Abstract
Background/purpose
Fibrin hydrogel is commonly used as hemostatic agent and scaffold but it is questionable for carrying antibiotics. Thus, this study aimed to investigate whether the fibrin hydrogel can be used to deliver the optimal concentration of ciprofloxacin against oral pathogen.
Materials and methods
The optimal concentration of ciprofloxacin was investigated from broth microdilution technique against three common oral bacteria. Ten times the bactericidal concentration of ciprofloxacin loaded to 0.4% fibrin hydrogel was observed by using a confocal laser scanning microscope and then was left in tris-buffer saline solution (TBS) for 0, 1, 12, 24, 72 and 168 h in parallel with the control group of ciprofloxacin loaded to 0.5% alginate hydrogel and ciprofloxacin solution. Spectrophotometer was used to analyze the accumulated drug release from the collected TBS, of which the measurement method was calibrated. The efficacy of the released ciprofloxacin was tested using an agar well diffusion assay. The inhibition zone of the released ciprofloxacin from fibrin hydrogel was statistically compared with 150 and 1500 μg/ml ciprofloxacin solution, while non-loaded fibrin hydrogel served as the control.
Results
The results revealed that minimum inhibitory concentration was 1–2 μg/ml and minimum bactericidal concentration was 4–15 μg/ml. The fibrin hydrogel gradually released ciprofloxacin until 168 h while the alginate hydrogel immediately liberated all the loaded ciprofloxacin within an hour. The agar well diffusion significantly showed greater clear zone in fibrin hydrogel loaded ciprofloxacin compared to non-loaded fibrin hydrogel but not with ciprofloxacin in TBS.
Conclusion
The results suggested that fibrin hydrogel can be used for local ciprofloxacin delivery without interfering the efficacy of ciprofloxacin.
Keywords: Ciprofloxacin, Fibrin hydrogel, Local antibiotic delivery
Introduction
Antibiotics used in dentistry can be administered systemically and locally for dentoalveolar infection and prophylaxis.1 Although oral administration shows good compliance for patients, it can lead to dramatic adverse effects rather than distribution to the infected area in the oral cavity.2 The drug usually endangers the vital organs prior to reaching the infected tooth. On the other hand, the efficacy of local antibiotics showed similar outcomes to systemic therapy.3 Bactericidal activity was comparable while the distribution to plasma was minimized.4
The local delivery of drug for dental treatment has used various carriers such as polymethylmethacrylate cement (PMMA),5 Hydroxyapatite tri-calciumphosphate (HA-TCP),6 and Collagen fiber.2 Fibrin hydrogel was also selected for multiple purposes including bone infection,7 periodontitis,8 and pulp diseases.9,10 On top of that, it was applied in tissue engineering for pulp regeneration of the immature permanent tooth.11 The use of fibrin hydrogel is convenient not only for facilitating blood clot formation but also for helping in oral tissue reconstruction through its properties of osteoinductive, biodegradable, and cell scaffold.2,12 Accordingly, the development of fibrin hydrogel delivering antibiotics can enhance the therapeutic efficiency, as well as, infectious prevention at the operating site.
Dental pulp infection requires a complete step of root canal treatment to eradicate bacteria and prevent further microorganism invasion. Root canal medicament is one of the important measures to disinfect the pulp13 especially since the regenerative technique for treating the immature permanent tooth relied on a thorough elimination of bacteria on the therapeutic agents only.14 Antibiotic medicament showed greater benefits compared to calcium hydroxide whose long-term application could reduce dentin strength and its bactericidal activity.15 The antibiotics unlikely affected hardness and showed greater antibacterial activity against facultative anaerobe.16 However, the high concentration of antibiotic paste is harmful to the stem cells from apical papilla which have the ability to regenerate the dental pulp.17,18 Therefore, the modifying antibiotic delivery by fibrin hydrogel has more advantages over the removing of bacteria.
This study aimed to observe the possibility of using fibrin hydrogel at suitable formula for pulp regeneration as a carrier for the antibiotic ciprofloxacin, which shows effectiveness toward pathogenic bacteria of the pulp diseases.19 Primarily, the release kinetics of ciprofloxacin was established and the bacterial activity was analyzed.
Materials and methods
Standard solution preparation
1000 μg/ml ciprofloxacin hydrochloride USP standard stock solution was prepared by dissolving 10 mg ciprofloxacin hydrochloride (Sigma–Aldrich, St. Louis, Missouri, USA) in 10 ml of tris-buffered saline solution (TBS) which consisted of TrisHCl 100 mM (Sigma–Aldrich) and NaCl 150 mM (Sigma–Aldrich) adjusted pH to 7.6 by using NaOH and HCl.
Minimal bactericidal concentration
The optimal concentration of ciprofloxacin against oral pathogen was analyzed by using broth dilution method. Three common bacteria found in the oral cavity including Streptococcus mutans (ATCC 25175), Enterococcus faecalis (ATCC 19433), and Streptococcus sobrinus (ATCC 27352) were used to study the antibacterial activity of ciprofloxacin. 1 × 106 CFU/ml of the freshly isolated bacteria were inoculated in the brain heart infusion broth (BHI; Difco Laboratories, Detroit, Michigan, USA). Ciprofloxacin was serially diluted with BHI from 0.062 to 100 μg/ml before loading the medium containing bacteria into the 96-well microtiter plate (Corning, New York, USA). Each well was filled with 100 μl of ciprofloxacin and 100 μl of bacteria. The control wells were loaded either without antibiotic or without bacteria. The bacteria were grown at 37 °C in humidifier chamber with 5% CO2 for 24 h. The minimum concentration of ciprofloxacin, that can inhibit the growth of bacteria, was the minimum inhibitory concentration (MIC). 20 μl of all the concentration demonstrating the growth inhibition were then incubated on the BHI agar (Difco Laboratories) at 37 °C in humidifier chamber with 5% CO2 for 24 h to observe the bactericidal concentration of ciprofloxacin. The minimum concentration which showed no colony formation on the agar was considered the minimum bactericidal concentration (MBC).
Hydrogel loaded with ciprofloxacin
0.4% Fibrin hydrogel was prepared from 1:1 mixture of fibrinogen 8 mg/ml in 2.5 mmol/L calcium ion solution and 2 NIH Unit/mL thrombin (Baxter Healthcare, Bangkok, Thailand) in TBS. 150 or 1500 μg/ml ciprofloxacin was added into the thrombin solution prior to the mixing step as in the previous study.12 0.4% alginate hydrogels (Sigma–Aldrich) were prepared to be used as a control by heating the aqueous sodium alginate (0.4% w/v) to 120 °C for 20 min and loaded with 150 μg/ml ciprofloxacin just prior to gel formation using calcium chloride. Both hydrogels with and without ciprofloxacin were loaded into 30 mm3 molds. 30 min after a complete gelation of the fibrin hydrogel, a dispersion of ciprofloxacin was analyzed using Olympus FV30S-SW confocal laser-scanning microscope (Olympus, Tokyo, Japan).
Method validation
Prior to measuring the ciprofloxacin released from the fibrin hydrogel, we examined the accuracy and precision of the method according to The United State Pharmacopoeia.20 The absorbance of ciprofloxacin (Sigma–Aldrich) in TBS was screened in the range between 200 and 400 nm using spectrophotometer (BioTek, Winooski, Vermont, USA) to identify λmax. The standard curve was established from 0, 5, 10, 15, and 20 μg/ml ciprofloxacin as mentioned above. Three different levels of drug concentrations including 5, 10, and 20 μg/ml in triplicates (n = 27) determined the accuracy and the precision (reproducibility). The relative standard deviation (RSD) of the measurement was calculated for inter-day and inter-person precision while the percentage of relative error or percent bias (%Bias) was used for proving the accuracy of the method. The Limit of Detection and Quantification (LOD and LOQ) were computed from the absorbance of blank solution as describing in the equation.
In vitro release
We examined the percentage of ciprofloxacin released from the fibrin hydrogel compared to the alginate hydrogels using spectrophotometer. The fibrin hydrogels with and without 150 μg/ml ciprofloxacin as already described were incubated in 1.5 ml tube containing 500 μl TBS at 37 °C in the dark. 150 μg/ml of ciprofloxacin in TBS were kept in parallel for observing the stability of ciprofloxacin, while the alginate hydrogel with and without 150 μg/ml ciprofloxacin were stored separately as a control. All solutions were prepared in triplicates and they were collected at 0, 1, 12, 24, 48 and 168 h. The collected solution were stored at −20 °C until observing the absorbance at 270 nm.
Efficacy of released ciprofloxacin
The efficacy of ciprofloxacin released from the fibrin hydrogel was compared with the ciprofloxacin in TBS using the agar well diffusion method. 150 and 1500 μg/ml ciprofloxacin loaded into fibrin hydrogel as previously described were added to 1 mm3 wells in the brain heart infusion agar (BHI agar; Becton Dickinson, Franklin Lakes, New Jersey, USA) which cultured 1–5 × 105 CFU/ml of bacteria which was already described. Concurrently, 150 and 1500 μg/ml ciprofloxacin in TBS were loaded into the wells having an equal dimension and located in the same agar. The agars of each bacteria were triplicated and incubated in 5% CO2 at 37 °C for 24 h prior to the measurement of inhibition zones. Calcium hydroxide paste was used as the positive control whereas, TBS and fibrin hydrogel were the negative control.
Statistical analysis
The normal distribution of data was proved and the differences of inhibition zones were analyzed by one-way analysis of variance test. Tukey's test was used as post-hoc analysis and 95% is the confident interval.
Results
Minimal bactericidal concentration
MIC and MBC value of the ciprofloxacin against all bacteria were described in Table 1. 15 μg/ml of ciprofloxacin was the minimal concentration which can eradicate all bacteria.
Table 1.
Concentration of ciprofloxacin against bacteria.
| Antibiotic |
S. mutans |
S. sorbrinus |
E. faecalis |
|||
|---|---|---|---|---|---|---|
| MIC (μg/ml) | MBC (μg/ml) | MIC (μg/ml) | MBC (μg/ml) | MIC (μg/ml) | MBC (μg/ml) | |
| Ciprofloxacin | 1 | 15 | 2 | 4 | 2 | 15 |
Fibrin hydrogel loaded with ciprofloxacin
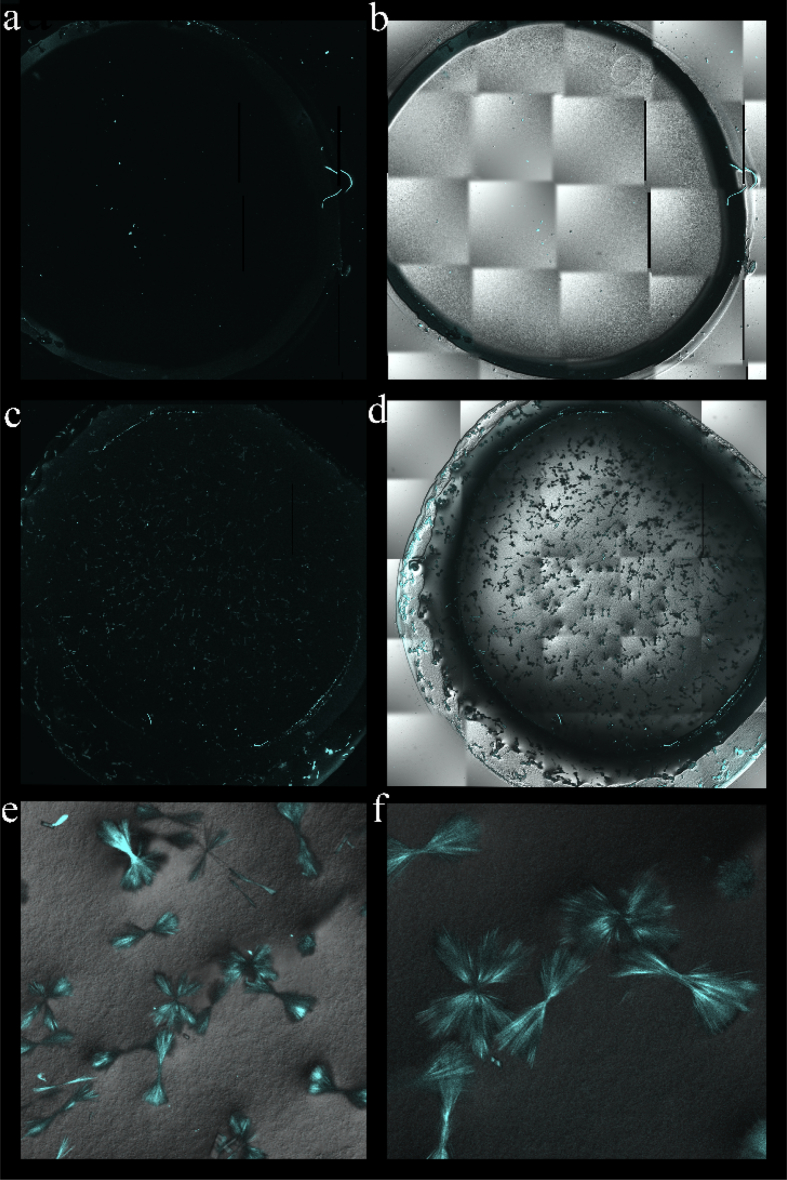
The microscopic analysis of fibrin hydrogel confirmed the addition of ciprofloxacin (Fig. 1a,b). The ability of ciprofloxacin to emit light demonstrated a consistent distribution of the insoluble particles (Fig. 1c), which were defined by superimposing the active fluorescence area on top of the crystalline drug in the fibrin hydrogel (Fig. 1d). The fine needle-like crystals had formed in the bundles or aggregates (Fig. 1e,f) following the incorporation of 1500 μg/ml ciprofloxacin but not with the low concentration of 150 μg/ml (data not shown).
Figure 1.
Confocal fluorescence microscope showing a dispersion of ciprofloxacin loaded into the fibrin hydrogel. a–b) No particle could be observed in the fibrin hydrogel alone. c–d) Meanwhile, the fibrin hydrogel loaded with 1500 μg/ml ciprofloxacin showed undissolved crystalline structure, which had intrinsic fluorescence. e) 20× and f) 40× magnification micrographs revealed the needle-like bundles and aggregates of the ciprofloxacin crystals. a & c: the fluorescence micrographs. b & d–f: the overlaid of phase contrast micrographs.
Method validation
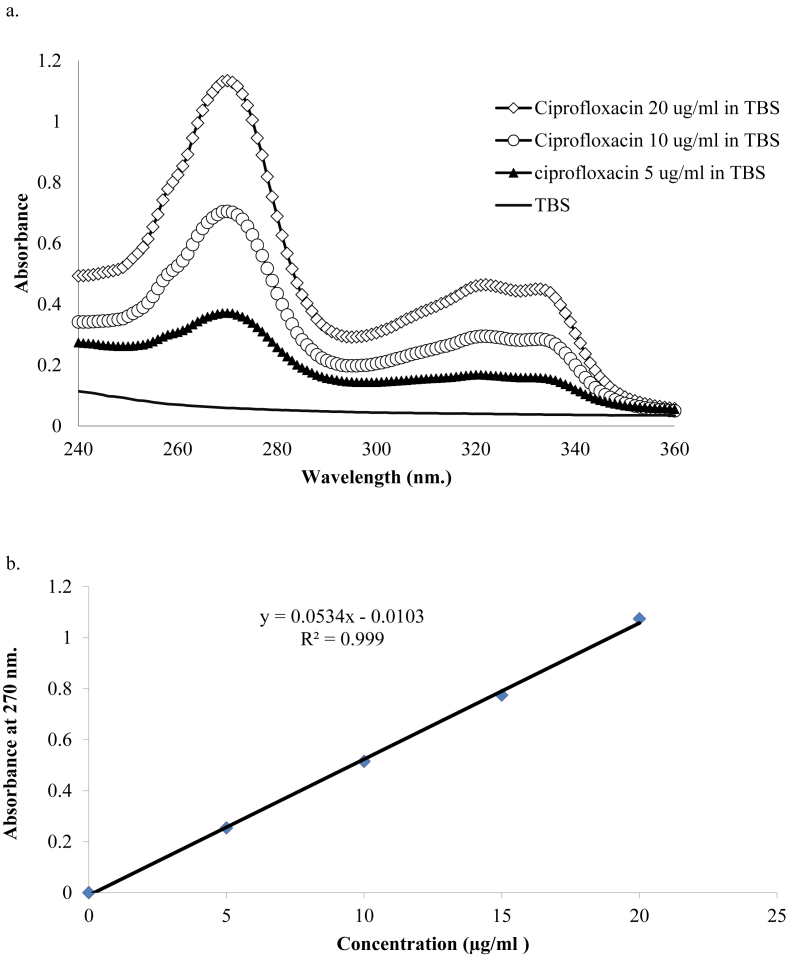
The spectral analysis of ciprofloxacin hydrochloride in TBS displayed maximum absorption at 270 nm (Fig. 2a) and the linear regression by using spectrophotometer showed r2 = 0.999 (Fig. 2b). Precision was determined by repeating the method three times and computing the percentage of relative standard deviation. It showed that the prepared ciprofloxacin at concentrations 5, 10, and 20 μg/ml were 0.27%, 1.39%, and 2.86% respectively. Likewise, the accuracy was accounted from the percentage bias of triplicate ciprofloxacin at same concentrations of which were −2.7591%, −0.8177%, and −0.9394% correspondingly. The LOD and LOQ were obtained from the calculation, which resulted in 0.50 μg/ml for the LOD and 1.26 μg/ml for the LOQ.
Figure 2.
Spectra absorption of ciprofloxacin in TBS. a) The screening wavelength between 240 and 360 nm showed the maximum absorbance at 270 nm, which should be used for further analysis. b) The concentration together with their corresponding absorbance were plotted the calibration curve, which presented the least square analysis and equation.
Release kinetics of ciprofloxacin
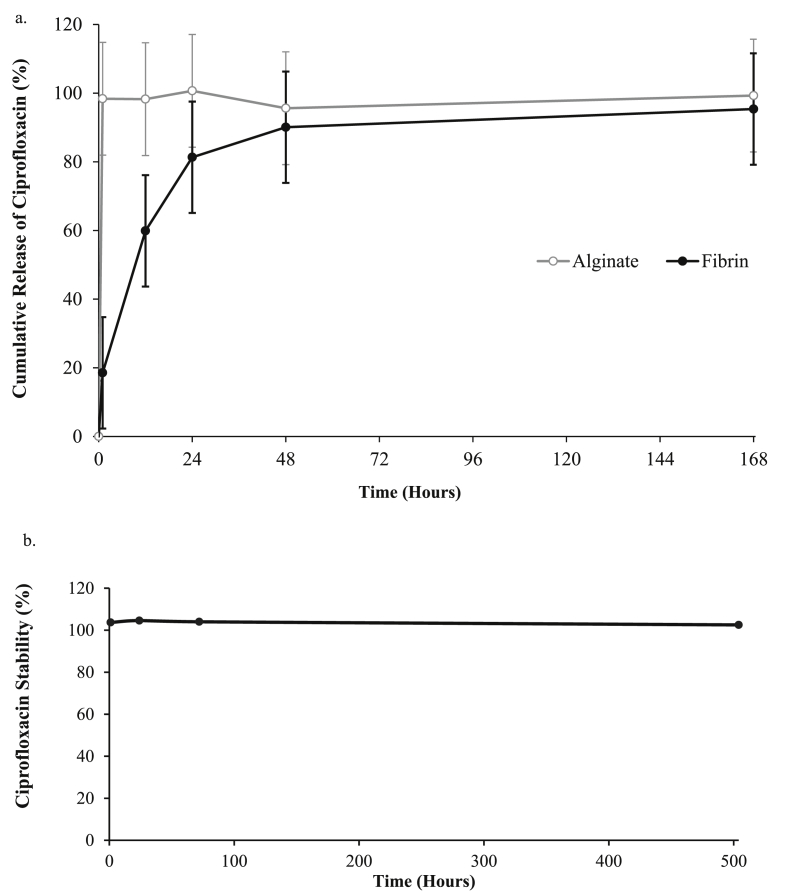
Ciprofloxacin released from the fibrin hydrogel was observed in parallel with the ciprofloxacin liberated from the alginate hydrogel while the ciprofloxacin dissolved in TBS was stored similarly to determine the stability of ciprofloxacin over the experimental period. The ciprofloxacin released from the fibrin hydrogel was gradually increase until 168 h, whilst the ciprofloxacin liberated from the alginate hydrogel reached the loaded concentration within an hour (Fig. 3a). The ciprofloxacin dissolved in TBS was stable until the end of the experiment (Fig. 3b).
Figure 3.
The percentage of ciprofloxacin released from (a) the fibrin hydrogel at 0, 1, 12, 24, 72 and 168 h and (b) the stability of ciprofloxacin in tris-buffered saline solution at 37 °C protected from light.
Susceptibility test by the released ciprofloxacin
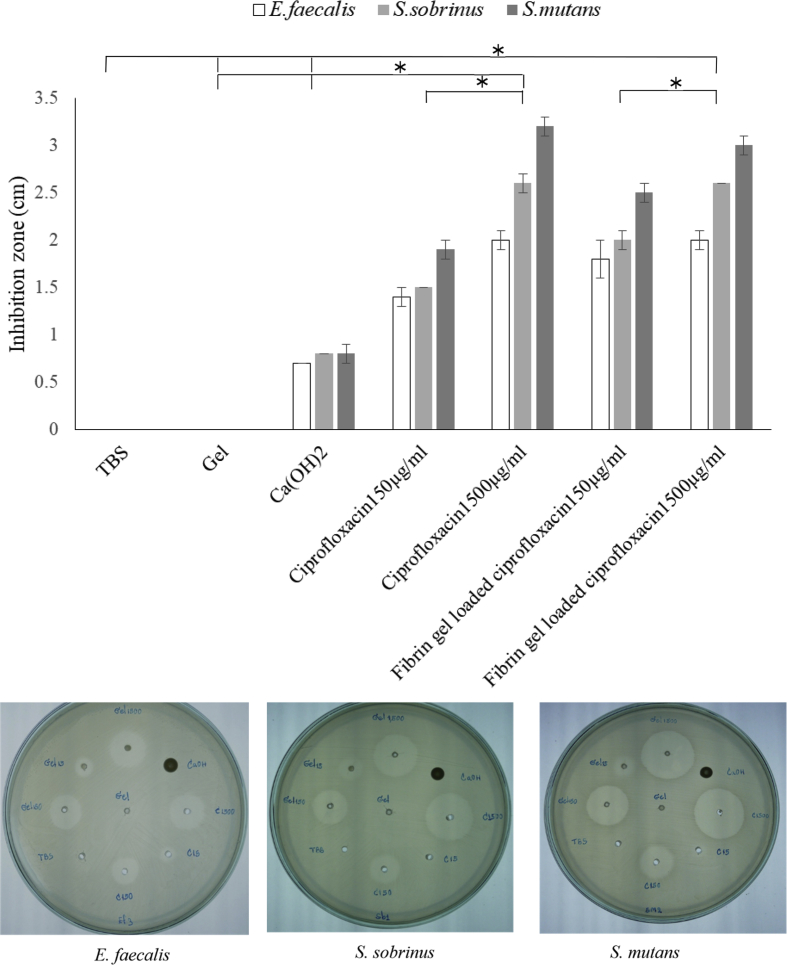
The efficacy of ciprofloxacin released from the fibrin hydrogel was tested by observing the ability to inhibit the growth of S. mutans, E. faecalis, and S. sobrinus in the agar. The results revealed that the released ciprofloxacin can impede the growth of all bacterial strains while only TBS and fibrin hydrogel could not. The ciprofloxacin showed a significant greater bactericidal activity compared to only having fibrin hydrogel, TBS or calcium hydroxide (Fig. 4). Meanwhile, the inhibition zone of the fibrin hydrogel loaded with ciprofloxacin at 150 and 1500 μg/ml was insignificantly different with the ciprofloxacin dissolved in TBS (Fig. 4). However, the efficacy was enhanced gradually in accordance with the concentration of ciprofloxacin by which the fibrin hydrogel and in the solution form.
Figure 4.
The antibacterial properties of ciprofloxacin delivered by a fibrin hydrogel compared to ciprofloxacin solution. The inhibition zone of 150 and 1500 μg/ml ciprofloxacin delivered by fibrin hydrogel were tested against Streptococcus mutans, Enterococcus faecalis, and Streptococcus sobrinus. Likewise, the standard ciprofloxacin solutions prepared in TBS at the same concentration were verified in parallel. Only TBS, fibrin hydrogel, and calcium hydroxide were loaded into the agar as the control. TBS: tris-buffered saline solution, * p-value < 0.05.
Discussion
Fibrin hydrogel showed satisfactory outcomes for maxillofacial and periodontal infection diseases.3 There is no evidence for endodontic treatment; however, recent studies showed that fibrin hydrogel presented appropriate properties for pulp regeneration.10,21 Our study aimed to investigate the efficacy of fibrin hydrogel for releasing ciprofloxacin, which is the antibiotic used in root canal medicament. The results showed that fibrin hydrogel can extend the releasing time of ciprofloxacin while its bactericidal activity was maintained.
Ciprofloxacin is classified as fluoroquinolone antibiotics, which has a broad spectrum against both gram-negative and gram-positive bacteria. In our study, the in vitro bacteriostatic and bactericidal activity of ciprofloxacin were 1–2 μg/ml and 4–15 μg/ml respectively which was around two-fold lower than previous studies found that the median MIC of ciprofloxacin against both gram-positive and gram-negative bacteria could be ranged from ≤0.015–1 mg/l.22, 23, 24 The reasons might be due to the amount or strain of bacteria used for testing and the acidity of culture medium were difference. However, the bactericidal activity was favorably efficient compared to the resistant strains.25
Various biological methods can be used to measure the ciprofloxacin concentration based on its properties involving the absorption of radiation and fluorescence.26 Using confocal laser-scanning microscope in this study showed an alternative option to monitor the ciprofloxacin. However, the methods seemed to satisfy when the saturated crystals were formed. The Alexa Fluor 405 channel was selected because it revealed the highest fluorescence intensity, which also superimposed on the crystalline ciprofloxacin found in the phase contrast image. The technique helped to confirm the loading of ciprofloxacin into the fibrin hydrogel and to understand the characteristic of ciprofloxacin distribution, which may further influence the release of ciprofloxacin.
The analysis of ciprofloxacin can be affected by many factors such as acid-base condition and solvent.26 Although the use of spectrophotometer to determine ciprofloxacin hydrochloride in ophthalmic solution has been validated already, our ciprofloxacin analysis is necessary due to a different diluent used. Spectral characteristic of ciprofloxacin in TBS showed the maximum absorption and less interference at 270 nm, which was slightly different from the other diluents.27 To determine the ciprofloxacin precisely, we used linear regression and LOQ to adjust the ciprofloxacin concentration being in the range where the percentage of relative standard deviation were acceptable.28 Moreover, all investigators can perform accurately at all concentrations since the percentage bias were in the range of ±5%.20 Hence, this method is valid for further experiments.
The release profile of both hydrogel showed an initial burst-release within 24 h which is common in the controlled release system due to a weak adsorption of drug molecules on the hydrogel surface.29 However, the diffusion controlled by fibrin hydrogel led to the slower release rate over 168 h compared to the alginate hydrogel, which was also claimed for a promising scaffold for oral tissue regeneration.30,31 In addition, our fibrin hydrogel similarly showed the drug–release profile like in the previous study of ampicillin liberated from the fibrin hydrogel.2 Many advantages of the fibrin hydrogel seem to overcome the limitation of other drug delivery system used in dental practices. The fibrin hydrogel can be modified into various forms while HA-TCP showed less adaptability and super slow release as 20% of drug discharged within 70 days. Unlike PMMA that provided more favorable drug release, the fibrin hydrogel is biodegradable.32
Local antibiotic delivery aims to improve the effectiveness of drug therapy by means of controlling drug exposure over the period, overcoming anatomical barrier, and preventing a premature degradation. Fibrin hydrogel in this study proved that it could rapidly and continuously release antibiotics to maintain the drug concentration within the therapeutic window in a longer duration. In addition, the molecules of ciprofloxacin were absorbed physically on the pore surfaces of fibrin hydrogel so the structures did not interfere the electrical and chemical bonds.33 As in our result, the ciprofloxacin released from fibrin hydrogel maintained its potency similar to directly applying the drug to inhibit the growth of bacteria, which were considered to be a cause of endodontic failure.34,35 By using fibrin hydrogel, the efficacy of antibiotic could be enhanced favorably corresponding to the increase of ciprofloxacin concentration. Likewise, the antibacterial effects of ciprofloxacin were greater than the standard root canal medication, calcium hydroxide, of which therapeutic mechanism is minimized due to environmental pH.
In conclusion, fibrin hydrogel tested in vitro demonstrated a promising local antibiotic delivery system for dental usage because it showed an ability to control ciprofloxacin release while maintaining drug efficacy. On top of that, the local antibiotic delivery using fibrin hydrogel could overcome the limits of standard root canal medicament, such as extending the duration of bactericidal activity and possessing regenerative potency. However, there are still many unpredictable factors in the living tissue that should be taken into account with this system. In the future study, the use of fibrin hydrogel should be further investigated in vivo for observing a clinical feasibility on root canal medicament.
Conflicts of interest
The authors declare that they have no conflict of interest.
Acknowledgements
Authors would like to acknowledge Faculty of Dentistry, Mahidol University to support this study. The authors also acknowledge Arthit Kaophimai and Thaniya Muadcheingka for their helps in microbiology study.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jds.2018.08.010.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Dar-Odeh N.S., Abu-hammad O.A., Al-Omiri M.K., Khraisat A.S., Shehabi A.A. Antibiotic prescribing practices by dentists: a review. Ther Clin Risk Manag. 2010;6:301–306. doi: 10.2147/tcrm.s9736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu B.G., Kwon I.C., Kim Y.H. Development of a local antibiotic delivery system using fibrin glue. J Contr Release. 1996;39:65–70. [Google Scholar]
- 3.Purucker P., Mertes H., Goodson J.M., Bernimoulin J.P. Local versus systemic adjunctive antibiotic therapy in 28 patients with generalized aggressive periodontitis. J Periodontol. 2001;72:1241–1245. doi: 10.1902/jop.2000.72.9.1241. [DOI] [PubMed] [Google Scholar]
- 4.Zilch H., Lambiris E. The sustained release of cefotaxim from a fibrin-cefotaxim compound in treatment of osteitis. Pharmacokinetic study and clinical results. Arch Orthop Trauma Surg. 1986;106:36–41. doi: 10.1007/BF00435650. [DOI] [PubMed] [Google Scholar]
- 5.Penn-Barwell J.G., Murray C.K., Wenke J.C. Local antibiotic delivery by a bioabsorbable gel is superior to PMMA bead depot in reducing infection in an open fracture model. J Orthop Trauma. 2014;28:370–375. doi: 10.1097/BOT.0b013e3182a7739e. [DOI] [PubMed] [Google Scholar]
- 6.Nayak A.K., Bhattacharya A., Sen K.K. Hydroxyapatite-antibiotic implantable minipellets for bacterial bone infections using precipitation technique: preparation, characterization and in-vitro antibiotic release studies. J Pharm Res. 2013;3:53–59. [Google Scholar]
- 7.Davis B., Sando G. Use of fibrin glue in maxillofacial surgery. J Otolaryngol. 1998;27:107–112. [PubMed] [Google Scholar]
- 8.Jacob S., Nath S. Fibrin sealant: a review of its applications in periodontal surgery. Int J Exp Dent Sci. 2015;4:40–46. [Google Scholar]
- 9.Friess W. Collagen in drug delivery and tissue engineering. Adv Drug Deliv Rev. 2003;55:1529–1530. [Google Scholar]
- 10.Ruangsawasdi N., Zehnder M., Weber F.E. Fibrin gel improves tissue ingrowth and cell differentiation in human immature premolars implanted in rats. J Endod. 2014;40:246–250. doi: 10.1016/j.joen.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 11.Ruangsawasdi N., Zehnder M., Patcas R., Ghayor C., Weber F.E. Regenerative dentistry: animal model for regenerative endodontology. Transfus Med Hemother. 2016;43:359–364. doi: 10.1159/000447644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruangsawasdi N., Zehnder M., Patcas R., Ghayor C., Siegenthaler B., Gjoksi B. Effects of stem cell factor on cell homing during functional pulp regeneration in human immature teeth. Tissue Eng Part A. 2017;23:115–123. doi: 10.1089/ten.TEA.2016.0227. [DOI] [PubMed] [Google Scholar]
- 13.Yousuf W., Khan M., Mehdi H. Endodontic procedural errors: frequency, type of error, and the most frequently treated tooth. Int J Dent. 2015;2015:1–7. doi: 10.1155/2015/673914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nerness A., Ehrlich Y., Spolnik K., Platt J., Yassen G. Effect of triple antibiotic paste with or without ethylenediaminetetraacetic acid on surface loss and surface roughness of radicular dentine. Odontology. 2016;104:170–175. doi: 10.1007/s10266-014-0191-0. [DOI] [PubMed] [Google Scholar]
- 15.Andreasen J.O., Farik B., Munksgaard E.C. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent Traumatol. 2002;18:134–137. doi: 10.1034/j.1600-9657.2002.00097.x. [DOI] [PubMed] [Google Scholar]
- 16.Stuart C.H., Schwartz S.A., Beeson T.J., Owatz C.B. Enterococcus faecalis: its role in root canal treatment failure and current concepts in retreatment. J Endod. 2006;32:93–98. doi: 10.1016/j.joen.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 17.Ruparel N., Teixeira F., Ferraz C., Diogenes A. Direct effect of intracanal medicaments on survival of stem cells of the apical papilla. J Endod. 2012;38:1372–1375. doi: 10.1016/j.joen.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 18.Ribeiro M.R.G., Thomaz E.B.A.F., Lima D.M., Leitao T.J., Bauer J., Souza S. Chlorhexidine prevents root dentine mineral loss and fracture caused by calcium hydroxide over time. Int J Dent. 2017;2017:1–7. doi: 10.1155/2017/1579652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoshino E., Kurihara-Ando N., Sato I. In-vitro antibacterial susceptibility of bacteria taken from infected root dentine to a mixture of ciprofloxacin, metronidazole and minocycline. Int Endod J. 1996;29:125–130. doi: 10.1111/j.1365-2591.1996.tb01173.x. [DOI] [PubMed] [Google Scholar]
- 20.2009 U.S. Pharmacopoeia-National Formulary [USP 32 NF 27] United States Pharmacopeial Convention, Inc; Rockville, Md: 2009. [1225] validation of compendial procedures; p. 1711–1712. [Google Scholar]
- 21.Galler K.M., Brandl F.P., Kirchhof S. Suitability of different natural and synthetic biomaterials for dental pulp tissue engineering. Tissue Eng Part A. 2018;24:234–244. doi: 10.1089/ten.TEA.2016.0555. [DOI] [PubMed] [Google Scholar]
- 22.Dziedzic A., Wojtyczka R.D., Kubina R. Inhibition of oral streptococci growth induced by the complementary action of berberine chloride and antibacterial compounds. Molecules. 2015;20:13705–13724. doi: 10.3390/molecules200813705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banavar Ravi S., Nirupad S., Chippagiri P., Pandurangappa R. Antibacterial effects of natural herbal extracts on streptococcus mutans: can they be potential additives in dentifrices? Int J Dent. 2017;2017:1–5. doi: 10.1155/2017/4921614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeiler H.J., Grohe K. The in vitro and in vivo activity of ciprofloxacin. Eur J Clin Microbiol. 1984;3:339–343. doi: 10.1007/BF01977490. [DOI] [PubMed] [Google Scholar]
- 25.Genaro A., Cunha M.L.R.S., Lopes C.A.M. Study on the susceptibility of Enterococcus faecalis from infectious processes to ciprofloxacin and vancomycin. J Venom Anim Toxins Incl Trop Dis. 2005;11:252–260. [Google Scholar]
- 26.Yang R., Fu Y., Li L.D., Liu J.M. Medium effects on fluorescence of ciprofloxacin hydrochloride. Spectrochim Acta Mol Biomol Spectrosc. 2003;59:2723–2732. doi: 10.1016/s1386-1425(03)00059-3. [DOI] [PubMed] [Google Scholar]
- 27.Cazedey E., Salgado H.R.N. Spectrophotometric determination of ciprofloxacin hydrochloride in ophthalmic solution. Anal Chem. 2012;2:74–79. [Google Scholar]
- 28.Motwani S., Chopra S., Ahmad F., Khar R. Validated spectrophotometric methods for the estimation of moxifloxacin in bulk and pharmaceutical formulations. Spectrochim Acta Mol Biomol Spectrosc. 2007;68:250–256. doi: 10.1016/j.saa.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 29.Huang X., Brazel C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J Contr Release. 2001;73:121–136. doi: 10.1016/s0168-3659(01)00248-6. [DOI] [PubMed] [Google Scholar]
- 30.Moshaverinia A., Chen C., Akiyama K. Alginate hydrogel as a promising scaffold for dental-derived stem cells: an in vitro study. J Mater Sci Mater Med. 2012;23:3041–3051. doi: 10.1007/s10856-012-4759-3. [DOI] [PubMed] [Google Scholar]
- 31.Chen L., Shen R., Komasa S. Drug-loadable calcium alginate hydrogel system for use in oral bone tissue repair. Int J Mol Sci. 2017;18:989–1006. doi: 10.3390/ijms18050989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gogia J., Meehan J., Di Cesare P., Jamali A. Local antibiotic therapy in osteomyelitis. Semin Plast Surg. 2009;23:100–107. doi: 10.1055/s-0029-1214162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vali Z., Scheraga H.A. Localization of the binding site on fibrin for the secondary binding site of thrombin. Biochemistry. 1988;27:1956–1963. doi: 10.1021/bi00406a023. [DOI] [PubMed] [Google Scholar]
- 34.Gomes B.P., Pinheiro E.T., Gade-Neto C.R. Microbiological examination of infected dental root canals. Oral Microbiol Immunol. 2004;19:71–76. doi: 10.1046/j.0902-0055.2003.00116.x. [DOI] [PubMed] [Google Scholar]
- 35.Haapasalo M.U.T., Endal U. Persistent, recurrent, and acquired infection of the root canal system post-treatment. Endod Top. 2003;6:29–56. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.