Abstract
Biomarkers remain the gold standard for assessing chemical exposure. However, silicone wristbands may provide some added benefits for characterizing personal exposures compared to single biomarker measurements such as decreased costs, non-invasive sampling, and increased ease of analysis. Previously, we validated their use in characterizing exposure to organophosphate flame retardants (PFRs). However, it is unclear whether these results would extend to chemicals like polybrominated diphenyl ethers (PBDEs), which biomagnify and have longer half-lives than PFRs in the body. This study sought to determine if accumulation of PBDEs on wristbands was correlated to serum biomarkers. Adult participants (n=30) provided serum samples and wore wristbands for 7 days. PBDEs and 6 novel brominated flame retardants (BFRs) were measured on wristbands, and serum samples were analyzed for PBDE biomarkers. Like most PBDE congeners, 5 of 6 novel BFRs were detected on wristbands frequently (≥90% of bands). In particular, decabromodiphenyl ethane (DBDPE) was detected in all wristbands in this study and was significantly correlated with BDE-209, suggesting a similar source and exposure pathway. Wristband levels of BDE-47, −99, −100, and −153 were significantly and positively associated with respective serum biomarkers (rs=0.39–0.57, p<0.05). This study demonstrates that silicone wristbands can accurately detect personal PBDE exposures.
Keywords: Wristbands, brominated flame retardants, serum biomarkers, exposure
Graphical Abstract
Introduction
Consumer products have been frequently treated with additive flame retardants chemicals (FRs) as a manner of adhering to flammability standards worldwide. Polybrominated diphenyl ethers (PBDEs) were once the most commonly used class of flame retardant in a wide range of products such as furniture, electronics, and building materials.1 However, due to extensive concerns about their persistence, bioaccumulative potential, and toxicity, PBDEs have been phased out globally over the last two decades, with the most recent being a U.S. voluntary phase out of DecaBDE in 2013.2 Despite the phase-out, PBDEs are still found in products in many homes, likely due to low turnover rates and recycling of furniture, particularly the PentaBDE commercial mixture in furniture. For example, some televisions purchased in the U.S. as recently as 2017 were found to contain DecaBDE within the plastic casing.3–5 As such, people are still being exposed to PBDEs, and serum biomarkers suggest exposure is widespread.6,7
A number of alternative brominated flame retardants (BFRs), some with similar structures to PBDEs, have been increasingly used as PBDE replacements. The PentaBDE commercial mixture, which was typically applied to furniture foam, has been replaced by other commercial mixtures such as Firemaster® 550 and 600 (FM550, FM600), which were recently characterized and shown to contain a variety of aryl phosphates and BFRs, including 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (EH-TBB) and bis(2-ethylhexyl)-tetrabromophthalate (BEH-TEBP).8,9 Octabromotrimethylphenylindane (OBIND), 2,4,6-tris(2,4,6-tribromophenoxy)-1,3,5-triazine (TTBP-TAZ), decabromodiphenyl ethane (DBDPE), and tetrabromobisphenol A bis(2,3-dibromopropyl ether) (TBBPA-DBPE) have been used as replacements for DecaBDE, which was reported to be applied to plastic casings of electronics, among other products.2,10,11
Toxicity of PBDEs has been extensively assessed over the last few decades.12,13 The individual BDE congeners associated with PBDE mixtures have been quantified in a number of environmental matrices such as air, sediment, dust, and human tissues, and negative health impacts such as thyroid hormone dysregulation and neurodevelopment deficits have been associated with exposure to these compounds in both human and animal studies.14–18 In general, less is known about the alternative BFRs that are used as PBDE replacements. Components of FM550 have been associated with endocrine and metabolic disruption in exposed rodents and activation of nuclear receptors regulating adipogenic pathways in in vitro models.19–21 They have also been measured in different environmental matrices and in human biospecimens.4,22,23 Very little is known about the toxicity potential of OBIND, DBDPE, TTBP-TAZ, and TBBPA-DBPE, although they have been detected in the environment, on product wipes, and in household dust.10,11,24–27 With such structural similarity to BDE-209, DBDPE is often assumed to have similar toxicity and has been measured at similar detection frequencies and concentrations to BDE-209 on hand wipes and in dust.28,29 In general, other than measurements in indoor air and house dust, little data is available on potential exposure to these alternative BFRs.
Historically, dust has been used as the primary external exposure measure for PBDEs in North America, as house dust has been shown to be significantly associated with serum PBDE measurements of residents.30,31 In some cases, dust levels have been used as proxies for internal dose to quantitatively assess and monitor individual exposures. Similarly, hand wipes have been used to measure exposure as a method of estimating hand-to-mouth contact and exposure via dermal absorption and have been associated with both serum and urinary biomarkers for flame retardants.32–34 To estimate inhalation exposure, both passive and active air samplers have been deployed in homes; however, personal air monitors have been shown to be generally more effective for sampling individual exposures across several micro-environments.35–37 While all of these matrices serve as important measures for various exposure pathways, they only capture a single pathway or microenvironment (e.g., home environment), and therefore necessitate the collection of numerous samples to capture an integrated picture of individual exposure. Biomarkers, which are typically used to determine internal doses, are invasive to collect (e.g., serum), analytically difficult to measure in biospecimen matrices, and expensive to analyze.
In recent years, silicone wristbands have been used as a method of assessing human exposure to various semi-volatile organic compounds in the environment such as FRs, plasticizers, industrial chemicals, and pesticides.38–43 The wristbands provide the potential for a noninvasive and inexpensive tool for quantitative assessment of personal exposure. These are advantageous over other exposure measures such as dust and air samplers because they capture exposure from multiple microenvironments over a multiday time period. They may also capture individual exposures from multiple routes (i.e., inhalation, dermal absorption, and hand-to-mouth contact), whereas these other measures only often sample one exposure pathway. In this way, wristbands may represent a more sophisticated tool for assessing personal exposures than other methods that are more commonly in use. Previous work in our laboratory has shown that the wristbands serve as a reliable tool for quantifying personal exposure to organophosphate FRs (PFRs), as positive and significant correlations between the PFRs on the wristbands and corresponding metabolites in pooled urine samples were observed.43 Since these compounds are rapidly metabolized (t1/2= hours to days), this result suggested that the wristbands served as a good measurement of short-term exposure. However, it is unclear how well the wristbands would reflect exposure for chemicals that have longer half-lives in the body, such as PBDEs (t1/2= months to years).44 Thus, we sought to examine if wristband levels would be associated with biomarkers for compounds that have longer half-lives in the body.
In the present study, our objective was to evaluate the relationship between PBDEs on silicone wristbands and serum biomarkers as well as analyze the wristbands for a variety of novel BFRs which may serve as replacements for the PBDE compounds. To our knowledge, this is the first study to examine the correlations between PBDEs on silicone wristbands with serum biomarkers, as well as the first to measure several DecaBDE replacements on the wristbands.
Materials and Methods
Study Design.
Study participants were recruited in August 2016 from the Duke University and Durham County communities in North Carolina, United States (n = 30) by convenience sampling. Participants were eligible for the study if they were at least 18 years old, lived in their homes for at least one calendar year, and were willing to provide samples for the study (wear a wristband, give a serum sample, and complete questionnaires). All study protocols and materials related to the study were approved by the Duke University Institutional Review Board, and every participant provided informed consent prior to taking part in any aspect of the study.
Wristband Collection.
Commercially available wristbands were purchased in a single size and black color (24hourwristbands.com, Houston, TX) and cleaned as described in Hammel et al., with two 12-hour Soxhlet extractions with 1:1 ethyl acetate/hexane (v/v) and 1:1 ethyl acetate/methanol (v/v) and passive drying in a fume hood.43 The wristbands were then wrapped in aluminum foil, which had been baked at 450°C, and placed in a labeled 40 mL amber jar. Participants were asked to wear the wristbands continuously for a 7-day period including during bathing, sleeping, or other daily activities. At the end of the study period, participants rewrapped the wristbands in clean aluminum foil and replaced it in the amber jar. Samples were stored at −20°C until extraction. Field blank wristbands (n = 4) were not deployed and were stored at room temperature until extraction.
Serum Collection.
Each participant was asked to provide a blood sample on day 1 of the study period. A trained phlebotomist collected the samples using venipuncture to obtain 6–10 mL of blood in a serum separator tube. The blood was allowed to clot on ice for an hour then centrifuged at 3,000 rpm for 5 minutes to separate the red blood cells from the serum. These samples were stored at −20°C until extraction. Although the sample was taken at the time at which wristbands were eployed (day 1), serum PBDE levels have been shown to be stable over the course of a year (ICCs=0.87–0.99) in a sample size of 52 for the congeners for which we analyzed.45 Therefore, we determined that the time of blood sample during the week-long sample period would not impact associations for exposure assessment.
Questionnaires.
Study participants completed 3 short online surveys on alternating days within the 7 days during which they wore the wristbands. The surveys were similar to those distributed in Hammel et al., and results were handled in a similar fashion, with responses being dichotomized based on the median reported value (or category).43 Briefly, participants were asked to provide information about demographics, time spent in various microenvironments, and a few daily activities such as handwashing frequency. Time spent in various microenvironments was averaged across each of the 3 surveys from the week. Handwashing frequency was queried on two separate days, and the lower reported range was used in analyses. Participants were additionally asked about the number of years they had lived in their current home (1–2, 3–4, 5–6, and 7+ years).
Wristband Extraction.
Wristband samples were extracted and analyzed for a suite of brominated flame retardants including 27 PBDEs, 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (EH-TBB), bis(2-ethylhexyl)-tetrabromophthalate (BEH-TEBP), octabromotrimethylphenylindane (OBIND), decabromodiphenyl ethane (DBDPE), tetrabromobisphenol A bis(2,3-dibromopropyl ether) (TBBPA-DBPE), and 2,4,6-tris(2,4,6-tribromophenoxy)-1,3,5-triazine (TTBP-TAZ) (Table S1, Figure S1). Using solvent-rinsed stainless steel scissors, each wristband and field blank was cut into 8 equal pieces to facilitate extraction then accurately weighed and placed into a labeled 50 mL glass centrifuge tube. The bands were spiked with 13C-EH-TBB (100 ng), 13C-BEH-TEBP (100 ng), 4’-fluoro-2,3’,4,6-tetrabromodiphenylether (FBDE-69; 50 ng), and 13C-decabromodiphenyl ether (13C-BDE-209; 100 ng) as internal standards. The samples were then extracted 3 times with 10 mL 1:1 hexane/acetone (v/v) via sonication extraction then concentrated to 1.0 mL using a Thermo Scientific SpeedVac Concentrator. These extracts were fractionated using Florisil® solid-phase extraction cartridges (Supelclean ENVI-Florisil, 6 mL, 500 mg bed weight; Supelco), eluting the first fraction (F1) with 8 mL hexane for brominated compounds and the second fraction (F2) with 10 mL ethyl acetate for organophosphate esters (data not included here). Each fraction was concentrated again to 1 mL using the SpeedVac Concentrator, with the F2 fractions transferred to autosampler vials and stored at −20 °C for future analysis. The F1 fractions were further purified by eluting through 12.0 g of deactivated silica gel (60–200 mesh), impregnated with 5% concentrated sulfuric acid by mass, using 1:1 hexane/dichloromethane (v/v) in 2 consecutive chromatography columns. Extracts were then concentrated to 1 mL using a nitrogen evaporator system and transferred to autosampler vials for analysis by gas chromatography/mass spectrometry (GC/MS) in electron capture negative chemical ionization (ECNI) mode. Recovery of surrogate standards was evaluated using 13C-2,2’,3,4,5,5’-hexachlorodiphenyl ether (13C-CDE141; 50 ng) for FBDE-69, 13C-EH-TBB, and 13C-BEH-TEBP and 4’-fluoro-2, 2’,3,3’,4,5,5’,6,6’-nonabromodiphenyl ether (FBDE-208; 100 ng) for 13C-BDE-209. Recoveries of FBDE-69, 13C-EH-TBB, 13C-BEH-TEBP, and 13C-BDE-209 were on average 105 ± 1%, 141 ± 8%, 51.2 ± 3%, and 61.1 ± 1%. Additionally, lab blanks (n = 4) were analyzed alongside field blanks (n = 4) and wristband samples for quality assurance and quality control.
TBBPA-DBPE was identified based on the presence of 79 and 81 m/z at a similar retention time as the authentic standard (Table S1, Figure S1). Quantification was performed using a standard from TCI (Tokyo, Japan).
Serum Extraction.
Serum was analyzed for PBDEs and extracted in a manner similar to Stapleton et al. and Hovander et al.46,47 Briefly, serum samples were accurately weighed and spiked with FBDE-69 (5 ng) and 13C-BDE-209 (10 ng) as internal standards. Samples were vortexed following the addition of 1 mL 6.0 M HCl and 5 mL isopropyl alcohol. Then, 10 mL of 1:1 hexane/methyl-tert-butyl ether (MTBE) was added to the samples, which were vortexed again. Samples were allowed to stand for one hour then centrifuged at 2,500 RPM for 20 minutes. Organic layers were removed following centrifugation and extracted twice more with 10 mL hexane:MTBE. After, 5 mL of a 1% KCl solution and 5 mL hexane:MTBE were added to the organic fractions, vortexed, and centrifuged again at 2500 RPM for 20 minutes. Again, the organic layers were removed following centrifugation and extracted twice more with 5 mL hexane:MTBE. Serum extracts were concentrated to 3 mL using a nitrogen evaporator then cleaned using solid-phase silica chromatography. The extracts were eluted through 10 g deactivated silica gel (60–200 mesh), impregnated with 40% sulfuric acid by mass, using 45 mL hexane. The eluent of each sample was again concentrated by nitrogen evaporator to 100 μL, transferred to autosampler vials, and spiked with 5.0 ng 13C-CDE-141 and 10 ng FBDE-208 to assess the recovery of FBDE-69 and 13C-BDE-209, respectively. The samples were analyzed using GC/ECNI-MS for 27 PBDEs. Average recovery of FBDE-69 and 13C-BDE-209 were 102 ± 30% and 69 ± 15%, respectively. Fetal bovine serum, which served as field blanks, and serum SRM 1958 (National Institute of Standards and Technology, Gaithersburg, MD) were extracted alongside the serum samples for quality assurance and quality control. One sample was lost during processing, leading to a sample size of 29. Measurements of SRM 1958 relative to the certified values were 82–123% for the PBDEs detected in serum samples within this study (BDE-28/33, −47, −99, −100, −153). PBDE masses in serum were normalized to total lipid content as well as to mass serum extracted. Total lipid (TL) content were calculated from serum triglycerides (TG) and total cholesterol (CHOL), which were measured via enzymatic techniques (Duke University Hospital Central Automated Laboratory, Durham, NC), using the equation TL=1.33*TG + 1.12*CHOL + 1.48 (g/L).48
Statistical Analyses.
Analyses were performed using SAS statistical software (version 9.4; SAS Institute Inc., Cary, NC). These were only conducted if analytes had >60% detection frequency. For samples with concentrations below the MDL, the concentrations were replaced by MDL divided by 2. All sample concentrations were blank-corrected based on the average field blank concentration. When the data were examined for normality, BFRs on wristbands and serum PBDEs were determined to be log-normally distributed. Therefore, Spearman correlation coefficients (rs) were reported to assess the associations within wristbands and between wristbands and serum biomarkers. To account for the use of parametric and non-parametric statistical analyses, Pearson correlation coefficients using log10-transformed concentrations were also reported (Supporting Information).
Linear regression models were used to examine additional associations between data collected via the questionnaire and PBDE levels in the serum and BFRs on the wristbands. Log10-transformed concentrations of analytes were used for the outcomes within the regression models; thus, beta coefficients were exponentiated and represent multiplicative change in the outcome variable relative to the reference category. PBDE concentrations on the wristbands were categorized into tertiles to examine relationships with serum levels, as a manner of reducing the potential bias from extreme observations and allowing for a more flexible shape of the relationship between the wristbands and serum. Results were assessed at α = 0.05 for significance in all analyses.
Analyses of serum biomarkers were performed with PBDE concentrations normalized to serum wet weight as well as total lipid content. Results between the two normalization methods were non-differentiable; therefore, the lipid-normalized serum concentrations and associations are presented here.
Evaluation of log KOA.
Data from this study and from Hammel et al. were used to examine associations between FR sorption to wristbands and their respective octanol-air partitioning coefficients (log KOA).43 Evaluating how FR sorption on wristbands relates to log KOA could provide additional insights for predicting which semi-volatile organic compounds (SVOCs), and possibly exposure pathways, are best captured by the wristbands. Wristband concentrations for FRs measured in our laboratory were normalized to mass per band per day. Compounds were included in the analysis if they were major components of FR application mixtures, defined as at least 1% by weight. Therefore, a subset of 6 PBDE congeners (BDE-47, −99, −100, −153, −154, and −209), all PFRs from Hammel et al., and novel BFRs detected here (EH-TBB, BEH-TEBP, DBDPE, TBBPA-DBPE, TTBP-TAZ) were included for a total of 15 compounds.8,49,50 BDE-85 meets the criteria of being >1% in the PentaBDE mixture; however, BDE-85 and −155 co-elute in our GC/MS method so it was excluded from this analysis. Log KOA values were calculated using the reported log KOW and Henry’s Law constants reported on the EPA Chemistry Dashboard.51 If experimental values were available, particular for the PBDEs, these were used instead of modeled data. Otherwise, the average predicted value given by the dashboard was used.
Results and Discussion
Of the 30 participants, approximately two-thirds were female, and the mean age was 34 years (range: 23–57 years; Table 1). Every participant completed the three questionnaires and provided all requested samples. The majority of participants (73%) had lived in their current home between 1–2 years.
Table 1.
Characteristics of cohort (n =30).
| Variables | Number (Percent) |
|---|---|
| Sex | |
| Male | 12 (40.0) |
| Female | 18 (60.0) |
| Age (years) | |
| 20–24 | 4(13.3) |
| 25–29 | 9 (30.0) |
| 30–35 | 10 (33.3) |
| 36+ | 7(23.3) |
| Avg times hands washed/day | |
| 1–3 | 4(13.3) |
| 4–6 | 11 (36.7) |
| 7–9 | 9(30.0) |
| 10+ | 6(20.0) |
| Avg hours of day spent in home | |
| ≤15 | 15(50.0) |
| >15 | 15(50.0) |
| Avg hours of day spent in work/school | |
| ≤5.5 | 15(50.0) |
| >5.5 | 15(50.0) |
| Avg hours of day spent in car | |
| ≤1 | 18 (60.0) |
| >1 | 12(40.0) |
BFRs in Individual Matrices.
Wristbands.
Our GC/ECNI-MS method sought to quantify 27 BDEs and 6 novel BFRs; however, only 26 of these BFRs were positively detected in the samples. Among these detected compounds, all BFRs were detected in >90% of samples with the exception of BDE-181, −190, −203, and −205 and OBIND (Table 2). BDE-47 was the most abundant compound measured on the wristbands (GM = 55.9 ng/g band), closely followed by EH-TBB (GM = 43.0 ng/g band). This finding was similar to Kile et al. who analyzed wristbands worn by children and also observed BDE-47 to be most abundant of PBDEs on wristbands with similarly high detection frequency for BDE-47, −99, −100, −154, and −153.42 Of the larger molecular weight compounds, BDE-209, DBDPE, and TBBPA-DBPE were each detected in every wristband at similar levels (GM=12.2, 12.2, and 14.2 ng/g band, respectively).
Table 2.
Geometric means and selected percentiles for BFRs in wristbands (n = 30) and PBDEs in serum (n = 29), with PBDEs reported >60% detection.
| Percentile | |||||||
|---|---|---|---|---|---|---|---|
| Matrix and Compound | Percent detect | MDL (1×10−2 ) | Geometric Mean |
25th | 50th | 75th | Maximum |
| Wristbands (ng/g band) | |||||||
| PBDEs | |||||||
| BDE-17 | 100 | 0.60 | 0.10 | 0.062 | 0.090 | 0.21 | 0.90 |
| BDE-25 | 100 | 2.42 | 0.47 | 0.26 | 0.49 | 0.89 | 3.32 |
| BDE-28,33 | 100 | 10.7 | 2.20 | 1.31 | 2.18 | 3.48 | 14.5 |
| BDE-30 | 96.7 | 2.02 | 0.12 | 0.064 | 0.090 | 0.21 | 0.90 |
| BDE-47 | 100 | 30.1 | 55.9 | 22.5 | 56.8 | 118 | 709 |
| BDE-49 | 100 | 0.48 | 0.50 | 0.19 | 0.50 | 1.23 | 2.61 |
| BDE-66 | 100 | 1.65 | 1.23 | 0.44 | 1.15 | 2.85 | 21.8 |
| BDE-75 | 100 | 7.68 | 1.99 | 1.04 | 1.49 | 2.95 | 23.3 |
| BDE-85,155 | 100 | 1.90 | 1.89 | 0.68 | 2.05 | 4.39 | 35.8 |
| BDE-99 | 100 | 3.73 | 35.4 | 17.6 | 35.0 | 67.2 | 576 |
| BDE-100 | 100 | 3.11 | 13.7 | 4.39 | 13.5 | 37.8 | 212 |
| BDE-138 | 100 | 1.46 | 0.34 | 0.12 | 0.27 | 0.74 | 6.29 |
| BDE-153 | 100 | 2.59 | 3.78 | 1.36 | 3.33 | 7.98 | 59.1 |
| BDE-154 | 100 | 2.48 | 3.26 | 1.07 | 3.12 | 5.78 | 68.8 |
| BDE-181 | 50 | 3.84 | 0.041 | 0.019 | 0.030 | 0.086 | 0.33 |
| BDE-183 | 100 | 1.56 | 0.46 | 0.20 | 0.33 | 0.77 | 7.98 |
| BDE-190 | 60 | 2.78 | 0.046 | 0.014 | 0.044 | 0.086 | 2.74 |
| BDE-191 | 100 | 1.98 | 0.51 | 0.28 | 0.46 | 0.98 | 2.62 |
| BDE-203,205 | 66.7 | 10.4 | 0.14 | 0.052 | 0.15 | 0.25 | 0.83 |
| BDE-209 | 100 | 66.0 | 12.2 | 6.78 | 12.8 | 21.2 | 60.6 |
| Novel BFRs | |||||||
| EH-TBB | 96.7 | 0.683 | 43.0 | 15.5 | 36.5 | 76.0 | 361 |
| BEH-TEBP | 90.0 | 4.40 | 32.6 | 26.3 | 50.6 | 146 | 1060 |
| OBIND | 40 | 5.88 | 0.098 | n.a. | n.a. | 0.083 | 2.46 |
| DBDPE | 100 | 56.7 | 12.2 | 5.43 | 7.83 | 31.3 | 222 |
| TBBPA-DBPE | 100 | 6.50 | 14.2 | 6.86 | 12.9 | 37.5 | 122 |
| TTBP-TAZ | 93.3 | 7.39 | 0.64 | 0.28 | 0.45 | 1.55 | 60.6 |
| Serum (ng/g lipid) | |||||||
| BDE-28,33 | 65.5 | 1.91* | 2.31 | 1.13 | 2.43 | 4.10 | 11.6 |
| BDE-47 | 100 | 1.45* | 6.80 | 3.35 | 5.78 | 12.3 | 174 |
| BDE-99 | 79.3 | 0.72* | 1.46 | 0.97 | 1.32 | 3.17 | 23.2 |
| BDE-100 | 65.5 | 0.72* | 1.44 | 0.53 | 1.48 | 2.78 | 27.6 |
| BDE-153 | 93.1 | 0.72* | 5.78 | 2.83 | 6.72 | 15.1 | 37.3 |
| BDE-154 | 62.1 | 0.72* | 1.19 | 0.45 | 1.29 | 2.73 | 6.72 |
| BDE-209 | 17.2 | 9.46* | - | - | - | - | - |
Serum MDLs are reported in ng/g lipid and not 1×10−2.
Correlations among BFRs detected within the wristbands were assessed for the most prominent PBDE congeners from Table 2 (Table 3). As expected, the PentaBDE components (BDE-28/33, −99, −100, −153, −154) were highly correlated on the wristbands (rs=0.70–0.92, p<0.01). A similar trend for PentaBDEs has been observed in hand wipes and dust as well as serum.5,52–54 BDE-209 was also significantly correlated with DBDPE (rs=0.62, p<0.01), which suggests they have a similar exposure source and provides further evidence for their co-application.5 In flatscreen TVs, DBDPE appears to have been used with BDE-209 based on product wipes and measurements in a plastic TV casing sample.5,10 DBDPE on the wristbands was also positively and significantly correlated with TTBP-TAZ levels (rs=0.39, p<0.05). With the phase-out of BDE-209, the co-application of DBDPE and TTBP-TAZ in electronics could explain similar exposure pathways, which was observed again in a small sample of recently analyzed television casings.5 This suggests that DBDPE was and continues to be co-applied with other halogenated FRs in electronics through different generations of electronic production (pre- and post-BDE-209 phase-out). Additionally, EH-TBB and BEH-TEBP were significantly correlated on the wristbands (rs=0.50, p<0.01), which was expected given their frequent use together in the Firemaster® mixtures and applications in products.8,55 In this study, BFR concentrations on bands were generally not impacted by other covariates such as age or sex of the participants or time spent in various microenvironments, although this is a small sample size (Table S2, S3).
Table 3.
Spearman correlation coefficients for BFRs measured on wristbands (n = 30).
| BDE-28,33 | BDE-47 | BDE-99 | BDE-100 | BDE-153 | BDE-154 | BDE-209 | EH-TBB | BEH-TEBP | DBDPE | TBBPA-DBPE | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BDE-47 | 0.89# | ||||||||||
| BDE-99 | 0.68# | 0.78# | |||||||||
| BDE-100 | 0.83# | 0.92# | 0.86# | ||||||||
| BDE-153 | 0.73# | 0.85# | 0.70# | 0.84# | |||||||
| BDE-154 | 0.70# | 0.84# | 0.81# | 0.93# | 0.92# | ||||||
| BDE-209 | 0.10 | 0.24 | 0.07 | 0.23 | 0.38* | 0.31 | |||||
| EH-TBB | 0.15 | 0.19 | 0.21 | 0.13 | −0.03 | 0.07 | −0.06 | ||||
| BEH-TEBP | −0.07 | 0.05 | −0.21 | −0.16 | −0.09 | −0.17 | −0.09 | 0.50# | |||
| DBDPE | 0.24 | 0.27 | 0.12 | 0.21 | 0.37* | 0.25 | 0.62# | 0.03 | 0.06 | ||
| TBBPA-DBPE | −0.28 | −0.29 | −0.37* | −0.34 | −0.15 | −0.21 | 0.28 | 0.05 | 0.11 | 0.13 | |
| TTBP-TAZ | −0.05 | 0.04 | −0.03 | −0.03 | 0.11 | 0.07 | 0.33 | 0.31 | 0.33 | 0.39* | 0.26 |
p<0.05
p<0.01
Serum.
Serum samples were analyzed for a suite of PBDE compounds. While the serum extraction method used here has only been optimized for PBDEs, we did attempt to screen for the novel BFRs in the serum extracts; however, no discernible peaks were present for novel BFRs. Therefore, it is possible that our extraction method may not have recovered these analytes. Only 6 BDE congeners were detected in >60% of the samples (Table 2). In general, the serum PBDEs were similar to or lower than other reports in the U.S. from recent years.6,7,56,57 While this decline in certain congeners like BDE-47 appears to reflect the PentaBDE phase-out, continued detection of these congeners in serum suggests persistent exposure, which is especially concerning since these congeners have half-lives on the order of years in serum.44
Relationship between Wristbands and Serum.
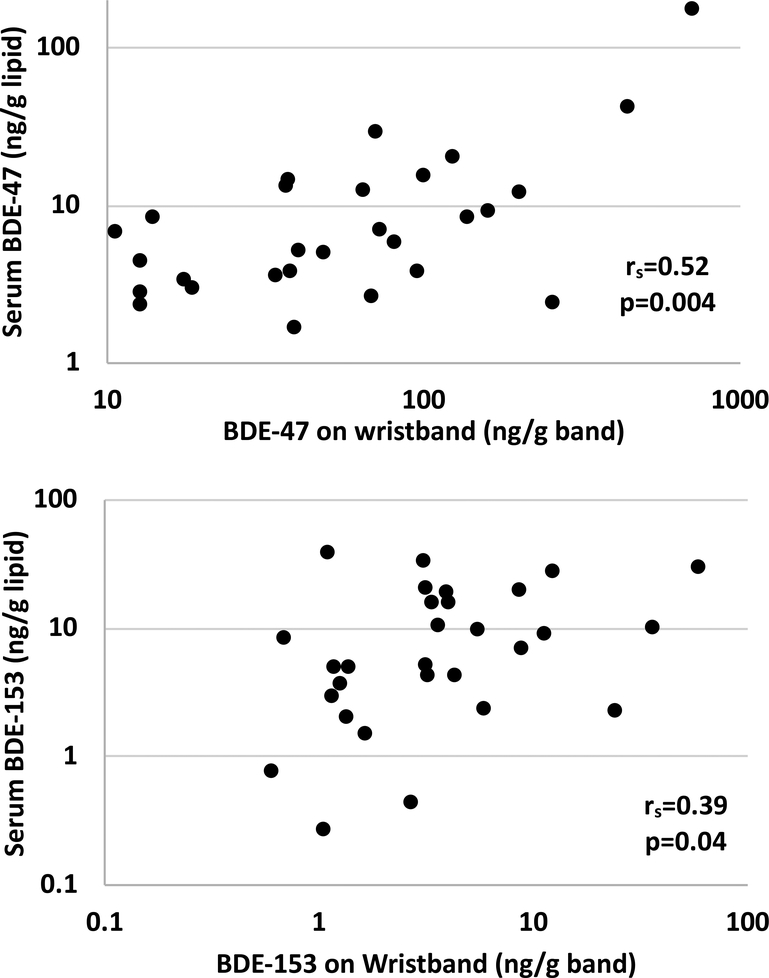
Correlations between wristbands and serum were assessed for the 6 BDE congeners detected in serum. Concentrations of BDE-47, −99, −100s, and −153 measured in paired wristbands and serum were positively and significantly correlated (rs=0.39–0.57, p<0.05; Table 4, Figure 1), suggesting that silicone wristbands worn for seven days capture relevant personal exposures to PBDEs. The wristband-serum relationships were also statistically significant between congeners, in particular BDE-47, −99, and −100, with wristband levels (rs=0.39–0.61, p<0.05; Table 4), which seems reasonable given their likely co-exposure from the PentaBDE commercial mixture. The half-lives of these particular BDEs in serum range from 1.6 to 6.5 years, with BDE-47 and −100 falling on the lower end of the range and BDE-153 having the longest estimated half-life.58 Although the wristbands were worn for only a 7-day period, these data suggest that the wristbands capture quantitative individual exposures to compounds with longer half-lives. The significant relationship between wristbands and serum is likely reflective of a chronic exposure to PBDEs in the home environment, which has been previously shown to be consistent at multiple time points in house dust over the course of a year.59 As dust ingestion is considered to be one of the primary pathways of exposure for PBDEs, it is unlikely that PBDE exposure fluctuates drastically over a short period of time, especially since these compounds were phased out over a decade prior to the sampling period and the participants in this study lived in their homes for at least a year. Similarly, serum PBDEs have been shown to be consistent over a year-long time period, suggesting that the time of blood collection during our study period would likely not impact associations observed between the wristbands and serum.45 Positive and significant correlations between PBDEs in paired house dust and serum samples have also been demonstrated previously.31,33 While PBDEs in the environment and serum may be declining over time, the decline would be gradual and expected to be proportional to prior exposure. Therefore, this decline is also not expected to impact the associations observed between wristbands and serum.
Table 4.
Spearman correlation coefficients for PBDEs measured in paired wristbands and serum (n = 29).
| Serum | |||||||
|---|---|---|---|---|---|---|---|
| BDE-28,33 | BDE-47 | BDE-99 | BDE-100 | BDE-153 | BDE-154 | ||
| Wristbands | BDE-28,33 | 0.28 | 0.41* | 0.34 | 0.39* | 0.29 | 0.43* |
| BDE-47 | 0.35 | 0.52# | 0.45* | 0.39* | 0.44# | 0.33 | |
| BDE-99 | 0.57# | 0.49# | 0.57# | 0.45* | 0.25 | 0.22 | |
| BDE-100 | 0.38* | 0.58# | 0.49# | 0.40* | 0.31 | 0.21 | |
| BDE-153 | 0.21 | 0.51# | 0.42* | 0.42* | 0.39* | 0.20 | |
| BDE-154 | 0.34 | 0.61# | 0.51# | 0.47# | 0.34 | 0.15 | |
| BDE-209 | −0.11 | 0.10 | 0.04 | −0.04 | 0.10 | −0.07 | |
p<0.05
p<0.01
Figure 1.
Associations between wristbands and serum biomarkers for BDE-47 and BDE-153.
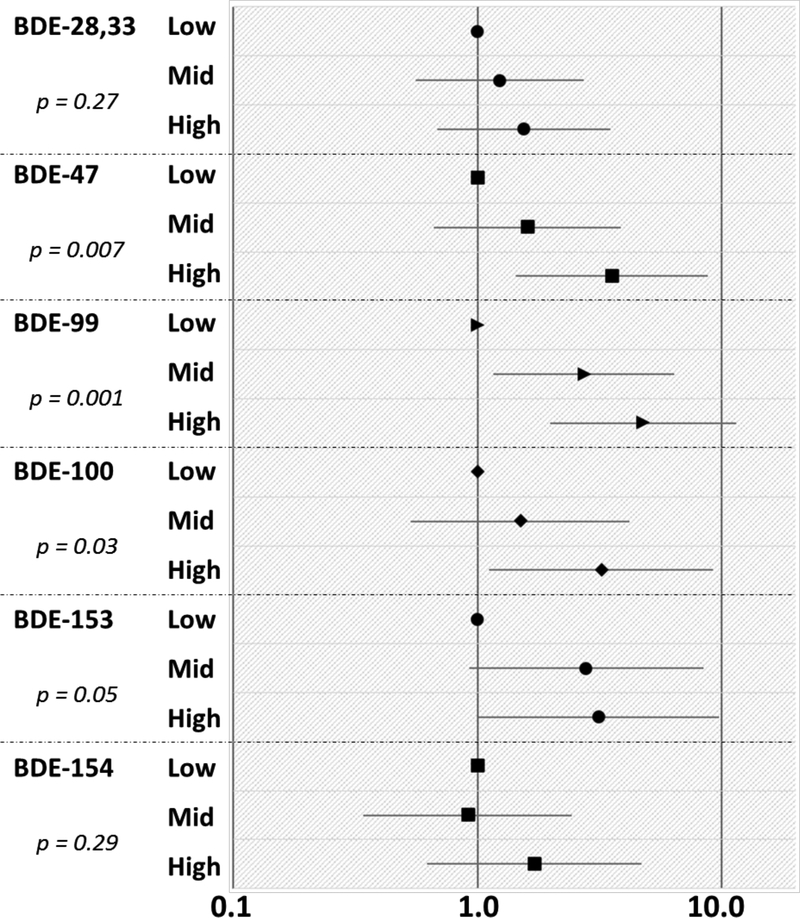
Associations between serum and wristbands were further analyzed by categorizing the BDE levels on the wristbands into tertiles to assess their sensitivity for measuring reflective changes in serum (Figure 2). In general, the trends observed suggest that low, middle, and high BDE levels on the wristbands do indeed reflect similar and significant associations with serum (p<0.05). For example, individuals within the highest tertile of BDE-99 on the wristbands had 3.75 times higher levels of BDE-99 in their serum, compared to the lowest wristband tertile (p=0.001; Figure 2). None of the serum biomarkers were associated with BDE-209 on the wristbands, which is unsurprising since BDE-209 is typically used in different applications from the PentaBDE congeners and may have differing pathways of exposure due to physicochemical properties.
Figure 2.
Multiplicative change (10β) and 95% confidence intervals in serum biomarkers based on the BDE compounds on wristbands categorized by tertiles. The low category for each congener served as the reference. P-values describe the p for trend.
In bivariate analyses, age was positively associated with serum BDE-100 and BDE-154 levels (p<0.05; Table S3). In contrast, during the NHANES 2003–2004 cycle, serum PBDEs were observed to decrease with age until around age 60 after which the levels increase again, possibly due to bioaccumulation or slower metabolism/excretion.60 Our age range was much narrower, with 63% of our study population falling between 25–35 years old (Table 1). Additionally, the NHANES study was prior to the phase-out of PBDEs in the U.S. so exposure patterns for these compounds may have changed since then, thereby explaining the opposite trend with age.
Associations with BDEs and sex, handwashing frequency, or time spent in various microenvironments were generally not observed in this study, with the exception of a suggestive increase in levels of serum BDEs-28/33, −47, and −99 for individuals who reported spending more time each day in their car (10β =2.1–2.4, p<0.1; Table S2, S3). This suggests that perhaps spending more time in a car on a daily basis could lead to an increase in PBDE exposure, or that cars may serve as a source of exposure to these compounds. We were unable to evaluate bathing differences in this study population since the majority of participants (70%) bathed between 6–8 times per week. Although we could not adjust for many potential confounding variables simultaneously due to the small sample size, we attempted to adjust the regression models examining the association between serum and wristbands (categorized in tertiles) for variables associated with serum (e.g., age). The relationship between wristbands and serum was not expected to be confounded by these variables since most were not significantly related to wristband PBDE levels, and the results were qualitatively similar (data not shown).
Wristband Concentrations and log KOA.
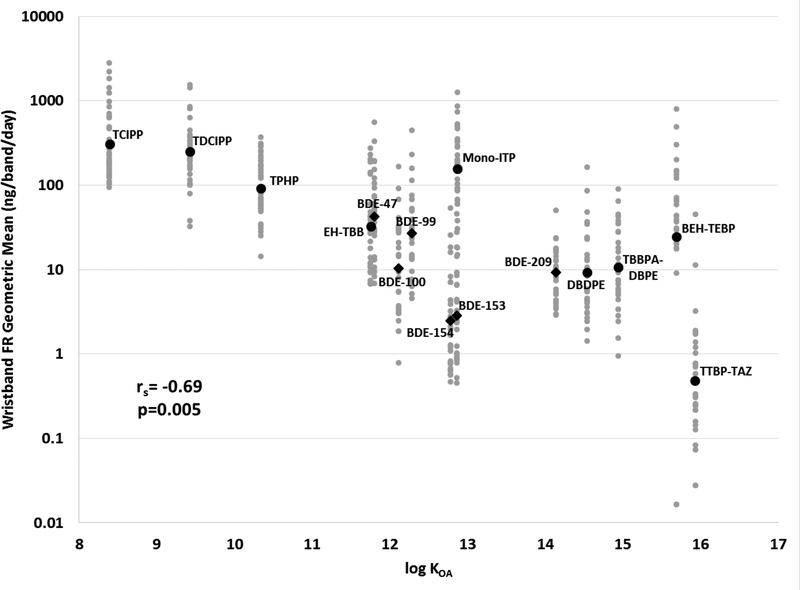
In general, the BFR concentrations measured on wristbands in this study were lower than the PFR concentrations measured in our previous study, which reflects the current trend in household dust.4,53,61,62 In wristbands, this trend could either reflect greater use of PFRs in products in the average indoor environment, or differences in emissions due to differences in their physicochemical properties (e.g., vapor pressure). When the geometric means of 15 FR compounds were plotted against their respective octanol-air partitioning coefficient (log KOA) values, a significant decreasing trend was observed (rs = −0.69, p=0.005; Figure 3). Each individual data point is also graphed with the geometric means, and it is evident that a decreasing geometric mean concentration on the wristbands is associated with an increase in the log KOA values. This suggests that lower molecular weight compounds with higher vapor pressures may be more abundant in the air and become absorbed by the wristbands at higher rates. However, it should be noted that in this analysis we were unable to account for application and emission rates from specific products, which would also be a factor in this trend.63
Figure 3.
Concentrations of 15 compounds measured on the wristbands per band per day based on log KOA of the compound. Each individual wristband is plotted in gray with the geometric mean plotted in black. Geometric means of BDE congeners are demarcated by diamonds with all other FRs are circles. The Spearman correlation coefficient was calculated from the geometric means and log KOA values.
Given their physicochemical properties, it is likely that the high molecular weight compounds such as BDE-209, DBDPE, and TBBPA-DBPE are particle-bound in indoor air (at 25°C) rather than present in the gas phase.64–67 Previous work in a different laboratory examining log KOA and binding to the wristbands reported that the wristbands effectively captured compounds in the log KOA range of 2 to 13; however, preparation of these samples included washing the wristbands with water and isopropanol which could have removed more particles and therefore compounds with higher log KOA values from the wristbands.68 Compounds in the log KOA range of 8 to 12 have been examined in the context of hand wipe samples in both adults and children, with dermal absorption suggested to be a primary exposure pathway via partitioning of semi-volatile organic compounds (SVOCs) from air directly to skin surface lipids.69,70 The fact that the wristbands capture exposure to compounds within this range of log KOA values prompts the question of whether the wristbands themselves may serve as a mimic of the skin surface and therefore capture the dermal absorption exposure pathway, regardless of whether the compounds are in the gas phase or bound to aerosols. As reported by Harner and Shoeib, 80% of BDE-153 would be bound to aerosols at 25°C compared to 17% of BDE-47 at the same temperature.67 Nonetheless, both of these compounds were significantly correlated between wristband BFR levels and serum biomarkers (rs=0.39 for BDE-153, 0.52 for BDE-47, p<0.05, Table 4). While exposure to PFRs have been generally accepted to be via inhalation and dermal absorption, the same has not been the case for PBDEs.71–75 Therefore, wristbands may have the potential to be used in examining the dermal absorption pathway for a large range of SVOCs, which has largely been underestimated in exposure assessment.
Limitations.
Our results should be examined in the context of several limitations. Only one paired wristband was collected per participant and therefore serves as a cross-sectional sample that attempts to predict a longer-term exposure. Additional studies should determine how wristband deployment periods may affect associations with serum biomarkers. The wristband and serum samples were all collected in August 2016 and from a generally homogenous population around Duke University and Durham County, NC. Because our study population was relatively small, we were unable to fully consider for potential confounders; however, the homogeneity of the population could be advantageous by reducing residual confounding. Nonetheless, the homogenous population may limit generalizability. Future studies with larger sample sizes should evaluate potential confounding variables. In our analyses, we assume serum biomarkers are the gold standard for assessing BDE exposure. As some of the compounds for which we analyzed (e.g., DBDPE) do not have reliable internal dose measurements, it prompts the possibility of using external exposure measures such as wristbands as a gold standard for assessing exposure. Given that the current study and previous studies have shown that wristbands hold the potential to enhance assessment of personal exposures, further work with true validation studies are needed to reduce the risk of exposure misclassification if used in future epidemiological studies.
Implications.
Wristbands could potentially be used as an inexpensive and non-invasive method of measuring personal exposure that might replace or complement the complexity of large-scale biomonitoring endeavors. This could provide opportunities for expanded monitoring during critical periods of development or susceptibility, which would be highly beneficial for epidemiological studies. Wristbands also present the potential of measuring integrated exposure to hundreds of compounds with a single sample, thereby allowing for the evaluation of individual exposure to mixtures. Oftentimes, biomarker analysis is limited to single chemicals or classes of compounds due to cost or availability of the biospecimen samples. Despite being considered the gold standard for accurately measuring personal exposure to environmental chemicals, biomarkers often involve invasive and difficult collection techniques and require cumbersome sample processing.76 In addition, the low levels at which biomarkers are often found in the body present an analytical challenge, and some environmental chemicals lack appropriate biomarkers altogether (e.g., those that are rapidly metabolized).77 With the ability to simultaneously sequester compounds from a wide range of physicochemical properties, the diversity of chemical exposures that can be measured with a single wristband sample has the potential to significantly increase the power of environmental epidemiological studies and collect crucial data on exposures to mixtures.
Our results suggest that silicone wristbands may be effective tools for accurately and quantitatively assessing individual PBDE exposure. Because they have been previously effectively used to capture PFR exposure, compounds with shorter half-lives and lower log KOA values compared to PBDEs, this study demonstrates that silicone wristbands are likely effective at capturing personal exposure for a wide range of SVOCs in which inhalation and dermal absorption are predominant exposure pathways.
Supplementary Material
Acknowledgements
Funding for this research was provided by the NIEHS (Grant R01ES016099) and the EPA (Grant 83564201). Additional support for ALP was provided by NIEHS (T32-ES021432). We also gratefully acknowledge the effort provided by our research participants.
Footnotes
All of the authors declare no competing financial interest.
Supporting Information
Additional info on the structures and properties of the novel BFRs (Table S1), univariate regression analyses with wristband BFRs (Table S2), univariate regression analyses with wristband and serum PBDEs (Table S3), Pearson correlation tables for log10-transformed BFRs on wristbands (Table S4), Pearson correlation tables for log10-transformed PBDEs for paired wristbands and serum (Table S5), and a merged extracted ion chromatogram for a wristband sample identifying novel BFRs (Figure S1).
References
- (1).Rahman F; Langford KH; Scrimshaw MD; Lester JN Polybrominated diphenyl ether (PBDE) flame retardants. Sci. Total Environ 2001, 275 (1–3), 1–17. [DOI] [PubMed] [Google Scholar]
- (2).U.S. EPA Design for the Environment. An Alternatives Assessment for the Flame Retardant Decabromodiphenyl Ether (DecaBDE); 2014.
- (3).Cooper EM; Kroeger G; Davis K; Clark CR; Ferguson PL; Stapleton HM Results from Screening Polyurethane Foam Based Consumer Products for Flame Retardant Chemicals: Assessing Impacts on the Change in the Furniture Flammability Standards. Environ. Sci. Technol 2016, 50 (19), 10653–10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Hammel SC; Hoffman K; Lorenzo AM; Chen A; Phillips AL; Butt CM; Sosa JA; Webster TF; Stapleton HM Associations between flame retardant applications in furniture foam, house dust levels, and residents’ serum levels. Environ. Int 2017, 107, 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Toxic Free Future; Clean Production Action. TV Reality: Toxic Flame Retardants in TVs; 2017. [Google Scholar]
- (6).Sjödin A; Wong L-Y; Jones RS; Park A; Zhang Y; Hodge C; Dipietro E; McClure C; Turner W; Needham LL; Patterson DG Serum concentrations of polybrominated diphenyl ethers (PBDEs) and polybrominated biphenyl (PBB) in the United States population: 2003–2004. Environ. Sci. Technol 2008, 42 (4), 1377–1384. [DOI] [PubMed] [Google Scholar]
- (7).Hoffman K; Lorenzo A; Butt CM; Hammel SC; Henderson BB; Roman SA; Scheri RP; Stapleton HM; Sosa JA Exposure to flame retardant chemicals and occurrence and severity of papillary thyroid cancer: A case-control study. Environ. Int 2017, 107, 235–242. [DOI] [PubMed] [Google Scholar]
- (8).Phillips AL; Hammel SC; Konstantinov A; Stapleton HM Characterization of Individual Isopropylated and tert -Butylated Triarylphosphate (ITP and TBPP) Isomers in Several Commercial Flame Retardant Mixtures and House Dust Standard Reference Material SRM 2585. Environ. Sci. Technol 2017, 51 (22), 13443–13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Stapleton HM; Sharma S; Getzinger G; Ferguson PL; Gabriel M; Webster TF; Blum A Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environ. Sci. Technol 2012, 46 (24), 13432–13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Abbasi G; Saini A; Goosey E; Diamond ML Product screening for sources of halogenated flame retardants in Canadian house and office dust. Sci. Total Environ 2016, 545–546, 299–307. [DOI] [PubMed] [Google Scholar]
- (11).Covaci A; Harrad S; Abdallah MA-E; Ali N; Law RJ; Herzke D; de Wit CA Novel brominated flame retardants: A review of their analysis, environmental fate and behaviour. Environ. Int 2011, 37 (2), 532–556. [DOI] [PubMed] [Google Scholar]
- (12).Hites RA Polybrominated Diphenyl Ethers in the Environment and in People: A Meta-Analysis of Concentrations. Environ. Sci. Technol 2004, 38 (4), 945–956. [DOI] [PubMed] [Google Scholar]
- (13).Akortia E; Okonkwo JO; Lupankwa M; Osae SD; Daso AP; Olukunle OI; Chaudhary A A review of sources, levels, and toxicity of polybrominated diphenyl ethers (PBDEs) and their transformation and transport in various environmental compartments. Environ. Rev 2016, 24 (3), 253–273. [Google Scholar]
- (14).Costa LG; de Laat R; Tagliaferri S; Pellacani C A mechanistic view of polybrominated diphenyl ether (PBDE) developmental neurotoxicity. Toxicol. Lett 2014, 230 (2), 282–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Hale RC; Alaee M; Manchester-Neesvig JB; Stapleton HM; Ikonomou MG Polybrominated diphenyl ether flame retardants in the North American environment. Environ. Int 2003, 29 (6), 771–779. [DOI] [PubMed] [Google Scholar]
- (16).Herbstman JB; Sjödin A; Kurzon M; Lederman SA; Jones RS; Rauh V; Needham LL; Tang D; Niedzwiecki M; Wang RY; Perera F Prenatal Exposure to PBDEs and Neurodevelopment. Environ. Health Perspect 2010, 118 (5), 712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Cowell WJ; Lederman SA; Sjödin A; Jones R; Wang S; Perera FP; Wang R; Rauh VA; Herbstman JB Prenatal exposure to polybrominated diphenyl ethers and child attention problems at 3–7 years. Neurotoxicol. Teratol 2015, 52 (Pt B), 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Zhou T; Taylor MM; DeVito MJ; Crofton KM Developmental exposure to brominated diphenyl ethers results in thyroid hormone disruption. Toxicol. Sci 2002, 66 (1), 105–116. [DOI] [PubMed] [Google Scholar]
- (19).Patisaul HB; Roberts SC; Mabrey N; McCaffrey KA; Gear RB; Braun J; Belcher SM; Stapleton HM Accumulation and endocrine disrupting effects of the flame retardant mixture Firemaster® 550 in rats: an exploratory assessment. J. Biochem. Mol. Toxicol 2013, 27 (2), 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Pillai HK; Fang M; Beglov D; Kozakov D; Vajda S; Stapleton HM; Webster TF; Schlezinger JJ Ligand binding and activation of PPARγ by Firemaster® 550: effects on adipogenesis and osteogenesis in vitro. Environ. Health Perspect 2014, 122 (11), 1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Belcher SM; Cookman CJ; Patisaul HB; Stapleton HM In vitro assessment of human nuclear hormone receptor activity and cytotoxicity of the flame retardant mixture FM 550 and its triarylphosphate and brominated components. Toxicol. Lett 2014, 228 (2), 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Hoffman K; Fang M; Horman B; Patisaul HB; Garantziotis S; Birnbaum LS; Stapleton HM Urinary tetrabromobenzoic acid (TBBA) as a biomarker of exposure to the flame retardant mixture Firemaster® 550. Environ. Health Perspect 2014, 122 (9), 963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Butt CM; Congleton J; Hoffman K; Fang M; Stapleton HM Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers. Environ. Sci. Technol 2014, 48 (17), 10432–10438. [DOI] [PubMed] [Google Scholar]
- (24).Lee SC; Sverko E; Harner T; Pozo K; Barresi E; Schachtschneider J; Zaruk D; DeJong M; Narayan J Retrospective analysis of “new” flame retardants in the global atmosphere under the GAPS Network. Environ. Pollut 2016, 217, 62–69. [DOI] [PubMed] [Google Scholar]
- (25).Guerra P; Alaee M; Jiménez B; Pacepavicius G; Marvin C; MacInnis G; Eljarrat E; Barceló D; Champoux L; Fernie K Emerging and historical brominated flame retardants in peregrine falcon (Falco peregrinus) eggs from Canada and Spain. Environ. Int 2012, 40, 179–186. [DOI] [PubMed] [Google Scholar]
- (26).Qi H; Li W-L; Liu L-Y; Zhang Z-F; Zhu N-Z; Song W-W; Ma W-L; Li Y-F Levels, distribution and human exposure of new non-BDE brominated flame retardants in the indoor dust of China. Environ. Pollut 2014, 195, 1–8. [DOI] [PubMed] [Google Scholar]
- (27).Ballesteros-Gómez A; de Boer J; Leonards PEG A Novel Brominated Triazine-based Flame Retardant (TTBP-TAZ) in Plastic Consumer Products and Indoor Dust. Environ. Sci. Technol 2014, 48 (8), 4468–4474. [DOI] [PubMed] [Google Scholar]
- (28).Liu X; Yu G; Cao Z; Wang B; Huang J; Deng S; Wang Y; Shen H; Peng X Estimation of human exposure to halogenated flame retardants through dermal adsorption by skin wipe. Chemosphere 2017, 168, 272–278. [DOI] [PubMed] [Google Scholar]
- (29).Van den Eede N; Dirtu AC; Ali N; Neels H; Covaci A Multi-residue method for the determination of brominated and organophosphate flame retardants in indoor dust. Talanta 2012, 89, 292–300. [DOI] [PubMed] [Google Scholar]
- (30).Watkins DJ; McClean MD; Fraser AJ; Weinberg J; Stapleton HM; Sjödin A; Webster TF Impact of Dust from Multiple Microenvironments and Diet on PentaBDE Body Burden. Environ. Sci. Technol 2012, 46 (2), 1192–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Johnson PI; Stapleton HM; Sjodin A; Meeker JD Relationships between Polybrominated Diphenyl Ether Concentrations in House Dust and Serum. Environ. Sci. Technol 2010, 44 (14), 5627–5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Watkins DJ; McClean MD; Fraser AJ; Weinberg J; Stapleton HM; Sjödin A; Webster TF Exposure to PBDEs in the office environment: Evaluating the relationships between dust, handwipes, and serum. Environ. Health Perspect 2011, 119 (9), 1247–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Stapleton HM; Eagle S; Sjödin A; Webster TF Serum PBDEs in a North Carolina toddler cohort: Associations with handwipes, house dust, and socioeconomic variables. Environ. Health Perspect 2012, 120 (7), 1049–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Hoffman K; Garantziotis S; Birnbaum LS; Stapleton HM Monitoring indoor exposure to organophosphate flame retardants: hand wipes and house dust. Environ. Health Perspect 2015, 123 (2), 160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Bohlin P; Jones KC; Strandberg B Occupational and indoor air exposure to persistent organic pollutants: a review of passive sampling techniques and needs. J. Environ. Monit 2007, 9 (6), 501–509. [DOI] [PubMed] [Google Scholar]
- (36).Allen JG; McClean MD; Stapleton HM; Nelson JW; Webster TF Personal exposure to polybrominated diphenyl ethers (PBDEs) in residential indoor air. Environ. Sci. Technol 2007, 41 (13), 4574–4579. [DOI] [PubMed] [Google Scholar]
- (37).Fromme H; Körner W; Shahin N; Wanner A; Albrecht M; Boehmer S; Parlar H; Mayer R; Liebl B; Bolte G Human exposure to polybrominated diphenyl ethers (PBDE), as evidenced by data from a duplicate diet study, indoor air, house dust, and biomonitoring in Germany. Environ. Int 2009, 35 (8), 1125–1135. [DOI] [PubMed] [Google Scholar]
- (38).O’Connell SG; Kincl LD; Anderson KA Silicone wristbands as personal passive samplers. Environ. Sci. Technol 2014, 48 (6), 3327–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Donald CE; Scott RP; Blaustein KL; Halbleib ML; Sarr M; Jepson PC; Anderson KA Silicone wristbands detect individuals’ pesticide exposures in West Africa. R. Soc. Open Sci 2016, 3 (8), 160433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Bergmann AJ; North PE; Vasquez L; Bello H; del Carmen Gastañaga Ruiz M; Anderson KA Multi-class chemical exposure in rural Peru using silicone wristbands. J. Expo. Sci. Environ. Epidemiol 2017, 27 (6), 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Aerts R; Joly L; Szternfeld P; Tsilikas K; De Cremer K; Castelain P; Aerts J-M; Van Orshoven J; Somers B; Hendrickx M; Andjelkovic M; Van Niewenhuyse A Silicone Wristband Passive Samplers Yield Highly Individualized Pesticide Residue Exposure Profiles. Environ. Sci. Technol 2018, 52 (1), 298–307. [DOI] [PubMed] [Google Scholar]
- (42).Kile ML; Scott RP; O’Connell SG; Lipscomb S; MacDonald M; McClelland M; Anderson KA Using silicone wristbands to evaluate preschool children’s exposure to flame retardants. Environ. Res 2016, 147, 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Hammel SC; Hoffman K; Webster TF; Anderson KA; Stapleton HM Measuring Personal Exposure to Organophosphate Flame Retardants Using Silicone Wristbands and Hand Wipes. Environ. Sci. Technol 2016, 50 (8), 4483–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Thuresson K; Höglund P; Hagmar L; Sjödin A; Bergman A; Jakobsson K Apparent half-lives of hepta- to decabrominated diphenyl ethers in human serum as determined in occupationally exposed workers. Environ. Health Perspect 2006, 114 (2), 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Makey CM; McClean MD; Sjödin A; Weinberg J; Carignan CC; Webster TF Temporal Variability of Polybrominated Diphenyl Ether (PBDE) Serum Concentrations over One Year. Environ. Sci. Technol 2014, 48 (24), 14642–14649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Stapleton HM; Sjödin A; Jones RS; Niehüser S; Zhang Y; Patterson DG Serum Levels of Polybrominated Diphenyl Ethers (PBDEs) in Foam Recyclers and Carpet Installers Working in the United States. Environ. Sci. Technol 2008, 42 (9), 3453–3458. [DOI] [PubMed] [Google Scholar]
- (47).Hovander L; Athanasiadou M; Asplund L; Jensen S; Wehler EK Extraction and Cleanup Methods for Analysis of Phenolic and Neutral Organohalogens in Plasma. J. Anal. Toxicol 2000, 24 (8), 696–703. [DOI] [PubMed] [Google Scholar]
- (48).Covaci A; Voorspoels S; Thomsen C; van Bavel B; Neels H Evaluation of total lipids using enzymatic methods for the normalization of persistent organic pollutant levels in serum. Sci. Total Environ 2006, 366 (1), 361–366. [DOI] [PubMed] [Google Scholar]
- (49).La Guardia MJ; Hale RC; Harvey E Detailed Polybrominated Diphenyl Ether (PBDE) Congener Composition of the Widely Used Penta-, Octa-, and Deca-PBDE Technical Flame-retardant Mixtures. Environ. Sci. Technol 2006, 40 (20), 6247–6254. [DOI] [PubMed] [Google Scholar]
- (50).Stapleton HM; Klosterhaus S; Eagle S; Fuh J; Meeker JD; Blum A; Webster TF Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ. Sci. Technol 2009, 43 (19), 7490–7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).US EPA Chemical Safety for Sustainability Research Program. EPA Chemistry Dashboard.
- (52).Stapleton HM; Kelly SM; Allen JG; Mcclean MD; Webster TF Measurement of polybrominated diphenyl ethers on hand wipes: Estimating exposure from hand-to-mouth contact. Environ. Sci. Technol 2008, 42 (9), 3329–3334. [DOI] [PubMed] [Google Scholar]
- (53).Stapleton HM; Misenheimer J; Hoffman K; Webster TF Flame retardant associations between children’s handwipes and house dust. Chemosphere 2014, 116, 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Zota AR; Rudel RA; Morello-Frosch RA; Brody JG Elevated house dust and serum concentrations of PBDEs in California: unintended consequences of furniture flammability standards? Environ. Sci. Technol 2008, 42 (21), 8158–8164. [DOI] [PubMed] [Google Scholar]
- (55).Stapleton HM; Allen JG; Kelly SM; Konstantinov A; Klosterhaus S; Watkins D; Mcclean MD; Webster TF Alternate and new brominated flame retardants detected in U.S. house dust. Environ. Sci. Technol 2008, 42 (18), 6910–6916. [DOI] [PubMed] [Google Scholar]
- (56).Hurley S; Goldberg D; Nelson DO; Guo W; Wang Y; Baek H-G; Park J-S; Petreas M; Bernstein L; Anton-Culver H; Reynolds P Temporal Evaluation of Polybrominated Diphenyl Ether (PBDE) Serum Levels in Middle-Aged and Older California Women, 2011–2015. Environ. Sci. Technol 2017, 51 (8), 4697–4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Buttke DE; Wolkin A; Stapleton HM; Miranda ML Associations between serum levels of polybrominated diphenyl ether (PBDE) flame retardants and environmental and behavioral factors in pregnant women. J. Expo. Sci. Environ. Epidemiol 2013, 23 (2), 176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Geyer HJ; Schramm K-W; Darnerud PO; Aune M; Feicht EA; Fried KW; Henkelmann B; Lenoir D; Schmid P; McDonald T Terminal elimination half-lives of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. Organohalogen Compd 2004, 66, 3820–3825. [Google Scholar]
- (59).Whitehead TP; Brown FR; Metayer C; Park J-S; Does M; Petreas MX; Buffler PA; Rappaport SM Polybrominated diphenyl ethers in residential dust: Sources of variability. Environ. Int 2013, 57–58, 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Sjödin A; Wong L-Y; Jones RS; Park A; Zhang Y; Hodge C; Dipietro E; McClure C; Turner W; Needham LL; Patterson DG Serum concentrations of polybrominated diphenyl ethers (PBDEs) and polybrominated biphenyl (PBB) in the United States population: 2003–2004. Environ. Sci. Technol 2008, 42 (4), 1377–1384. [DOI] [PubMed] [Google Scholar]
- (61).Brommer S; Harrad S Sources and human exposure implications of concentrations of organophosphate flame retardants in dust from UK cars, classrooms, living rooms, and offices. Environ. Int 2015, 83, 202–207. [DOI] [PubMed] [Google Scholar]
- (62).Cequier E; Ionas AC; Covaci A; Marcé RM; Becher G; Thomsen C Occurrence of a Broad Range of Legacy and Emerging Flame Retardants in Indoor Environments in Norway. Environ. Sci. Technol 2014, 48 (12), 6827–6835. [DOI] [PubMed] [Google Scholar]
- (63).Okeme JO; Saini A; Yang C; Zhu J; Smedes F; Klánová J; Diamond ML Calibration of polydimethylsiloxane and XAD-Pocket passive air samplers (PAS) for measuring gas- and particle-phase SVOCs. Atmos. Environ 2016, 143, 202–208. [Google Scholar]
- (64).Weschler CJ Indoor/outdoor connections exemplified by processes that depend on an organic compound’s saturation vapor pressure. Atmos. Environ 2003, 37 (39–40), 5455–5465. [Google Scholar]
- (65).Weschler CJ; Salthammer T; Fromme H Partitioning of phthalates among the gas phase, airborne particles and settled dust in indoor environments. Atmos. Environ 2008, 42 (7), 1449–1460. [Google Scholar]
- (66).Cousins IT; Mackay D Gas−Particle Partitioning of Organic Compounds and Its Interpretation Using Relative Solubilities. Environ. Sci. Technol 2001, 35 (4), 643–647. [DOI] [PubMed] [Google Scholar]
- (67).Harner T; Shoeib M Measurements of Octanol−Air Partition Coefficients (Koa) for Polybrominated Diphenyl Ethers (PBDEs): Predicting Partitioning in the Environment. J. Chem. Eng. Data 2002, 47 (2), 228–232. [Google Scholar]
- (68).Anderson KA; Points GL; Donald CE; Dixon HM; Scott RP; Wilson G; Tidwell LG; Hoffman PD; Herbstman JB; O’Connell SG Preparation and performance features of wristband samplers and considerations for chemical exposure assessment. J. Expo. Sci. Environ. Epidemiol 2017, 27 (6), 551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Weschler CJ; Nazaroff WW Semivolatile organic compounds in indoor environments. Atmos. Environ 2008, 42 (40), 9018–9040. [Google Scholar]
- (70).Weschler CJ; Nazaroff WW SVOC exposure indoors: fresh look at dermal pathways. Indoor Air 2012, 22 (5), 356–377. [DOI] [PubMed] [Google Scholar]
- (71).Mäkinen MSE; Mäkinen MR; Koistinen JT; Pasanen AL; Pasanen PO; Kalliokoski PJ; Korpi AM Respiratory and dermal exposure to organophosphorus flame retardants and tetrabromobisphenol A at five work environments. Environ. Sci. Technol 2009, 43 (3), 941–947. [DOI] [PubMed] [Google Scholar]
- (72).Abou-Elwafa Abdallah M; Pawar G; Harrad S Human dermal absorption of chlorinated organophosphate flame retardants; implications for human exposure. Toxicol. Appl. Pharmacol 2016, 291, 28–37. [DOI] [PubMed] [Google Scholar]
- (73).Babich MA CPSC Staff Preliminary Risk Assessment of Flame Retardant FR Chemicals in Upholstered Furniture Foam; Bethesda, MD, 2006.
- (74).Schreder ED; Uding N; La Guardia MJ Inhalation a significant exposure route for chlorinated organophosphate flame retardants. Chemosphere 2016, 150, 499–504. [DOI] [PubMed] [Google Scholar]
- (75).Frederiksen M; Stapleton HM; Vorkamp K; Webster TF; Jensen NM; Sørensen JA; Nielsen F; Knudsen LE; Sørensen LS; Clausen PA; Nielsen JB Dermal uptake and percutaneous penetration of organophosphate esters in a human skin ex vivo model. Chemosphere 2018, 197, 185–192. [DOI] [PubMed] [Google Scholar]
- (76).Holland NT; Pfleger L; Berger E; Ho A; Bastaki M Molecular epidemiology biomarkers - Sample collection and processing considerations. In Toxicology and Applied Pharmacology; 2005; Vol. 206, pp 261–268. [DOI] [PubMed] [Google Scholar]
- (77).Pearce N; de Sanjose S; Boffetta P; Kogevinas M; Saracci R; Savitz D Limitations of biomarkers of exposure in cancer epidemiology. Epidemiology 1995, 6 (2), 190–194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.