Abstract
Tribbles homolog 3 (TRIB3) mediating signaling pathways are closely related to blood pressure regulation. Our previous findings suggested a greater benefit on vascular outcomes in patients carrying TRIB3 (251, A > G, rs2295490) G allele with good glucose and blood pressure control. And TRIB3 (rs2295490) AG/GG genotypes were found to reduce primary vascular events in type 2 diabetic patients who received intensive glucose treatment as compared to those receiving standard glucose treatment. However, the effect of TRIB3 genetic variation on antihypertensives was not clear in essential hypertension patients. A total of 368 patients treated with conventional dosage of antihypertensives (6 groups, grouped by atenolol/bisoprolol, celiprolol, doxazosin, azelnidipine/nitrendipine, imidapril, and candesartan/irbesartan) were enrolled in our study. Genetic variations were successfully identified by sanger sequencing. A linear mixed model analysis was performed to evaluate blood pressures among TRIB3 (251, A > G) genotypes and adjusted for baseline age, gender, body mass index, systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol and other biochemical factors appropriately. Our data suggested that TRIB3 (251, A > G) AA genotype carriers showed better antihypertensive effect than the AG/GG genotype carriers [P = 0.014 for DBP and P = 0.042 for mean arterial pressure (MAP)], with a maximal reduction of DBP by 4.2 mmHg and MAP by 3.56 mmHg after azelnidipine or nitrendipine treatment at the 4th week. Similar tendency of DBP-change and MAP-change was found for imidapril (ACEI) treatment, in which marginally significances were achieved (P = 0.073 and 0.075, respectively). Against that, we found that TRIB3 (251, A > G) AG/GG genotype carriers benefited from antihypertensive therapy of ARBs with a larger DBP-change during the period of observation (P = 0.036). Additionally, stratified analysis revealed an obvious difference of the maximal blood pressure change (13 mmHg for the MAP between male and female patients with AA genotype who took ARBs). Although no significant difference in antihypertensive effect between TRIB3 (251, A > G) genotypes in patients treated with α, β-ADRs was observed, we found significant difference in age-, sex-dependent manner related to α, β-ADRs. In conclusion, our data supported that TRIB3 (251, A > G) genetic polymorphism may serve as a useful biomarker in the treatment of hypertension.
Keywords: TRIB3, rs2295490, polymorphism, antihypertensive agents, essential hypertension
Introduction
Essential hypertension (EH) can be defined as a rise in blood pressure for unknown cause. It can increase risks for cerebral, cardiac, and renal events. It usually clusters with other cardiovascular risk factors such as aging, being overweight, insulin resistance, diabetes, and hyperlipidaemia (Messerli et al., 2007; Sun et al., 2019). Human tribbles homolog 3 (TRIB3) is organized as four exons, encoding a 358-amino-acid protein. TRIB3 proteins disrupts insulin signaling by binding directly to Akt and blocking activation of the kinase, which contributes to insulin resistance and other physiological processes of vessel (Du et al., 2003; Prudente and Trischitta, 2015). AKT mediates the phosphatidylinositol 3′-kinase-protein kinase B-endothelial nitric oxide (NO) synthase (PI3K-AKT-eNOS)-dependent pathway in the cardiovascular protective effect of insulin, which is crucial for NO synthase activation, eventually leading to increased NO production, vasodilation and blood flow (Yu et al., 2011). Other signaling molecules such as bone morphogenetic proteins type II receptor (BMRPII) and bone morphogenetic proteins (BMP) also interact with TRIB3 and modulate the vascular effect (Kiss-Toth et al., 2004; West et al., 2004; Chan et al., 2007). Accumulating evidences supported that some drugs or active materials affect vascular functions by regulating TRIB3. For example, the amiloride derivative phenamil promotes contractile phenotype of vascular smooth muscle cells (vSMCs) by activating TRIB3 gene transcription (Chan et al., 2011; Kim et al., 2014). Homocysteine, which is significantly associated with hypertension could up-regulate the expression of TRIB3, thus leading to the endothelial dysfunction (Chan et al., 2011; Zou et al., 2011).
A single nucleotide polymorphism (SNP) variant in human TRIB3 exon 2, which results in a glutamine (Q) to arginine (R) missense mutation in a conserved motif at position 84, confers stronger Akt binding, resulting in reduced Akt phosphorylation (Fischer et al., 2017). Since Prudente S first reported the relationship between TRIB3 missense Q84R (rs2295490) polymorphism and insulin resistance or its related cardiovascular events (Prudente et al., 2005), numerous clinical studies have confirmed the risk of genetic variant associated with vascular events, especially the deleterious role of TRIB3 R84 (Gong et al., 2009; Formoso et al., 2011; Prudente et al., 2015; Zhang et al., 2015). Among patients with metabolic syndrome and the control group, patients with TRIB3 RR84 genotype had significantly higher level of serum semaphorin 3E than those with QQ84 or QR84 genotypes (Qin et al., 2017), individuals with RR84 genotype also showed further decreased serum obestatin. Increased serum semaphorin 3E or decreased serum obestatin might in part exacerbate insulin resistance and carotid atherosclerosis (Cui et al., 2012). In human endothelial cells, data demonstrate that the TRIB3 R84 variant impairs insulin signaling and NO production (Andreozzi et al., 2008). These vascular disease-related studies suggested a possible link between TRIB3 and EH.
Studies showed that imidapril mediated various actions, such as vasodilation, anticoagulation, hypotension, atherosclerosis and other cardiovascular protection via bradykinin-eNOS-NO pathway (Kobayashi et al., 2000; Chen et al., 2003). Celiprolol triggered PI3K-Akt and increased eNOS expression (Kobayashi et al., 2003), and doxazosin also increased the expression of eNOS accompanying with NO production via cAMP/cGMP/adrenalin signaling pathway (Stojkov et al., 2013, 2014). Azelnidipine significantly increased eNOS expression levels in the brain as well as in the heart and aorta (Kimura et al., 2007). Zhang, et al. demonstrated that valsartan significantly down-regulated the expression of TRIB3 mRNA level and improved the cardiac function in rats with diabetic cardiomyopathy (Zhang et al., 2006).
These data indicate that TRIB3 has an integral role in regulating the blood pressure, and its genetic variation (251, A > G) may cause a variability of antihypertensive drug therapy. Our previous findings have suggested a greater benefit on vascular outcomes in patients carrying TRIB3 (rs2295490) G allele with good glucose and blood pressure control (He et al., 2016). TRIB3 (rs2295490) AG/GG genotypes were found to reduce primary vascular events in patients who received intensive glucose treatment as compared to those receiving standard glucose treatment in type 2 diabetic patients (He et al., 2018). However, to our knowledge, there is no study to evaluate the association between TRIB3 Q84R polymorphism and the effect of antihypertensive drugs in patients with EH.
Patients and Methods
Patients
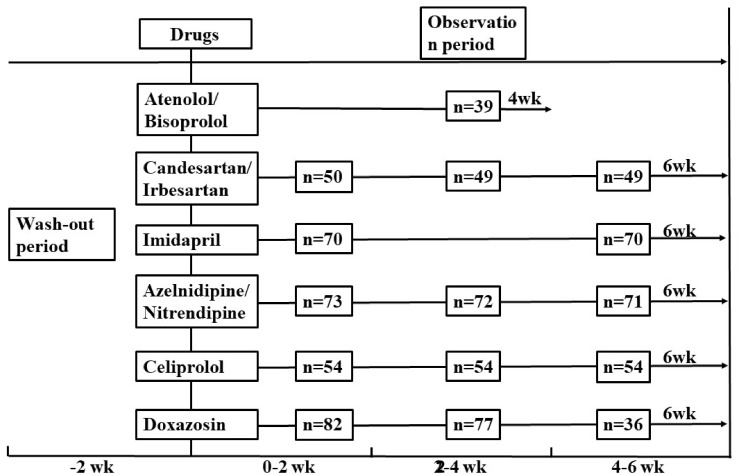
The study protocol was approved by the Ethical Committee of the Institute of Clinical Pharmacology, Central South University (Hunan, China). Clinical study was registered at the Chinese Clinical Trial Register with the registration number ChiCTR-RO-120026121. All the patients participating in the study were given written informed consent. The clinical data and DNA samples were graciously provided by Shanghai Institute of Hypertension, Ruijin Hospital affiliated with Shanghai Jiao Tong University. According to study design, all the patients fulfilled the following inclusion and exclusion criteria: male or female patients, heart rates within 55∼90 beats/min, systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg were included. Patients with secondary hypertension, coronary heart disease, diabetes, obesity (BMI.30 kg/m2), stroke, renal or liver dysfunction, malignant tumor or pregnancy and those whose blood pressure measurements above 180/110 mmHg or remaining lower than 140/90 mmHg during the wash-out period, were withdrawn from the study. In our study, a total of 368 unrelated subjects with primary mild to moderate EH, aged from 26 to 81 years were recruited for genetic screening. All the patients were enrolled after a run-in period of 2 weeks and assigned to receive the drugs for 4 weeks or more. The patients were followed up every 2 weeks at the weekend, and details of the protocol were shown in Table 1. According to the study protocol, 86 samples were excluded from 454 eligible patients due to the small sample size, not contactable and fail to meet inclusion criteria. At last, a total of 368 patients were included in our study. The medications include β-adrenoceptor antagonists (β-ADRs, atenolol, bisoprolol and celiprolol), α-adrenoceptor antagonist (α-ADR, doxazosin), calcium channel blockers (CCBs, azelnidipine and compound nitrendipine), ACE-inhibitor (ACEI, imidapril) and angiotensin receptor blocker (ARB, candesartan and irbesartan). All patients received monotherapy. Details are shown in Figure 1.
Table 1.
The protocols of the related drugs to a total of 368 eligible patients in the study.
| Sample | Treatment | Case | |
|---|---|---|---|
| Drugs and dose | size (N) | course (W) | report |
| Celiprolol (200 mg/d) | 54 | 6 | ETW |
| Atenolol (25 mmg/d)/ bisoprolol (5 mmg/d) | 39 | 4 | EQW |
| Doxazosin (2 mmg/d) | 82 | 6 | ETW |
| Azelnidipine (2 mmg/d)/nitrendipine + atenolol (5 + 10 mmg/d) | 73 | 6 | ETW |
| Candesartan (8 mmg/d) + irbesartan (150 mmg/d) | 50 | 6 | ETW |
| Imidapril (5 mmg/d) | 70 | 6 or 8 | ETW or EQW |
N = number; W = week; ETW = every two weeks; EQW = every four weeks.
Figure 1.
Study protocol and visit time to 368 patients with related drugs in the study. wk, week.
Blood Pressure and Heart Rate Measurement
Blood pressure determinations were performed on the morning after a light breakfast with subjects in the seated position and following a 30 min quiet resting period. Blood pressure and heart rate were measured by trained nurses, with an automatic blood pressure monitor with intellisense, which allows the detection of alteration of the heart rate by greater than or equal to beat/min and the blood pressure by greater than or equal to 1 mmHg. Detected results were then validated by supervisors with the mercury sphygmomanometer. The blood pressure values were determined by the average of three measurements taken every 10 min. Values of SBP and DBP were defined by Korotkoff phase I and V, respectively. Pulse pressure was calculated as PP = (SBP- DBP); mean arterial pressure (MAP) was calculated as MBP = DBP + (PP/3) (Wain et al., 2011).
Genotyping Procedure for TRIB3
Tribbles homolog 3 (251, A > G) genotypes were determined by sanger sequencing. The PCR primers for TRIB3 (251, A > G) were shown as follows: the sense primer, 5′GTTGCCCCTGAGCCCACCTACT3′; and the antisense primer, 5′TCCCTGGATGCTTCCCCACTAA3′. The product length was 286 bp. The reaction mixture (25 μl) contained: 10 × PCR buffer (2.5 μl), 10 × dNTP (2.5 μl), 10 μM for each of the sense and antisense primers (0.5 μl), H2O (16.8 μl), gDNA (2 μl), Taq polymerase (0.2 μl). Temperature cycling was proceeded as follows: initial denaturation for 5 min at 94°C, followed by 36 cycles of denaturation 30 s at 94°C, annealing at 57°C for 30 s, and elongation at 72°C for 30 s, and a terminal extension for 5 min. Genotypes were determined without knowledge of the status of patients, Ten percent blinded, random DNA samples from the patients were genotyped twice, with 100% concordance. And sequencing was assisted by Shanghai Majorbio Bio-pharm Technology Company.
Statistical Analysis
All the analyses were performed with SPSS (version 19.0 for windows; Chicago, IL, United States). Allele frequencies were determined by the genotypes of all the participants. Continuous data were presented as mean values ± standard error (SE), and categorical variable was shown as frequencies and percentages. The Hardy–Weinberg equilibrium analysis was carried out for the genotypes of participants using the χ2-test. Difference in baseline characteristics among the phenotypes were assessed by independent-samples T-test.
The linear mixed model was used to examine the efficacy of TRIB3 (251, A > G). This allowed the use of time-dependent and time-independent covariates within the model to be analyzed. Furthermore, this model has been found to work well for small sample sizes with missing data (Patel et al., 2007; Chen et al., 2015). The outcome measures included ΔSBP, ΔDBP and ΔMAP. These measures were analyzed individually, with the main effects of genotype, follow-up time and their interaction, as well as subjects’ intercept serving as random effects. The best fit model was decided after checking the smallest Akaike Information Criterion (AIC), and the Schwarz Bayesian Criterion (BIC) values. The covariance structure adopted for the final model was the Unstructured (UN) which means each variance and each covariance is estimated uniquely from the data. Models were additionally adjusted for the fixed effects of baseline age, sex, body mass index (BMI), SBP, DBP, total cholesterol, heart rate, triglyceride, high-density lipoprotein (HDL), low-density lipoprotein (LDL), fasting blood-glucose (FBG) and other biochemical factors appropriately. The linear mixed model analysis was done by using the PASW 19.0 for Windows (SPSS Inc., Chicago, IL, United States). Functions in nlme package of R statistical computing environment (R Foundation —for Statistical Computing, Vienna, Austria) were used to confirm the analysis. Stratified Analysis was used to address whether significant interaction was present between age-genotype and sex-genotype. A two-tailed P-value < 0.05 was considered significant.
Results
Baseline Characteristics, Genotyping Results and Outline of Major Findings
In this study, 368 patients were genotyped unambiguously for TRIB3 (251, A > G) genetic variation. Our data showed that the frequency of TRIB3 (251, A > G) G allele was 18.5% in Chinese population. Genotype frequencies of TRIB3 (251, A > G) AA, AG and GG were 66.0, 31.0 and 3.0%, respectively. All the allele frequencies were in Hardy-Weinberg equilibrium (P > 0.05). Owing to the low frequency of the GG genotype, we combined the AG and GG genotypes as one group for the following analysis. Baseline characteristics of the patients stratified by TRIB3 genotypes are shown in Table 2. No significant differences between AA and AG+GG genotype groups in age, BMI, HR, BP or other clinical characteristics, such as FBG, triglyceride, cholesterol, HDL and LDL, etc. were observed.
Table 2.
Baseline characteristics of patients with essential hypertension stratified by TRIB3 genotype.
| Variable |
TRIB3 |
P-value | |
|---|---|---|---|
| AA (n) | AG+GG (n) | ||
| Age, year | 57.0 ± 8.4(243) | 56.5 ± 8.6(125) | 0.524 |
| BMI, kg/m2 | 25.2 ± 3.0(242) | 25.1 ± 3.6(125) | 0.723 |
| HR, bpm | 75.2 ± 7.5(243) | 75.1 ± 7.3(125) | 0.781 |
| SBP, mm Hg | 149.3 ± 10.3(243) | 151.2 ± 10.5(125) | 0.485 |
| DBP, mm Hg | 98.0 ± 4.3(243) | 98.3 ± 4.8(125) | 0.485 |
| MAP, mm Hg | 115.1 ± 5.3(243) | 115.9 ± 5.6(125) | 0.388 |
| PP, mm Hg | 51.3 ± 9.3(243) | 52.8 ± 9.7(125) | 0.100 |
| ALT, umol/L | 31.5 ± 17.3(212) | 31.8 ± 27.7(101) | 0.336 |
| BUN, mmol/L | 5.2 ± 1.2(222) | 6.5 ± 9.3(109) | 0.471 |
| UCr, mmol/L | 79.8 ± 16.6(237) | 79.1 ± 17.3(122) | 0.610 |
| UA, mmol/L | 320.4 ± 80.5(180) | 328.1 ± 89.6(82) | 0.246 |
| FBG, mmol/L | 5.2 ± 0.98(210) | 5.4 ± 1.2(105) | 0.739 |
| TG, mmol/ | 1.78 ± 1.6(236) | 2.33 ± 1.3(120) | 0.653 |
| CHO, mmol/L | 5.1 ± 1.0(237) | 5.4 ± 1.3(121) | 0.101 |
| HDL, μmol/L | 1.4 ± 0.3(221) | 1.4 ± 0.3(111) | 0.740 |
| LDL, umol/L | 3.4 ± 1.1(144) | 3.6 ± 1.0(78) | 0.374 |
Data expressed as mean ± SD. BMI indicates body mass index; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; MAP, mean arterial pressure; ALT, alanine aminotransferase; BUN, blood urea nitrogen; UCr, urine creatinine; UA, uric acid; FBG, fasting blood-glucose; TG, triglyceride; CHO, cholesterol; HDL, high-density lipoprotein; LDL, low density lipoprotein.
Different Pressure Response to TRIB3 (251, A > G) for Antihypertensive Drugs
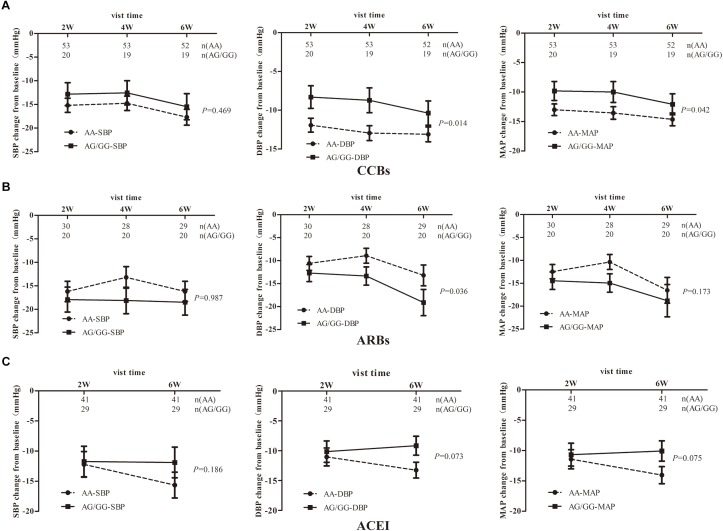
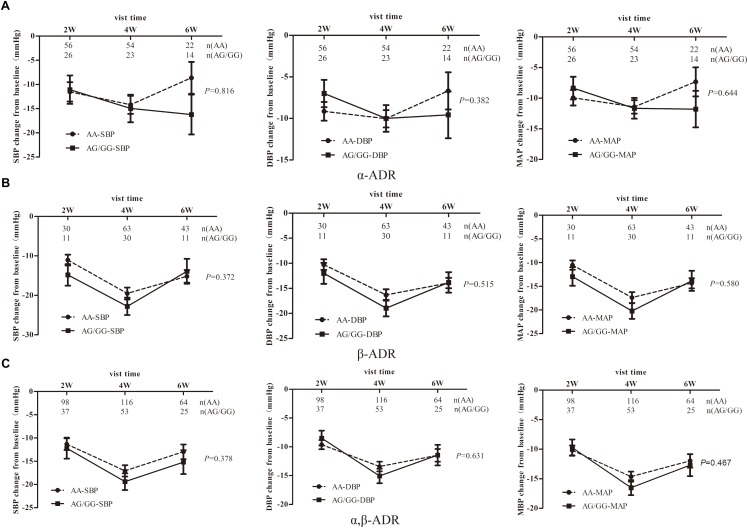
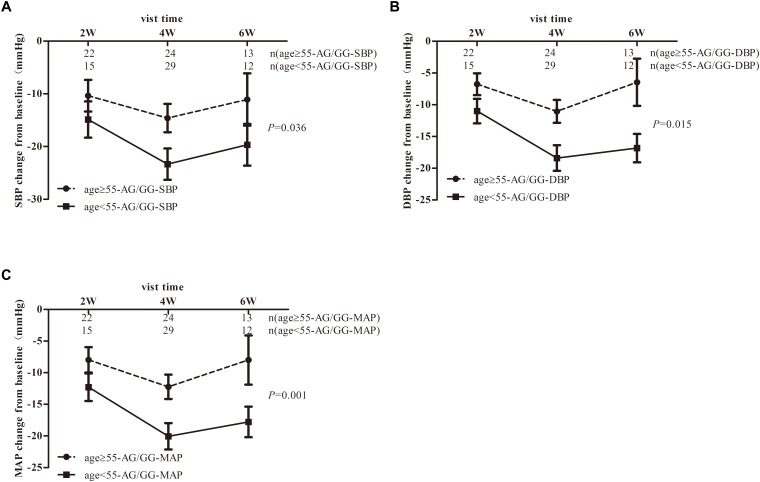
As shown in Figure 2A, the DBP-change and MAP-change from baseline response to TRIB3 (251, A > G) variant was significantly reduced (AA genotype compared to AG/GG genotype carriers) after CCBs administration. A similar tendency of DBP-change and MAP-change was found for imidapril (ACEI) treatment (Figure 2C), in which a marginally significant difference was achieved (P = 0.073 and 0.075, respectively). Inversely, DBP change from baseline after ARBs prescription was significantly increased for the AA genotype carriers compared to AG/GG genotype (Figure 2B). In the stratified analysis, there were significant differences in BP-change (blood pressure, include SBP or DBP or MAP) of AG/GG carriers between older and younger age or male and female (Supplementary Figures S1, S2). Nevertheless, no association between TRIB3 (251, A > G) genetic polymorphism and α-ADR or β-ADR drug response was observed in our study cohort (Figure 3A,B).
Figure 2.
The relationship between TRIB3 (251, A > G) genetic polymorphism and the hypotensive effects of the antihypertensives monotherapy in EH patients. Panels (A–C) respectively depicts that BP-changes from baseline in EH patients carrying the TRIB3 (251, A > G) AA and AG/GG genotypes after treatment with the drugs of CCBs, ARBs and ACEIs for 6 weeks. P-value were calculated by the linear mixed model and adjusted for baseline age, BMI, gender, SBP, DBP, total cholesterol, heart rate, triglyceride, HDL, LDL, FBG and other biochemical factors appropriately. Error bar indicated 95% confidence interval.
Figure 3.
The relationship between TRIB3 (251, A > G) genetic polymorphism and the hypotensive effects of α,β-ADRs monotherapy in EH patients. Panels (A–C) respectively depicts that BP- changes from baseline in EH patients carrying the TRIB3 (251, A > G) AA and AG/GG genotypes after treatment with α-ADR, β-ADR and α, β-ADRs for 6 weeks. P-value were adjusted for baseline age, BMI, gender, SBP, DBP, total cholesterol, heart rate, triglyceride, HDL, LDL, FBG and other biochemical factors appropriately. Error bar indicated 95% confidence interval.
Age-, Sex-Dependent Manner Related to α, β-ADRs on Blood Response
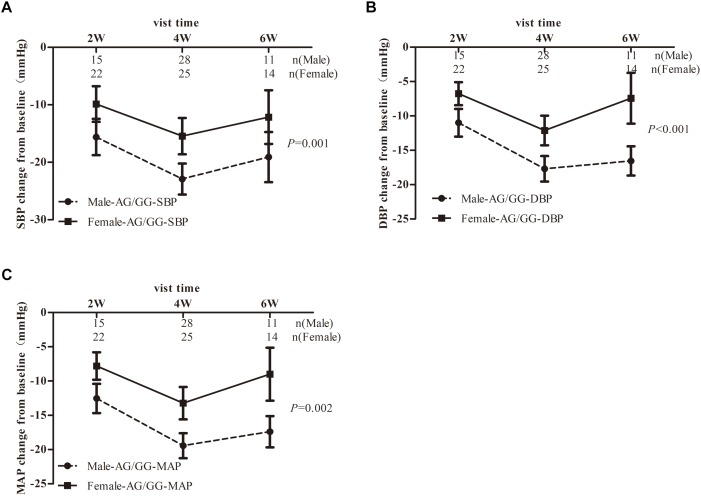
As studies showed that α, β-ADRs were all playing a role in the cAMP/cGMP/adrenalin signaling pathways to induce the transcription of eNOS in a time-dependent manner[18], we combined α, β-ADRs treatment groups in the analysis model. However, there was no significant change between AG/GG and AA genotype in the linear mixed model (Figure 3C). Stratified analysis was then used to explore the difference in SBP-, DBP-, MAP-change from baseline after α, β-ADRs therapy. In the sex stratification analysis, BP-change from baseline was significantly reduced for male compared to female in AG/GG genotype (Figure 4). And in the analysis of age stratification, we took 50,55,60 as the boundaries for analysis respectively, and found that 55 was the most appropriate cut-off value. The results showed that people with age younger than 55 years old had more significant antihypertensive effects in AG/GG genotype carriers (Figure 5).
Figure 4.
Blood pressure response to α,β-ADR blocker therapy in EH patients stratified by sex dependent of TRIB3 genotype. Panels (A–C) respectively depicts that SBP-, DBP-, MAP- changes from baseline in EH patients carrying the TRIB3 (251, A > G) AA and AG/GG genotypes after treatment with α, β-ADRs for 6 weeks. P-value were adjusted for baseline age, BMI, SBP, DBP, total cholesterol, heart rate, triglyceride, HDL, LDL, FBG and other biochemical factors appropriately. Error bar indicated 95% confidence interval.
Figure 5.
Blood pressure response to α,β-ADR blocker therapy in EH patients stratified by age-specific to TRIB3 genotype. Panels (A–C) respectively depicts that SBP-, DBP-, MAP- changes from baseline in EH patients carrying the TRIB3 (251, A > G) AA and AG/GG genotypes after treatment with α, β-ADRs for 6 weeks. P-value were adjusted for baseline BMI, gender, SBP, DBP, total cholesterol, heart rate, triglyceride, HDL, LDL, FBG and other biochemical factors appropriately. Error bar indicated 95% confidence interval. The stratification based on a boundary of age-55 showed the largest difference in blood pressure decrease after analysis of age-50, 55, 60.
Discussion
In this study we showed that TRIB3 (251, A > G) genetic variation had a significant clinical relevance to the efficacy of certain class of antihypertensive drugs in individuals with EH treatment. It has been demonstrated that a better effect on BP response was observed in TRIB3 Q84R (rs2295490, 251 A > G) AA genotype carriers than AG/GG carriers after taking a routine dosage of ACEI and CCBs antihypertensives. After stratification analysis for age and sex, we found a more significant difference between these two genotype carriers. Studies have shown that CCBs and ACEI have an excellent antioxidant stress effect via increasing eNOS expression and NO synthesis (Kanno et al., 2001; Koyama et al., 2007). TRIB3, has been identified affecting insulin action by binding to and inhibiting Akt phosphorylation, which also regulated eNOS and NO. In human and mice, insulin can relax bladder via activating PI3K-AKT-eNOS pathway and lead to a three folds increase in NO synthesis than baseline in AA-but not in TRIB3 AG/GG genotype carriers (Leiria et al., 2013). Meanwhile, insulin had almost no effect on NO synthesis for AG/GG genotype carriers in human umbilical vein endothelial cells (Andreozzi et al., 2008). Taken together, our results are consistent with the deleterious role of TRIB3 G allele on NO production, which may lead to a weaker response to ACEI and CCBs antihypertensives.
On the contrary, a better response to ARBs was found on AG/GG genotype carriers than AA genotype carriers. This phenomenon may be interpreted as the different mechanisms of Angiotensin. The published literature has reported that Ang-(1-7) can elevate blood pressure by promoting pro-inflammatory and pro-thrombotic effects. Moreover, Ang IV can activate AT4R, and may contribute to cardiac damage (Ruiz-Ortega et al., 2007; Velkoska et al., 2011). Likewise, Yang et al. (2010) has demonstrated that Ang IV increased blood pressure and reduced renal blood flow through counteracting the presence of AT2 receptors in mice. Other studies revealed that Ang IV can ameliorate AngII-induced cardiac injury via AT4R and protect against acute cerebral ischemia via Ang IV-AT4R, NO-dependent mechanisms (Faure et al., 2006; Yang et al., 2011). As mentioned above, an Ang-(1-7) triggered mechanism involving AT1R-, AT4R-, mass receptors and cGMP-PKG-NO pathway may cause the different antihypertensive effect of ACEI and ARB among individuals induced by TRIB3 (251, A > G) genetic polymorphism. In addition, ARB could down-regulate TRIB3 mRNA level and a dual function of ARB on eNOS expression may also lead to the opposite relationship between TRIB3 G allele and antihypertensive effects of ACEI and ARBs (Zhang et al., 2006; Myojo et al., 2014).
In the ARB and ACEI treatment groups, we noticed that the difference in antihypertensive efficacy between different carriers of TRIB3 genotypes became more significant as time went on, which may be due to an increase in local eNOS mRNA expression and a decrease in angiotensin II in the LV, leading to the amelioration of myocardial remodeling in hypertensive condition (Kobayashi et al., 2000). Reversed results of ARBs were also shown in the sex stratification. In this drug treatment group, female of AA genotype but not those with G allele have stronger antihypertensive efficacy compared to male. In the treatment of ACEIs, older (age ≥ 55) patients with AA genotype have stronger antihypertensive efficacy compared to younger patients (age < 55). The specific mechanism needs more in-depth discussion and exploration.
In EH patients treated with α, β-ADRs, we did not observe significant difference of antihypertensive effect on response to TRIB3 genotypes. However, it is interesting to note that TRIB3 (251, A > G) variance was associated with α, β-ADRs monotherapy in Chinese population with EH in an age-dependent and sex-specific manner. And between the age groups and the sex groups, there is a significant difference in BP-change from baseline only limited to patients with TRIB3 (251, A > G) AG/GG genotype, while the mechanism is unclear. In addition, our study has shown that among G allele carriers, males or youngers are more likely to have a better antihypertensive effect (Figure 4, 5). This may be attributed to the inconsistent distribution of adrenoceptor in a sex-dependent manner. The regulation of estrogen may also result in greater adrenoceptor blocker reactivity in males than in females (Luksha et al., 2005; Al-Gburi et al., 2017). Researches showed that aging decreases cardiac beta-adrenergic responsiveness in model systems and in humans in vivo with multiple mechanisms including downregulation and decreased agonist binding of beta 1-receptors, uncoupling of beta 2-receptors, and abnormal G protein-mediated signal transduction (White et al., 1994).
Reduction in BP by 1–4 mm was also a question be worthy to think about, in the research of Lewington et al. (2002) a 2 mm Hg lower usual SBP was associated with approximately 10% lower stroke mortality and about 7% lower mortality from ischaemic heart disease or other vascular causes in middle age. In general, a 20 mm Hg difference in usual SBP is approximately equivalent in its hazards to a 10 mm Hg difference in usual DBP (Lewington et al., 2002). Therefore, we make hypothesis that for every 1 mm Hg reduction in diastolic blood pressure in the general normotensive population, the mortality rate of vascular-related events can be reduced by 7–10%. For patients with high blood pressure, a decrease of several mmHg of BP may not bring such a big benefit, but we can still assume the benefit exists.
In recent years it became evident that the vast majority of genetic variants in genes of importance for drug treatment are rare, with minor allele frequencies <1%. Also, recent approximations based on analyses of population-scale sequencing projects estimate that such rare variants account for 30–40% of the interindividual variability in drug response (Lauschke and Ingelman-Sundberg, 2018). Some low-frequency genetic variants detected by re-sequencing of TRIB3 can partially account for cardiovascular clinical outcomes in diabetes. But the association was especially strong among individuals who also carried the common R84 variant (Prudente et al., 2015). Other studies reported that the variation at 5′-untranslated region of the gene may have a strong impact on translational efficiency and serve an important function on gene expression regulating (Hughes, 2006). Several additional infrequent nonsynonymous single-nucleotide polymorphisms have been identified in the genomic region encompassing the human TRIB3 gene (dbSNP2) whose function and potential biological relevance have never, so far, been addressed. These may lead us to speculate that rare and common variants in TRIB3 may act together to alter drug efficacy.
Our study was a well-controlled drug clinical trial in which patients received monotherapy. Nevertheless, some limitations should be considered in the interpretation of our findings. On the one hand, diet habits, lifestyle factors may affect the antihypertensive effect of drugs. Such as a daily mean of 30 min of vigorous exercise, a DASH style diet, modest alcohol intake up to 10 g/day, non-narcotic analgesic use less than once per week, and intake of 400 μg/day or more of supplemental folic acid may lead to lower risk of hypertension (Forman et al., 2009). These factors also have the potential to make patients have a distinguishing antihypertensive effect. However, our results do not exclude the influence of these factors because of the lack of these information. On the other hand, polymorphisms of other genes may also be involved in the interaction between heredity and drug efficacy or affect vascular function (He et al., 2015; Shen et al., 2019).
Conclusion
In conclusion, the results of the present study suggest that the TRIB3 (251, A > G) polymorphism was associated with the response of antihypertensive drugs therapy in Chinese EH patients,. Although this data may prove TRIB3 (251, A > G) genetic variation is useful in clinical therapy, combining other genetic markers to identify individuals at high risk of cardiovascular abnormalities may bring a better clinical outcome. Further pharmacogenomics studies on TRIB3 merit attention.
Author Contributions
JZ, FH, JL, LX, and WZ were responsible for the study design. JZ, FH, and BS were responsible for the experiments. JZ and FH did the data processing and statistical analysis. JZ, FH, and BS wrote and interpreted the manuscript. FH and BS were responsible for data management and contributed reagents and materials. RL, YG, HR, YS, XC, ZL, HZ, SD, and HX calibrated and discussed the manuscripts.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to the Shanghai Institute of Hypertension in Ruijin Hospital for supporting our clinical trials. We also thank Dr. Tang jie for her advice on revision of language.
Funding. The work was supported by National Scientific Foundation of China (Nos. 81573511, 81874329, 81522048, and 81273595), the National Key R&D Plan (No. 2016YFC0905000), the ‘863’ Project (No. 2012AA02A518).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.00236/full#supplementary-material
Blood pressure response to CCBs, ARBs, and ACEI therapy in EH patients stratified by age-specific to TRIB3 genotype. A–C respectively depicts that BP changes from baseline in EH patients carrying the TRIB3 (251, A>G) AA and AG/GG genotypes after treatment with CCBs, ARBs, and ACEI for 6 weeks respectively. P-value were adjusted for baseline BMI, sex, SBP, DBP, total cholesterol, heart rate, triglyceride, HDL, LDL, FBG, and other biochemical factors appropriately. Error bar indicated 95% confidence interval. The stratification based on a boundary of age-55 showed the largest difference in blood pressure decrease after analysis of age-50, 55, 60.
Blood pressure response to CCBs, ARBs, and ACEI therapy in EH patients stratified by sex dependent of TRIB3 genotype. A–C respectively depicts that BP changes from baseline in EH patients carrying the TRIB3 (251, A>G) AA and AG/GG genotypes after treatment with CCBs, ARBs, and ACEI for 6 weeks respectively. P-value were adjusted for baseline age, BMI, SBP, DBP, total cholesterol, heart rate, triglyceride, HDL, LDL, FBG, and other biochemical factors appropriately. Error bar indicated 95% confidence interval.
References
- Al-Gburi S., Deussen A., Zatschler B., Weber S., Künzel S., El-Armouche A., et al. (2017). Sex-difference in expression and function of beta-adrenoceptors in macrovessels: role of the endothelium. Basic Res. Cardiol. 112:29. 10.1007/s00395-017-0617-2 [DOI] [PubMed] [Google Scholar]
- Andreozzi F., Formoso G., Prudente S., Hribal M. L., Pandolfi A., Bellacchio E., et al. (2008). TRIB3 R84 variant is associated with impaired insulin-mediated nitric oxide production in human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 28 1355–1360. 10.1161/atvbaha.108.162883 [DOI] [PubMed] [Google Scholar]
- Chan M. C., Nguyen P. H., Davis B. N., Ohoka N., Hayashi H., Du K., et al. (2007). A novel regulatory mechanism of the bone morphogenetic protein (BMP) signaling pathway involving the carboxyl-terminal tail domain of BMP type II receptor. Mol. Cell Biol. 27 5776–5789. 10.1128/mcb.00218-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M. C., Weisman A. S., Kang H., Nguyen P. H., Hickman T., Mecker S. V., et al. (2011). The amiloride derivative phenamil attenuates pulmonary vascular remodeling by activating NFAT and the bone morphogenetic protein signaling pathway. Mol. Cell Biol. 31 517–530. 10.1128/mcb.00884-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Iwai M., Wu L., Suzuki J., Min L. J., Shiuchi T., et al. (2003). Important role of nitric oxide in the effect of angiotensin-converting enzyme inhibitor imidapril on vascular injury. Hypertension 42 542–547. 10.1161/01.hyp.0000092440.52239.39 [DOI] [PubMed] [Google Scholar]
- Chen Y. L., Pan A. W., Hsiung P. C., Chung L., Lai J. S., Shur-Fen Gau S, et al. (2015). Life adaptation skills training (LAST) for persons with depression: a randomized controlled study. J. Affect. Disord. 185 108–114. 10.1016/j.jad.2015.06.022 [DOI] [PubMed] [Google Scholar]
- Cui A. D., Gai N. N., Zhang X. H., Jia K. Z., Yang Y. L., Song Z. J. (2012). Decreased serum obestatin consequent upon TRIB3 Q84R polymorphism exacerbates carotid atherosclerosis in subjects with metabolic syndrome. Diabetol. Metab. Syndr. 4:52. 10.1186/1758-5996-4-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K., Herzig S., Kulkarni R. N., Montminy M. (2003). TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science 300 1574–1577. 10.1126/science.1079817 [DOI] [PubMed] [Google Scholar]
- Faure S., Chapot R., Tallet D., Javellaud J., Achard J. M., Oudart N. (2006). Cerebroprotective effect of angiotensin IV in experimental ischemic stroke in the rat mediated by AT(4) receptors. J. Physiol. Pharmacol. 57 329–342. [PubMed] [Google Scholar]
- Fischer Z., Das R., Shipman A., Fan J. Y., Pence L., Bouyain S., et al. (2017). A Drosophila model of insulin resistance associated with the human TRIB3 Q/R polymorphism. Dis. Model. Mech. 10 1453–1464. 10.1242/dmm.030619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman J. P., Stampfer M. J., Curhan G. C. (2009). Diet and lifestyle risk factors associated with incident hypertension in women. JAMA 302 401–411. 10.1001/jama.2009.1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formoso G., Di Tomo P, Andreozzi F., Succurro E., Di Silvestre S., Prudente S., et al. (2011). The TRIB3 R84 variant is associated with increased carotid intima-media thickness in vivo and with enhanced MAPK signalling in human endothelial cells. Cardiovasc. Res. 89 184–192. 10.1093/cvr/cvq255 [DOI] [PubMed] [Google Scholar]
- Gong H. P., Wang Z. H., Jiang H., Fang N. N., Li J. S., Shang Y. Y., et al. (2009). TRIB3 functional Q84R polymorphism is a risk factor for metabolic syndrome and carotid atherosclerosis. Diabetes Care 32 1311–1313. 10.2337/dc09-0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F., Liu M., Chen Z., Liu G., Wang Z., Liu R., et al. (2016). Assessment of human tribbles homolog 3 genetic variation (rs2295490) effects on type 2 diabetes patients with glucose control and blood pressure lowering treatment. EBioMedicine 13 181–189. 10.1016/j.ebiom.2016.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F., Luo J., Zhang Z., Luo Z., Fan L., He Y., et al. (2015). The RGS2 (-391, C > G) genetic variation correlates to antihypertensive drug responses in Chinese patients with essential hypertension. PLoS One 10:e0121483. 10.1371/journal.pone.0121483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F., Shu Y., Wang X., Liu X., Liu G., Chen Z., et al. (2018). Intensive glucose control reduces the risk effect of TRIB3, SMARCD3, and ATF6 genetic variation on diabetic vascular complications. Front. Pharmacol. 9:1422. 10.3389/fphar.2018.01422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes T. A. (2006). Regulation of gene expression by alternative untranslated regions. Trends Genet 22 119–122. 10.1016/j.tig.2006.01.001 [DOI] [PubMed] [Google Scholar]
- Kanno S., Wu Y. J., Lee P. C., Billiar T. R., Ho C. (2001). Angiotensin-converting enzyme inhibitor preserves p21 and endothelial nitric oxide synthase expression in monocrotaline-induced pulmonary arterial hypertension in rats. Circulation 104 945–950. 10.1161/hc3401.093155 [DOI] [PubMed] [Google Scholar]
- Kim S., Hata A., Kang H. (2014). Down-regulation of miR-96 by bone morphogenetic protein signaling is critical for vascular smooth muscle cell phenotype modulation. J. Cell Biochem. 115 889–895. 10.1002/jcb.24730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y., Hirooka Y., Sagara Y., Sunagawa K. (2007). Long-acting calcium channel blocker, azelnidipine, increases endothelial nitric oxide synthase in the brain and inhibits sympathetic nerve activity. Clin. Exp. Hypertens. 29 13–21. 10.1080/10641960601096745 [DOI] [PubMed] [Google Scholar]
- Kiss-Toth E., Bagstaff S. M., Sung H. Y., Jozsa V., Dempsey C., Caunt J. C., et al. (2004). Human tribbles, a protein family controlling mitogen-activated protein kinase cascades. J. Biol. Chem. 279 42703–42708. 10.1074/jbc.M407732200 [DOI] [PubMed] [Google Scholar]
- Kobayashi N., Hara K., Watanabe S., Higashi T., Matsuoka H. (2000). Effect of imidapril on myocardial remodeling in L-NAME–induced hypertensive rats is associated with gene expression of NOS and ACE mRNA. Am. J. Hypertens. 13 199–207. 10.1016/S0895-7061(99)00117-X [DOI] [PubMed] [Google Scholar]
- Kobayashi N., Mita S., Yoshida K., Honda T., Kobayashi T., Hara K., et al. (2003). Celiprolol activates eNOS through the PI3K-Akt pathway and inhibits VCAM-1 Via NF-κB induced by oxidative stress. Hypertension 42 1004–1013. 10.1161/01.hyp.0000097547.35570.70 [DOI] [PubMed] [Google Scholar]
- Koyama Y., Takeishi Y., Takahashi H., Shishido T., Arimoto T., Niizeki T., et al. (2007). Azelnidipine inhibits H2O2-induced cell death in neonatal rat cardiomyocytes. Cardiovasc. Drugs Ther. 21 69–72. 10.1007/s10557-007-6008-4 [DOI] [PubMed] [Google Scholar]
- Lauschke V. M., Ingelman-Sundberg M. (2018). How to consider rare genetic variants in personalized drug therapy. Clin. Pharmacol. Ther. 103 745–748. 10.1002/cpt.976 [DOI] [PubMed] [Google Scholar]
- Leiria L. O., Sollon C., Báu F. R., Mónica F. Z., D’Ancona C. L., De Nucci G., et al. (2013). Insulin relaxes bladder via PI3K/AKT/eNOS pathway activation in mucosa: unfolded protein response-dependent insulin resistance as a cause of obesity-associated overactive bladder. J. Physiol. 591 2259–2273. 10.1113/jphysiol.2013.251843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewington S., Clarke R., Qizilbash N., Peto R., Collins R. (2002). Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360 1903–1913. 10.1016/S0140-6736(02)11911-8 [DOI] [PubMed] [Google Scholar]
- Luksha L., Poston L., Gustafsson J. A., Aghajanova L., Kublickiene K. (2005). Gender-specific alteration of adrenergic responses in small femoral arteries from estrogen receptor-beta knockout mice. Hypertension 46 1163–1168. 10.1161/01.HYP.0000185648.48498.c1 [DOI] [PubMed] [Google Scholar]
- Messerli F. H., Williams B., Ritz E. (2007). Essential hypertension. Lancet 370 591–603. 10.1016/s0140-6736(07)61299-9 [DOI] [PubMed] [Google Scholar]
- Myojo M., Nagata D., Fujita D., Kiyosue A., Takahashi M., Satonaka H., et al. (2014). Telmisartan activates endothelial nitric oxide synthase via Ser1177 phosphorylation in vascular endothelial cells. PLoS One 9:e96948. 10.1371/journal.pone.0096948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A., Advance Collaborative Group. MacMahon S., Chalmers J., Neal B., Woodward M.,et al. (2007). Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 370 829–840. 10.1016/s0140-6736(07)61303-8 [DOI] [PubMed] [Google Scholar]
- Prudente S., Bailetti D., Mendonca C., Mannino G. C., Fontana A., Andreozzi F., et al. (2015). Infrequent TRIB3 coding variants and coronary artery disease in type 2 diabetes. Atherosclerosis 242 334–339. 10.1016/j.atherosclerosis.2015.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudente S., Hribal M. L., Flex E., Turchi F., Morini E., De Cosmo S., et al. (2005). The functional Q84R polymorphism of mammalian Tribbles homolog TRB3 is associated with insulin resistance and related cardiovascular risk in Caucasians from Italy. Diabetes 54 2807–2811. 10.2337/diabetes.54.9.2807 [DOI] [PubMed] [Google Scholar]
- Prudente S., Trischitta V. (2015). The TRIB3 Q84R polymorphism, insulin resistance and related metabolic alterations. Biochem. Soc. Trans. 43 1108–1111. 10.1042/bst20150115 [DOI] [PubMed] [Google Scholar]
- Qin R. R., Song M., Li Y. H., Wang F., Zhou H. M., Liu M. H., et al. (2017). Association of increased serum sema3E with TRIB3 Q84R polymorphism and carotid atherosclerosis in metabolic syndrome. Ann. Clin. Lab. Sci. 47 47–51. [PubMed] [Google Scholar]
- Ruiz-Ortega M., Esteban V., Egido J. (2007). The regulation of the inflammatory response through nuclear factor-kappab pathway by angiotensin IV extends the role of the renin angiotensin system in cardiovascular diseases. Trends Cardiovasc. Med. 17 19–25. 10.1016/j.tcm.2006.10.003 [DOI] [PubMed] [Google Scholar]
- Shen J., Liu M., Xu J., Sun B., Xu H., Zhang W. (2019). ARL15 overexpression attenuates high glucose-induced impairment of insulin signaling and oxidative stress in human umbilical vein endothelial cells. Life Sci. 220 127–135. 10.1016/j.lfs.2019.01.030 [DOI] [PubMed] [Google Scholar]
- Stojkov N. J., Baburski A. Z., Bjelic M. M., Sokanovic S. J., Mihajlovic A. I., Drljaca D. M., et al. (2014). In vivo blockade of alpha1-adrenergic receptors mitigates stress-disturbed cAMP and cGMP signaling in Leydig cells. Mol. Hum. Reprod. 20 77–88. 10.1093/molehr/gat052 [DOI] [PubMed] [Google Scholar]
- Stojkov N. J., Janjic M. M., Kostic T. S., Andric S. A. (2013). Orally applied doxazosin disturbed testosterone homeostasis and changed the transcriptional profile of steroidogenic machinery, cAMP/cGMP signalling and adrenergic receptors in Leydig cells of adult rats. Andrology 1 332–347. 10.1111/j.2047-2927.2012.00035.x [DOI] [PubMed] [Google Scholar]
- Sun B., He F., Sun L., Zhou J., Shen J., Xu J., et al. (2019). Cause-specific risk of major adverse cardiovascular outcomes and hypoglycemic in patients with type 2 diabetes: a multicenter prospective cohort study. Endocrine 63 44–51. 10.1007/s12020-018-1715-0 [DOI] [PubMed] [Google Scholar]
- Velkoska E., Dean R. G., Griggs K., Burchill L., Burrell L. M. (2011). Angiotensin-(1-7) infusion is associated with increased blood pressure and adverse cardiac remodelling in rats with subtotal nephrectomy. Clin. Sci. 120 335–345. 10.1042/cs20100280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wain L. V., Verwoert G. C., O’Reilly P. F., Shi G., Johnson T., Johnson A. D., et al. (2011). Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat. Genet. 43 1005–1011. 10.1038/ng.922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J., Fagan K., Steudel W., Fouty B., Lane K., Harral J., et al. (2004). Pulmonary hypertension in transgenic mice expressing a dominant-negative BMPRII gene in smooth muscle. Circ. Res. 94 1109–1114. 10.1161/01.res.0000126047.82846.20 [DOI] [PubMed] [Google Scholar]
- White M., Roden R., Minobe W., Khan M. F., Larrabee P., Wollmering M., et al. (1994). Age-related changes in beta-adrenergic neuroeffector systems in the human heart. Circulation 90 1225–1238. 10.1161/01.CIR.90.3.1225 [DOI] [PubMed] [Google Scholar]
- Yang H., Zeng X. J., Wang H. X., Zhang L. K., Dong X. L., Guo S., et al. (2011). Angiotensin IV protects against angiotensin II-induced cardiac injury via AT4 receptor. Peptides 32 2108–2115. 10.1016/j.peptides.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Yang R., Walther T., Gembardt F., Smolders I., Vanderheyden P., Albiston A. L., et al. (2010). Renal vasoconstrictor and pressor responses to angiotensin IV in mice are AT1a-receptor mediated. J. Hypertens. 28 487–494. 10.1097/HJH.0b013e3283343250 [DOI] [PubMed] [Google Scholar]
- Yu Q., Gao F., Ma X. L. (2011). Insulin says NO to cardiovascular disease. Cardiovasc. Res. 89 516–524. 10.1093/cvr/cvq349 [DOI] [PubMed] [Google Scholar]
- Zhang W., Yang Z., Li X., Wen J., Zhang H., Wang S., et al. (2015). The functional Q84R polymorphism of TRIB3 gene is associated with diabetic nephropathy in Chinese type 2 diabetic patients. Gene 555 357–361. 10.1016/j.gene.2014.11.031 [DOI] [PubMed] [Google Scholar]
- Zhang W., Zhong M., Tang M. X., Ma X., Miao Y., Sun H., et al. (2006). Effect of valsartan on Tribble 3 gene expression in rats with experimental diabetic cardiomyopathy. Zhonghua Xin Xue Guan Bing Za Zhi 34 212–216. [PubMed] [Google Scholar]
- Zou T., Liu W. J., Li S. D., Zhou W., Yang J. F., Zou C. G. (2011). TRB3 mediates homocysteine-induced inhibition of endothelial cell proliferation. J. Cell Physiol. 226 2782–2789. 10.1002/jcp.22554 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Blood pressure response to CCBs, ARBs, and ACEI therapy in EH patients stratified by age-specific to TRIB3 genotype. A–C respectively depicts that BP changes from baseline in EH patients carrying the TRIB3 (251, A>G) AA and AG/GG genotypes after treatment with CCBs, ARBs, and ACEI for 6 weeks respectively. P-value were adjusted for baseline BMI, sex, SBP, DBP, total cholesterol, heart rate, triglyceride, HDL, LDL, FBG, and other biochemical factors appropriately. Error bar indicated 95% confidence interval. The stratification based on a boundary of age-55 showed the largest difference in blood pressure decrease after analysis of age-50, 55, 60.
Blood pressure response to CCBs, ARBs, and ACEI therapy in EH patients stratified by sex dependent of TRIB3 genotype. A–C respectively depicts that BP changes from baseline in EH patients carrying the TRIB3 (251, A>G) AA and AG/GG genotypes after treatment with CCBs, ARBs, and ACEI for 6 weeks respectively. P-value were adjusted for baseline age, BMI, SBP, DBP, total cholesterol, heart rate, triglyceride, HDL, LDL, FBG, and other biochemical factors appropriately. Error bar indicated 95% confidence interval.