22–25 In the study procedures, careful observation and recording of tasks are performed continually without interference. Complex tasks are broken into simple steps to help detect redundant motion.
This manuscript describes an observational study of clinical research coordinators in a pediatric ED to evaluate the time spent on patient screening, enrollment, and all other tasks. We leveraged the time-and-motion methodology to investigate the workflow of clinical trial enrollment in the pediatric ED to examine socio-technical aspects of technology and to identify areas to improve efficiency.
2. MATERIALS AND METHODS
2.1. Study Design
We performed a prospective, observational time and motion study in an urban, tertiary care pediatric ED to evaluate the research team’s workflow. Approval of ethics for this study was given by the Cincinnati Children’s Hospital Medical Center (CCHMC) institutional review board (study ID: 2012–2771) and a waiver of consent was authorized.
2.2. Setting
CCHMC is a level 1 trauma center with 628 beds. The ED and urgent care sites have approximately 120,000 annual patient visits. All patient data is recorded electronically via the EHR and it has been active in the ED since 2009. All orders, clinical documentation, and patient notes are electronic.
2.3. Current ED Clinical Trials During the Study Period
The ED currently manages numerous single and multi-center trials with a successful track record of meeting study enrollment goals. Active trials during the study period included: (1) The performance of the AppyScore™ test in the evaluation of possible acute appendicitis in children, adolescents and young adults presenting to the ED; (2) Exploring a cessation intervention for low income smokers in an emergency setting; (3) STAT-ED: suicidal teens accessing treatment after an ED visit; (4) Catalyzing ambulatory research on pneumonia etiology and diagnostic innovations in emergency medicine (CARPE DIEM); (5) An assessment of HIV knowledge among adolescents utilizing the pediatric ED; (6) Clinical decision rules to discriminate bruising caused by physical child abuse from bruising caused by accidental trauma; along with multiple other studies from outside divisions occurring in the ED.
2.4. Participants
The ED has a team of eight CRCs and two undergraduate students that are responsible for eligibility screening, consent and the conduct of all enrollment procedures for research participants in the ED. There are CRCs present in the ED seven days a week. CRCs recruit for sixteen hours a day Monday through Thursday, fourteen hours a day on Fridays and Sundays and ten hours a day on Saturdays. Each study has a lead CRC that is responsible for the overall management of the study. All eight CRCs were included in the study. The CRCs varied in experience from 6 months to 8 years.
2.5. Study Procedures
Observations were conducted from 12/12/2013 to 1/3/2014, including morning (8am-12pm), afternoon (1pm-5pm) and evening (6pm-10pm) shift. One CRC was shadowed at a time while he/she enrolled patients in the ED in each session and his/her activities were recorded in 30-second increments; the CRC to shadow during a shift was chosen randomly. The activity categories are listed in Figure 1, with associated sub-categories. The numbers of patients screened, approached and enrolled were also recorded.
Figure 1.
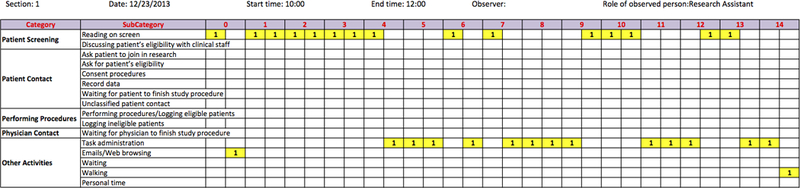
A sample sheet for recording CRC workflow activities in 15 minutes (the major activities the CRC was engaged in during the 30-second period were marked with yellow color and labelled with a “1” by the observer).
Similar to previous studies,24, 26 we used observation based time-and-motion study to conduct the investigation in the CCHMC ED. One graduate student (observer) was hired to track how the observed CRC allocated his/her time during 120-minute observation periods at 30-second intervals/increments. During the observation period, the observer shadowed one CRC to observe the patient recruitment workflow and measure the time the CRC had to spend in each activity. One or two major activities the CRC was engaged in were chosen by the observer during the 30-second period and recorded to the data sheet (Figure 1). The major categories for activities include Patient Screening, Patient Contact, Performing Procedures, Physician Contact and Other Activities (Table 1). Each category has several subcategories. The observer shadowed and followed the CRCs step by step without conversation during the observation periods to minimize the Hawthorne effect.27, 28
Table 1.
Time and percentage over all time spent on workflow activities.
| Category | Subcategory | Minutes per Section (±SD) | Percentage |
|---|---|---|---|
| Patient Screening (31.62%) |
Reading EHR content on screen | 36.0 (±20.18) | 29.4 |
| Discussing patient’s eligibility with clinical staff | 2.67 (±2.01) | 2.18 | |
| Patient Contact (18.67%) |
Ask patient to join in research | 4.03 (±2.72) | 3.30 |
| Ask for patient’s eligibility | 0.07 (±0.25) | 0.05 | |
| Consent procedures | 4.73 (±3.65) | 3.87 | |
| Record data | 8.67 (±11.29) | 7.09 | |
| Waiting for patient to finish study procedure | 3.83 (±5.13) | 3.13 | |
| Unclassified patient contact | 1.50 (±2.42) | 1.23 | |
| Performing Procedures (17.63%) |
Performing procedures/Logging eligible patients | 18.5 (±12.39) | 15.2 |
| Logging ineligible patients | 3.03 (±4.10) | 2.48 | |
| Physician Contact (1.09%) |
Waiting for physician to finish study procedure | 1.33 (±2.05) | 1.09 |
| Other Activities (30.99%) |
Administrative Tasks | 14.2 (±7.46) | 11.6 |
| Emails/Web browsing | 2.97 (±5.03) | 2.43 | |
| Waiting | 7.70 (±9.34) | 6.30 | |
| Walking | 12.6 (±5.87) | 10.3 | |
| Personal time | 0.47 (±1.50) | 0.38 |
The workflow (Figure 2) for CRCs conducting patient recruitment was studied. During the ES section, a CRC had to spend time reviewing the chart on the computer screen to determine the patient’s initial eligibility for trials. In some situations, the CRC would discuss the patient’s eligibility with clinical staff first and then determine whether go further to contact patients. The CRC would then approach patients and their families who initially seemed eligible, introduce the study, verify patient eligibility and then ask patients and their families to join in the research. If the patient family was willing to participate in the research consent was obtained. After consent was obtained, the CRC would begin study procedures. The procedures may include data collection from the family or medical staff and/or specimen collection from the patient. All patients screened for study participation, enrolled or not, were documented electronically into the patient’s EHR and/or research database by the CRC. The time for logging eligible/ineligible patients, administrative tasks (e.g., data entry, follow up phone calls), and email/web browsing on the computer screen were recorded as subcategories. Walking, waiting and personal time were also recorded.
Figure 2.
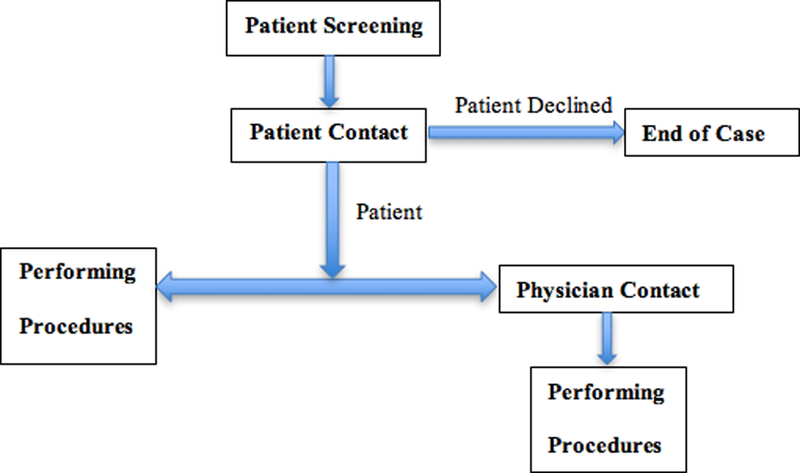
Workflow for CRCs in ED.
2.6. Data Analysis
The primary outcome was time spent in each activity. Time spent was summarized with mean and standard deviation. The categories of workflow activities were Patient Screening, Patient Contact, Performing Procedures, Physician Contact, and Other Activities. Subcategories are defined in Table 1. Secondary outcomes included suggestions of workflow improvement, areas to increase the efficiency of clinical trial enrollment, number of patients screened and enrolled, and activities in subcategories. Chi-squared was used to compare eligibility enrollment.
3. RESULTS
3.1. Study Data
We completed 30 hours of CRC observation (fifteen 120-minute observations), including 5 morning, 5 afternoon, and 5 evening shifts. The observations were conducted on 8 CRCs within the two-week time frame. Table 1 shows how CRCs allotted their time: approximately one third (31.62%) of the total time spent on Patient Screening; another one third (30.99%) of time spent on other activities. The Other Activities time period is large, however, CRCs are limited by the number of patients that arrive in the PED during a shift and frequently use down time performing other tasks (e.g. Administrative Tasks). The time spent on Patient Contact and Performing Procedures are 18.67% and 17.63%, respectively. The CRCs spent most of their time on Reading on Screen (29.44%) among the subcategories. The observation also shows that the CRCs spent a notable amount of time Walking (10.3%) and Waiting (6.3%).
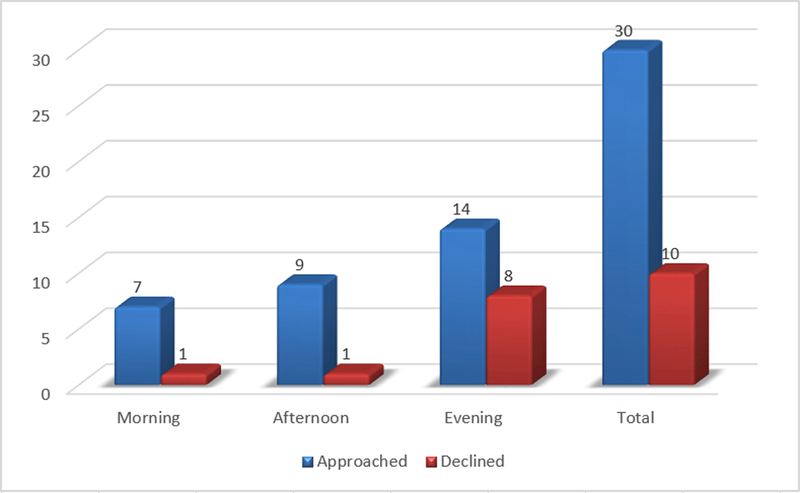
Table 2 shows the descriptive statistics on patient recruitment. The CRCs approached 30 out of 197 screened patients and enrolled 20 subjects (enrollment 66.7%). Table 3 shows the comparison of patient enrollment for the morning, afternoon and evening shifts. Among the 10 declined cases, 8 occurred in the evening, 1 in the morning and 1 in the afternoon. The decline rates for morning, afternoon and evening were statistically significantly different (p=4.626E-8).
Table 2.
Numbers on patients screened, approached, declined and enrolled during the observation periods.
| Section | Shift Information | Enrollment Information | ||||||
|---|---|---|---|---|---|---|---|---|
| Date | Start Time | End Time | Day of Week | Screened | Approached | Declined | Enrolled | |
| 1 | 12/23/2013 | 10:00 | 12:00 | Monday | 2 | 1 | 0 | 1 |
| 2 | 12/23/2013 | 19:00 | 21:00 | Monday | 13 | 5 | 2 | 3 |
| 3 | 12/26/2013 | 15:00 | 17:00 | Thursday | 17 | 1 | 0 | 1 |
| 4 | 12/26/2013 | 19:00 | 21:00 | Thursday | 9 | 3 | 2 | 1 |
| 5 | 12/27/2013 | 10:00 | 12:00 | Friday | 6 | 0 | 0 | 0 |
| 6 | 12/27/2013 | 15:00 | 17:00 | Friday | 39 | 1 | 0 | 1 |
| 7 | 12/28/2013 | 18:00 | 20:00 | Saturday | 15 | 3 | 3 | 0 |
| 8 | 12/30/2013 | 15:00 | 17:00 | Monday | 14 | 2 | 1 | 1 |
| 9 | 12/30/2013 | 20:00 | 22:00 | Monday | 10 | 2 | 1 | 1 |
| 10 | 12/31/2013 | 10:00 | 12:00 | Tuesday | 8 | 2 | 1 | 1 |
| 11 | 12/31/2013 | 15:00 | 17:00 | Tuesday | 11 | 3 | 0 | 3 |
| 12 | 01/02/2014 | 10:00 | 12:00 | Thursday | 5 | 3 | 0 | 3 |
| 13 | 01/02/2014 | 17:00 | 19:00 | Thursday | 11 | 1 | 0 | 1 |
| 14 | 01/03/2014 | 10:00 | 12:00 | Friday | 16 | 1 | 0 | 1 |
| 15 | 01/03/2014 | 15:00 | 17:00 | Friday | 21 | 2 | 0 | 2 |
| Total | 197 | 30 | 10 | 20 | ||||
Table 3.
Statistics of the morning, afternoon and evening shifts on patient recruitment.
| Section Time | Screened | Approached | Declined | Enrolled | Decline Rate |
|---|---|---|---|---|---|
| Morning | 37 | 7 | 1 | 6 | 14.29% |
| Afternoon | 102 | 9 | 1 | 8 | 11.11% |
| Evening | 58 | 14 | 8 | 6 | 57.14% |
| Total | 197 | 30 | 10 | 20 | 33.33% |
4. DISCUSSION
While a lot of research studies take place in the PED, there are limited studies examining the CRCs and their processes. Patient Screening and Performing Procedures (e.g. logging eligible patients) occupied 50% of the CRCs’ time. Although some procedures cannot be reduced by computerized methods (e.g. prepping laboratory samples), screening and logging patients’ eligibility including refusal reasons could be partially automated. This time could be potentially reduced by computerized approaches such as automated ES and documenting.29, 30
The CRCs spent a notable amount of time (10.3%) in Walking, suggesting that relocating the workspace could be helpful. Since the study was performed, the CRC’s workplace was relocated to be more central to the ED operations (Figure 3). Finally, the waiting time (6.3%) could be greatly reduced by setting up an automated alert to inform the CRCs of patients’ activities (e.g., patient undergoing radiographs or tests) so that they could choose a more opportune time to approach the patients without waiting and repeated walking.
Figure 3.
Workplace relocation after study completion.
We observed that the CRCs spent 29.4% of the time reading the screen to identify eligible patients, the most of any single task performed. For a general pediatric clinical trial, if a CRC spends approximately 30% of their time screening patients, the screening process can result in an annual operating cost of approximately $13,000. Since clinical trials have coded inclusion criteria (e.g., age), and providers continually file clinical note data during a visit, it is possible to use an algorithm to decrease the initial screening time. Rule-based alerts are increasing in popularity, but are not implemented in our ED. Patient screening is an integral area where time could be saved using automated methods.29 Implementation of screening algorithms into the CRC workflow could help improve screening and possibly improve patient recruitment into research studies.
Regarding patient enrollment, we observed statistically significant difference of decline rates between shifts. The enrollment time slots are independent of enrollment rate. Patients were more easily enrolled in the morning and afternoon, and tended to decline in the evening. Patient volumes are highest in the afternoons and evenings, and higher volume is associated with increased wait time. As wait times increase, patient satisfaction decreases,31 which may explain the reluctance to agree to participate and contribute to the higher study decline rate for the evening shift.32, 33 Future work will be to investigate the difference of decline rates including how this may influence participation.34 The findings could suggest improvement areas in recruitment planning to increase the efficiency of clinical trial enrollment.
In this study we investigated the processes of patient recruitment in the pediatric ED to identify inefficiencies that may be streamlined to improve workflow. Using the time-and-motion methodology, we identified several workflow areas that could be improved by computerized approaches such as automated ES and documenting. In addition, the study suggested a notable amount of time in repeated walking, which was mitigated by the relocation of workspace. The findings could suggest improvement areas in recruitment planning to increase the efficiency of clinical trial enrollment.
Acknowledgments
Funding/Support: This work was supported by NIH grants 5R00LM010227–05, 1-R01-LM012230–01 and 1U01HG008666.
Abbreviations:
- ED
emergency department
- EHR
electronic health record
- ES
eligibility screening
- CCHMC
Cincinnati Children’s Hospital Medical Center
- CRC
Clinical Research Coordinator
5. REFERENCES
- 1.Ibrahim GM, Chung C and Bernstein M. Competing for patients: an ethical framework for recruiting patients with brain tumors into clinical trials. J Neurooncol. 2011; 104: 623–7. [DOI] [PubMed] [Google Scholar]
- 2.Penberthy LT, Dahman BA, Petkov VI, et al. Effort required in eligibility screening for clinical trials. J Oncol Pract. 2012; 8: 365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wynn L, Miller S, Faughnan L, et al. Recruitment of infants with sickle cell anemia to a Phase III trial: data from the BABY HUG study. Contemp Clin Trials. 2010; 31: 558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandes CM, Price A and Christenson JM. Does reduced length of stay decrease the number of emergency department patients who leave without seeing a physician? J Emerg Med. 1997; 15: 397–9. [DOI] [PubMed] [Google Scholar]
- 5.Hampers LC, Cha S, Gutglass DJ, et al. Language barriers and resource utilization in a pediatric emergency department. Pediatrics. 1999; 103: 1253–6. [DOI] [PubMed] [Google Scholar]
- 6.Kini NM and Strait RT. Nonurgent use of the pediatric emergency department during the day. Pediatr Emerg Care. 1998; 14: 19–21. [DOI] [PubMed] [Google Scholar]
- 7.Petersen LA, Burstin HR, O’Neil AC, et al. Nonurgent emergency department visits: the effect of having a regular doctor. Med Care. 1998; 36: 1249–55. [DOI] [PubMed] [Google Scholar]
- 8.Taylor RG, Hounchell M, Ho M, et al. Factors associated with participation in research conducted in a pediatric emergency department. Pediatr Emerg Care. 2015; 31: 348–52. [DOI] [PubMed] [Google Scholar]
- 9.Nigrovic LE, Lee LK, Hoyle J, et al. Prevalence of clinically important traumatic brain injuries in children with minor blunt head trauma and isolated severe injury mechanisms. Arch Pediatr Adolesc Med. 2012; 166: 356–61. [DOI] [PubMed] [Google Scholar]
- 10.Freedman SB, Adler M, Seshadri R, et al. Oral ondansetron for gastroenteritis in a pediatric emergency department. N Engl J Med. 2006; 354: 1698–705. [DOI] [PubMed] [Google Scholar]
- 11.Huppert JS, Taylor RG, St Cyr S, et al. Point-of-care testing improves accuracy of STI care in an emergency department. Sex Transm Infect. 2013; 89: 489–94. [DOI] [PubMed] [Google Scholar]
- 12.Beauharnais CC, Larkin ME, Zai AH, et al. Efficacy and cost-effectiveness of an automated screening algorithm in an inpatient clinical trial. Clin Trials. 2012; 9: 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butte AJ, Weinstein DA and Kohane IS. Enrolling patients into clinical trials faster using RealTime Recuiting. Proc AMIA Symp. 2000: 111–5. [PMC free article] [PubMed] [Google Scholar]
- 14.Embi PJ, Jain A, Clark J, et al. Effect of a clinical trial alert system on physician participation in trial recruitment. Arch Intern Med. 2005; 165: 2272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Embi PJ, Jain A, Clark J, et al. Development of an electronic health record-based Clinical Trial Alert system to enhance recruitment at the point of care. AMIA Annu Symp Proc. 2005: 231–5. [PMC free article] [PubMed] [Google Scholar]
- 16.Hendrich A, Chow MP, Skierczynski BA, et al. A 36-hospital time and motion study: how do medical-surgical nurses spend their time? Perm J. 2008; 12: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pizziferri L, Kittler AF, Volk LA, et al. Primary care physician time utilization before and after implementation of an electronic health record: a time-motion study. J Biomed Inform. 2005; 38: 176–88. [DOI] [PubMed] [Google Scholar]
- 18.Ampt A and Westbrook JI. Measuring nurses’ time in medication related tasks prior to the implementation of an electronic medication management system. Stud Health Technol Inform. 2007; 130: 157–67. [PubMed] [Google Scholar]
- 19.Mamykina L, Vawdrey DK, Stetson PD, et al. Clinical documentation: composition or synthesis? J Am Med Inform Assoc. 2012; 19: 1025–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meguerditchian AN, Krotneva S, Reidel K, et al. Medication reconciliation at admission and discharge: a time and motion study. BMC Health Serv Res. 2013; 13: 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poissant L, Pereira J, Tamblyn R, et al. The impact of electronic health records on time efficiency of physicians and nurses: a systematic review. J Am Med Inform Assoc. 2005; 12: 505–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thorpe-Jamison PT, Culley CM, Perera S, et al. Evaluating the impact of computer-generated rounding reports on physician workflow in the nursing home: a feasibility time-motion study. J Am Med Dir Assoc. 2013; 14: 358–62. [DOI] [PubMed] [Google Scholar]
- 23.Westbrook JI, Li L, Georgiou A, et al. Impact of an electronic medication management system on hospital doctors’ and nurses’ work: a controlled pre-post, time and motion study. J Am Med Inform Assoc. 2013; 20: 1150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yen K, Shane EL, Pawar SS, et al. Time motion study in a pediatric emergency department before and after computer physician order entry. Ann Emerg Med. 2009; 53: 462–8.e1. [DOI] [PubMed] [Google Scholar]
- 25.Zhu JN, Weiland TJ, Taylor DM, et al. An observational study of emergency department intern activities. Med J Aust. 2008; 188: 514–9. [DOI] [PubMed] [Google Scholar]
- 26.Hollingsworth JC, Chisholm CD, Giles BK, et al. How do physicians and nurses spend their time in the emergency department? Ann Emerg Med. 1998; 31: 87–91. [DOI] [PubMed] [Google Scholar]
- 27.Mayo E The human problems of an industrial civilization. New York: The Macmillan Co., 1933. [Google Scholar]
- 28.Roethlisberger FJ, Dickson WJ, Wright HA, et al. Management and the worker : an account of a research program conducted by the Western Electric Company, Hawthorne Works, Chicago. Cambridge, Mass: Harvard University Press, 1939. [Google Scholar]
- 29.Ni Y, Kennebeck S, Dexheimer JW, et al. Automated clinical trial eligibility prescreening: increasing the efficiency of patient identification for clinical trials in the emergency department. J Am Med Inform Assoc. 2015; 22: 166–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ni Y, Wright J, Perentesis J, et al. Increasing the efficiency of trial-patient matching: automated clinical trial eligibility pre-screening for pediatric oncology patients. BMC Med Inform Decis Mak. 2015; 15: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moskop JC, Sklar DP, Geiderman JM, et al. Emergency department crowding, part 1--concept, causes, and moral consequences. Ann Emerg Med. 2009; 53: 605–11. [DOI] [PubMed] [Google Scholar]
- 32.Carter EJ, Pouch SM and Larson EL. The relationship between emergency department crowding and patient outcomes: a systematic review. J Nurs Scholarsh. 2014; 46: 106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stang AS, McCusker J, Ciampi A, et al. Emergency department conditions associated with the number of patients who leave a pediatric emergency department before physician assessment. Pediatr Emerg Care. 2013; 29: 1082–90. [DOI] [PubMed] [Google Scholar]
- 34.Ni Y, Beck AF, Taylor R, et al. Will they participate? predicting patients’ response to clinical trial invitations in a pediatric emergency department. [Epub ahead of print]. J Am Med Inform Assoc. 2016. April 27. [DOI] [PMC free article] [PubMed] [Google Scholar]


