Abstract
Background/purpose
The application of ozone as an adjunctive treatment represents a new approach in the management of chronic periodontitis. The purpose of this study was to evaluate the clinical, biochemical and microbiological efficacy of ozone treatment as an adjunct to scaling and root planing (SRP) in generalized chronic periodontitis (GCP) patients.
Materials and methods
Eighteen patients (9 males and 9 females; aged from 28 to 47 years, mean age of 40 ± 6.51 years) with GCP were recruited in the study. In a split mouth design, two quadrants in each patient were randomly allocated to SRP-alone or SRP-ozone therapy (SRP + OT) groups by coin toss method. Subgingival plaque and gingival crevicular fluid (GCF) samples were collected at baseline, following 1st and 3rd months. The clinical parameters were monitored at baseline and after 3 months. Microbiological parameters were analyzed by quantitative-PCR and GCF biomarkers were determined by ELISA. Results were analyzed statistically.
Results
Statistically significant improvements in all clinical parameters were accompanied by a reduction in microbiological and biochemical parameters in both treatment groups. SRP treatment resulted in a significant reduction of Porphyromonas gingivalis (Pg) at 1st month and Tannerella forsythia (Tf) and Prevotella intermedia (Pi) at 3 months. Following SRP treatment the interleukin (IL)-8 levels were significantly reduced at month 1. There were no significant differences between two treatments for any of the parameters.
Conclusion
Within the limitations of this study, adjunctive ozone therapy did not provide additional benefits to clinical, microbiological and biochemical parameters over SRP in chronic periodontitis patients.
Keywords: Chronic periodontitis, Ozone, Periodontal pathogens, Pro-inflammatory cytokines, Scaling and root planing
Introduction
Periodontitis is an infectious disease characterized by pocket formation and/or gingival recession that result in inflammation and destruction of the periodontal tissues.1
The primary goal of periodontal treatment is the elimination or reduction in the number of pathogenic bacteria and inflammation, decrease in the probing pocket depth (PPD), and improvement in the clinical attachment level (CAL). Scaling and root planing (SRP) is the most common non-surgical periodontal treatment. However, SRP does not completely eliminate the residual pockets and periodontopathogenic bacteria, particularly at the furcation, root concavities, interproximal areas, and sites with deeper pockets that are inaccessible to periodontal instruments.2, 3, 4 In order to overcome these problems, adjunctive therapies, such as topical antiseptics or local or systemic antibiotics have been successfully researched at various levels to improve the SRP outcome in the management of periodontal disease.5 Conflicting results have been reported regarding the effectiveness of adjunctive therapies, and there is no consensus on the best method to improve the outcome of mechanical treatment.6, 7, 8, 9, 10
The application of ozone as an adjunctive treatment represents a new approach in the management of periodontitis and can be an alternative treatment option. Gaseous ozone (O3-triatomic oxygen) is the third-strongest oxidizing agent worldwide. It influences the cellular and humoral immune system by stimulating the proliferation of immunocompetent cells and the synthesis of immunoglobulin. Biologically active substances, such as interleukins, leukotrienes, and prostaglandins, that are beneficial in reducing inflammation and wound healing, are synthesized following ozone application.11
Ozone has been used in the field of dentistry for various procedures, such as the management of early caries lesions, ulcerations, and herpetic lesions of the oral mucosa; sterilization of root canals and cavities; and reduction of periodontal pockets depth. Several studies have demonstrated significant improvements in the clinical periodontal parameters following SRP along with the application of ozonated water in aggressive periodontitis patients.12,13 Recently, both gaseous and aqueous ozone have been used to complement the treatment of periodontal diseases.12, 13, 14, 15 However, limited clinical and microbiological data are available regarding the effectiveness of adjunctive ozone therapy (OT) in patients with generalized chronic periodontitis (GCP).
In this study, we aimed to determine if the application of gaseous ozone, as an adjunct to SRP, to residual pockets in patients with GCP undergoing initial periodontal therapy alters the clinical periodontal parameters, gingival crevicular fluid (GCF) levels of inflammatory cytokines, and periodontal pathogens compared to SRP alone.
Materials and methods
Study design and population
This split-mouth clinical trial was conducted in accordance with the applicable ethical principles, including the World Medical Association Declaration of Helsinki,16 and was independently approved by the Ethical Committee of Ankara University (36290600/105). All the study subjects provided informed consent after they were given detailed information regarding the nature, potential risks, and benefits of study participation.
From January 2017 to December 2017, 18 patients (9 males and 9 females; mean age 40 ± 6.51 years) diagnosed with generalized chronic periodontitis (GCP) based on the current international classification of the American Academy of Periodontology17 were recruited from among individuals referred to the Gazi University, Faculty of Dentistry, and Department of Periodontology.
Sample size calculation
Sample size calculation was performed regarding the primary outcome variable, PPD. Power analysis calculations indicated a minimum requirement of 18 patients, considering a clinical difference of 1 mm in the PPD between the two treatment methods with a 95% confidence interval (alpha = 0.05) and intragroup standard deviation of 2 mm with a power of 80%.18
Inclusion and exclusion criteria
The inclusion criteria for patient selection were as follows: 1) good general health (patients without systemic involvement and those not taking any medication); 2) no periodontal treatment within 6 months from the enrollment date; 3) no antibiotics or anti-inflammatory medication taken within 6 months from the enrollment date; 4) no smoking; 5) not pregnant or lactating at the time of the study; 6) no contraindication for periodontal treatment and ozone application; 7) presenting ≥30% of the sites with bone loss in whole mouth, and at least non-adjacent teeth with ≥3 sites with PD ≥ 5 mm in at least 2 quadrants.
The screened subjects were excluded if they had less than 16 teeth, had partial denture, or fixed prosthodontics.
Clinical parameters
The following parameters were recorded in a sequential order: Plaque index (PI),19 gingival index (GI),20 bleeding on probing (BOP), and PPD. Clinical attachment changes were not assessed because of uncertainty in measurements due to treatment modalities and short follow-up period.21All the clinical parameters were measured at 6 sites per tooth (mesio-buccal, mid-buccal, disto-buccal, mesio-palatal, mid-palatal, and disto-palatal), excluding the third molars, using a Williams periodontal probe (Nordent Manufacturing Inc., IL, USA) calibrated in millimeters for diagnosis of GCP. Further, all the parameters were recorded for the sampling areas in each group. The periodontal clinical examinations were performed at baseline (prior to therapy) and after 3 months by the same calibrated examiner (A.U), experienced in clinical trial, who was blinded to treatment assignment.
Intra-examiner calibration was achieved by the examination of 10 patients with GCP. For each subject, the examiner measured the PPD at 6 aspects of each tooth in the maxilla for three times, 3 days apart, before study initiation. The intra-examiner reproducibility for PPD measurements was assessed, and the interclass correlation coefficient was 0.95.
Primary and secondary outcomes
Changes in the values of PI, GI, PPD, BOP, bacterial species, and cytokines at 3 months after the therapy were defined as the primary outcomes. The secondary outcomes were the differences in the PI, GI, PPD, BOP, counts and proportions of the bacterial species analyzed, and values of cytokines of both the groups.
Treatment procedure
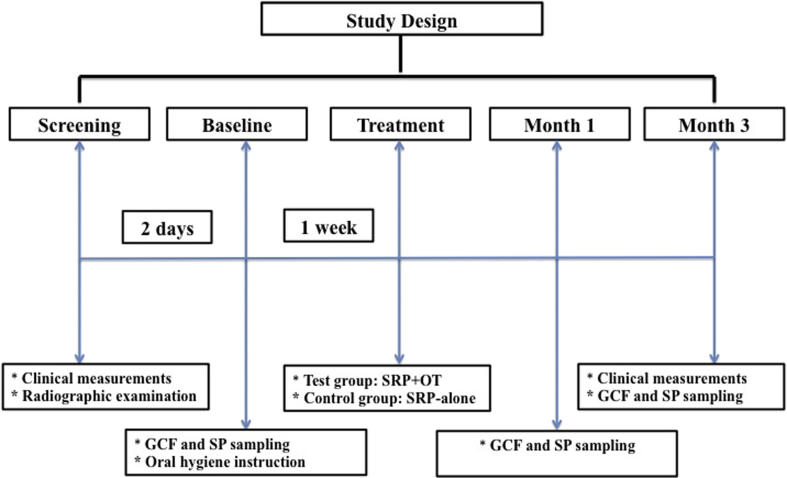
The study design is presented in Fig. 1. Before study initiation, samples of GCF and subgingival plaque (SP) were collected at 2nd day following diagnosis. In same appointment, the participants were provided standard oral hygiene instructions, using the modified Bass technique, toothbrush, toothpaste, and interdental tools. Pre- or post-operative antibiotics or non-steroidal anti-inflammatory drugs were not prescribed.
Figure 1.
Study design. GCF: Gingival crevicular fluid, SP: Subgingival plaque sample, SRP: Scaling and root planning, OT: Ozone therapy.
Two quadrants with at least 3 proximal sites presenting PPD ≥5 mm in each patient were randomly allocated to the test group (SRP followed by OT, n = 18) or the control group (SRP-alone, n = 18) based on the coin toss method by an experienced investigator (B.K) who was not involved in data collection or any study procedure.
In each patient, SRP was performed under local anesthesia using sharp Gracey and universal curettes (Hu-Friedy, Chicago, IL, USA) and ultrasonic instruments (EMS Minimaster, Nyon, Switzerland) at 1 week after sampling. The SRP was performed in a single appointment for each quadrant. The SRP process involved minimum 20 strokes per tooth (about 5–6 min per tooth). Gaseous ozone was applied immediately after SRP at a fixed concentration of 2100 ppm with 80% oxygen 3 times for 30 s (every 3rd day) for 1 week, using a device (Ozone DTA Ozone Generator, Denta Tec Dental AS, Norway) equipped with a periodontal tip (PA Probe, Ozone Generator, Denta Tec Dental AS, Norway) as per the manufacturer's instructions.
Gingival crevicular fluid and subgingival plaque sampling
The GCF and SP sampling was performed at the 3 deepest pockets with a probing depth ≥5 mm in each treatment site with no endodontic or furcation involvement. All the samples were collected at baseline as well at the 1 and 3 months following treatment.
Before sampling, the area was isolated with cotton rolls to prevent saliva contamination; thereafter, the area was slightly air-dried. GCF was collected using standardized sterile paper strips (Periopaper, ProFlow Inc., Amityville, NY, USA). Filter paper strips were gently inserted into the crevice until a minimum resistance was felt and left there for 30 s.22 Strips contaminated with blood or saliva were discarded. The volume of fluid in each strip was determined using a calibrated Periotron™ 6000 (Proflow Inc., Amityville, NY, USA). The 3 strips from each site were pooled and transferred into a sterile microtube.
Immediately after GCF sampling, the SP samples were collected from the same sites for GCF using 2 standardized sterile paper points (No. 30). One was inserted at a 45° angle and the other was inserted parallel to the tooth axis and left in place for 30 s. Following removal, the paper points were transferred into a screw-capped sterile vial.
The collected samples were stored at −20 °C until analyses.
ELISA and microbiological analyses
Each tube containing the sample strips was eluted with 250 μL Hank's buffer containing 0.1% bovine serum albumin (Sigma, St Louis, MO, USA) via centrifugation (2000 × g; 4 °C) for 5 min. After removing the strips, the supernatants were divided into 4 aliquots for determining each cytokine. The tumor necrosis factor- α (TNF-α), interleukin 1 (IL)-β, IL-6, and IL-8 concentrations were measured using commercially available enzyme-linked immunosorbent assay (ELISA) (RayBiotech Inc., Norcross, GA). The assays were conducted as per the manufacturer's instructions. After color development stopped, the optical density was measured at 450 nm. The total amount of cytokines was determined in picograms (pg). The concentration in each sample was calculated by dividing the total amount of cytokines by the sample volume (pg/μL).
DNA extraction
The Insta-Gene Matrix Kit (Bio-Rad Labs, California, USA) was used for bacterial DNA extraction. According to the manufacturer's instructions the DNA samples were centrifuged for 3 min at 13,200 rpm, then 350 μl of supernatant was taken off and the pellet was re-suspended in 200 μl of Instagene Matrix (Bio-Rad Labs, California, USA). The suspension was incubated at 56 °C for 30 min and at 100 °C for 8 min afterwards the samples were vortexed for 10 s and centrifuged for 3 min at 13,200 rpm. 150 μl of sample was taken for further analyses.
Quantitative- polymerase chain reaction (qPCR)
In order to quantify the total amount of bacteria and specific bacterial populations in the samples, qPCR was performed with the CFX 96 Real Time System (Bio-Rad, Hercules, CA, USA). All bacterial DNA samples were performed qPCR to assess Aggregatibacter actinomycetemcomitans (Aa) ATCC 43718, Porphyromonas gingivalis (Pg) ATCC 33277, Prevotella intermedia (Pi) ATCC 25611, Tannerella forsythia (Tf) ATCC 43037 and total bacterial amount. Universal primers, 338F (5′-ACTCCTACGGGAGGCAGCAG–3′) and 518r (5′-ATTACCGCGGCTGCTGG–3′) were used to quantify total bacterial amount in syber green based assay (SYBR). In SYBR reactions, each reaction well was containing 12.5 μl Mastermix (MESA Blue PCR Master Mixfor SYBR assay, Eurogentec, Belgium), 5.5 μl Milli Q and 1 μl of forward and reverse primers.
The Taqman 5’ nuclease assay qPCR method is used for detection and quantification of bacterial DNA. Primers of specific bacterial sequential were used to assess bacterial amount in Taq reactions (Table 1). Each taqman reaction was containing 12.5 μl Mastermix (Eurogentec, Seraing, Belgium), 4.5 μl Milli Q and 1 μl of each primer and probe. 5 μl of extracted DNA was used as template both in SYBR and Taq reactions.
Table 1.
Specific primers and probes used in the qPCR.
| Periodontal Pathogens | Primer/TaqMan probe sequence (5′-3′) |
|---|---|
| Aggregatibacter actinomycetemcomitans | |
| Forward primer | 5′- GAACCTTACCTACTCTTGACATCCGAA-3′ |
| Reverse primer | 5′-TGCAGCACCTGTCTCAAAGC-3′ |
| Probe | FAM-AGAACTCAGAGATGGGTTTGTGCCTTAGGG-TAMRA |
| Porphyromonas gingivalis | |
| Forward primer | 5′-GCGCTCAACGTTCAGCC-3′ |
| Reverse primer | 5′-CACGAATTCCGCCTGC-3′ |
| Probe | FAM-CACTGAACTCAAGCCCGGCAGTTTCAA-TAMRA |
| Prevotella intermedia | |
| Forward primer | 5′-CGGTCTGTTAAGCGTGTTGTG-3′ |
| Reverse primer | 5′-CACCATGAATTCCGCATACG-3′ |
| Probe | FAM-TGGCGGACTTGAGTGCACGC-TAMRA |
| Tannerella forsythensis | |
| Forward primer | 5′-GGGTGAGTAACGCGTATGTAACCT-3′ |
| Reverse primer | 5′-ACCCATCCGCAACCAATAAA-3′ |
| Probe | FAM- CCCGCAACAGAGGGATAACCCGG-TAMRA |
| SYBR | |
| Forward primer | 5′- ACT-CCT-ACG-GGA-GGC-AGC-AG-3′ |
| Reverse primer | 5′-ATTACCGCGGCTGCTGG-3′ |
As a standard for the qPCR, a fragment of the 16S rRNA gene of each bacterium was cloned into a plasmid with the pGEM-T easy vector system (Promega, Madison, WI, USA). Plasmids were isolated from the clones with the High Pure Plasmid Isolation Kit (Roche Diagnostics GmbH, Mannheim, Germany). The concentration of the plasmid was determined with the GeneQuant RNA/DNA calculator (Amersham Pharmacia Biotech/GE Healthcare, Diegem, Belgium) at a wavelength of 260 nm. A 10-fold dilution series of this plasmid was used in each qPCR run to construct the standard curve. For Aa cultivation standards were used instead of a plasmid standard. To achieve this Aa was grown overnight; the concentration of this overnight culture was checked spectrophotometrically and by microbial plating. Afterwards DNA was extracted by using the QIAamp DNA mini kit (Qiagen GmbH, Hilden, Germany) following the manufacturers’ instruction.
Cycling conditions for both SYBR and Taqman assay were the same; 2 min at 50 °C initially, followed by a denaturation step for 10 min at 95 °C, then 45 cycles of 95 °C for 15 s and 60 °C for 60 s.
Statistical analyses
Data analyses were performed using Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA) version 17 software. Shapiro–Wilk test was used to test the normality of distribution for continuous variables. Continuous variables are expressed as mean and standard deviation (SD) values. The significance of the differences in the median values between the SRP and SRP + OT groups was evaluated using Wilcoxon Sign Rank test. Further, the differences in the measurement times were compared using Wilcoxon Sign Rank test. Degrees of association between the variables were calculated using Spearman's correlation analyses. P values < 0.05 were considered statistically significant. Bonferroni correction was applied for all possible multiple comparisons for controlling Type I error.
Results
All the subjects completed the entire study. Healing was uneventful in all cases. No adverse effects related to OT were reported by any subject. Postoperative complications, such as infections, suppuration, and abscesses, were not observed.
Clinical findings
The mean clinical data for the sampling areas are shown in Table 1. There were no significant differences in the groups with respect to PI, GI, BOP, and PPD (P > 0.05) at baseline. As compared to that at baseline, all the parameters were significantly reduced at 3 months after the therapy in both the groups (P < 0.05). However, no significant differences among the treatments were evident in any of the periodontal parameters at 3rd months (P > 0.05) (Table 2).
Table 2.
Clinical parameters of sampling areas.
| SRP + OT (n = 18) (Mean ± SD) | Between Groups P valuesa | SRP-alone (n = 18) (Mean ± SD) | ||
|---|---|---|---|---|
| Sampling Areas Measurements | PI | |||
| Baseline | 1.23 ± 0.46 | N.S | 1.27 ± 0.43 | |
| Within the group | P = 0.003* | P = 0.005* | ||
| Month 3 | 0.73 ± 0.30 | N.S | 0.78 ± 0.34 | |
| GI | ||||
| Baseline | 1.58 ± 0.33 | N.S | 1.61 ± 0.32 | |
| Within the group | P = 0.002* | P = 0.004* | ||
| Month 3 | 1.03 ± 0.28 | N.S | 1.14 ± 0.24 | |
| PPD (mm) | ||||
| Baseline | 5.87 ± 1.13 | N.S | 5.91 ± 1.05 | |
| Within the group | P = 0.002* | P = 0.002* | ||
| Month 3 | 3.96 ± 0.95 | N.S | 3.98 ± 0.92 | |
| Interproximal sites with PPD >5 mm (%) | ||||
| Baseline | 39.87 ± 7.74 | N.S | 40.47 ± 7.66 | |
| Within the group | P = 0.001* | P = 0.001* | ||
| Month 3 | 27.97 ± 6.14 | N.S | 26.18 ± 6.13 | |
| BOP (%) | ||||
| Baseline | 69.44 ± 12.54 | N.S | 67.42 ± 18.95 | |
| Within the group | P = 0.001* | P = 0.001* | ||
| Month 3 | 15.55 ± 18.60 | N.S | 19.44 ± 22.15 | |
*P < 0.05, Mann Whitney-U test.
SRP: Scaling and root planning, OT: Ozone therapy, PI: Plaque index, GI: Gingival index, PD: probing depth, BOP: Bleeding on probing, SD: Standard deviation, N.S: Non significant.
Comparison of PI, GI, PD, BOP values between SRP + OT and SRP-alone groups by Mann Whitney-U test.
GCF volume
Table 3 shows the mean changes in the GCF volume at the follow-up visits compared to that at baseline. Both treatment methods caused a consistent reduction in the GCF volume at all time points, although this decrease was not statistically significant (P > 0.05). No statistically significant difference was observed in the GCF volume between the treatments at any time point in the study (P > 0.05).
Table 3.
Changes in GCF cytokines levels over time for the SRP + OT and SRP-alone groups.
| Baseline |
1st month |
3rd Month |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Between Groups P values a |
Mean ± SD | Within Groups | Between Groups P values a |
Mean ± SD | Within Groups | Between Groups P values a |
||
| GCF volume | SRP + OT (n = 18) | 0.009 ± 0.008 | N.S | 0.006 ± 0.011 |
B vs 1st M 0.031* |
N.S | 0.005 ± 0.003 | N.S | N.S |
| SRP-alone (n = 18) | 0.008 ± 0.004 | 0.005 ± 0.003 |
B vs 1st M 0.041* |
0.005 ± 0.004 | N.S | ||||
| TNF-α(pg/μL) | SRP + OT (n = 18) | 5.079 ± 4.04 | N.S | 1.553 ± 0.22 | N.S | N.S | 1.167 ± 0.22 | N.S | N.S |
| SRP-alone (n = 18) | 5.868 ± 2.61 | 2.989 ± 0.70 | 0.038* | 1.772 ± 0.43 | N.S | ||||
| IL-1β (pg/μL) | SRP + OT (n = 18) | 15.808 ± 2.35 | N.S | 12.091 ± 1.88 | N.S | N.S | 11.041 ± 1.72 | N.S | N.S |
| SRP-alone (n = 18) | 13.783 ± 2.02 | 12.625 ± 2.26 | N.S | 11.325 ± 1.66 | N.S | ||||
| IL-6 (pg/μL) | SRP + OT (n = 18) | 6.088 ± 0.69 | N.S | 5.325 ± 0.71 | N.S | N.S | 2.083 ± 0.50 | N.S | N.S |
| SRP-alone (n = 18) | 6.884 ± 0.88 | 5.351 ± 0.57 | N.S | 2.483 ± 0.45 |
1st vs 3rd M 0.046* |
||||
| IL-8 (pg/μL) | SRP + OT (n = 18) | 10.087 ± 0.75 | N.S | 9.333 ± 0.72 | N.S | N.S | 9.158 ± 0.74 | N.S | N.S |
| SRP-alone (n = 18) | 12.383 ± 0.72 | 10.010 ± 0.74 |
B vs 1st M 0.013* |
8.275 ± 0.71 | N.S | ||||
*P < 0.05, Wilcoxon Sign Rank test.
SRP: Scaling and root planning, OT: Ozone therapy, TNF-α: Tumor necrosis factor-alpha, IL-1β: Interleukin-1beta, IL-6: Interleukin-6, IL-8: Interleukin-8.
SD: Standard deviation, N.S: Non significant, B: Baseline, M: Month.
Comparison of GCF volume, TNF-α, IL-1β, IL-6 and IL-8 values between SRP + OT and SRP-alone groups by Wilcoxon Sign Rank test.
GCF cytokine levels
The GCF levels of each cytokine at baseline and after the therapy in each group are highlighted in Table 3. The GCF cytokine levels did not differ significantly between the treatment modalities at baseline (P > 0.05). The results indicated no significant alterations in the cytokine concentrations over time between the SRP + OT and SRP-alone groups (P > 0.05). In the SRP-alone group, the IL-8 and TNF-α levels significantly decreased at 1 month after the therapy compared to those at baseline (P = 0.038 and P = 0.013, respectively). A statistically significant difference was observed between the IL-6 concentration at baseline and that at the 3-month follow-up in the SRP-alone group (P = 0.046).
Microbiological results
The SRP + OT and SRP-alone treatment modalities decreased the levels of Aa, Pg, Tf, and Pi over time. The levels of Pg and Tf decreased significantly at the 1-month follow up as compared to that at baseline in the SRP-alone group (P = 0.006 and P = 0.028, respectively). The levels of Tf (P = 0.004) and Pi (P = 0.013) had decreased significantly at 3 months in the SRP-alone group (P < 0.05). The total bacterial counts had decreased significantly in the SRP-alone group at the 1-month follow-up (P = 0.003). Statistically significant differences were observed in the baseline and 3-month follow values of Pi (P = 0.049) and total bacterial counts (P = 0.05) in the SRP + OT group (P < 0.05). The Aa levels did not change significantly in the groups (P > 0.05). The microbiological parameters of the groups were not significantly altered during the study period (P > 0.05) (Table 4).
Table 4.
Changes in microbiological values over time for the SRP + OT and SRP-alone groups.
| Baseline |
1st month |
3rd Month |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Between Groups P valuesa |
Mean ± SD | Within Groups | Between Groups P valuesa |
Mean ± SD | Within Groups | Between Groups P valuesa |
||
| P.gingivalis | SRP + OT (n = 18) | 2.61E ± 0.12 | N.S | 2.12E ± 0.067 | N.S | N.S | 1.98E ± 0.18 | N.S | N.S |
| SRP-alone (n = 18) | 3.08E ± 0.12 | 2.05E ± 0.014 |
B vs 1st M 0.006* |
2.03E ± 0.17 | N.S | ||||
| A. actinomycetemcomitans | SRP + OT (n = 18) | 0.31E ± 0.01 | N.S | 0.15E ± 0.05 | N.S | N.S | 0.08E ± 0.02 | N.S | N.S |
| SRP-alone (n = 18) | 0.40E ± 0.01 | 0.27E ± 0.06 | N.S | 0.21E ± 0.07 | N.S | ||||
| P. intermedia | SRP + OT (n = 18) | 2.31E ± 0.15 | N.S | 1.57E ± 0.15 | N.S | N.S | 1.12E ± 0.15 |
B vs 3rd M 0.049* |
N.S |
| SRP-alone (n = 18) | 2.74E ± 0.11 | 1.73E ± 0.16 | N.S | 1.37E ± 0.15 |
B vs 3rd M 0.013* |
||||
| T. forsythensis | SRP + OT (n = 18) | 1.98E ± 0.13 | N.S | 1.61E ± 0.12 | N.S | N.S | 0.84E ± 0.09 | N.S | N.S |
| SRP-alone (n = 18) | 2.65E ± 0.46 | 1.58E ± 0.12 |
B vs 1st M 0.028* |
1.48E ± 0.09 |
B vs 3rd M 0.004* |
||||
| Total bacterial counts | SRP + OT (n = 18) | 4.44E ± 0.35 | N.S | 4.10E ± 0.63 | N.S | N.S | 4.15E ± 0.27 |
B vs 3rd M 0.05* |
N.S |
| SRP-alone (n = 18) | 4.56E ± 0.29 | 3.98E ± 0.36 |
B vs 3st M 0.003* |
4.26E ± 0.35 | N.S | ||||
*P < 0.05, Wilcoxon Sign Rank test.
SRP: Scaling and root planning, OT: Ozone therapy, A. actinomycetemcomitans: Aggregatibacter actinomycetemcomitans, P. gingivalis: Porphyromonas gingivalis, P. intermedia: Prevotella intermedia, T. forsythensis:Tannerella forsythensis.
SD: Standard deviation, N.S: Non significant, B: Baseline, M: Month.
Comparison of microbiological values between SRP + OT and SRP-alone groups by Wilcoxon Sign Rank test.
Correlations
In the SRP + OT group, there was a positive correlation between the baseline PI scores and baseline GCF values of Pg and Tf at baseline, respectively (r = 0.718, P = 0.009) (r = 0.639, P = 0.025). Moreover, the IL-8 value was positively correlated with the initial PPD (r = 0.577, P = 0.049); a positive correlation also existed between the baseline plaque index score and IL-6 values (r = 0.622, P = 0.030).
At 3 months after the therapy, PI values (r = 0.603, P = 0.38) were positively correlated with Tf values for the SRP-alone group (P < 0.05). In addition, there were positive correlations of the mean GI values with TNF-α (r = 0.674, P = 0.16), as well as the scores of PPD with IL-6 (r = 0.756, P = 0.004) and IL-1β (r = 0.694, P = 0.18) was positively correlated (P < 0.05).
In the SRP + OT group, the scores of PPD were correlated with IL-1β (r = 0.818, P = 0.001), the mean PI values were correlated with IL-8 (r = 0.697, P = 0.012), and the GI values were correlated with TNF-α (r = 0.607, P = 0.036) (P < 0.05) at the 3-month follow up.
Discussion
Elimination of pathogen-containing biofilms remains the primary goal of periodontal treatment.2 Non-surgical mechanical treatment is the most important stage of periodontal treatment.3 The complete removal of supragingival and subgingival calculus with SRP is extremely challenging with a closed approach.23,24 In areas deeper than 5 mm, removal of the full calculus occurred only to the extent of 11%: therefore, if the depth of the pocket is > 5 mm after non-surgical periodontal treatment, the probability of failure is high enough for an indication for surgical pocket removal or reduction. Persistent periodontal pockets are common in chronic periodontitis patients undergoing initial periodontal treatment and are a predictive risk factor for disease progression and increased attachment loss.24
The use of OT in the field of dentistry has been discussed in several studies.12, 13, 14, 15 However, limited evidence is available regarding the clinical application of OT as an adjunctive alternative treatment for periodontal diseases.
In the present study, both SRP-alone and SRP + OT treatment modalities resulted in significant improvements in all clinical parameters. It is noteworthy that there was no significant difference between the treatment methods after 3 months with respect to the periodontal parameters. Although the antibacterial effect of OT was very strong and long lasting, the pocket depth reduction was similar to that in the SRP-alone quadrants. Brauneret et al.25 compared the clinical periodontal status between patients who were instructed to use ozone water with SRP in patients and concluded that ozone water rinse cannot be used as a substitute for professional dental plaque removal. In addition, ozone oil caused inflammatory symptoms and reduced the accompanying immunologic and morphological changes.26 The author observed a reduction in GI, PI, and PPD, that helped in restoring the normal state of the gingiva within 3 days by reducing the bleeding in the gingiva and the normal color returned following the ozone therapy.26 Similar to our results, the findings by Skurska et al.14 showed a significant reduction in the inflammatory indices of patients who received gaseous ozone; however, these were not significantly different from those of patients who did not undergo OT. A recent study has demonstrated that patients with chronic periodontitis who underwent OT with SRP had a greater improvement in their parameters than who underwent only SRP. However, these effects were mild and statistically non-significant.27 We found that adjunctive therapy with ozone failed to exert any additional positive effects on the studied clinical parameters when compared to SRP treatment alone in patients with chronic periodontitis; this is in agreement with previous reports.13,14 In addition, no further ozone application was performed in the SRP + OT group during the re-examination period. These results can be attributable to the insufficient penetration and localized effect on the tissue following one-time application of gaseous ozone.
The synthesis of inflammatory mediators, such as IL-1β, TNF-α, TGF-β, prostaglandin E2, and growth factors depends on nuclear factor kappa B that is stimulated by the gaseous form of ozone.28,29 These cytokines play a crucial in the pathogenesis of the inflammatory process. The mean levels of TNF-α, IL-1, IL-6, and IL-8 in the GCF in patients with generalized chronic periodontitis were higher than those in the periodontally healthy subjects; these results are consistent with previous results.30, 31, 32 Many authors have reported that levels of these cytokines in the GCF decreased after non-surgical periodontal therapy.33, 34, 35 However, in our study, patients with GCP showed only a slight decrease in IL-1β, TNF-α, and IL-8 at 3 months after SRP as compared to the baseline values. Only the IL-6 levels were significantly different. After additional ozone application procedures following SRP, the GCP patients had decreased levels of IL-1β, TNF-α, IL-6, and IL-8. The changes in the cytokine levels were not statistically significant. A previous study has reported that following SRP with additional ozone therapy, the matrix metalloproteinase levels increased in patients with chronic periodontitis, while they decreased in those with aggressive periodontitis.36
After 3 months, the levels of these cytokines were usually similar to the baseline values. It is difficult to compare our results to previous reports because to our knowledge, no studies have evaluated these cytokines in the GCF of chronic periodontitis patients after OT. Our analyses showed that additional application of ozone to the pockets following SRP in patients with periodontitis had no significant effect on the treatment outcome. Neither the clinical indices nor the GCF levels of cytokines differed between patients who and did not undergo OT. The study outcome may have been affected by conditions wherein ozone was applied to the pockets. It was not possible to assess the depth of ozone penetration and determine the actual ozone effect in the deep pockets using a different ozone appliance equipped with periodontal tips.
Several antimicrobial agents have been used as adjuncts to mechanical debridement to further suppress periodontal pathogens because of the microbial etiology of periodontitis.5,13,37 It is well known that ozone can kill bacteria, fungi, and viruses in the gaseous or aqueous phase because it is a strong oxidizer of cell walls and cytoplasmic membranes of bacteria.38,39 Different forms of ozone were used as adjuncts to mechanical debridement in periodontitis treatment periodontitis. In the present study, the SRP-alone and SRP + OT groups showed statistically significant reductions in the mean total number of bacteria in the subgingival plaque during the entire study period. Our data indicated that all putative periodontal pathogens of the species that were analyzed had decreased after 1 and 3 months of both treatments. The reduction was selective, that is, Pi were markedly reduced in both groups, while Aa seemed unaffected. Several explanations have been suggested, including the possibility that those bacteria can reside in the soft tissues or in the root surface irregularities and dentinal tubules. Our findings showed that the value at baseline and at the 3-month follow up for the periodontopathogenic bacteria were similar in both the treatment groups. If we partly eliminated the periodontopathogenic bacteria from the periodontal pocket, they may re-grow. This can be a possible explanation for these findings. These results seem to contradict previous reports13,40, 41, 42 that have shown that non-surgical mechanical therapy could reduce the prevalence and levels of the main periodontal pathogens. In the present study, SRP-alone was unable to achieve significant reductions in the periodontal pathogens. However, the lack of significant reductions within and between the groups may be attributable to our small sample size.
Nagayoshi et al.43 showed that ozonated water (0.5–4 mg/L) was highly effective in killing both gram-positive and gram-negative oral microorganisms despite some researchers suggesting that ozone in the aqueous or gaseous form has an incomplete efficacy for eliminating the viable bacteria.44,45 Moreover, a high level of biocompatibility of aqueous ozone has been detected in human oral epithelial cells, gingival fibroblast cells, and periodontal cells.46
Similar to our results, the findings of Bezrukova et al.47 show that both gaseous and aqueous ozone therapy reduced the growth of Aa, Tf, Treponema denticola (Td), Pg, and Pi; however, no information was provided about the application time or dose in their study. Recently, it has been reported that ozone nano-bubble water has antimicrobial activity against specific periodontal pathogens.13 The ozone nano-bubble group showed statistically significant reductions in the mean total number of bacteria in the subgingival plaque over the study period. Regarding each periodontal pathogen, Hayakuma et al. did not find significant reductions in the mean number and percentage of Pg and Tf at any follow-up in their study groups.13
A review of the literature shows that the method of ozone delivery to the periodontal tissues differs between studies; three13,27,48 utilized ozonized/ozonated water, two14,49 utilized gaseous ozone, and the other two50,51 utilized ozonized oil with varying concentration of ozone. There are differences in the study designs and application methods for ozone; therefore, an absolute comparison of our results with these previous reports is difficult.
Within the limitation of this study, the number of patients can be enough for reach significant results, but not possible to define important differences in all clinical parameters, and microbiologic/biochemical effects. This study was designed as split-mouth. A split-mouth design would be a poor choice for trials that compare drugs or antiseptics that are topically applied to the dentition because any treatment effect could be carried across to other segments of the dentition that are used for comparison. This was an important limitation of this study. For the detection and quantification of the periodontopathic bacteria, quantitative polymerase chain reaction (qPCR) was used in this study. This technique detects both viable and non-viable bacteria. It is difficult to assess the effectiveness of the antimicrobial agents because of the detection of non-viable microorganisms following antimicrobial therapy. Most of the enrolled subjects had moderate periodontitis. Moreover, the depth of PPD and CAL were not categorized because of the small sample size. Future interventional studies are warranted to define the ozone application effect in more severe cases of chronic periodontitis, categorized based on PPD and CAL depth (e.g., shallow, mild to moderate, and deep). Future studies with larger samples and longer follow-up periods that compare different applications of ozone agents for treating chronic periodontitis would yield interesting results.
In conclusion, SRP has a beneficial effect on the periodontal status of patients with chronic periodontitis. Gaseous ozone application following SRP contributes to minor or unknown improvements in the clinical periodontal parameters. In addition, the microbiologic and biochemical effectiveness of ozone application is limited in terms of clinic practice. Following the determination of the optimal concentration and application frequency, the use of gaseous ozone adjunct to SRP in sites with deep pockets may be a considerable clinical option.
Conflicts of interest
The authors declare that they have no conflict of interest.
Acknowledgments
This study was funded by Gazi University Research Board, with project number 03/201010.
References
- 1.Savage A., Eaton K.A., Moles D.R., Needleman I. A systematic review of definitions of periodontitis and methods that have been used to identify this disease. J Clin Periodontol. 2009;36:458–467. doi: 10.1111/j.1600-051X.2009.01408.x. [DOI] [PubMed] [Google Scholar]
- 2.Van der Weijden G.A., Timmerman M.F. A systematic review on the clinical efficacy of subgingival debridement in the treatment of chronic periodontitis. J Clin Periodontol. 2002;29:55–71. doi: 10.1034/j.1600-051x.29.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 3.Heitz-Mayfield L.J., Trombelli L., Heitz F., Needleman I., Moles D. A systematic review of the effect of surgical debridement vs non-surgical debridement for the treatment of chronic periodontitis. J Clin Periodontol. 2002;29:92–102. doi: 10.1034/j.1600-051x.29.s3.5.x. [DOI] [PubMed] [Google Scholar]
- 4.Nagarakanti S., Gunupati S., Chava V.K., Reddy B.V.R. Effectiveness of subgingival irrigation as an adjunct to scaling and root planing in the treatment of chronic periodontitis: a systematic review. J Clin Diagn Res. 2015;9:ZE06–ZE09. doi: 10.7860/JCDR/2015/13862.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quirynen M., Teughels W., De Soete M., van Steenberghe D. Topical antiseptics and antibiotics in the initial therapy of chronic adult periodontitis: microbiological aspects. J Periodontol 2000. 2002;28:72–90. doi: 10.1034/j.1600-0757.2002.280104.x. [DOI] [PubMed] [Google Scholar]
- 6.Sigusch B.W., Pfitzner A., Albrecht V., Glockmann E. Efficacy of photodynamic therapy on inflammatory signs and two selected periodontopathogenic species in a beagle dog model. J Periodontol. 2005;76:1100–1105. doi: 10.1902/jop.2005.76.7.1100. [DOI] [PubMed] [Google Scholar]
- 7.Atieh M.A. Photodynamic therapy as an adjunctive treatment for chronic periodontitis: a meta-analysis. Laser Med Sci. 2010;25:605–613. doi: 10.1007/s10103-009-0744-6. [DOI] [PubMed] [Google Scholar]
- 8.Sgolastra F., Petrucci A., Severino M., Graziani F., Gatto R., Monaco A. Adjunctive photodynamic therapy to non-surgical treatment of chronic periodontitis: a systematic review and meta-analysis. J Clin Periodontol. 2013;40:514–526. doi: 10.1111/jcpe.12094. [DOI] [PubMed] [Google Scholar]
- 9.Vohra F., Akram Z., Safi S.H. Role of antimicrobial photodynamic therapy in the treatment of aggressive periodontitis: a systematic review. Photodiagn Photodyn Ther. 2016;13:139–147. doi: 10.1016/j.pdpdt.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Souza E., Medeiros A.C., Gurgel B.C., Sarmento C. Antimicrobial photodynamic therapy in the treatment of aggressive periodontitis: a systematic review and meta-analysis. Laser Med Sci. 2016;31:187–196. doi: 10.1007/s10103-015-1836-0. [DOI] [PubMed] [Google Scholar]
- 11.Azarpazhooh A., Limeback H. The application of ozone in dentistry: a systematic review of literature. J Dent. 2008;36:104–116. doi: 10.1016/j.jdent.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Ramzy M.I., Gonna H.E., Mostafa M.I., Zaki B.M. Management of aggressive periodontitis using ozonized water. Egypt Med JNRC. 2005;6:229–245. [Google Scholar]
- 13.Hayakumo S., Arakawa S., Mano Y., Izumi Y. Clinical and microbiological effects of ozone nano- bubble water irrigation as an adjunct to mechanical subgingival debridement in periodontitis patients in a randomized controlled trial. Clin Oral Invest. 2013;17:379–388. doi: 10.1007/s00784-012-0711-7. [DOI] [PubMed] [Google Scholar]
- 14.Skurska A., Pietruska M.D., Paniczko-Drężek A. Evaluation of the influence of ozonotherapy on the clinical parameters and MMP levels in patients with chronic and aggressive periodontitis. Adv Med Sci. 2010;55:297–307. doi: 10.2478/v10039-010-0048-x. [DOI] [PubMed] [Google Scholar]
- 15.Kshitish D., Laxman V.K. The use of ozonated water and 0.2% chlorhexidine in the treatment of periodontitis patients: a clinical and microbiologic study. Indian J Dent Res. 2010;21:341–348. doi: 10.4103/0970-9290.70796. [DOI] [PubMed] [Google Scholar]
- 16.World Medical Association Inc Declaration of Helsinki. Ethical principles for medical research involving human subjects. J Indian Med Assoc. 2009;107:403–405. [PubMed] [Google Scholar]
- 17.Armitage G.C. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Novaes A.B., Schwartz-Filho H.O., de Oliveira R.R., Feres M., Sato S., Figueiredo L.C. Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: microbiological profile. Laser Med Sci. 2012;27:389–395. doi: 10.1007/s10103-011-0901-6. [DOI] [PubMed] [Google Scholar]
- 19.Silness J., Loe H. Periodontal Disease in Pregnancy. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 20.Loe H., Silness J. Periodontal disease in pregnancy. Prevalence and severity. Acta Odontol Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 21.Badersten A., Nilveus R., Egelberg J. Effect of nonsurgical periodontal therapy-III. Single versus repeated instrumentation. J Clin Periodontol. 1984;11:114–124. doi: 10.1111/j.1600-051x.1984.tb00839.x. [DOI] [PubMed] [Google Scholar]
- 22.Hartroth B., Seyfahrt I., Conrads G. Sampling of periodontal pathogens by paper points: evaluation of basic parameters. Oral Microbiol Immunol. 1999;14:326–330. doi: 10.1034/j.1399-302x.1999.140510.x. [DOI] [PubMed] [Google Scholar]
- 23.Deas D.E., Moritz A.J., Sagun R.S., Jr., Gruwell S.F., Powell C.A. Scaling and root planing vs. conservative surgery in the treatment of chronic periodontitis. Periodontol 2000. 2016;71:128–139. doi: 10.1111/prd.12114. [DOI] [PubMed] [Google Scholar]
- 24.Heitz-Mayfield L.J., Lang N.P. Surgical and nonsurgical periodontal therapy. Learned and unlearned concepts. Periodontol 2000. 2013;62:218–231. doi: 10.1111/prd.12008. [DOI] [PubMed] [Google Scholar]
- 25.Brauner A.W. Periodontology: new methods. Ozone Sci Eng. 1992;14:165–176. [Google Scholar]
- 26.Menabde G.T., Natroshvili N.D., Natroshvili T.D. Ozonotherapy for the treatment of periodontitis. Georgian Med News. 2006;134:43–46. [PubMed] [Google Scholar]
- 27.Al Habashneh R., Alsalman W., Khader Y. Ozone as an adjunct to conventional nonsurgical therapy in chronic periodontitis: a randomized controlled clinical trial. J Periodont Res. 2015;50:37–43. doi: 10.1111/jre.12177. [DOI] [PubMed] [Google Scholar]
- 28.Bocci V. Ozone as Janus: this controversial gas can be either toxic or medically useful. Mediat Inflamm. 2004;13:3–11. doi: 10.1080/0962935062000197083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bocci V. Scientific and medical aspects of ozone therapy. State of the art. Arch Med Res. 2006;37:425–435. doi: 10.1016/j.arcmed.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Graves D.T., Cochran D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol. 2003;74:391–401. doi: 10.1902/jop.2003.74.3.391. [DOI] [PubMed] [Google Scholar]
- 31.Ejeil A.L., Gaultier F., Igondjo-Tchen S. Are cytokines linked to collagen breakdown during periodontal disease progression? J Periodontol. 2003;74:196–201. doi: 10.1902/jop.2003.74.2.196. [DOI] [PubMed] [Google Scholar]
- 32.Beklen A., Ainola M., Hukkanen M., Gürgan C., Sorsa T., Konttinen Y.T. MMPs, IL-1, and TNF are regulated by IL-17 in periodontitis. J Dent Res. 2007;86:347–351. doi: 10.1177/154405910708600409. [DOI] [PubMed] [Google Scholar]
- 33.Holmlund A., Hänström L., Lerner U.H. Bone resorbing activity and cytokine levels in gingival crevicular fluid before and after treatment of periodontal disease. J Clin Periodontol. 2004;31:475–482. doi: 10.1111/j.1600-051X.2004.00504.x. [DOI] [PubMed] [Google Scholar]
- 34.O'connell P.A., Taba M., Jr., Nomizo A. Effects of periodontal therapy on glycemic control and inflammatory markers. J Periodontol. 2008;79:774–783. doi: 10.1902/jop.2008.070250. [DOI] [PubMed] [Google Scholar]
- 35.Thunell D.H., Tymkiw K.D., Johnson G.K. A multiplex immunoassay demonstrates reductions in gingival crevicular fluid cytokines following initial periodontal therapy. J Periodontal Res. 2010;45:148–152. doi: 10.1111/j.1600-0765.2009.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pietruska M.D., Paniczko-Drężek A., Dolińska E. Evaluation of the influence of ozonotherapy on the clinical parameters and MMP levels in patients with chronic and aggressive periodontitis. Adv Med Sci. 2010;55:297–307. doi: 10.2478/v10039-010-0048-x. [DOI] [PubMed] [Google Scholar]
- 37.Mombelli A. Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontol 2000. 2018;76:85–96. doi: 10.1111/prd.12147. [DOI] [PubMed] [Google Scholar]
- 38.Arita M., Nagayoshi M., Fukuizumi T. Microbicidal efficacy of ozonated water against Candida albicans adhering to acrylic denture plates. Oral Microbiol Immunol. 2005;20:206–210. doi: 10.1111/j.1399-302X.2005.00213.x. [DOI] [PubMed] [Google Scholar]
- 39.Kim J.G., Yousef A.E., Dave S. Application of ozone for enhancing the microbiological safety and quality of foods: a review. J Food Prot. 1999;62:1071–1087. doi: 10.4315/0362-028x-62.9.1071. [DOI] [PubMed] [Google Scholar]
- 40.Cugini M.A., Haffajee A.D., Smith C., Kent R.L., Socransky S.S. The effect of scaling and root planing on the clinical and microbiological parameters of periodontal diseases: 12 month results. J Clin Periodontol. 2000;27:30–36. doi: 10.1034/j.1600-051x.2000.027001030.x. [DOI] [PubMed] [Google Scholar]
- 41.Colombo A.P.V., Teles R.P., Torres M.C. Effects of non-surgical mechanical therapy on the subgingival microbiota of Brazilians with untreated chronic periodontitis: 9-month results. J Periodontol. 2005;76:778–784. doi: 10.1902/jop.2005.76.5.778. [DOI] [PubMed] [Google Scholar]
- 42.Colombo A.P.V., Bennet S., Cotton S.L. Impact of periodontal therapy on the subgingival microbiota of severe periodontitis: comparison between good responders and individuals with refractory periodontitis using the human oral microbe identification microarray. J Periodontol. 2012;83:1279–1287. doi: 10.1902/jop.2012.110566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagayoshi M., Fukuizumi T., Kitamura C., Yano J., Terashita M., Nishihara T. Efficacy of ozone on survival and permeability of oral microorganisms. Oral Microbiol Immunol. 2004;19:240–246. doi: 10.1111/j.1399-302X.2004.00146.x. [DOI] [PubMed] [Google Scholar]
- 44.Muller P., Guggenheim B., Schmidlin P.R. Efficacy of gasiform ozone and photodynamic therapy on a multispecies oral biofilm in vitro. Eur J Oral Sci. 2007;115:77–80. doi: 10.1111/j.1600-0722.2007.00418.x. [DOI] [PubMed] [Google Scholar]
- 45.Walker J.T., Bradshaw D.J., Fulford M.R., Marsh P.D. Microbiological evaluation of a range of disinfectant products to control mixed-species biofilm contamination in a laboratory model of a dental unit water system. Appl Environ Microbiol. 2003;69:3327–3332. doi: 10.1128/AEM.69.6.3327-3332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huth K.C., Jakob F.M., Saugel B. Effect of ozone on oral cells compared with established antimicrobials. Eur J Oral Sci. 2006;114:435–440. doi: 10.1111/j.1600-0722.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 47.Bezrukova I.V., Petrukhina N.B., Voinov P.A. Experience in medical ozone use for root canal treatment. Stomatologiia (Mosk) 2005;84:20–22. [PubMed] [Google Scholar]
- 48.Katti S.S., Chava V.K. Effect of ozonised water on chronic periodontitis - a clinical study. J Int Oral Health. 2013;5:79–84. [PMC free article] [PubMed] [Google Scholar]
- 49.Yilmaz S., Algan S., Gursoy H., Noyan U., Kuru B.E., Kadir T. Evaluation of the clinical and antimicrobial effects of the Er:YAG laser or topical gaseous ozone as adjuncts to initial periodontal therapy. Photomed Laser Surg. 2013;31:293–298. doi: 10.1089/pho.2012.3379. [DOI] [PubMed] [Google Scholar]
- 50.Patel P.V., Patel A., Kumar S., Holmes J.C. Effect of subgingival application of topical ozonated olive oil in the treatment of chronic periodontitis: a randomized, controlled, double blind, clinical and microbiological study. Minerva Stomatol. 2012;61:381–398. [PubMed] [Google Scholar]
- 51.Shoukheba M.Y.M., Ali S.A. The effects of subgingival application of ozonated olive oil gel in patient with localized aggressive periodontitis. A clinical and bacteriological study. Tanta Dent J. 2014;11:63–73. [Google Scholar]