Abstract
Amikacin liposome inhalation suspension (ALIS; Arikayce®) [formerly known as liposomal amikacin for inhalation, or LAI] is a liposomal formulation of the aminoglycoside antibacterial drug amikacin. The ALIS formulation, administered via inhalation following nebulization, is designed to facilitate targeted and localized drug delivery to the lungs while minimizing systemic exposure. Based on the prespecified primary endpoint analysis of the ongoing phase III CONVERT trial, ALIS has been approved in the USA for use as part of a combination antibacterial drug regimen against Mycobacterium avium complex (MAC) lung disease that is treatment refractory (i.e. an active infection present despite ≥ 6 consecutive months of a multidrug regimen) in adult patients who have limited or no alternative treatment options. In the CONVERT trial, once-daily ALIS as an add-on to guidelines-based therapy (GBT) significantly increased the odds of achieving sputum culture conversion by month 6 compared with GBT alone in patients with treatment-refractory MAC lung disease. The addition of ALIS to GBT was associated with an increased risk of respiratory adverse events compared with GBT alone; however, serious adverse events were experienced by a similar proportion of patients in the two treatment groups. In conclusion, although current evidence for efficacy is limited to microbiological outcomes (with clinical benefit yet to be established), available data suggest that ALIS is a useful option for the treatment of patients with MAC lung disease who have not responded to conventional therapy and for whom there are limited or no alternative treatment options available.
Amikacin liposome inhalation suspension: clinical considerations in MAC lung disease
| A liposomal formulation of amikacin, administered once daily via inhalation following nebulization |
| Designed to facilitate targeted and localized drug delivery to the lungs while minimizing systemic exposure |
| As an add-on to guidelines-based therapy (GBT), ALIS significantly increases the odds of achieving sputum culture conversion compared with GBT alone |
| Acceptable tolerability; carries a black box warning pertaining to a risk of increased respiratory adverse events (leading to hospitalizations in some cases) |
Introduction
Non-tuberculous mycobacteria (NTM) comprise a group of widespread environmental bacteria that can cause infection at a range of sites throughout the body, particularly in individuals with impaired immunity or other risk factors [1, 2]. NTM lung disease, the most common manifestation of NTM infection, is a chronic, frequently progressive and potentially life-threatening condition. It most often occurs in individuals with chronic underlying lung disorders, such as bronchiectasis, chronic obstructive pulmonary disease (COPD), cystic fibrosis or prior tuberculosis. Clinical symptoms of NTM lung disease vary in intensity between individuals and are generally non-specific, but they commonly include a chronic cough, sputum production, fatigue and weight loss. Disease progression can lead to deteriorating lung function (often with permanent damage) [3], reduced physical function and health-related quality of life [4], together with an increased risk of mortality [5, 6].
In developed countries, the prevalence of NTM lung disease has been increasing [7, 8]. In North America, annual rates of disease are approximately 5–10/100,000 individuals [8], although the rates are much higher (> 40/100,000) in individuals aged ≥ 65 years [7, 8]. Approximately 80% of cases of NTM lung disease in the USA are caused by Mycobacterium avium complex (MAC) species (primarily M. avium and Mycobacterium intracellulare) [9].
Treatment of NTM lung disease, including MAC infections, is challenging, with microbiological eradication typically requiring a multidrug antibacterial regimen over a prolonged period [1, 2]. American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) guidelines for the treatment of MAC lung disease recommend that, for patients with macrolide-susceptible MAC isolates, treatment involves a three-drug regimen composed of a macrolide, ethambutol and a rifamycin (rifampin or rifabutin) [1]. The primary microbiological goal of therapy is 12 months of consistently negative sputum cultures while on treatment. For patients who fail to achieve sustained culture conversion, alternative therapeutic options are limited. ATS/IDSA treatment guidelines recommend that for patients with advanced or previously treated disease a course of an intravenous aminoglycoside (streptomycin or amikacin) can be added to the regimen [1]. However, the utility of these intravenous aminoglycosides is often limited due to the risk of systemic toxicities, notably renal and auditory toxicities [1, 10, 11].
Amikacin liposome inhalation suspension (ALIS; Arikayce®) [formerly known as liposomal amikacin for inhalation, or LAI] is a liposomal formulation of amikacin that was designed for nebulization and administration through inhalation, thereby facilitating targeted and localized drug delivery to the lungs while minimizing systemic exposure [12]. Based on the prespecified 6-month primary endpoint analysis of the ongoing, multinational, phase III CONVERT trial [13], ALIS has been approved by the US FDA (under the Limited Population Pathway for Antibacterial and Antifungal Drugs) for use as part of a combination antibacterial drug regimen for the treatment of MAC lung disease in adult patients who have not achieved negative sputum cultures despite ≥ 6 consecutive months of a multidrug background regimen therapy and who have limited or no alternative treatment options [14]. ALIS is the first therapeutic agent to be approved in the USA specifically for the treatment of MAC lung disease.
This article reviews the therapeutic efficacy, safety and tolerability of ALIS in its use as add-on therapy for treatment-refractory MAC lung disease in adults. Relevant pharmacokinetic and antibacterial activity data are also summarized.
Antibacterial Activity
The antibacterial agent amikacin is a polycationic, semisynthetic, broad-spectrum aminoglycoside [14]. As for other aminoglycoside antibacterials, the primary mechanism of action of amikacin involves binding of the 30S ribosomal subunit, leading to inhibition of bacterial protein synthesis [14]. Amikacin has well-established bactericidal activity against a broad range of bacterial species, including Gram-negative and Gram-positive species [15]. Of note, amikacin has displayed potent in vitro activity against clinical isolates of MAC and other NTM species [16, 17]. In a US study of 462 consecutive clinical MAC isolates, 96.2% of isolates had amikacin minimum inhibitory concentrations (MICs) of ≤ 32 μg/mL and 48.9% of isolates had MICs of ≤ 8 μg/mL [16].
ALIS, the liposomal amikacin formulation, is able to penetrate M. avium biofilms in vitro and concentration-dependently reduce viable cell counts at concentrations ≥ 16 μg/mL [18]. It was also shown in an in vitro study that ALIS has activity against intracellular mycobacteria, effectively reducing cell counts of M. avium and Mycobacterium abscessus in infected macrophages [19]. Furthermore, in the study, ALIS appeared to be more effective than free amikacin against intracellular M. avium and M. abscessus [19]. Uptake of ALIS into macrophages had no apparent detrimental effects on macrophage function in vitro [20].
In vivo activity of inhaled, nebulized ALIS against M. avium was demonstrated in a study using a mouse model of respiratory infection [19]. In the study, ALIS administered through inhalation was associated with a reduction in the M. avium burden in the mice that was comparable to that observed with parenterally administered free amikacin [19]. Furthermore, in clinical trials in patients with treatment-refractory NTM lung disease, the addition of ALIS to guidelines-based therapy (GBT) was associated with higher response rates in microbiological endpoints compared with GBT alone (see Sect. 5).
Resistance
Development of resistance to amikacin has been observed infrequently. In mycobacteria, acquired resistance to amikacin most commonly involves modification of the drug target via mutations in the 16S rRNA gene (e.g. A1408G mutation) [16, 17]. In the CONVERT phase III clinical trial (see Sect. 5.1), 23 (10.3%) of 224 patients in the ALIS plus GBT arm and 3 (2.7%) of 112 patients in the GBT alone arm had MAC isolates with a post-baseline amikacin MIC > 64 μg/mL; 7 (26.9%) of these 26 patients subsequently had MAC isolates with an amikacin MIC < 64 μg/mL [13]. Data on mutational resistance are not available for the CONVERT trial.
Drug Delivery
In the ALIS formulation, amikacin (i.e. the active component) is encapsulated in liposomes composed of the natural lipids dipalmitoylphosphatidylcholine (DPPC) and cholesterol [14]. ALIS liposomes are relatively small in size (~ 300 nm in diameter) [21] and have a targeted concentration of amikacin 70 mg/mL with a lipid-to-amikacin weight ratio in the range of 0.60–0.79 [14]. This high drug-to-lipid ratio, which is achieved through a proprietary process, was designed to provide efficient drug delivery and enables the recommended dose in MAC lung disease patients (see Sect. 7) to be administered in ~ 14 min [14].
ALIS is to be administered only using the Lamira™ Nebulizer System [14], a product-specific nebulizer based on Pari Pharma’s eFlow® nebulizer [22]. The Lamira™ system has been optimized for the administration of the ALIS formulation [22]. Compared with the original eFlow® device, the holes in the aerosol head membranes of the Lamira™ system have a slightly different geometry. The Lamira™ system also has a larger medication reservoir compared with the original eFlow® [22].
During nebulization, ~ 70% of the amikacin dose remains encapsulated within liposomes while ~ 30% of the dose is released as free amikacin [13]. Thus, ALIS is delivered to the lungs as a combination of free and liposomal amikacin. When using ALIS with the Lamira™ system, the nebulized aerosol droplets have a mass median aerodynamic diameter of ~ 4.7 μm [14], within the respirable range (< 5 μm), enabling good distribution in the lungs (see Sect. 4).
Pharmacokinetic Properties of ALIS
In patients with NTM lung disease, nebulization and inhalation of ALIS resulted in 43% of the loaded dose being deposited in the lungs [23]. ALIS liposomes distributed to both the central and peripheral lung regions (at a respective ratio of 2.05 immediately following dosing); however, deposition into cavitary and air-trapped areas appeared to be limited. One hour post dose, 79% of the initially deposited dose was retained in the lungs; 53% was retained at 24 h post dose [23]. Uptake of ALIS into macrophages has also been demonstrated, both in vitro and in vivo [18–20]. Compared with free amikacin, ALIS was associated with fourfold increased amikacin uptake into human macrophages in vitro [18]. In a study in rats, ALIS inhalation was associated with five- to eight-fold increased amikacin uptake into alveolar macrophages in vivo relative to inhaled free amikacin [18].
With nebulization and inhalation, ALIS is delivered directly to the lung, resulting in post-dose sputum concentrations that are substantially higher than the peak concentrations (Cmax) observed in serum (Table 1). High variability [coefficient of variation (CV) > 100%] was observed in amikacin concentrations in sputum, with variation in ALIS bioavailability expected to primarily result from differences in nebulizer efficiency and the individual patient’s airway pathology [14]. By 48–72 h after a single dose of ALIS, sputum concentrations of amikacin were ~ 5% of those observed 1–4 h post dose [14].
Table 1.
Sputum and serum exposures of ALIS
| Sputum concentrations at 1–4 h post inhalation after dosing for: | |
| 1 month | 1720 μg/g |
| 3 months | 884 μg/g |
| 6 months | 1300 μg/g |
| Mean serum exposure after 3 months | |
| Cmax | 2.8 μg/mL (range, 1.0–4.4 μg/mL) |
| AUC24 | 23.5 μg·h/mL (range, 8.0–46.5 μg·h/mL) |
The table shows sputum and serum amikacin exposures with once-daily inhalation of ALIS (590 mg) in Mycobacterium avium complex lung disease patients [14]
ALIS amikacin liposome inhalation suspension, AUC24 area under the concentration-time curve from 0 to 24 h, Cmax maximum concentration
Systemic exposures of amikacin after inhalation of ALIS are low (Table 1), with little to no accumulation following multiple once-daily doses [13, 22]. Notably, the serum Cmax (4.4 μg/mL) and area under the concentration-time curve from 0 to 24 h (AUC24; 46.5 μg·h/mL) upper range values observed in MAC lung disease patients (n = 12) following 3 months of once-daily inhaled ALIS were markedly lower than the mean Cmax (~ 76 μg/mL) and AUC24 (154 μg·h/mL) values observed in healthy adult subjects following intravenous administration of amikacin sulfate at the approved dosage (15 mg/kg/day) [14].
Amikacin is not metabolized to any appreciable extent [14]. The apparent serum half-life of amikacin following administration of ALIS by inhalation in MAC lung disease patients ranged from ~ 5.9 to 19.5 h. Systemically absorbed amikacin is primarily eliminated via glomerular filtration. However, whereas urine excretion accounts for 94% of intravenously administered amikacin sulfate, only 7.4% of the ALIS total dose is excreted in urine, providing further evidence that systemic exposure of amikacin following ALIS inhalation is low. Elimination of unabsorbed ALIS in the lungs likely occurs through cellular turnover and expectoration [14].
Therapeutic Efficacy of ALIS
Pivotal Phase III CONVERT Trial
Evidence for the efficacy of ALIS as add-on therapy for treatment-refractory MAC lung disease is primarily drawn from the ongoing, randomized, open-label, multinational, phase III CONVERT trial [13]. Patients enrolled in CONVERT were adults (aged ≥ 18 years) with MAC lung disease and MAC-positive sputum cultures despite ≥ 6 consecutive months of stable GBT. Patients were required to have MAC isolates with an amikacin MIC ≤ 64 μg/mL on culture screening, whereas patients with MAC isolates with an amikacin MIC > 64 μg/mL were excluded. Other key exclusion criteria included cystic fibrosis, immunodeficiency syndromes, active pulmonary tuberculosis and neuromuscular disorders [13].
In the trial, 336 patients were randomized (2:1) to receive once-daily ALIS as add-on to GBT, or GBT alone [13]. ALIS (amikacin 590 mg) was administered through inhalation using an investigational eFlow® nebulizer; GBT was administered according to guideline recommendations. Sputum samples were collected monthly during the trial, in duplicate or triplicate [13].
The primary endpoint of the trial was the proportion of patients achieving culture conversion, defined as having three consecutive monthly MAC-negative sputum cultures (covering all samples collected), by month 6 [13]. For patients who achieved culture conversion and remained culture negative at month 6, study treatment was continued for 12 months from the first month that defined culture conversion. Patients who had not achieved culture conversion by month 6 left the trial at month 8, although they may have been eligible to enter an open-label extension study (see below). To account for multiplicity, secondary endpoints in CONVERT were assessed using a hierarchical procedure [13].
Patients in the CONVERT intent-to-treat population (224 in the ALIS plus GBT group, 112 in the GBT alone group) had a mean age of 64.7 years [13]. In general, demographics and baseline characteristics were well balanced across the ALIS plus GBT and GBT alone groups, although there was a slight imbalance between groups in the proportion of female patients (73.7 vs. 60.7%, respectively) and in the median duration of MAC lung disease (4.5 vs. 3.3 years). In terms of underlying lung disease, 62.5% of patients overall had bronchiectasis only, 14.3% had COPD only and 11.9% had bronchiectasis and COPD. At enrolment, 89.9% of patients were receiving GBT or had stopped within the prior 3 months; 66.3% of patients were on a regimen that included a macrolide, ethambutol and a rifamycin (with or without other GBT agents) [13].
Once-daily ALIS as an add-on to GBT was associated with a significant (p < 0.001) increase in the proportion of patients achieving sputum culture conversion in the CONVERT trial, with conversion rates by month 6 of 29.0% in the ALIS plus GBT group versus 8.9% in the GBT alone group (Table 2) [13]. In a posthoc analysis (available in a poster presentation [24]), culture conversion rates in the ALIS plus GBT group were generally similar across geographical regions [USA, 29/93 (31.2%); Japan, 9/34 (26.5%); rest of the world, 26/81 (32.1%)], except in Asian countries other than Japan [1/15 (6.7%)] where lower patient numbers limit data interpretation. Culture conversion rates in the ALIS plus GBT group were also similar across patient subgroups based on the MAC isolate amikacin MIC at baseline over the range of 8–64 μg/mL, ranging from 28.6 to 34.5% according to the posthoc analysis [13]. Among patients who had MAC isolates with post-baseline amikacin MIC > 64 μg/mL (see Sect. 2.1), 2 of 23 patients in the ALIS plus GBT group and 0 of 3 patients in the GBT alone group achieved culture conversion (although one of the patients who converted in the ALIS plus GBT group subsequently had a MAC isolate with an amikacin MIC > 64 μg/mL) [13].
Table 2.
Culture conversion by month 6 in the CONVERT trial [13]
| ALIS + GBT | GBT alone | Adjusted odds ratio | |
|---|---|---|---|
| Total no. of patients (ITT population) | 224 | 112 | |
| Patients with culture conversiona (%) | 65 (29.0*) | 10 (8.9) | 4.22 (95% CI 2.08–8.57) |
ALIS amikacin liposome inhalation suspension, GBT guidelines-based therapy, ITT intent-to-treat
*p < 0.001 vs. GBT alone
aCulture conversion defined as having three consecutive monthly Mycobacterium avium complex-negative sputum cultures by month 6
For the first secondary endpoint under the hierarchical testing procedure, no significant difference was observed between the ALIS plus GBT and GBT alone groups in the change from baseline to month 6 in the six-minute walk test (6MWT) distance [least-squares mean (LSM) change, − 1.5 vs. + 1.5 m; p = 0.74] [13]. In contrast, a prespecified exploratory analysis in the overall study population (i.e. across both treatment groups) found that patients who achieved culture conversion did have an improvement in the 6MWT distance from baseline to month 6 compared with non-converters (LSM change, + 16.8 vs. − 7.9 m; p = 0.011) [13].
Interim results (available in an abstract and poster presentation [25]) from the CONVERT extension study support the findings of the main CONVERT trial. At the data cut-off for a prespecified interim analysis, 133 patients who had not achieved culture conversion by month 6 in CONVERT (59 patients from the ALIS plus GBT group, 74 patients from the GBT alone group) had enrolled in the extension study [25]. In the extension study, patients who had been randomized to ALIS plus GBT in CONVERT continued the same treatment for another 12 months and patients who had been randomized to GBT alone had ALIS added to their therapy for 12 months. At the time of the interim analysis, 3 (6.1%) of 49 assessable patients continuing on ALIS plus GBT and 17 (27.4%) of 62 assessable patients who had ALIS added to their therapy achieved culture conversion ≤ 6 months into the extension study [25].
Phase II Trial
The potential of ALIS as add-on therapy to GBT in the treatment of MAC lung disease was earlier shown in a randomized, double-blind, placebo-controlled, phase II proof-of-concept study of ALIS in patients (n = 89) with treatment-refractory NTM (MAC or M. abscessus) lung disease [26]. In the study, patients were randomized (1:1) to receive once-daily ALIS or placebo (empty liposomes), to be added to their ongoing GBT for 84 days (double-blind phase), after which all patients could receive open-label ALIS plus GBT for an additional 84 days (open-label phase). Prior to randomization, patients were stratified based on the presence (19% of patients) or absence (81%) of cystic fibrosis and the predominant mycobacterial species at screening [MAC (64%) or M. abscessus (36%)] [26].
Although the trial failed to meet its primary endpoint (change on a semiquantitative mycobacterial growth scale from baseline to day 84), in a secondary endpoint analysis it was found that a greater proportion of patients randomized to ALIS plus GBT [14/44 (31.8%)] than patients randomized to placebo plus GBT [4/45 (8.9%)] had negative sputum cultures at day 84 (p = 0.006) [26]. Overall, sputum culture conversion was achieved by 17 patients who had received ALIS in the trial (in either the double-blind or the open-label phase), with most cases occurring in non-cystic fibrosis patients with MAC infections. In contrast to what was observed in the CONVERT trial, ALIS treatment was associated with an improvement in the 6MWT distance compared with placebo (change from baseline to day 84, + 20.6 vs. − 25.0 m; p = 0.017) [26].
Tolerability of ALIS
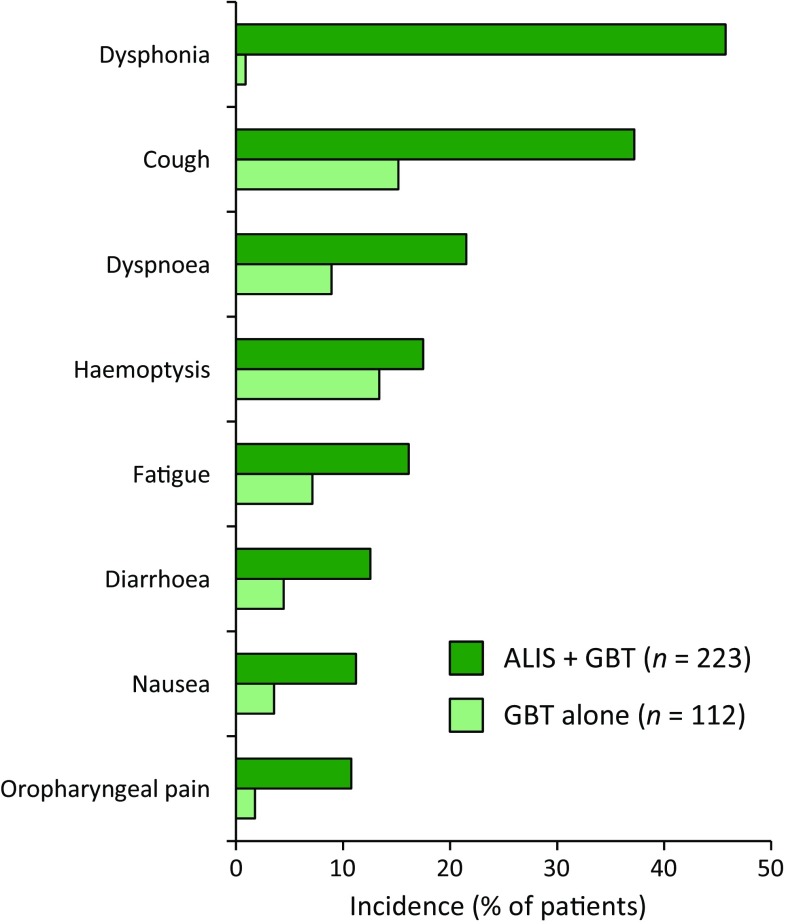
Based on clinical trial data [13, 25, 26], ALIS (as add-on therapy to GBT) has an acceptable tolerability profile in patients with treatment-refractory MAC lung disease. Treatment-emergent adverse events (TEAEs) observed in clinical trials [including in the pivotal phase III CONVERT trial (Fig. 1)] were most commonly respiratory in nature.
Fig. 1.
Most common treatment-emergent adverse events (occurring in ≥ 10% of patients in either treatment group) in the CONVERT trial [13]. ALIS amikacin liposome inhalation suspension, GBT guidelines-based therapy
In the CONVERT trial (Sect. 5.1), > 90% of patients in both the ALIS plus GBT (98.2%) and GBT alone (91.1%) groups experienced one or more TEAEs, with 20.2% and 17.9% of patients in the respective groups experiencing serious TEAEs [13]. In the ALIS plus GBT group 17.5% of patients experienced TEAEs that led to permanent discontinuation of ALIS. Six patients (2.7%) in the ALIS plus GBT group had TEAEs resulting in death compared with five patients (4.5%) in the GBT alone group [13]; one of the deaths in the ALIS plus GBT group (due to lung infection) was considered possibly related to ALIS treatment [22].
Respiratory TEAEs, mostly of mild to moderate severity, were reported in 87.4% of patients in the ALIS plus GBT group and in 50.0% of patients in the GBT alone group in CONVERT [13]. The US prescribing information carries a boxed warning that ALIS has been associated with an increased risk of respiratory adverse reactions, with events including hypersensitivity pneumonitis, haemoptysis, bronchospasm and exacerbation of underlying pulmonary disease reported at a higher frequency in patients treated with ALIS plus GBT than in patients receiving GBT alone; some respiratory events in patients receiving ALIS led to hospitalization [14]. If hypersensitivity pneumonitis occurs, ALIS should be discontinued; if other respiratory adverse events occur during use of ALIS, patients should be managed as is medically appropriate [14].
Ototoxicity-related adverse events have been observed in patients administered ALIS in clinical trials [14]. In CONVERT, ototoxicity-related events (i.e. deafness, dizziness, presyncope, tinnitus and vertigo) were reported in 17.0% of patients in the ALIS plus GBT group compared with 9.8% of patients treated with GBT alone. The imbalance observed between the groups was primarily driven by events of tinnitus (in 7.6 vs. 0.9% of patients in the respective groups) and dizziness (6.3 vs. 2.7%) [14].
Adverse events related to nephrotoxicity were observed infrequently in clinical trials investigating ALIS, with such events occurring in a similar proportion of patients (< 10% for combined nephrotoxicity-related TEAEs) in ALIS-treated and control groups [13, 14, 25, 26]. Nonetheless, given the potential for nephrotoxicity with aminoglycosides, close monitoring may be required for patients treated with ALIS who have known or suspected renal dysfunction [14].
Dosage and Administration of ALIS
ALIS is approved in the USA for use as part of a combination antibacterial drug regimen for the treatment of MAC lung disease in adult patients who have not achieved negative sputum cultures despite ≥ 6 consecutive months of a multidrug background regimen therapy and who have limited or no alternative treatment options [14]. ALIS is supplied as a suspension (amikacin 590 mg/8.4 mL) in a unit-dose vial. The recommended dosage of ALIS is once-daily inhalation of the nebulized contents of one vial; only the Lamira™ Nebulizer System is to be used for nebulization. Patients who are using a bronchodilator should use the bronchodilator prior to ALIS dosing. For patients with known hyper-reactive airway disease, COPD, asthma or bronchospasm, pre-treatment with short-acting selective beta-2 agonists should be considered [14].
ALIS is contraindicated in patients with a known hypersensitivity to any aminoglycoside [14]. Additionally, concomitant use of ALIS with medications associated with ototoxicity, nephrotoxicity or neurotoxicity should be avoided due to a potential risk of pharmacodynamic drug interactions (Sect. 6) [14]. Local prescribing information should be consulted for full details regarding the use of ALIS, including further information on warnings and precautions and on the use of the Lamira™ Nebulization System.
Current Status of ALIS in MAC Lung Disease
GBT has been shown to be effective in the treatment of MAC lung disease, with a majority of patients responding to therapy, albeit with treatment-related adverse events frequently a concern [27, 28]. However, for patients who fail to respond to conventional treatment, alternative therapeutic options are limited. Resectional surgery may be appropriate in some cases; however, guidelines regarding surgery are limited, and recommendations are largely based on expert opinion [1, 2]. In terms of the use of therapeutic agents, ATS/IDSA treatment guidelines (published in 2007) recommend that for advanced or previously treated MAC lung disease a course of intravenous streptomycin or amikacin can be used in addition to a standard three-drug regimen of a macrolide, ethambutol and a rifamycin [1]. However, the challenges of achieving effective drug concentrations at the site of infection while managing risks of systemic toxicities can limit the utility of these intravenous aminoglycosides in the treatment of MAC lung disease [1, 29].
The liposomal amikacin formulation ALIS was designed to facilitate targeted and localized drug delivery to the lungs while minimizing systemic exposure [12]. Preclinical studies demonstrated that ALIS can effectively penetrate MAC biofilms (Sect. 2), and that ALIS has enhanced uptake into alveolar macrophages (i.e. an important site where mycobacteria can survive and persist [30]) relative to free amikacin (Sect. 4), highlighting further potential benefits of the liposomal formulation.
As demonstrated in the pivotal, randomized, phase III CONVERT trial in patients with treatment-refractory MAC lung disease, the addition of once-daily ALIS to GBT significantly increased the odds of achieving sputum culture conversion by month 6 (Sect. 5.1). In CONVERT, ALIS appeared to have efficacy in the treatment of patients with MAC isolates with amikacin MICs in the range of 8 to 64 μg/mL, whereas there was limited evidence of efficacy against isolates with an amikacin MIC > 64 μg/mL. Thus, determining the amikacin MIC of MAC isolates may be useful for guiding treatment decisions relating to the use of ALIS [24].
Data from an earlier phase II trial (Sect. 5.2) and available data from the CONVERT extension study support the findings from CONVERT (Sect. 5.1). Furthermore, the CONVERT and extension trials are ongoing, with final results awaited. Of particular interest will be data relating to the sustainability and the durability of the culture conversion response (i.e. respectively, the maintenance of culture-negative status during continued treatment, and after treatment is stopped) and data relating to any potential clinical benefit of ALIS treatment. One limitation of currently available data is that, although ALIS as add-on therapy to GBT was associated with a strong microbiological response, a clinical benefit of ALIS treatment is yet to be demonstrated, highlighted by the lack of a significant between-group difference in 6MWT results at 6 months in CONVERT (Sect. 5.1). However, a prespecified exploratory analysis involving the whole study population did show that culture conversion was associated with a significant improvement in 6MWT distance, indicating that there is a clinical benefit from achieving culture conversion and supporting the validity of culture conversion at month 6 as a meaningful surrogate endpoint. Furthermore, final results from the CONVERT trial are expected to include data on 6MWT results when assessed over a longer timeframe.
Another limitation of the CONVERT trial was that variation in methods used to assess lung disease at screening did not permit the analysis of outcomes in patients based on the presence or absence of cavitary disease [13]. The presence of cavitary disease has been associated with a reduced likelihood of response to treatment for MAC lung disease [1]. Furthermore, drugs administered through inhalation are more likely to have poor deposition into cavitary areas, as has been observed for ALIS (Sect. 4). In the phase II trial (Sect. 5.2), 76% of patients overall had evidence of cavitary disease (with or without primarily nodular bronchiectasis), including 11 (65%) of 17 patients who achieved sputum culture conversion having received ALIS [26]. Nonetheless, further investigation of the effectiveness of ALIS in the treatment of MAC lung disease patients with cavitary disease would be of interest.
Although limited to a relatively small number of patients (< 400 ALIS recipients), currently available clinical trial data suggest that ALIS (as add-on therapy to GBT) has an acceptable tolerability profile in patients with MAC lung disease (Sect. 6). The addition of ALIS to GBT was associated with an increased risk of respiratory adverse events compared with GBT alone. However, adverse events related to renal toxicity or ototoxicity (which are commonly associated with aminoglycoside systemic toxicity) were observed relatively infrequently and, with the exception of tinnitus and dizziness, were generally observed with similar incidences across treatment groups in CONVERT. Furthermore, pharmacokinetic analyses indicate that there is little or no accumulation of amikacin in serum with extended daily ALIS administration (Sect. 4).
In conclusion, given the limitations of currently available data on ALIS in terms of safety and clinical effectiveness (with evidence for efficacy being limited to microbiological outcomes and clinical benefit yet to be established), use of ALIS should be reserved for adult patients with MAC lung disease who have not responded to conventional therapy and for whom there are limited or no alternative treatment options available. However, in this limited population, ALIS appears to be a useful treatment option for consideration, particularly given that treatment-refractory MAC lung disease is a life-threatening condition.
Data selection drug name: 124 records identified
| Duplicates removed | 21 |
| Excluded during initial screening (e.g. press releases; news reports; not relevant drug/indication; preclinical study; reviews; case reports; not randomized trial) | 28 |
| Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 45 |
| Cited efficacy/tolerability articles | 4 |
| Cited articles not efficacy/tolerability | 26 |
| Search Strategy: EMBASE, MEDLINE and PubMed from 1946 to present. Clinical trial registries/databases and websites were also searched for relevant data. Key words were: amikacin liposome inhalation suspension, Arikayce, Arikace, ALIS, nontuberculous mycobacterium. Records were limited to those in the English language. Searches last updated 04 March 2019. | |
Acknowledgments
During the peer review process, the manufacturer of ALIS was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Matt Shirley is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Footnotes
The manuscript was reviewed by:, A. Barac Faculty of Medicine, University of Belgrade, Belgrade, Serbia; M.M. Johnson, Division of Pulmonary Medicine, Mayo Clinic Florida, Jacksonville, FL, USA; W-J. Koh, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea.
The original version of this article was revised due to a retrospective Open Access request.
Change history
4/3/2019
The article Amikacin Liposome Inhalation Suspension: A Review in Mycobacterium avium Complex Lung Disease.
References
- 1.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 2.Johnson MM, Odell JA. Nontuberculous mycobacterial pulmonary infections. J Thorac Dis. 2014;6(3):210–220. doi: 10.3978/j.issn.2072-1439.2013.12.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park HY, Jeong B-H, Chon HR, et al. Lung function decline according to clinical course in nontuberculous mycobacterial lung disease. Chest. 2016;150(6):1222–1232. doi: 10.1016/j.chest.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Mehta M, Marras TK. Impaired health-related quality of life in pulmonary nontuberculous mycobacterial disease. Respir Med. 2011;105(11):1718–1725. doi: 10.1016/j.rmed.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Diel R, Lipman M, Hoefsloot W. High mortality in patients with Mycobacterium avium complex lung disease: a systematic review. BMC Infect Dis. 2018;18(1):206. doi: 10.1186/s12879-018-3113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marras TK, Vinnard C, Zhang Q, et al. Relative risk of all-cause mortality in patients with nontuberculous mycobacterial lung disease in a US managed care population. Respir Med. 2018;145:80–88. doi: 10.1016/j.rmed.2018.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winthrop K, Adjemian J, Mirsaeidi M, et al. Incidence and prevalence of nontuberculous mycobacterial lung disease in US Medicare beneficiaries, 2008–2015 [abstract 780 and poster]. In: ID week. 2018.
- 8.Prevots DR, Marras TK. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med. 2015;36(1):13–34. doi: 10.1016/j.ccm.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prevots DR, Shaw PA, Strickland D, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. 2010;182(7):970–976. doi: 10.1164/rccm.201002-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peloquin CA, Berning SE, Nitta AT, et al. Aminoglycoside toxicity: daily versus thrice-weekly dosing for treatment of mycobacterial diseases. Clin Infect Dis. 2004;38(11):1538–1544. doi: 10.1086/420742. [DOI] [PubMed] [Google Scholar]
- 11.Rybak MJ, Abate BJ, Kang SL, et al. Prospective evaluation of the effect of an aminoglycoside dosing regimen on rates of observed nephrotoxicity and ototoxicity. Antimicrob Agents Chemother. 1999;43(7):1549–1555. doi: 10.1128/AAC.43.7.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meers P, Neville M, Malinin V, et al. Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic Pseudomonas aeruginosa lung infections. J Antimicrob Chemother. 2008;61(4):859–868. doi: 10.1093/jac/dkn059. [DOI] [PubMed] [Google Scholar]
- 13.Griffith DE, Eagle G, Thomson R, et al. Amikacin liposome inhalation suspension for treatment-refractory lung disease caused by Mycobacterium avium complex (CONVERT): a prospective, open-label, randomized study. Am J Respir Crit Care Med. 2018;198(12):1559–1569. doi: 10.1164/rccm.201807-1318OC. [DOI] [PubMed] [Google Scholar]
- 14.US FDA. Arikayce® (amikacin liposome inhalation suspension): US prescribing information. 2018. http://www.accessdata.fda.gov/drugsatfda_docs/label/2018/207356s000lbl.pdf. Accessed 19 Feb 2019.
- 15.National Institutes of Health—US National Library of Medicine. Amikacin sulfate injection: US prescribing information. 2018. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6ec3129b-c53b-4bdb-913d-a2d0060fa140. Accessed 19 Feb 2019.
- 16.Brown-Elliott BA, Iakhiaeva E, Griffith DE, et al. In vitro activity of amikacin against isolates of Mycobacterium avium complex with proposed MIC breakpoints and finding of a 16S rRNA gene mutation in treated isolates. J Clin Microbiol. 2013;51(10):3389–3394. doi: 10.1128/JCM.01612-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown-Elliott BA, Nash KA, Wallace RJ., Jr Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clin Microbiol Rev. 2012;25(3):545–582. doi: 10.1128/CMR.05030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Leifer F, Rose S, et al. Amikacin liposome inhalation suspension (ALIS) penetrates non-tuberculous mycobacterial biofilms and enhances amikacin uptake into macrophages. Front Microbiol. 2018;9:915. doi: 10.3389/fmicb.2018.00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rose SJ, Neville ME, Gupta R, et al. Delivery of aerosolized liposomal amikacin as a novel approach for the treatment of nontuberculous mycobacteria in an experimental model of pulmonary infection. PLoS One. 2014;9(9):e108703. doi: 10.1371/journal.pone.0108703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malinin V, Neville M, Eagle G, et al. Pulmonary deposition and elimination of liposomal amikacin for inhalation and effect on macrophage function after administration in rats. Antimicrob Agents Chemother. 2016;60(11):6540–6549. doi: 10.1128/AAC.00700-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weers J, Metzheiser B, Taylor G, et al. A gamma scintigraphy study to investigate lung deposition and clearance of inhaled amikacin-loaded liposomes in healthy male volunteers. J Aerosol Med Pulm Drug Deliv. 2009;22(2):131–138. doi: 10.1089/jamp.2008.0693. [DOI] [PubMed] [Google Scholar]
- 22.Insmed Ltd. Amikacin liposome inhalation suspension: Antimicrobial Drugs Advisory Committee briefing materials. 2018. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM615723.pdf. Accessed 19 Feb 2019.
- 23.Olivier KN, Maass-Moreno R, Whatley M, et al. Airway deposition and retention of liposomal amikacin for inhalation in patients with pulmonary nontuberculous mycobacterial disease [abstract A3732 and poster] Am J Respir Crit Care Med. 2016;193:A3732. [Google Scholar]
- 24.Brown-Elliott BA, Eagle G, Wallace Jr RJ, et al. Amikacin liposome inhalation suspension (ALIS) add-on therapy for refractory Mycobacterium avium complex (MAC) lung disease: effect of in vitro amikacin susceptibility on sputum culture conversion [abstract 805 and poster]. In: ID week. 2018.
- 25.Winthrop KL, Eagle G, Morimoto K, et al. Extension study of Amikacin Liposome Inhalation Suspension (ALIS) for treatment-refractory lung disease caused by Mycobacterium avium complex (MAC): interim analysis [abstract and poster] Chest. 2018;154(4 Suppl):182A–183A. doi: 10.1016/j.chest.2018.08.160. [DOI] [Google Scholar]
- 26.Olivier KN, Griffith DE, Eagle G, et al. Randomized trial of liposomal amikacin for inhalation in nontuberculous mycobacterial lung disease. Am J Respir Crit Care Med. 2017;195(6):814–823. doi: 10.1164/rccm.201604-0700OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwak N, Park J, Kim E, et al. Treatment outcomes of Mycobacterium avium complex lung disease: a systematic review and meta-analysis. Clin Infect Dis. 2017;65(7):1077–1084. doi: 10.1093/cid/cix517. [DOI] [PubMed] [Google Scholar]
- 28.Wallace RJ, Jr, Brown-Elliott BA, McNulty S, et al. Macrolide/azalide therapy for nodular/bronchiectatic Mycobacterium avium complex lung disease. Chest. 2014;146(2):276–282. doi: 10.1378/chest.13-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weers J. Inhaled antimicrobial therapy—barriers to effective treatment. Adv Drug Deliv Rev. 2015;85:24–43. doi: 10.1016/j.addr.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Awuh JA, Flo TH. Molecular basis of mycobacterial survival in macrophages. Cell Mol Life Sci. 2017;74(9):1625–1648. doi: 10.1007/s00018-016-2422-8. [DOI] [PMC free article] [PubMed] [Google Scholar]