Abstract
Background: The purpose of this study is to provide a critical review of current evidence for the impact of time to initiation of chemoradiation on overall survival in patients with newly diagnosed high-grade gliomas treated with radiation and concurrent temozolomide chemotherapy.
Methods: A literature search was conducted using PubMed/MEDLINE and EMBASE databases. Studies were included if they provided separate analysis for patients treated with current standard of care: radiation and concurrent temozolomide. Bias assessment was performed for each included study using the Newcastle-Ottawa Assessment Scale, with Karnofsky Performance Status (KPS) and extent of resection used for comparability.
Results: The initial search yielded 575 citations. Based on the inclusion/exclusion criteria, a total of 10 retrospective cohort studies were included in this review for a total of 30,298 patients. Of these, one study described an indirect relationship between time to initiation of treatment and overall survival. One study found decreased survival only with patients with significantly longer time to treatment. Four studies found no significant effect of time to treatment on overall survival. The four remaining studies found that patients with moderate time to initiation had the best overall survival.
Conclusion: This review provides evidence that moderate time to initiation of chemoradiotherapy in patients with high-grade gliomas does not lead to a significant decrease in overall survival, though the effect of significant delays in treatment initiation remains unclear.
Keywords: gliobastoma, high-grade glioma, chemoradiation, timing, wait time
Introduction
Glioblastoma (GBM) is the most common primary central nervous system tumor in adults, accounting for 45.2% of malignant primary brain tumors in the United States (1). The current standard of care that provides the greatest life expectancy in these patients became standard of care following the publication by Stupp et al. and includes maximal safe tumor resection followed by radiation therapy with concurrent temozolomide (TMZ) for 6 weeks and six subsequent cycles of adjuvant TMZ (2). In the European Organisation for Research and Treatment of Cancer (EORTC) and National Cancer Institute of Canada (NCIC) randomized study, this regimen (referred to as the Stupp protocol) was associated with an increase in median survival of GBM patients from 12.1 to 14.6 months when compared to the previous standard of radiotherapy alone (3). In this trial, patients had a median time from diagnosis to start of chemoradiotherapy of 5 weeks (range: 1.7–12.9 weeks), however the optimal timing of initiation of chemoradiation has not been well elucidated.
Studies of optimal timing of radiation therapy in breast, lung, and head and neck cancers have consistently shown an indirect correlation between time to initiation of radiation and recurrence risk (4–7). For aggressive malignancies such as GBM with rapid doubling time, it would be expected that longer time to initiation of treatment could allow for further tumor growth and progression (8). Indeed, studies have shown areas of increased contrast enhancement between the time of tumor resection and the time of therapy initiation consistent with tumor progression in 82% of patients (9).
Due to ethical concerns, no prospective trials have been conducted to address the question of optimal timing of treatment initiation in patients with GBM. Several retrospective studies that have attempted to address this question have yielded conflicting results (10). Some studies have found that increasing time from surgical resection to initiation of treatment is correlated with worse overall survival (11–13), while other studies have found no association between the timing of treatment and patient outcomes (14–16). Some studies have even shown a potential benefit to moderately increased time to treatment initiation, though a mechanism for this phenomenon has not been well established (17, 18). Many of the aforementioned studies took place prior to the initiation of the Stupp protocol in 2005 and all systematic reviews and meta-analyses on the topic include studies that were done prior to this time period.
The purpose of this study is to provide a critical review of the current evidence for the impact of time to treatment (TT) initiation of chemoradiation on overall survival of patients with GBM who were treated with the current standard of concurrent radiation and TMZ.
Methods
This systematic review adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol (19).
Search Strategy
The goal of this search was to identify all published works evaluating the effect of timing of initiation of post-operative chemoradiotherapy in patients with high-grade gliomas (grade III/IV) treated with the current standard of care. The databases used for this search included the U.S. National Library of Medicine (PubMed/MEDLINE) and Excerpta Medica Database (EMBASE). All searches were limited to January 2005–June 2018, as the EORTC/NCIC randomized study was published in 2005 (2). Key words used in the search algorithm included: glioma, glioblastoma, radiotherapy, chemoradiotherapy, timing, early, and delay. Specific search algorithms were designed in accordance with the author and an institutional research librarian (Table S1). All citations of the articles selected in the initial screening of search results were manually evaluated for eligibility as well.
Selection Criteria
Eligibility criteria included publications that evaluated overall survival as it related to time between surgical resection and initiation of chemoradiotherapy in adult patients with newly diagnosed high-grade gliomas (grade III/IV). Publications were eligible if they included patients who underwent adjuvant treatments other than adjuvant chemoradiation with TMZ, as long as patients who underwent combined radiation therapy with temozolomide therapy were analyzed separately. Publications were excluded if they included patients with recurrent gliomas, patients who did not undergo a neurosurgical procedure (either biopsy, subtotal or gross total resection), or patients who did not undergo combined radiation and temozolomide therapy within the analysis.
Titles and abstracts were reviewed from the initial search and excluded publications that were clearly inappropriate. After duplications were removed, all remaining publications underwent full-text inspection to evaluate eligibility based on the aforementioned criteria.
Data Collection
The following information was collected from each study: study period, total sample size, patient ages, Karnofsky performance status (KPS), tumor histology, extent of resection, and adjuvant chemotherapy and radiation regimens. Each of the included publications divided patients into different subgroups based on the time between surgery and initiation of therapy. Hazard Ratios with 95% confidence interval were collected from each study. Any additional factors that were found to be significantly associated with overall survival were recorded as well. For those studies that included it, information regarding factors associated with early and/or delayed treatment initiation was also recorded.
Risk of Bias Assessment
Bias assessment for individual studies was evaluated using the Newcastle-Ottawa Quality Assessment Scale for Cohort studies, which evaluates studies based on selection (maximum of 4), comparability (maximum of 2), and outcome (maximum of 3) (20, 21). Factors included in the “comparability” category included control for KPS and extent of resection, as each of these factors has consistently been shown in multiple studies to be highly associated with prognosis (22–24).
Results
A total of 575 citations resulted from the initial database search of which 32 were selected for full-text inspection following exclusion based on title/abstract and removal of duplications. Of these 32 publications, 10 met inclusion criteria and were included in this systematic review. Reasons for exclusion include publication type, not assessing variable of interest, study period prior to 2005, study period includes patients treated after 2005 but without separate analysis of patients receiving the Stupp protocol, and cohort that does not include post-operative high-grade glioma (HGG) patients (Figure 1). With these 10 retrospective cohort studies combined, a total of 30,298 patients were assessed. Three of the studies evaluated patients who had been enrolled in various clinical trials for correlation between TT and overall survival (OS) (25–27). Nine of the studies were done exclusively in patients with grade IV gliomas, and one study cohort was a mix of grade III and grade IV gliomas with the majority of patients being grade IV. Information regarding the study period, total number of patients, patient ages, KPS scores, tumor histology, extent of resection, chemotherapy regimen, radiation dosages, median TT and TT subgroups for each study can be found in Table 1.
Figure 1.
Literature search strategy, results, and selection criteria. The search was performed on July 3, 2018. HGG, high-grade gliomas.
Table 1.
Study characteristics of all studies included in this systematic review.
| Study period | Patients (n) | Patient ages (median [range]) | KPS (median) | Histology (% GBM) | Extent of resection | Chemo regimen (% receiving TMZ) | Radiation | Median TT (days) | TT subgroups | |
|---|---|---|---|---|---|---|---|---|---|---|
| Adeberg et al. (26) | 2004–2011 | 177 | 58.8 [20.3–75.9] | 90 | 100 | • Biopsy: 4% • STR: 60% • GTR: 36% |
86.4% | 60 Gy | 31* | < 24 d > 24 d |
| Blumenthal et al. (25) | 2011 | 1,395 | 58 * [19–87] | 90 | 100 | • STR: 42.9% • GTR: 53.0% • Other: 4.1% |
100 | 60 Gy | 26* | ≤ 3 wks 3–4 wks > 4 wks |
| Han et al. (27) | 2004–2010 | 198 | 55.1 [21.3–80] | 90 | 100 | • Biopsy: 16.7% • STR: 47.9% • GTR: 33.8% |
100 | 60 Gy | 29.5 | < 30 d 30–34 d > 34 d |
| Louvel et al. (28) | 2005–2011 | 692 | Mean: 57.5 ± 10.8 | 34.2% ≤ 70 65.8% >70 | 100 | • Biopsy: 0% • STR: 34.5% • GTR: 65.5% |
100 | 60 Gy | 42 | < 1.5 mos > 1.5 mos |
| Nathan et al. (29) | 2005–2014 | 2,535 | 58* | Not reported | 77 | Not reported | 100 | 60 Gy | 35.7* | 0–4 wks 4–6 wks 6–13 wks |
| Noel et al. (15) | 2006 | 400 | 60.5 [22.7–85.6] | Not reported | 100 | • Biopsy: 36% • STR: 23% • GTR: 41% |
100 | 60 Gy [median] | 41 | 2–4 wks 5 wks 6 wks 7 wks ≥ 8 wks |
| Osborn et al. (30) | 2010–2012 | 11,652 | 61 [IQR: 53–69] | Not reported | 100 | • Biopsy: 0% • STR: 55.1% • GTR: 44.9% |
100** | Not reported | 30 | ≤ 24 d 25–30d 31–37d > 37d |
| Pollom et al. (31) | 2010–2013 | 12,738 | 61–69 | Not reported | 100 | • Biopsy: 22% • STR: 37% • GTR: 41% |
100** | 27% < 60 Gy 66% ≥ 60 Gy | 29 | < 15d 15–21d 22–28 d 29–35 d 36–42 d > 42 d |
| Sun et al. (32) | 2005–2015 | 218 | 58 [21–86] | 80 | 100 | Not reported | 100 | 60 Gy | 27 | < 27 > 27 |
| Wang et al. (33) | 1996–2014 | 447 | 23.5% < 50 76.5% ≥ 50 | 80.3% ≥ 70 19.7% > 70 | 100 | • Biopsy: 21.5% • STR: 14.5% • GTR: 64% |
61% | 10.3% < 36 Gy 9.8% 36–54 Gy 79.9% > 54 Gy | 34% < 21 33.7% 21–32 32.2% > 32 | < 21 d 21–32 d > 32 d |
Median reported as average of medians for groups involved, as data regarding the entire cohort was unavailable
Type of chemotherapy not recorded, but assumed to be TMZ by authors given time period.
D, days; wks, weeks; mos, months; KPS, karnofsky performance Score; GBM, glioblastoma multiforme; TMZ, temozolomide; WT, wait time; STR, subtotal resection; GTR, gross total resection.
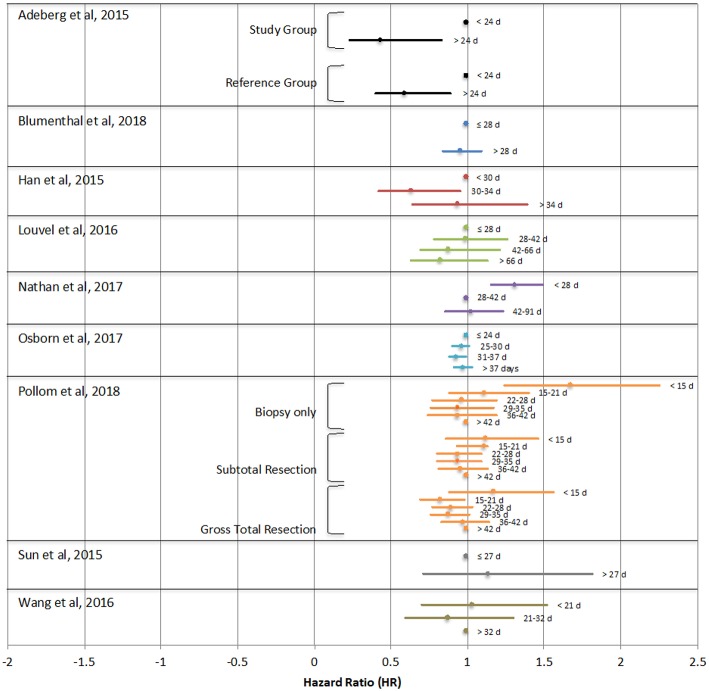
Of the studies analyzed, one study found improved survival with early initiation of treatment (within 15–21 days) compared to longer time to initiation (>42 days) only in patients who underwent gross total resection, though the opposite was true for patient who underwent biopsy only (31). One study found poorer survival only in a small subset of patients with particularly long TT (>6 weeks) (32). Four studies found no statistically significant effects of TT on OS (15, 25, 28, 30). Adeberg et al, Han et al, Nathan et al, and Wang et al. each found that the greatest survival was in patients with a slight delay to chemoradiotherapy initiation of >24 days, 30–34 days, 4–13 weeks, or 21–32 days respectively (26, 27, 29, 33). Figure 2 demonstrates the hazard ratio of death of study groups reported in each study relative to their respective reference points, which are indicated by HR of 1. Noel et al is not indicated in this figure, as it did not report HR as it relates to TT. This study found no statistically significant differences in median survival in patients with TT of 2–4 weeks, 5 weeks, 6 weeks, 7 weeks or ≥8 weeks (15).
Figure 2.
Hazard ratios (HR) of overall survival as they relate to time between surgical resection and initiation of chemoradiotherapy reported in each study. The point depicted with HR = 1 was used as the reference group. Any HR > 1 denotes an increased risk of death. Noel et al. is not indicated in this figure as this study did not report hazard ratios as they relate to treatment time.
Other variables found to be significantly associated with survival included O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation status in 3 studies (25, 26, 30), recursive partitioning analysis (RPA) classification in 3 studies (25, 28, 33), sex in 4 studies (25, 28–30), age in 4 studies (27, 29, 30, 32), KPS in 2 studies (27, 33), and extent of resection in 4 studies (27, 28, 30, 33). Additionally, Osborn et al found significant associations between survival and Charlson/Deyo comorbidity score, non-white race, tumor size, and facility type (academic vs. non-academic) (30). Wang et al also found significant associations between overall survival and total RT dose and use of TMZ (33). The hazard ratios for each of these associations are detailed in Table 2.
Table 2.
This table depicts the values that were found to be significantly related to overall survival in each study that reported these variables as well as their corresponding hazard ratios and 95% confidence intervals.
| Factors associated with OS on multivariate analysis | Hazard ratio (95% CI) | P-value | |
|---|---|---|---|
| Adeberg et al. (26) | MGMT promoter methylation | 0.43 (0.18,0.99) | 0.048 |
| Blumenthal et al. (25) | RPA IV (vs. III) | 1.65 (1.37, 1.99) | <0.001 |
| RPA V (vs. III) | 2.91 (2.34, 3.61) | <0.001 | |
| MGMT unmethylated | 1.72 (1.48, 2.00) | <0.001 | |
| Male sex | 1.31 (1.14, 1.50) | <0.001 | |
| Han et al. (27) | Age | 1.03 (1.02,1.05) | <0.001 |
| KPS | 3.64 (1.55,8.55) | 0.003 | |
| Biopsy (vs. STR/GTR) | 2.93 (1.93, 4.45) | <0.001 | |
| Louvel et al. (28) | Male sex | 1.28 (1.06,1.55) | 0.012 |
| RTOG-RPA class 5–6 | 1.31 (1.08,1.58) | 0.005 | |
| Total resection (vs. partial) | 0.75 (0.62,0.91) | 0.004 | |
| Nathan et al. (29) | Age at craniotomy | 1.031 (1.026,1.036) | <0.0001 |
| Female sex | 0.837 (0.742,0.944) | 0.0038 | |
| Osborn et al. (30) | Age > 60 | 1.68 (1.61,1.75) | <0.001 |
| Charlson/Deyo 1 (vs. 0) | 1.17 (1.10,1.24) | <0.001 | |
| Charlson/Deyo ≥ 2 (vs. 0) | 1.37 (1.27,1.47) | <0.001 | |
| Female gender | 0.90 (0.87,0.94) | <0.001 | |
| Other race (vs. white) | 0.68 (0.60,0.78) | <0.001 | |
| Tumor size 3–5cm (vs. < 3) | 1.09 (1.03,1.16) | <0.001 | |
| Tumor size >5cm (vs. < 3) | 1.13 (1.06,1.20) | <0.001 | |
| MGMT methylation | 0.72 (0.65,0.81) | <0.001 | |
| GTR (vs. STR) | 0.82 (0.79,0.86) | <0.001 | |
| Academic facility | 0.91 (0.87,0.95) | <0.001 | |
| Sun et al. (32) | Age | 1.018 (1.001,1.036) | 0.049 |
| Wang et al. (33) | KPS < 70 | 3.586 (1.644,7.822) | 0.001 |
| Biopsy only (vs. GTR) | 2.510 (1.327,4.747) | 0.005 | |
| RPA class IV (vs. III) | 3.467 (1.351,8.898) | 0.01 | |
| RPA class V/VI (vs. III) | 3.650 (1.077,12.369) | 0.001 | |
| Total RT dose < 36 Gy (vs. >54) | 4.671 (2.241,9.737) | 0.001 | |
| No temozolomide | 3.823 (1.694,8.627) | 0.001 |
MGMT, O6-methylguanine-DNA methyltransferase; RPA, recursive partitioning analysis; KPS, Karnofsky performance status; STR, subtotal resection; GTR, gross total resection; RT, radiation therapy.
Five of the included studies analyzed factors that are significantly associated with early and/or delayed treatment, which are outlined in Table 3. In Han et al. it was found that patients with biopsy only were significantly more likely to start treatment earlier and younger patients were more likely to start treatment later. Louvel et al. reported that patients were more likely to have longer TT if they had a carmustine wafer implantation during surgery. Patients in this cohort with shorter TT were more likely to be RPA class 5 or 6, have neurologic deficits, or have post-operative epileptic seizures. In Osborn et al. patients with shorter TT were more likely to be treated at non-academic facilities, be of white race, have lager tumors, and have subtotal resection (vs. GTR). In Wang et al. patients with shorter TT were more likely to be older, have lower KPS, have biopsy only, have a higher RPA class or have a 3-dimensional conformal RT or 2-dimensional RT technique. Pollom et al. (31) found associations with longer TT in patients who were black/African-American, had Medicaid/government insurance/no insurance, lived in a metropolitan area, or lived >50 miles from the treatment facility. Patients in this cohort were more likely to have a shorter TT if they had a higher income.
Table 3.
This table depicts all of the variables that were found to be statistically significantly associated with longer or shorter TT in the five studies that reported this analysis.
| Association | Variable | P value | |
|---|---|---|---|
| Han et al. (27) | Shorter TT | Biopsy only | 0.006 |
| Longer TT | Younger age | 0.02 | |
| Louvel et al. (28) | Shorter TT | Carmustine wafer implantation | <0.001 |
| Longer TT | RPA class 5–6 Neurologic deficit Post-operative seizures |
<0.001 <0.001 0.049 |
|
| Osborn et al. (30) | Shorter TT | Non-academic treatment facility White race Larger tumor size STR (vs. GTR) |
0.002 <0.001 <0.001 <0.001 |
| Wang et al. (33) | Shorter TT | Older age Lower KPS Biopsy only Higher RPA class RT technique 3D conformal or 2D |
0.006 <0.001 <0.001 <0.001 0.007 |
| Pollom et al. (31) | Shorter TT | Black/African American race Medicaid/Gov't insurance/no insurance Metropolitan area > 50 miles from treatment facility |
0.006 0.001 0.003 0.05 |
| Longer TT | Higher income | 0.03 |
TT, treatment time; RPA, recursive partitioning analysis; STR, sub-total resection; GTR, gross total resection; KPS, Karnofsky Performance Status; RT, radiation therapy.
Results of bias scoring using the Newcastle-Ottawa Quality Assessment Scale for cohort studies is outlined in Table 4. Three studies received the maximum total score of 9, indicating the lowest risk of bias (27, 31, 32). The lowest score was 6/9 given to Nathan et al. indicating the highest risk of bias (29) (Table 4).
Table 4.
This table depicts the bias score calculated for each study based on the Newcastle-Ottawa scale, with 9 as the highest score.
| Adeberg et al. (26) | Blumenthal et al. (25) | Han et al. (27) | Louvel et al. (28) | Nathan et al. (29) | Noel et al. (15) | Osborn et al. (30) | Pollom et al. (31) | Sun et al. (32) | Wang et al. (33) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Selection (Max = 4) | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Comparability (Max = 2) | 1 | 0 | 2 | 1 | 0 | 0 | 1 | 2 | 2 | 0 |
| Outcome (Max = 3) | 3 | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 3 | 3 |
| Total (Max = 9) | 7 | 7 | 9 | 8 | 6 | 7 | 8 | 9 | 9 | 7 |
A higher score correlates with a lower risk of bias. Factors included for comparability include Karnofsky Performance Status and extent of resection.
Discussion
The question of optimal timing of treatment initiation following surgical resection in patients with newly diagnosed glioblastoma has been investigated in several retrospective cohort studies, which has yielded varying results. Several studies have demonstrated decreased overall survival in these patients with increased wait time (11–13), while others have demonstrated no effect (14, 16, 34) and a third group of studies show a favorable outcome with delayed initiation of radiation therapy (17, 18). However, the majority of these studies were conducted prior to the initiation of the Stupp protocol in 2005. Many of those that were written after 2005 include subjects both before and after the Stupp era and do not provide separate analyses of Stupp patients. The addition of temozolomide to the treatment regimen for patients with glioblastoma represents an important change in the care of these patients and provided a significant survival benefit, particularly in patients with MGMT promoter methylation (2, 35). This systematic review aimed to provide an analysis of retrospective studies that only included patients receiving the current standard of care to best answer the question of optimal timing of chemoradiation therapy in glioblastoma patients in the modern era.
This study does not support an optimum time for initiation of chemoradiotherapy following surgical resection in patients with newly diagnosed HGG. The study in this systematic review with the highest number of patients evaluated (n = 12,738) that also received a maximum score of 9 on the risk of bias assessment found significantly improved survival in patients with a time to treatment initiation of 15–21 days in patients who underwent gross total resection (31). Five of the other publications reviewed in this study similarly found benefit to slightly longer times to treatment initiation, including the studies with the 2nd and 3rd largest sample sizes among these studies (29, 30) (n = 11,625 and 2,535, respectively). There are several possible explanations for the worse outcomes seen in patients with shorter TT. There is concern that starting radiation before the patient has fully recovered from surgery could result in impaired healing and an increase in radiation side effects (36–38). It is also probable that patients who start treatment sooner after surgery are chosen to do so based on the judgment of the clinician that they have more aggressive disease or worse functional status as a result of their disease. Indeed, several of the publications evaluated in this study found that patients with the shortest TT were more likely to have undergone less extensive surgery (27, 30, 33), have higher age (27, 33), have postoperative neurologic deficits, (28), have lower KPS (33), or have larger tumor size (30) compared to patients who started treatment later. All of these factors are known to have significant impact on prognosis in glioblastoma and could have contributed to the poorer survival of the early treatment group seen in several of these publications (22–24). Of the remaining four studies, three of them found no significant impact of TT on overall survival (15, 25, 28), while the 4th study, Sun et al. found that there was no survival impact with moderate TT in treatment, though significant TT > 42 days may be associated with worse outcomes (32). Although there is some regional variation, the most recent data from 2005 to 2014 showed that the majority of patients in the United States begin chemoradiotherapy within 6 weeks of surgical resection (29). With a malignancy as devastating as GBM, delays in treatment can be a concern for both patients and providers. Given the fairly narrow window in which patients are typically treated, it may be difficult to discern any significant differences in survival based on treatment timing. This systematic review provides some evidence that, in the era of the Stupp protocol, there is at least no evidence that moderate TT worsen overall patient outcomes and it is reasonable to continue the standard of treatment initiation within 6 weeks after the patient has recovered from surgery.
Each of the publications reviewed in this study suffer from the well-known limitations of retrospective studies. As ethical reasons restrict the possibility of conducting a prospective randomized trial to address this question, there are several confounding factors that have an unknown level of influence in the results of these studies. Ideally, data designed to best answer this question would include a large cohort of patients who are matched for several prognostic factors including age, extent of resection, and functional status to minimize confounders. Several of the studies included in this review attempted to simulate such a cohort by creating regression models to account for several of these prognostic factors and Pollom et al. even analyzed the data separately for patients who had biopsy only, sub-total, and gross total resections (31). Additionally, as novel treatments (such as systemic agents, immunotherapy or tumor-treating fields) are developed that could potentially improve survival of GBM patients, TT may or may not have a greater impact on OS (39). Of note, the design of several of these studies makes it difficult to evaluate the effects of significant TT in patients that may be vulnerable to treatment delays. Some of the studies had strict cutoffs and did not include patients with significant delays (11, 15, 25, 31) while others did not employ a cutoff for TT, but analyzed patients with TT >33 weeks in the same group as patients with a TT of 5 weeks, making it difficult to draw conclusions regarding this subpopulation (32). Patients who may be subject to delays in treatment, such as those who participate in inpatient rehabilitation programs after surgery and are unable to have any cancer treatment until the program is completed, may still have an impact on overall survival related to this delay and further investigation is warranted to draw a conclusion regarding this population.
This systematic review has several limitations. Due to the small number of publications that met the inclusion criteria of this study and the differing ways in which each group analyzed their data, it was not possible to create a mathematical model for evaluation of possible publication bias. The tool used to assess risk of bias for individual studies, the Newcastle-Ottawa Scale, has shown reliability between individual reviewers but has still been criticized for a paucity of evidence regarding validity of the tool (40, 41). Given the unlikelihood of a prospective trial to address this topic, a collaborative effort among institutions to review the current evidence in the Stupp protocol era is the best chance of providing an answer. Establishing a standard for grouping patients by TT and method of analysis in the future could provide a large population of studies that are directly comparable to one another.
Author Contributions
KTW performed the initial literature search, evaluated all of the resulting articles, performed the analysis and figure generations, and wrote the manuscript. LL assisted with data representation and verification of literature search results as well as editing of the manuscript. YL assisted with editing of the final manuscript. MM assisted with literature search and editing of the manuscript. KAW generated the idea to perform this review and provided guidance to the primary author through each step of the process.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. The project described in this publication was supported by the University of Rochester Clinical & Translational Science Award TL1 TR002000 from the National Center for Advancing Translational Sciences of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.00186/full#supplementary-material
This table depicts the search algorithms used for this systematic review and the number of results obtained for each database. For the EMBASE database, searches are built from the bottom up, starting with phrase #1. The final search is bolded at the top (#15) and includes all of the prior phrases.
References
- 1.Thakkar JP, Dolecek TA, Horbinski C, Ostrom QT, Lightner DD, Barnholtz-Sloan JS, et al. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev. (2014) 23:1985–96. 10.1158/1055-9965.EPI-14-0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Engl J Med. (2005) 352:987–96. 10.1056/NEJMoa043330 [DOI] [PubMed] [Google Scholar]
- 3.Davis ME. Glioblastoma: overview of disease and treatment. Clin J Oncol Nurs. (2016) 20(5 Suppl.):S2–8. 10.1188/16.CJON.S1.2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi N, Baumann M, Flentjie M, Kellokumpu-Lehtinen P, Senan S, Zamboglou N, et al. Predictive factors in radiotherapy for non-small cell lung cancer: present status. Lung Cancer. (2001) 31:43–56. 10.1016/S0169-5002(00)00156-2 [DOI] [PubMed] [Google Scholar]
- 5.Froud PJ, Mates D, Jackson JS, Phillips N, Andersen S, Jackson SM, et al. Effect of time interval between breast-conserving surgery and radiation therapy on ipsilateral breast recurrence. Int J Radiation Oncol Biol Phys. (2000) 46:363–72. 10.1016/S0360-3016(99)00412-5 [DOI] [PubMed] [Google Scholar]
- 6.Ampil FL, Buechter KJ, Bairnsfather LE, Shockley WW. Timing and dosage of postoperative radiotherapy for squamous cell carcinoma of the upper aerodigestive tract. J Oral Maxillofac Surg. (1993) 51:1194–97. 10.1016/S0278-2391(10)80287-3 [DOI] [PubMed] [Google Scholar]
- 7.Bastit L, Blot E, Debourdeau P, Menard J, Bastit P, Le Fur R. Influence of the delay of adjuvant postoperative radiation therapy on relapse and survival in oropharyngeal and hypopharyngeal cancers. Int J Radiat Oncol Biol Phys. (2001) 49:139–46. 10.1016/S0360-3016(00)01376-6 [DOI] [PubMed] [Google Scholar]
- 8.Kirkby NF, Jefferies SJ, Jena R, Burnet NG. A mathematical model of the treatment and survival of patients with high-grade brain tumours. J Theor Biol. (2007) 245:112–24. 10.1016/j.jtbi.2006.09.007 [DOI] [PubMed] [Google Scholar]
- 9.Farace P, Amelio D, Ricciardi GK, Zoccatelli G, Magon S, Pizzini F, et al. Early MRI changes in glioblastoma in the period between surgery and adjuvant therapy. J Neurooncol. (2013) 111:177–85. 10.1007/s11060-012-0997-y [DOI] [PubMed] [Google Scholar]
- 10.Han SJ, Englot DJ, Birk H, Molinaro AM, Chang SM, Clarke JL, et al. Impact of timing of concurrent chemoradiation for newly diagnosed glioblastoma: a critical review of current evidence. Neurosurgery. (2015) 62(Suppl. 1):160–5. 10.1227/NEU.0000000000000801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irwin C, Hunn M, Purdie G, Hamilton D. Delay in radiotherapy shortens survival in patients with high grade glioma. J Neurooncol. (2007) 85:339–43. 10.1007/s11060-007-9426-z [DOI] [PubMed] [Google Scholar]
- 12.Glinski B, Urbanski J, Hetnał M, Małecki K, Jarosz M, Mucha-Małecka A, et al. Prognostic value of the interval from surgery to initiation of radiation therapy in correlation with some histo-clinical parameters in patients with malignant supratentorial gliomas. Contemp Oncol (Pozn). (2012) 16:34–7. 10.5114/wo.2012.27334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Do V, Gebski V, Barton M. The effect of waiting for radiotherapy for grade III/IV gliomas. Radiother Oncol. (2000) 57: 131–6. 10.1016/S0167-8140(00)00257-7 [DOI] [PubMed] [Google Scholar]
- 14.Lai R, Hershman DL, Doan T, Neugut AI. The timing of cranial radiation in elderly patients with newly diagnosed glioblastoma multiforme. Neuro Oncol. (2010) 12:190–8. 10.1093/neuonc/nop004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noel G, Huchet A, Feuvret L, Maire JP, Verrelle P, Le Rhun E, et al. Waiting times before initiation of radiotherapy might not affect outcomes for patients with glioblastoma: a French retrospective analysis of patients treated in the era of concomitant temozolomide and radiotherapy. J Neurooncol. (2012) 109:167–75. 10.1007/s11060-012-0883-7 [DOI] [PubMed] [Google Scholar]
- 16.Lutterbach J, Weigel P, Guttenberger R, Hinkelbein W. Accelerated hyperfractionated radiotherapy in 149 patients with glioblastoma multiforme. Radiother Oncol. (1999) 53:49–52. 10.1016/S0167-8140(99)00128-0 [DOI] [PubMed] [Google Scholar]
- 17.Blumenthal DT, Won M, Mehta MP, Curran WJ, Souhami L, Michalski JM, et al. Short delay in initiation of radiotherapy may not affect outcome of patients with glioblastoma: a secondary analysis from the radiation therapy oncology group database. J Clin Oncol. (2009) 27:733–9. 10.1200/JCO.2008.18.9035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wehming FM, Wiese B, Nakamura M, Bremer M, Karstens JH, Meyer A. Malignant glioma grade 3 and 4: how relevant is timing of radiotherapy? Clin Neurol Neurosurg. (2012) 114:617–21. 10.1016/j.clineuro.2011.12.024 [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. PLoS Med. (2009) 6:e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viswanathan M, Ansari MT, Berkman ND, Chang S, Hartling L, McPheeters M, et al. Assessing the risk of bias of individual studies in systematic reviews of health care interventions. Agency for Healthcare Research and Quality Methods Guide for Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality (US) (2012). [PubMed] [Google Scholar]
- 21.Cota GF, de Sousa MR, Fereguetti TO, Rabello A. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa, ON: Ottawa Hospital Research Institute; (2000). [Google Scholar]
- 22.Mazaris P, Hong X, Altshuler D, Schultz L, Poisson LM, Jain R, et al. Key determinants of short-term and long-term glioblastoma survival: a 14-year retrospective study of patients from the Hermelin Brain Tumor Center at Henry Ford Hospital. Clin Neurol Neurosurg. (2014) 120:103–12. 10.1016/j.clineuro.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 23.Brown TJ, Brennan MC, Li M, Church EW, Brandmeir NJ, Rakszawski KL, et al. Association of the extent of resection with survival in glioblastoma: a systematic review and meta-analysis. JAMA Oncol. (2016) 2:1460–9. 10.1001/jamaoncol.2016.1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma X, Lv Y, Liu J, Wang D, Huang Q, Wang X, et al. Survival analysis of 205 patients with glioblastoma multiforme: clinical characteristics, treatment and prognosis in China. J Clin Neurosci. (2009) 16:1595–8. 10.1016/j.jocn.2009.02.036 [DOI] [PubMed] [Google Scholar]
- 25.Blumenthal DT, Won M, Mehta MP, Gilbert MR, Brown PD, Bokstein F, et al. Short delay in initiation of radiotherapy for patients with glioblastoma-effect of concurrent chemotherapy: a secondary analysis from the NRG Oncology/Radiation Therapy Oncology Group database. Neuro Oncol. (2018) 20:966–74. 10.1093/neuonc/noy017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adeberg S, Bostel T, Harrabi S, Bernhardt D, Welzel T, Wick W, et al. Impact of delays in initiating postoperative chemoradiation while determining the MGMT promoter-methylation statuses of patients with primary glioblastoma. BMC Cancer. (2015) 15:558. 10.1186/s12885-015-1545-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han SJ, Rutledge WC, Molinaro AM, Chang SM, Clarke JL, Prados MD, et al. The Effect of timing of concurrent chemoradiation in patients with newly diagnosed glioblastoma. Neurosurgery. (2015) 77:248–53; discussion 253. 10.1227/NEU.0000000000000766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Louvel G, Metellus P, Noel G, Peeters S, Guyotat J, Duntze J, et al. Delaying standard combined chemoradiotherapy after surgical resection does not impact survival in newly diagnosed glioblastoma patients. Radiother Oncol. (2016) 118:9–15. 10.1016/j.radonc.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 29.Nathan JK, Brezzell AL, Kim MM, Leung D, Wilkinson DA, Hervey-Jumper SL. Early initiation of chemoradiation following index craniotomy is associated with decreased survival in high-grade glioma. J Neurooncol. (2017) 135:325–33. 10.1007/s11060-017-2577-7 [DOI] [PubMed] [Google Scholar]
- 30.Osborn VW, Lee A, Garay E, Safdieh J, Schreiber D. Impact of timing of adjuvant chemoradiation for glioblastoma in a large hospital database. Neurosurgery. (2017) 83:915–21. 10.1093/neuros/nyx497 [DOI] [PubMed] [Google Scholar]
- 31.Pollom EL, Fujimoto DK, Han SS, Harris JP, Tharin SA, Soltys SG. Newly diagnosed glioblastoma: adverse socioeconomic factors correlate with delay in radiotherapy initiation and worse overall survival. J Radiat Res. (2018) 59(suppl_1): p. i11–i18. 10.1093/jrr/rrx103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun MZ, Oh T, Ivan ME, Clark AJ, Safaee M, Sayegh ET, et al. Survival impact of time to initiation of chemoradiotherapy after resection of newly diagnosed glioblastoma. J Neurosurg. (2015) 122:1144–50. 10.3171/2014.9.JNS14193 [DOI] [PubMed] [Google Scholar]
- 33.Wang TJ, Jani A, Estrada JP, Ung TH, Chow DS, Soun JE, et al. Timing of adjuvant radiotherapy in glioblastoma patients: a single-institution experience with more than 400 patients. Neurosurgery. (2016) 78:676–82. 10.1227/NEU.0000000000001036 [DOI] [PubMed] [Google Scholar]
- 34.Hulshof MC, Koot RW, Schimmel EC, Dekker F, Bosch DA, González González D. Prognostic factors in glioblastoma multiforme. 10 years experience of a single institution. Strahlenther Onkol. (2001) 177:283–90. 10.1007/s00066-001-0834-2 [DOI] [PubMed] [Google Scholar]
- 35.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. New Engl J Med. (2005) 352:997–1003. 10.1056/NEJMoa043331 [DOI] [PubMed] [Google Scholar]
- 36.Patel DM, Agarwal N, Tomei KL, Hansberry DR, Goldstein IM. Optimal timing of whole-brain radiation therapy following craniotomy for cerebral malignancies. World Neurosurg. (2015) 84:412–9. 10.1016/j.wneu.2015.03.052 [DOI] [PubMed] [Google Scholar]
- 37.Lawrence YR, Blumenthal DT, Matceyevsky D, Kanner AA, Bokstein F, Corn BW. Delayed initiation of radiotherapy for glioblastoma: how important is it to push to the front (or the back) of the line? J Neurooncol. (2011) 105:1–7. 10.1007/s11060-011-0589-2 [DOI] [PubMed] [Google Scholar]
- 38.Pirzkall A, McGue C, Saraswathy S, Cha S, Liu R, Vandenberg S, et al. Tumor regrowth between surgery and initiation of adjuvant therapy in patients with newly diagnosed glioblastoma. Neuro-Oncology. (2009) 11:842–52. 10.1215/15228517-2009-005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. (2017) 318:2306–16. 10.1001/jama.2017.18718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hartling L, Milne A, Hamm MP, Vandermeer B, Ansari M, Tsertsvadze A, et al. Testing the Newcastle Ottawa Scale showed low reliability between individual reviewers. J Clin Epidemiol. (2013) 66:982–993. 10.1016/j.jclinepi.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 41.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This table depicts the search algorithms used for this systematic review and the number of results obtained for each database. For the EMBASE database, searches are built from the bottom up, starting with phrase #1. The final search is bolded at the top (#15) and includes all of the prior phrases.