Abstract
The bed nucleus of the stria terminalis (BNST) is part of the limbic-hypothalamic system important for behavioral responses to stress, and glutamate transmission within this region has been implicated in the neurobiology of alcoholism. Herein, we used a combination of immunoblotting, neuropharmacological and transgenic procedures to investigate the role for metabotropic glutamate receptor 5 (mGlu5) signaling within the BNST in excessive drinking. We discovered that mGlu5 signaling in the BNST is linked to excessive alcohol consumption in a manner distinct from behavioral or neuropharmacological endophenotypes that have been previously implicated as triggers for heavy drinking. Our studies demonstrate that, in male mice, a history of chronic binge alcohol-drinking elevates BNST levels of the mGlu5-scaffolding protein Homer2 and activated extracellular signal-regulated kinase (ERK) in an adaptive response to limit alcohol consumption. Male and female transgenic mice expressing a point mutation of mGlu5 that cannot be phosphorylated by ERK exhibit excessive alcohol-drinking, despite greater behavioral signs of alcohol intoxication and reduced anxiety, and are insensitive to local manipulations of signaling in the BNST. These transgenic mice also show selective insensitivity to alcohol-aversion and increased novelty-seeking, which may be relevant to excessive drinking. Further, the insensitivity to alcohol-aversion exhibited by male mice can be mimicked by the local inhibition of ERK signaling within the BNST. Our findings elucidate a novel mGluR5-linked signaling state within BNST that plays a central and unanticipated role in excessive alcohol consumption.
SIGNIFICANCE STATEMENT The bed nucleus of the stria terminalis (BNST) is part of the limbic-hypothalamic system important for behavioral responses to stress and alcohol, and glutamate transmission within BNST is implicated in the neurobiology of alcoholism. The present study provides evidence that a history of excessive alcohol drinking increases signaling through the metabotropic glutamate receptor 5 (mGlu5) receptor within the BNST in an adaptive response to limit alcohol consumption. In particular, disruption of mGlu5 phosphorylation by extracellular signal-regulated kinase within this brain region induces excessive alcohol-drinking, which reflects a selective insensitivity to the aversive properties of alcohol intoxication. These data indicate that a specific signaling state of mGlu5 within BNST plays a central and unanticipated role in excessive alcohol consumption.
Keywords: anxiety, bed nucleus of the stria terminalis, binge-drinking, extracellular signal-regulated kinase, Homer protein, mGlu5 receptor
Introduction
Individual variation in alcohol sensitivity is a contributing factor to alcoholism vulnerability (Krystal et al., 2003; Trim et al., 2009). The inverse relation between alcohol-sensitivity and addiction vulnerability is well exemplified in pharmacogenetics studies, in which alcoholism vulnerability has been associated with dysregulated excitatory neurotransmission, particularly through ionotropic NMDA glutamate receptors (NMDARs; Krystal et al., 2003). In murine models, binge alcohol-drinking elicits robust, behaviorally relevant, increases in both NMDAR and Group1 metabotropic glutamate receptor (mGluRs; subtypes mGlu1 and mGlu5) signaling within structures embedded within the extended amygdala circuit, including the shell subregion of the nucleus accumbens (NAsh) and the central nucleus of the amygdala (CeA). Intact receptor scaffolding by Homer2 is required for the capacity of mGlu1/5 to regulate alcohol sensitivity and intake (Szumlinski et al., 2005, 2008b; Cozzoli et al., 2009, 2012, 2014, 2016); Lum et al., 2014. Homer2 scaffolds mGlu1/5 by interacting with a TPPSPF motif on the C-terminus of the receptor that is targeted by proline-directed kinases, including extracellular signal-regulated kinase (ERK). ERK-dependent phosphorylation of mGlu5(T1123/S1126) increases receptor affinity for Homer EVH1 binding to facilitate mGlu5-dependent gating of slow inward currents through NMDARs (Beneken et al., 2000; Orlando et al., 2009; Hu et al., 2012; Park et al., 2013). The importance of ERK-dependent receptor cross talk for cocaine-induced neuroplasticity within the NAsh was highlighted previously (Park et al., 2013). Here we identified a novel role for ERK signaling in the bed nucleus of the stria terminalis (BNST) regulating alcohol intake.
The BNST is an important hub within the extended amygdala, interconnecting stress and reward subcircuits, which is implicated in alcoholism-related behaviors (cf. Erb et al., 2001; Kash, 2012; Zamora-Martinez and Edwards, 2014; Lovinger and Kash, 2015). Alcohol exposure increases ERK activation throughout the extended amygdala, including the BNST, and systemic treatment with ERK inhibitors increases alcohol intake in mice (Faccidomo et al., 2009). Neurons in the BNST receive glutamatergic innervation from several limbic regions and exhibit mGlu5- and NMDAR-dependent synaptic plasticity in response to non-contingent alcohol (cf. Erb et al., 2001; Kash, 2012; Wills et al., 2012; Lovinger and Kash, 2015). Although relatively little is known regarding how voluntary alcohol intake influences BNST excitatory neurotransmission, binge alcohol-drinking increases immunohistochemical indices of cellular activity within this region, which is associated with anxiety during alcohol withdrawal (Lee et al., 2015).
The present study first tested the hypothesis that a history of binge alcohol-drinking augments BNST indices of glutamate signaling by conducting immunoblotting procedures on BNST tissue from C57BL/6J (B6) mice that underwent our 30 d, single-bottle (20% ethanol v/v) drinking-in-the-dark (DID) procedure (same as the mice used by Cozzoli et al., 2012, 2014, 2016). We next used an adeno-associated virus (AAV) strategy to examine the functional relevance of Homer scaffolding within the BNST for alcohol intake and related the effects to sensitivity to alcohol's sedative-hypnotic properties. We also used neuropharmacological procedures to confirm a necessary role for ERK within the BNST for binge-drinking and the negative affective valence of high-dose alcohol. Last and critically, we then characterized the alcohol phenotype of, and conducted BNST-targeted neuropharmacological studies in, male and female mice heterozygous (GRM5TS/AA) or homozygous (GRM5AA/AA) for alanine substitutions at T1123 and S1126 of mGluR5 that prevent phosphorylation of the receptor by proline-directed kinases, including ERK (Park et al., 2013). This was done to determine whether mGlu5(T1123/S1126) within the BNST might be a target of ERK regulating the propensity to binge-drink. Additional behavioral experiments related the alcohol-drinking phenotype of the mGlu5 mutants to behavioral endophenotypes associated with alcoholism, notably reactivity to stressors, as well as, examined the generalization of our observed effects to other drugs of abuse or non-drug reinforcers/rewards. Through this study, we identify the BNST as a likely neural locus in which the conditional potentiation of mGlu5-Homer scaffolding by ERK-dependent phosphorylation of mGlu5(T1123/S1126) functions to curb excessive alcohol-drinking by gating the aversiveness of high-dose alcohol intoxication.
Materials and Methods
Animals
Adult (8 weeks of age) male inbred C57BL/6J (B6) mice were purchased from Jackson Laboratories, single-housed and adapted to a temperature-controlled (25°C) and humidity-controlled (71%) colony room under a 12 h reverse light cycle (lights off at 11:00 h) for at least 2 d before surgical procedures. Heterozygous breeder pairs of transgenic mice with alanine substitution mutations at T1123 and S1126 sites on mGlu5 were obtained from the laboratory of Dr. P. F. Worley (Johns Hopkins University School of Medicine; Hu et al., 2012; Park et al., 2013) and used to generate wild-type (GRM5TS/TS), heterozygous mutant (GRM5TS/AA) and homozygous mutant (GRM5AA/AA) offspring. Heterozygous breeder pairs of Homer1-null mutant (Homer1−/− or KO) mice (Yuan et al., 2003) were also obtained from the Worley laboratory. Mice bred in-house remained multi-housed with same-sex littermates throughout experimentation unless slated (i.e., for home-cage alcohol consumption at which time they were single-housed). All studies of mutant mice used littermates from no less than three different litters and the mice were tested between 7 and 15 weeks of age. Food and water were available ad libitum, with the exception of the 2 h binge-drinking period as described in the next subsection. All studies were approved by the IACUC of the University of California Santa Barbara.
Home-cage alcohol-drinking procedures
The procedures used to determine alcohol intake under limited-access (Cozzoli et al., 2009, 2012, 2014, 2016; Lum et al., 2014), as well as both alcohol and saccharin intake under continuous-access conditions (Szumlinski et al., 2008b; Cozzoli et al., 2009; Goulding et al., 2011; Haider et al., 2015), in the home-cage were similar to those described by our group previously. Continuous-access procedures involved simultaneous presentation of four 50 ml sipper tubes containing 0, 3, 6, and 12% (v/v) alcohol or 0, 0.0625, 0.125, 0.25% (w/v) saccharin for 7 consecutive days. In both cases, the volume consumed over a 24 h period was determined by the difference in bottle weight, measured once daily (Szumlinski et al., 2005, 2008b).
For the majority of our limited-access, alcohol-drinking studies, we used modified DID binge-drinking procedures that involved the 2 h presentation of a single 50 ml sipper tube containing 20% (v/v) alcohol. This paradigm consistently generates high alcohol intakes across days and typically results in blood alcohol concentrations (BACs) ≥80% mg (Cozzoli et al., 2012, 2014, 2016; Lum et al., 2014), which satisfies the NIAAA criterion for binge-drinking. Based on prior results indicating that the effects of AAV-mediated manipulations of CeA Homer2 expression manifest only at high alcohol concentrations (Cozzoli et al., 2014), we used a similar multi-bottle DID procedure for the present AAV study in B6 mice, which involved the 2 h presentation of four 50 ml sipper tubes containing 5, 10, 20 and 40% (v/v) alcohol (Cozzoli et al., 2014; Lee et al., 2016). For both DID procedures, at 3 h after lights out, the home-cage water bottles were temporarily substituted with alcohol-containing sipper tubes for a 2 h period. As indicated above, the mice used in the immunoblotting study were presented with 20% alcohol for 30 consecutive days. In the AAV study, 5, 10, 20 and 40% alcohol was presented for 7 consecutive days. In the neuropharmacological studies, microinjections did not commence until 20% alcohol consumption stabilized (<10% variability across 3 consecutive presentations), which occurred typically within 3–5 d. All alcohol solutions were reformulated weekly and bottles refreshed as required. Sipper tubes were weighed before and immediately after each 2 h drinking session to ascertain the volume of alcohol consumed by each animal.
For all home-cage drinking experiments, subjects were weighed once a week to calculate alcohol or saccharin intake as a function of the animal's body weight (in kg).
Immunoblotting
B6 mice were killed 24 h following 30 consecutive days of alcohol or water drinking under single-bottle DID procedures (same mice as in Cozzoli et al., 2012) and the BNST was dissected out over ice. The BNST tissue was homogenized in a medium consisting of 0.32 m sucrose, 2 mm EDTA, 1% w/v SDS, 50 μm phenylmethylsulfonyl fluoride, and 1 μg/ml leupeptin, pH 7.2, and 50 mm sodium fluoride, 50 mm sodium pyrophosphate, 20 mm 2-glycerol phosphate, 1 mm p-nitrophenyl phosphate, and 2 μm microcystin-LR were included to inhibit phosphatases. Samples were then subjected to low-speed centrifugation at 10,000 × g for 20 min. Protein determinations were performed on the supernatant using the Bio-Rad DC protein assay according to the manufacturer's instructions, and homogenates were stored at −80°C until immunoblotting was completed. In this study, protein samples (5–30 μg) were exposed to SDS-PAGE and Tris-acetate gradient gels (3–8%; Invitrogen) were used for separation of all our proteins. Proteins were transferred to PVDF membranes and pre-blocked with PBS containing 0.1% (v/v) Tween 20 and either 5% (w/v) nonfat dried milk powder (for non-phosphorylated proteins) or 5% (w/v) bovine serum albumin (for all phosphorylated proteins) for at least 1 h before overnight incubation with primary antibodies. The following polyclonal antibodies were used: rabbit anti-Homer 2a/b (1:250 dilution; Abcam) and anti-Homer 1b/c (1:1000 dilution; Santa Cruz Biotechnology), mouse anti-mGlu5 (1:500 dilution; BD Transduction Laboratories), rabbit anti-GluN2a and anti-GluN2b (1:500 dilution; Calbiochem), rabbit anti-PI3K antibody (1:4000 dilution; Millipore) and rabbit anti-p(Tyr)p85 PI3K binding motif (1:250; Cell Signaling Technology), rabbit Akt (1:1000; Cell Signaling Technology) and p(Thr308)-Akt (1:500; Cell Signaling Technology), rabbit anti-ERK (1:4000) and mouse p(Tyr204)-ERK (1:2000; both from Santa Cruz Biotechnology), rabbit anti-PKCε and p(Ser729)-PKCε (1:1000; Santa Cruz Biotechnology). Membranes were washed, incubated with a horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:20,000 to 1:70,000 dilution; Millipore) or anti-mouse secondary antibody (1:20,000 to 1:70,000 dilution; Jackson ImmunoResearch) for 90 min. Immunoreactive bands were sensed by enhanced chemiluminescence using either ECL Plus (GE Healthcare) or Pierce SuperSignal West Femto (ThermoFisher Scientific). A rabbit anti-calnexin polyclonal primary antibody (Stressgen) was also used to index protein loading and transfer. The levels of immunoreactivity for all proteins were quantified with NIH ImageJ, and the immunoreactivity for each protein was first normalized to that of its appropriate calnexin signal to control for uneven protein loading/transfer and the protein/calnexin ratios of each of the samples on a gel were then normalized to the mean of the control samples for each individual gel (n = 3–4 per gel).
Surgical procedures
The surgical procedures for implanting bilateral guide cannulae (20-gauge, 10 mm long; Small Parts) into the BNST of either B6 mice or GRM5TS/TS and GRM5AA/AA mice were similar to those used in prior neuropharmacological studies by our group (Cozzoli et al., 2009, 2012, 2014, 2016; Lum et al., 2014). Mice were anesthetized by inhalation of 1–2% isoflurane with oxygen as the carrier gas and fixed to a stereotaxic device with tooth and ear bars adapted for mice. The animal's skull was exposed and leveled, and holes were drilled based on a set of coordinates starting from the bregma for the BNST with the arms at a 20° angle (AP: 0 mm; ML: + 3.00 mm; DV: −2.1 mm), according to the mouse brain atlas of Paxinos and Franklin (2004). The skull was prepared for polymer resin application, and then guide cannulae were lowered such that the tips of the cannulae were 2 mm above the intended region. A resin mound was light-cured around the guide cannulae for stabilization. The incision was closed with a tissue adhesive and dummy cannulae (24-gauge, 10 mm long) prevented cannulae blockage or contamination. After surgery, the animals are allowed to recover for a minimum of 3 d before commencing drinking procedures. The location of the microinjections was verified using standard histological approaches on Nissl-stained coronal sections. Only mice with correct placement of microinjector tips within the more dorsal aspects of the BNST were used in the statistical analyses of the results.
Intra-BNST drug infusion
The procedures to infuse pharmacological agents into the BNST of B6 mice or GRM5TS/TS/GRM5AA/AA mice were identical to those used in our previous neuropharmacological studies of binge-drinking (Cozzoli et al., 2009, 2012, 2014, 2016; Lum et al., 2014). Drugs were infused at a rate of 0.25 μl/min for 1 min (vol = 0.25 μl/side). In brief, mice were lightly restrained for removal of the dummy cannulae and sterilized microinjectors (33-gauge, threaded through a 24-gauge wire for stability, 12 mm long) were lowered bilaterally. Drugs were infused at a rate of 0.25 μl/min for 1 min (vol = 0.25 μl/side) and microinjectors left in place for an additional minute to allow for spread. Upon completion of the microinjection, injectors were removed, and the dummy cannulae replaced. For drinking studies, the mice were returned to their home cage for immediate alcohol bottle presentation. For other behavioral studies, the mice were placed immediately into the testing apparatus.
The following drugs and drug doses were infused and were selected based on their ability to inhibit binge alcohol intake or to interrupt some measure of drug reward when infused intracranially: the MEK inhibitor U0126 [1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene; 0, 0.095, 0.95 or 9.5 ng/side, Tocris Bioscience; Koya et al., 2009], the mGluR5-negative allosteric modulator MTEP [3-((2-Methyl-1,3-thiazol-4-yl)ethynyl)pyridine hydrochloride; 30 μg/side, Tocris Bioscience; Cozzoli et al., 2012, 2014], the GluN2b-containing NMDAR antagonist ifenprodil [(1R*,2S*)-erythro-2-(4-benzylpiperidino)-1-(4-hydroxyphenyl)-1-propanol hemi-tartrate; 11 ng/side, Tocris Bioscience; Wang et al., 2010], the mGlu1-negative modulator JNJ-16259685 [(3,4-dihydro-2H-pyrano[2,3-b]quinolin-7-yl)-(cis-4-methoxycyclohexyl)-methanone; 30 pg/side, Tocris Biosciences; Lum et al., 2014], the Class 1 PI3K inhibitor GDC-0941 [(2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1ylmethyl)-4-morpholin-4-yl-thieno(3,2-d)pyrimidine bismesylate; 0.58, 5.8, and 58 ng/side, Axon Medchem; Cozzoli et al., 2014], and the nonselective PI3K inhibitor wortmannin [(1S,6bR,9aS,11R,11bR)11-(acetyloxy)-1,6b,7,8,9a,10,11,11b-octahydro-1-(methoxymethyl)-9a,11b-dimethyl-3H-furo(4,3,2-de)indeno(4,5,-h)-2-h)-2-benzopyran-3,6,9-trione, 50 μg/side; Tocris Bioscience; Cozzoli et al., 2009, 2012, 2014]. To conserve animal numbers, the neuropharmacological studies used within-subjects designs, with no animal receiving more than four microinjections and with the doses counterbalanced across subjects within each treatment/genotype as detailed in the Experimental Design subsection below. No carryover effects of any manipulation were observed on intervening drinking days.
Following behavioral testing, the brains from mice tested under our neuropharmacological procedures were drop-fixed in 4% paraformaldehyde for a minimum of 7 d at room temperature. Then, the brains were sectioned (50–75 μm thick) on a vibratome at the level of the BNST and the sections mounted onto gel-coated slides. The slides were stained with cresyl violet and examined under a light microscope to determine the location of the microinjector tip. Any animals exhibiting one or both tip placements outside of the anterior-dorsal BNST were excluded from data analyses.
Intra-BNST AAV infusion
The specific details related to the construction of the AAVs carrying Homer2b and Homer1a cDNAs, as well as the AAV carrying an shRNA against Homer2b, used in this study are detailed in prior reports (Szumlinski et al., 2004, 2005, 2008b; Cozzoli et al., 2009, 2014; Goulding et al., 2011), as is the in vivo validation of the efficacy of our AAVs for altering Homer protein expression in brain (Klugmann and Szumlinski, 2008; Ary et al., 2013; Cozzoli et al., 2014; Haider et al., 2015).
Homer2b and Homer1a were expressed as an N-terminal fusion protein with the hemagglutinin (HA) tag in a recombinant AAV backbone containing the 1.1 kb cytomegalovirus immediate early enhancer/chicken β-actin (CBA) promoter (AAV–Homer2b). The same backbone encoding Renilla green fluorescent protein (hrGFP) was used as control (AAV–GFP). AAV pseudotyped vectors (virions containing a 1:1 ratio of AAV1 and AAV2 capsid proteins with AAV2 inter-trigeminal regions) were generated as described previously (Klugmann et al., 2005). Briefly, human embryonic kidney 293 cells were transfected with the AAV cis-plasmid, the AAV1 (pH21) and AAV2 (pRV1) helper plasmids (pF6), and the adenovirus helper plasmid by standard calcium phosphate transfection methods. For generation of Homer2b-specific small hairpin RNAs (shRNAs), we searched the Homer2b mRNA using an shRNA design algorithm (Ambion) and identified the targets sequences H2b#1 (5-GUGUGAAUAUGUCUCUGAGTT-3) and H2b#2 (5-CACAGAGUGCUGCCAAUGUTT-3). We generated sense and antisense oligonucleotides corresponding to these targets, annealed them, and cloned them into an AAV plasmid that simultaneously drives the expression of the shRNAs and hrGFP as described previously (Franich et al., 2008). In this cassette, shRNAs for knockdown of Homer2b were driven by the human U6 promoter inserted upstream of the CBA promoter in the AAV–GFP vector (Franich et al., 2008). This bicistronic strategy allowed for easy identification of cells transduced by the shRNA-expressing vector. Genomic titers ranged from 3.6 to 4.9 × 1011 and were determined using a Prism 7700 sequence detector system (Applied Biosystems) with primers designed to woodchuck post-transcriptional regulatory element (During et al., 2003).
Infusion of AAVs occurred in B6 mice under 1% isoflurane anesthesia at a rate of 0.05 μl/min over 5 min (total volume = 0.25 μl/side) and microinjectors were left in place for an additional 5 min postinfusion to allow for spread. Behavioral testing commenced at 3 weeks postinfusion to allow sufficient time for maximal neuronal transduction and localization of neuronal transduction by our AAVs carrying cDNA for Homer2b (cDNA-H2b) or cDNA for Homer2a (cDNA-H1a) was determined by immunohistochemical staining for the HA tag. For AAVs carrying the GFP reporter (cDNA-GFP) or an shRNA against Homer2b (shRNA-H2b), neuronal transduction and localization was examined by GFP immunofluorescence. Neuronal transduction/localization was conducted on coronal sections (50 μm) through the BNST as described previously for other brain regions (Szumlinski et al., 2004, 2005, 2008b; Cozzoli et al., 2009, 2014; Ary et al., 2013) and only animals exhibiting bilateral neuronal transduction within the anterior-dorsal BNST were included in the data analyses.
Operant responding for sucrose and alcohol
A distinct cohort of freely-fed GRM5TS/TS and GRM5AA/AA mice were assayed for sucrose and alcohol reinforcement under operant-conditioning procedures. The mice were trained in 30 min operant conditioning procedures in standard, two-lever, operant chambers, equipped with a reservoir located on the wall between the two levers into which the reinforcer solution was delivered (Med Associates). Depression of the active lever resulted in the activation of the infusion pump, delivery of 20 μl of the reinforcer solution into the reservoir and a 20 s activation of an amber stimulus light above the active lever and tone. During the 20 s cue presentation, depression of the active lever had no further consequences and throughout the session responding on the inactive lever had no programmed consequences. Mice were initially trained to lever-press for a 10% (w/v) sucrose solution under an FI20 (FR1; 20 s timeout) schedule of reinforcement until mice earned a minimum of 10 reinforcers/session with <10% variability in responding across 3 consecutive days. All subjects met this qualification within 14–21 d of training. The response requirement was then increased to FR2 and then FR5 (with 20 s timeout), with advancement requiring stable responding across 3 consecutive days (Szumlinski et al., 2005). Following lever-response training for sucrose reinforcement, alcohol was substituted for sucrose and the dose–response function for alcohol reinforcement (5, 10, 20, and 30% v/v) was determined under the FR5 reinforcement schedule with responding for each alcohol dose assessed across 5–7 d and the average of the last 3 d of responding for each dose was calculated for each animal and used in the statistical analyses of the data.
Blood alcohol concentrations
BACs were determined in GRM5TS/TS and GRM5AA/AA mice following their fifth alcohol presentation under DID procedures to determine the correlation between intake and BAC (Cozzoli et al., 2016). For this, blood (75–100 μl) was sampled by submandibular vein puncture immediately upon bottle removal. To relate the excessive alcohol-drinking phenotype exhibited by GRM5AA/AA mice to alcohol metabolism, GRM5TS/TS and GRM5AA/AA mice were injected intraperitoneally with 3.0 g/kg alcohol. As in our prior study of Homer2 knock-out mice (Szumlinski et al., 2005), blood was sampled from the infraorbital sinus at 5, 15, and 30 min after the alcohol injection. In both studies, plasma was assayed using an Analox Analyzer according to the manufacturer's instructions.
Assays of the sedative-hypnotic effects of alcohol
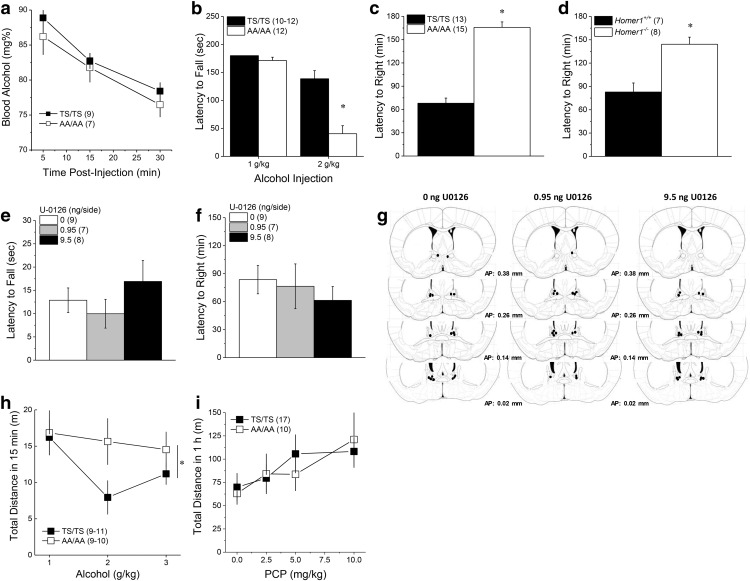
To determine the effects of the mGlu5 phospho-mutation upon indices of the sedative-hypnotic effects of alcohol, the intoxication expressed by GRM5TS/TS and GRM5AA/AA mice were compared using a fixed-speed (10 rpm) rotarod assay (IIT Life Science). An identical rotarod procedure was used in male B6 mice to determine the effects of locally inhibiting ERK within the BNST upon alcohol-induced motor incoordination, with the exception that mice were infused with different doses of the MEK1/2 inhibitor U0126 before testing. The rotarod procedures used were identical to those described previously by our group (Quadir et al., 2016, 2017). GRM5TS/TS, GRM5AA/AA or B6 mice were initially habituated to walking on the apparatus in a 2 min session during which mice were placed immediately back onto the rotarod if they fell. Following habituation, mice underwent a series of 3 min training trials, during which mice remained on the floor of the apparatus if they fell until the next trial began. When an animal could remain on the rotarod for three 3 min trials, it was considered “trained” and this was observed within 3–4 training sessions with no genotypic or sex differences. In the study of the phospho-mutant mice, male and female animals were injected intraperitoneally with either 1 or 2 g/kg alcohol and ∼15 min postinjection, each mouse was subjected to three 3 min rotarod testing trials (30 s apart), during which a mouse was left on the floor of the apparatus if it fell until the next trial began and the latency of each fall was recorded. In the neuropharmacological study, male B6 mice were microinjected with either 0, 0.95, or 9.50 ng/side of U0126, before an intraperitoneal injection of 2 g/kg alcohol. Then ∼15 min postinjection, the mice were tested in the same manner as the mutant animals. The average latency to fall from the three test sessions was used as an index of alcohol intoxication.
We also used a regain of righting reflex procedure to examine for genotypic differences in the sedative effect of high-dose alcohol, as well as the effects of ERK inhibition upon alcohol-induced sedation. As conducted in prior studies of Homer2 knock-out mice (Szumlinski et al., 2005), a separate cohort of male and female GRM5TS/TS and GRM5AA/AA mice were injected intraperitoneally with 5 g/kg alcohol and immediately placed in empty home cages. Once immobile (∼2–3 min), the mice were placed onto their backs for the duration of the study. The time taken for the animals to right themselves and place all four paws on the floor of the testing cage was determined by behavioral observation using a stopwatch. To probe a working model in which the facilitation of mGlu5-Homer crosslinking functions to reduce sensitivity to the sedative-hypnotic effects of alcohol (Szumlinski et al., 2005, 2008a), we compared also male Homer1+/+ and Homer1−/− mice for their ability to regain their righting reflex using identical procedures. Finally, to test the hypothesis that ERK-dependent signaling within the BNST regulates the sedative hypnotic effects of alcohol, we also assayed the effects of infusing 0, 0.95, or 9.5 ng/side U0126 upon the ability of B6 mice to regain their righting reflex. For this latter experiment, mice were microinjected with U0126 before intraperitoneal alcohol injection and behavioral testing.
Place-conditioning and locomotor activity
To determine genotypic differences in the perception of alcohol's interoceptive effects as appetitive or aversive, we conducted an alcohol-induced place-conditioning study in GRM5TS/TS, GRM5TS/AA, and GRM5AA/AA male and female mice. For alcohol-induced place-conditioning, distinct groups of animals received intraperitoneal injections of alcohol (1, 2, or 3 g/kg; vol = 20 ml/kg; Szumlinski et al., 2008b). To determine whether or not the effects of the mGlu5 mutation on alcohol-conditioning generalized to a different drug of abuse, a distinct cohort of male and female GRM5TS/TS, GRM5TS/AA and GRM5AA/AA mice underwent cocaine place-conditioning procedures (3, 10, or 30 mg/kg; vol = 10 ml/kg; a generous gift from National Institute on Drug Abuse, Bethesda, MD; Ary et al., 2013). To localize the effects of interrupting ERK signaling upon alcohol aversion to the BNST, male B6 mice were conditioned with 3 g/kg alcohol using procedures identical to those used in the study of the phospho-mutant mice. However, before testing for the conditioned response, mice were microinjected with either 0 or 9.5 ng/side U016.
The apparatus and procedures to induce place-conditioning were similar to those described in published work (Szumlinski et al., 2008a; Ary et al., 2013). To elicit place-conditioning, mice were conditioned in a two-compartment apparatus where the compartments were tactilely (floor texture) and visually (wall pattern) distinct and the time spent in each compartment was recorded using a digital video-tracking system (ANY Maze, Stoelting). Conditioning commenced with a 15 min habituation session to familiarize the animals to the entire apparatus. For each conditioning session, mice were confined to one compartment for 15 min. On alternating days, the mice were injected with an equivalent volume of saline and confined for 15 min to the other compartment. The day following the final pairing (8 pairings for alcohol; 4 pairings for cocaine), animals again had ad libitum access to the entire apparatus in a drug-free state during a 15 min post-conditioning test. The amount of time spent on the drug-paired versus saline-paired compartment during the post-conditioning test served to index the direction and magnitude of the conditioned response.
The locomotor activity exhibited by the mice during the alcohol-conditioning sessions was also recorded and the distance traveled during the first conditioning session (i.e., acute locomotor response to alcohol) and the change in locomotor activity with repeated alcohol-pairing (i.e., locomotor sensitization) were determined. The data pertaining to genotypic differences in cocaine-induced locomotion were published by Park et al. (2013) and thus, not presented herein.
PCP-induced locomotion
To determine whether or not the genotypic difference in alcohol-induced locomotion generalized to another nonselective NMDA receptor antagonist, we determined the dose–response function for phencyclidine (PCP)-induced locomotion using a within-subjects design. For this, a separate cohort of GRM5TS/TS and GRM5AA/AA mice were injected intraperitoneally, once daily, with increasing doses of PCP (0, 0.25, 0.5, and 1.0 mg/kg) and mice were placed in locomotor activity chambers where their distance traveled (in meters) was recorded for 1 h using digital video-tracking (ANY Maze, Stoelting).
Behavioral screen for negative affect
Negative affect is an endophenotype associated with the propensity to consume excessive amounts of alcohol (Blaine and Sinha, 2017; cf. Becker, 2017). As GRM5AA/AA mice exhibited an excessive alcohol-drinking phenotype (see Results), we assayed the behavior of male and female GRM5TS/TS, GRM5 TA/AA, and GRM5 AA/AA mice in forced swim, reactivity to novelty, and elevated plus-maze tests using procedures identical to those published previously by our group (Szumlinski et al., 2004, 2005; Ary et al., 2013; Guo et al., 2016). Mice were assayed in all of the above tests, with the order of testing randomized across cohorts of animals to avoid carryover effects. To localize the effects of interrupting ERK signaling on negative affect to the BNST, a cohort of male B6 mice were microinjected with 0, 0.95, or 9.5 ng/side U0126 and then assayed, in random order, for behavior in the reactivity to novelty and elevated plus-maze tests, before testing in the forced swim test. The specific details of each procedure is described below.
Porsolt/forced swim test.
“Behavioral despair” was assessed via a Porsolt swim test, where floating serves as a measure of learned helplessness (Porsolt et al., 2001). As previously described by our group (Ary et al., 2013), mice were placed into a pool (30 cm in diameter; 45 cm high) filled with room-temperature water up to 35 cm and allowed to swim for a total of 15 min. In 30 s intervals, behavior was scored by an experimenter as either swimming (front and/or back paws mobile), floating (both front and back paws immobile), or climbing (swimming with forepaws making contact with the walls of the pool). Latency to first float was also determined using a stopwatch.
Reactivity to a novel object.
To assess for effects of our manipulations on neophobia-related anxiety, a novel-object test was conducted. Mice were placed into an activity monitor containing one small, inedible object for 2 min. Animals were allowed to explore the object in the tub and both the number of contacts the mice made with the object and the amount of time the mice spent investigating the object (as defined by forepaw or snout contact with an object) were recorded by an experimenter.
Elevated plus maze.
Animals were placed on the center intersection of a four-arm radial plus maze with two white open arms and two black walled arms 24 cm high. Each arm measured 123 cm long × 5 cm wide. Latency to first open-arm entry, number of open-arm entries, and total time spent in an open arm were monitored for the 4 min trial by a trained observer who was blind to the drinking history of the mice. Differences in the amount of time spent in an open versus enclosed arm were also used to assess anxiety.
Experimental design and statistical analysis
Immunoblotting.
For the initial immunoblotting studies, the effects of binge-drinking on protein expression and indices of kinase activation were determined using two-tailed t tests conducted on the calnexin-normalized data between male water-drinking B6 mice and male B6 mice with a 30 d history of drinking under DID procedures.
AAV-mediated changes in Homer2-scaffolding within BNST.
To determine the effects of mimicking the alcohol-induced increase in BNST Homer2 expression (using cDNA-H2b) and the effects of disrupting Homer2 scaffolding within the BNST (using shRNA-H2b or cDNA-Homer1a), the data from male B6 mice infused with these AAV constructs were compared with those from mice infused with a GFP control AAV. This comparison was evaluated using ANOVAs, with the between-subjects factor of AAV (GFP, cDNA-H2b, shRNA-H2b, cDNA-H1a) and the within-subjects factor of Injection (for locomotion; 2 levels: 0 vs 3 g/kg) or Concentration (for drinking; 4 levels: 5, 10, 20, and 40% v/v). This latter analysis was used to examine AAV effects on Day 1 of binge-drinking to index the initiation of binge-drinking, as well as on the average alcohol intake from each concentration over the course of binge-drinking. One-way ANOVAs across the between-subjects factor of AAV were used to examine the effects of altering Homer2 scaffolding upon alcohol intake under continuous-access conditions and an AAV by Saccharin Concentration ANOVA was used to examine the effects of our AAV manipulations upon the dose-intake function on Day 1 of saccharin availability, as well as for the average saccharin intake. The AAV experiments commenced with n = 10–12/AAV and the final sample sizes ranged from n = 9–11 as a result of subject attrition because of transduction failure, inability to localize microinjector tip in BNST tissue or the development of illness that did not respond to nursing care.
Transgenic studies of the behavioral effect of blocking ERK-dependent phosphorylation of mGluR5 using GRM5TS/TS, GRM5TS/AA, and GRM5AA/AA−mice.
To determine the role played by ERK-dependent phosphorylation of mGlu5 in regulating alcohol intake and alcoholism-related behavior, a series of experiments compared the phenotypes of GRM5TS/TS, GRM5TS/AA, and GRM5AA/AA mice. Additional experiments examined for the generalization of the GRM5AA/AA phenotype to either other drugs of abuse or to non-drug rewards/reinforcers. All of these experiments used approximately equal numbers of male and female mice (±2) from each genotype and all initial analyses included Sex as a between-subjects factor. However, with the exception of the elevated plus maze, analyses did not reveal any significant interactions between the Sex and Genotype factors. Thus, with the exception of the data for the elevated plus maze, the data were collapsed across Sex and analyzed for Genotype effects and interactions as detailed in the paragraph below.
We first assayed mGlu5 mutant mice for alcohol and saccharin intake using simple home-cage drinking procedures. The data generated from these experiments were analyzed using a mixed-model ANOVA, with the between-subjects factor of Genotype (2 or 3 levels, depending upon the specific experiment) and the within-subjects factors of Concentration (3–4 levels, depending on the specific experiment). We next compared for genotypic differences in alcohol and saccharin self-administration under operant-conditioning procedures to index motivation for alcohol and a non-alcohol reinforcer, respectively. These data were analyzed also by a mixed model ANOVA, with the between-subjects factor of Genotype (GRM5TS/TS, GRM5TS/AA, GRM5AA/AA) and the within-subjects factors of Concentration (3–4 levels, depending on the reinforcer) or Lever (active vs inactive). We then examined for genotypic differences in alcohol metabolism using a Genotype (GRM5TS/TS vs GRM5AA/AA) by Time (5, 15, 30 min) ANOVA, with repeated measures of the Time factor. We related the alcohol-drinking phenotype of GRM5TS/TS and GRM5AA/AA mice to their sensitivity to alcohol intoxication using a rotarod assay. Mice were injected with one of two alcohol doses in this test and the data were analyzed using a between-subjects Genotype (GRM5TS/TS vs GRM5AA/AA) × Dose (1 vs 2 g/kg alcohol) ANOVA. The role for mGlu5-Homer cross-linking in alcohol-induced sedation was tested by comparing the amount of time taken by GRM5TS/TS versus GRM5AA/AA and by Homer1+/+ and Homer1−/− mice to regain their righting reflex and the data were analyzed separately for each genotype using two-tailed t tests. To relate the differences between GRM5TS/TS and GRM5AA/AA mice in alcohol-induced sedation to their perception of alcohol's effects as appetitive or aversive, the dose–response function for alcohol-induced place-conditioning was determined. To examine for the generalization of alcohol-conditioning effects to a different drug of abuse, a separate cohort of mice were tested for cocaine-induced place-conditioning. In both experiments, the time spent in the drug-paired versus -unpaired compartment was analyzed using a mixed-model Genotype (2 or 3 levels, depending on drug) × Dose (3 levels) × Side (paired vs unpaired) ANOVA, with repeated measures on the Side factor, followed by t test comparisons of time spent in each compartment to determine the presence/absence of a conditioned response.
To examine for genotypic differences in the locomotor response to the acute versus repeated injection with the various alcohol doses, mixed model Genotype × Dose × Injection ANOVA was conducted, with repeated measures on the Injection factor (8 pairings/injections). As this analysis failed to indicate any injection-dependent change in locomotion, the data for Injection 1 was reanalyzed alone to index genotypic differences in the acute locomotor response to alcohol. The locomotor response to cocaine was published previously by Park et al. (2013). To determine whether or not the genotypic difference in alcohol-induced locomotion generalized to another nonselective NMDA receptor antagonist, a within-subjects design was used to generate a dose–response function for PCP-induced locomotion. Thus, these data were analyzed using a Genotype (GRM5TS/TS vs GRM5AA/AA) by Dose (0, 0.25, 0.5, 1.0 mg/kg) ANOVA, with repeated measures on the Dose factor.
To relate the alcohol-drinking phenotype of GRM5TS/TS and GRM5AA/AA mice to endophenotypes associated with alcoholism, mice were subjected to a behavioral test battery that included various tests of negative affect (e.g., Porsolt forced swim test, elevated plus maze, novel object encounter). The data from these assays were analyzed using univariate ANOVAs along the Genotype factor (GRM5TS/TS, GRM5TS/AA, and GRM5AA/AA).
Neuropharmacological studies in B6 and mGlu5 phospho-mutant mice.
To test the functional relevance of alcohol-induced changes in BNST ERK and PI3K activity for binge-drinking by male B6 mice and to examine the BNST as a locus underpinning the potentiating effects of mutations of T1123 and S1126 on mGlu5 upon alcohol intake, a series of distinct neuropharmacological studies were conducted. The effects of our neuropharmacological manipulations on the binge-alcohol intake of B6 mice were analyzed separately for each drug using repeated-measures ANOVAs across the dose factor (2–4 levels, depending upon the specific experiment), whereas those conducted on GRM5 mutant mice included the additional between-subjects factor of Genotype (GRMTS/TS vs GRM5AA/AA). The initial sample sizes for the neuropharmacological studies of the mGlu5 wild-type and mutant mice were Finally, to confirm ERK-dependent signaling within the BNST in regulating basal anxiety-like behavior, as well as the sedative-hypnotic and rewarding properties of alcohol, a series of neuropharmacological studies were conducted in male B6 mice and the effects of our neuropharmacological manipulations were analyzed using ANOVA with the between-subjects factors of U0126 dose (2–3 levels, depending upon the experiment).
For all ANOVAs, significant interactions were deconstructed along the relevant factor to examine for main effects and group differences followed-up using LSD post hoc tests or t tests, when appropriate. All analyses were two-tailed and α = 0.05.
Results
Alcohol increases Homer2 expression in the BNST
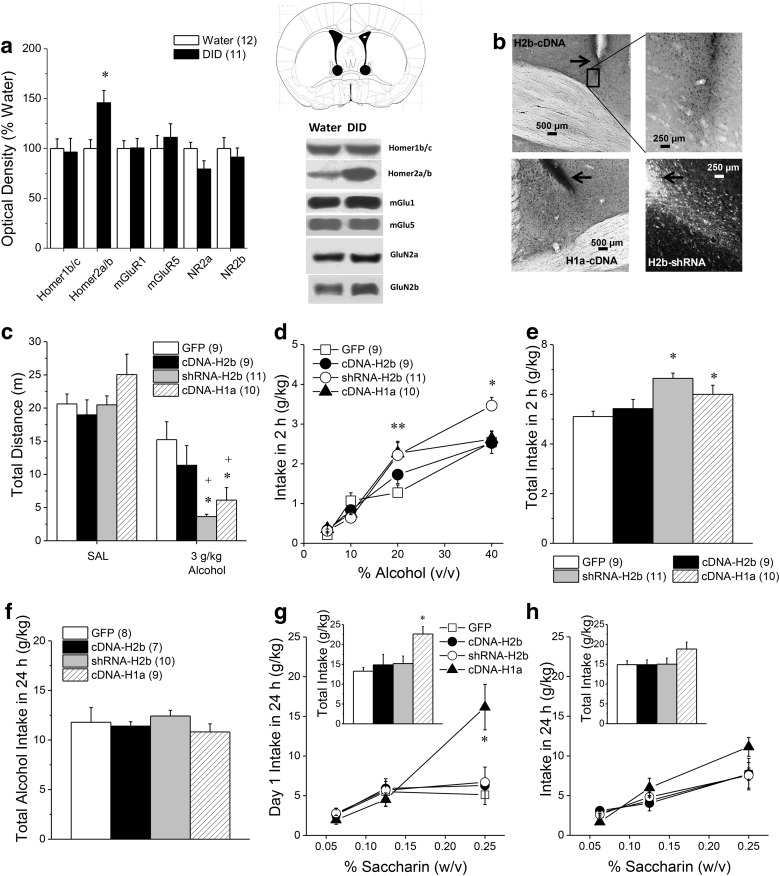
We first immunoblotted for changes in glutamate receptor-related proteins in BNST tissue obtained from B6 mice with a history of binge-drinking under DID procedures (average daily intake = 4.4 g/kg; range = 3.0–6.5 g/kg; same as the mice used by Cozzoli et al., 2012, 2014, 2016). Homer2a/b expression was elevated by ∼50% within the BNST of alcohol-binge drinking mice versus water-drinking controls (Fig. 1a; t(20) = 3.11, p = 0.005). However, in contrast to prior results for the NAsh and CeA (Cozzoli et al., 2012, 2014; Lum et al., 2014), we detected no binge-induced change in BNST levels of Homer1b/c, mGlu1/5 or GluN2a/b (Fig. 1a; t tests, all p values >0.15).
Figure 1.
Disruption of Homer2 scaffolding within the BNST promotes behavioral sensitivity to high-dose alcohol. a, Relative to water-drinking controls (Water), mice with a 30 d history of 20% (v/v) alcohol intake under 2 h DID procedures exhibited elevated protein expression of Homer2a/b within BNST, but no change in Homer1b/c, mGlu1/5, or GluN2a/b levels. *p < 0.05 versus water (t tests). Representative immunoblots are also shown for the proteins examined and a diagram depicting the tissue dissection within the anterior-dorsal BNST. b, Representative micrographs of immunostaining of the HA tag within the BNST of B6 mice infused with an AAV carrying Homer2b cDNA (cDNA-H2b) or with an AAV carrying Homer1a cDNA (cDNA-H1a), as well as immunofluorescence of the GFP tag in mice infused with an AAV carrying an shRNA against Homer2b (shRNA-H2b). Arrows in b depict the tip of the microinjector. c, Intra-BNST infusion of shRNA-H2b or cDNA-H1a augmented the motor-impairing effects of an acute intraperitoneal injection of 3 g/kg alcohol, whereas no saline-alcohol differences in locomotion were observed in GFP or cDNA-H2b mice. d, Examination of the alcohol dose-intake function under 2 h DID procedures revealed a shift upward in shRNA-H2b and cDNA-H1a mice, relative to GFP controls, with both shRNA-H2b and cDNA-H1a elevating the intake of 20% alcohol and shRNA-H2b elevating the intake of 40% alcohol. e, Thus, the average total alcohol intake under 2 h DID procedures was greater in shRNA-H2b and cDNA-H1a mice versus controls. f, In contrast, intra-BNST AAV infusion did not influence the average total alcohol intake when alcohol was made continuously available in the home cage. Sample sizes are for a–f are indicated in parentheses in their corresponding panels. g, However, when saccharin was substituted for alcohol, the cDNA-H1a mice exhibited a shift upward in the saccharin dose-intake function (0.0625, 0.125, 0.25%), as well as greater total saccharin intake (inset), when assessed on Day 1 of continuous access procedures. h, However, intra-BNST AAV infusion did not influence significantly the dose–response function for the average saccharin intake when the data from the entire 7 d drinking period was considered, nor did AAV infusion alter total saccharin intake (inset). The sample sizes for g and h are indicated in parentheses in f because the same animals were tested for alcohol and saccharin consumption under continuous-access procedures. *p < 0.05 versus GFP; **both groups p < 0.05 versus GFP (LSD post hoc tests), +p < 0.05 versus saline (t tests).
Interrupting homer crosslinking in the BNST promotes alcohol sensitivity and binge-intake
Homer2 expression within NAsh and CeA is required for the manifestation of binge-drinking (Cozzoli et al., 2009, 2012, 2014), and Homer2 in NAsh regulates alcohol intoxication (Szumlinski et al., 2005, 2008a). Thus, we tested the functional relevance of BNST Homer2 for alcohol-related behaviors using AAV-mediated gene delivery approaches. Despite evidence for robust neuronal transduction (Fig. 1b), intra-BNST infusion of an AAV carrying Homer2b cDNA (cDNA-H2b) did not influence alcohol-related behavior in any of our assays (Fig. 1c–e). In contrast, disrupting Homer2 expression via infusion of an AAV carrying an shRNA against Homer2b (shRNA-H2b) augmented behavioral sensitivity to high-dose alcohol, as evidenced by greater alcohol-induced inhibition of locomotor activity upon bolus administration (Fig. 1c; AAV × Injection: F(3,35) = 2.92, p = 0.04; one-way ANOVA for 0 g/kg alcohol, p = 0.79; one-way ANOVA for 3.0 g/kg; F(3,33) = 5.34, p = 0.01, LSD post hoc tests, p values < 0.05). Further, examination of the dose–response function for binge-alcohol intake indicated that disrupting Homer2 expression elevated the binge-intake of high-dose alcohol (Fig. 1d; AAV × Concentration: F(9,90) = 2.66, p = 0.009), and this effect was observed even on the first day of binge-drinking (data not shown; AAV effect: F(3,30) = 3.50, p = 0.03; Dose effect: F(3,90) = 94.77, p < 0.0001; AAV × Dose, p = 0.36). Deconstruction of the interaction for the average alcohol intake along with Concentration factor revealed that both shRNA-H2b and cDNA-H1a elevated the intake of 20% alcohol (Fig. 1d; one-way ANOVA: F(3,33) = 2.91, p = 0.05; LSD post hoc tests, p values <0.05) and shRNA-H2b elevated the intake of 40% alcohol (Fig. 1d; one-way ANOVA: F(3,33) = 4.16, p = 0.02; LSD post hoc tests, p = 0.04). As such, the average total alcohol intake under 2 h DID procedures was greater in shRNA-H2b and cDNA-H1a mice, versus GFP controls and cDNA-H2b mice (Fig. 1e; one-way ANOVA: F(3,30) = 5.49, p = 0.004; LSD post hoc tests, p values <0.05).
Constitutively expressed Homer proteins, including Homer2, possess two functional domains: (1) an N-terminal EVH1 domain mediates binding to proline-rich sequences in Group 1 mGluRs, as well as intracellular ion channels, TrpC channels, and potassium channels; and (2) a C-terminal coiled coil/leucine zipper domain that mediates oligomerization (cf. Shiraishi-Yamaguchi and Furuichi, 2007; Constantin, 2016). Accordingly, Homer2 can bind and crosslink signaling proteins to enhance transduction (Kammermeier et al., 2000). The immediate early gene form of Homer1 (Homer1a) possesses only the N-terminal EVH1 domain and functions as a natural dominant-negative to disrupt crosslinking. AAV expression of Homer1a cDNA in the BNST mimicked Homer2 knockdown (Fig. 1c–e). Thus, interrupting Homer2 crosslinking within the BNST enhances both the initiation and maintenance of binge-drinking, as well as sensitivity to alcohol's sedative effects.
The effects of interrupting Homer2 crosslinking in the BNST are selective for binge alcohol-drinking procedures
AAV-mediated disruption of Homer2 scaffolding within the BNST did not influence alcohol intake when alcohol became continuously available concurrently with water (Fig. 1f; one-way ANOVA, p = 0.79), nor did it influence water intake (data not shown). When saccharin was substituted for alcohol, fluid intake increased, but only in cDNA-H1a mice and this effect was only apparent on the first day of saccharin availability (Fig. 1g,h; for Day 1: Saccharin Concentration effect: F(2,60) = 11.95, p < 0.0001; AAV effect: F(3,30) = 4.92, p = 0.007; AAV × Saccharin Concentration: F(6,60) = 3.52, p = 0.005; 7 d average: AAV × Saccharin Concentration ANOVA, all p values >0.15). This saccharin effect contrasts with the cDNA-H1a effect upon binge-alcohol intake, which remained elevated in cDNA-H1a mice throughout study (Fig. 1d).
These results demonstrate the behavioral specificity of the effect of disrupting BNST Homer2 crosslinking for binge alcohol-drinking and argue that the potentiation of binge-drinking does not reflect general changes in alcohol taste-reactivity or a dysregulation of factors influencing the oral consumption of natural reinforcers (e.g., thirst/osmotic balance).
Inhibiting ERK activity within the BNST promotes binge-drinking
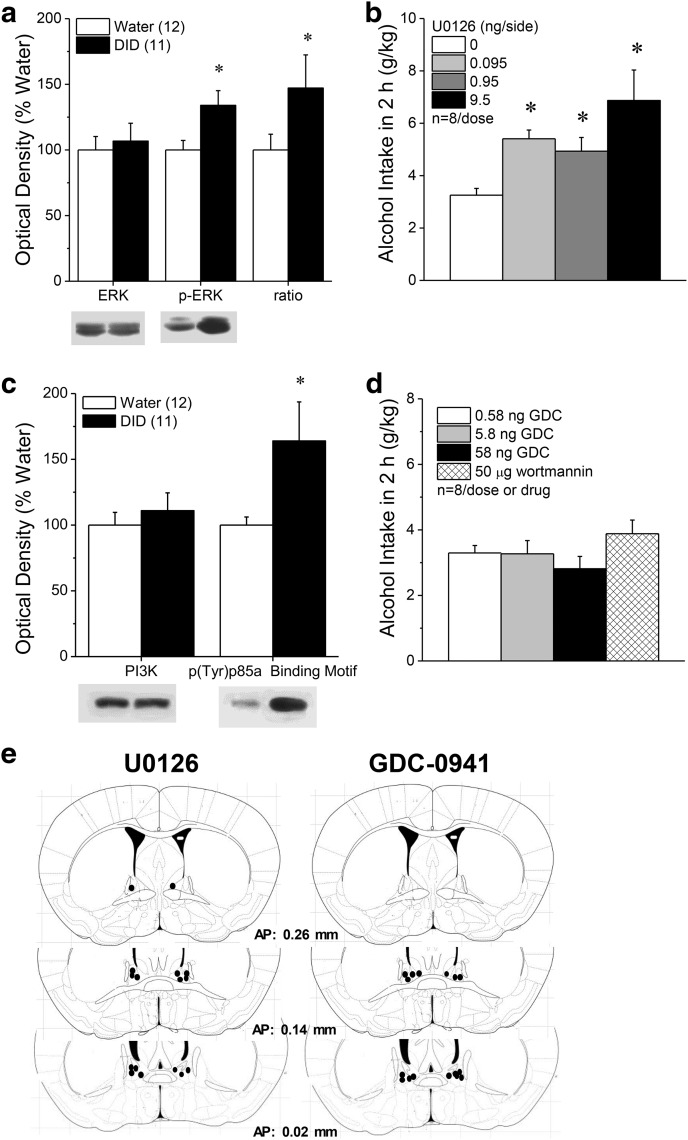
ERK activity in the BNST is induced by acute oral alcohol consumption (cf. Zamora-Martinez and Edwards, 2014), and systemic ERK inhibitors increase oral alcohol self-administration (Faccidomo et al., 2009). Immunoblotting BNST tissue for ERK(pY204) revealed increased ERK activity in B6 mice with a month-long history of binge-drinking [Fig. 2a; phospho-(Tyr204)ERK (p-ERK): t(21) = 2.60, p = 0.02; p-ERK–ERK ratio: t(21) = 2.38, p = 0.04], with no change in total ERK (t test, p > 0.35). Examining the potential relevance of ERK activity in the BNST for binge-drinking, the localized infusion of the selective MEK1/2 inhibitor U0126 dose-dependently augmented binge-alcohol intake in B6 mice (Fig. 2b; F(3,18) = 5.68, p = 0.006; n = 7; post hoc t tests).
Figure 2.
Inhibition of ERK within the BNST promotes binge-drinking. a, Relative to water-drinking controls (Water), mice with a 30 d history of binge-drinking under modified DID procedures exhibited higher total and relative expression of p-ERK within the BNST, with no change in total ERK. b, Intra-BNST infusions (0.25 μl/side) of the ERK inhibitor, U0126, dose-dependently elevated binge-alcohol intake under single-bottle DID procedures, with significant U0126 effects observed at all doses tested (*p < 0.05 vs 0 ng/side, post hoc t tests). c, Chronic binge-drinking also increased the expression of the PI3K activation marker p(Tyr)p85α PI3K binding motif within BNST tissue, without influencing total PI3K expression (t test, p > 0.60). d, However, intra-BNST fusions of the PI3K antagonists GDC-0941 or wortmannin did not impact binge-drinking under our single-bottle DID procedures in B6 mice. e, Diagram summarizing locations of the microinjector tips within the BNST of the B6 mice infused with U0126 versus those infused with GDC-0941. Sample sizes used in the statistical analyses of the immunoblotting data are indicated in parentheses in their respective panels.
To probe the selectivity of the ERK effect, we indexed the relative activity of several other kinases implicated in the neurobiology of binge-drinking, including PI3K, Akt and PKCε in this BNST tissue (Cozzoli et al., 2009, 2012, 2014, 2016; Lum et al., 2014). We detected increased expression of p(Y)p85α PI3K binding motif (Fig. 2c; t(19) = 2.18, p = 0.04), with no effect on total PI3K expression (t test, p > 0.60), suggesting increased PI3K activation (Zhang et al., 2006). However, BNST infusion of the PI3K inhibitors GDC-0941 and wortmannin did not alter binge-alcohol intake in B6 mice (Fig. 2d), despite localization of the microinjector tips within the BNST (Fig. 2e). Finally, in contrast to ERK and PI3K, we detected no significant changes in the activational state of Akt or PKCε within the BNST (Table 1; t tests, all p values >0.35). From these data, we conclude that ERK activation within BNST is part of a natural adaptation that selectively suppresses binge-drinking.
Table 1.
Summary of the immunoblotting results for the expression and activational state of Akt and PKCϵ within the BNST
| Water (12) | DID (11) | Water DID | |
|---|---|---|---|
| Akt | 100 ± 8.2 | 93.5 ± 8.7 |  |
| p(Ser473)-Akt | 100 ± 11.1 | 95.0 ± 8.6 |  |
| p-Akt:Akt ratio | 100 ± 12.4 | 109.4 ± 19.7 | |
| PKCϵ | 100 ± 8.4 | 108.3 ± 13.2 |  |
| p(Ser729)-PKCϵ | 100 ± 14.0 | 114.7 ± 15.1 |  |
| p-PKCϵ:PKCϵ ratio | 100 ± 13.4 | 117.5 ± 20.4 |
Analysis failed to indicate any significant group differences. Sample sizes are indicated in parentheses with representative immunoblots presented in each of the corresponding cells.
Interrupting mGluR5 phosphorylation increases alcohol consumption
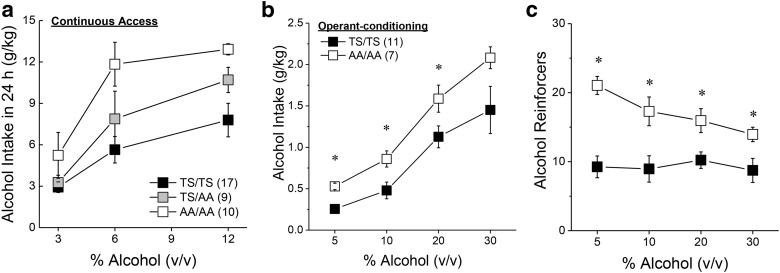
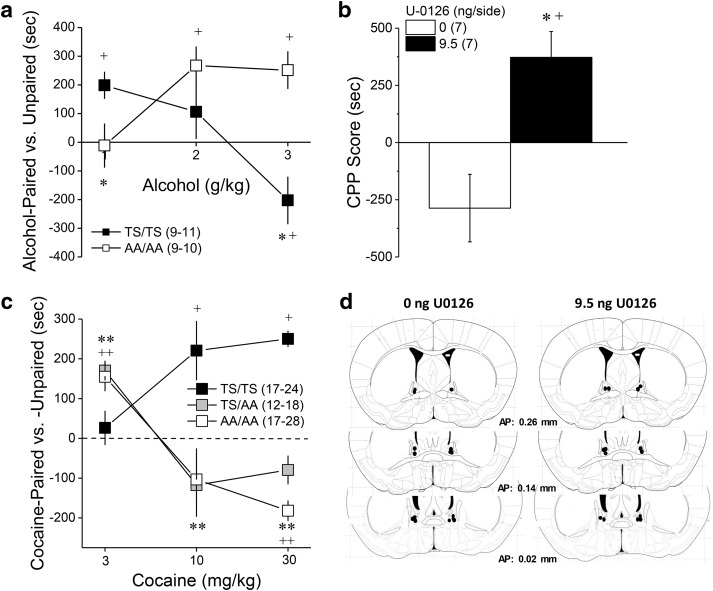
ERK phosphorylates many proteins including mGlu5(T1123/S1126) (Beneken et al., 2000; Orlando et al., 2009; Hu et al., 2012; Park et al., 2013), which increases the affinity of Homer EVH1 binding by as much as 40-fold (Park et al., 2013). Accordingly, we determined whether mGlu5(T1123/S1126) might be a target of ERK involved in suppressing binge-drinking by characterizing the alcohol phenotype of mice heterozygous (GRM5TS/AA) or homozygous (GRM5AA/AA) for alanine substitutions at T1123 and S1126 of mGlu5 (Park et al., 2013). We first examined alcohol preference and intake under simple, continuous-access procedures and observed a genotype-dependent increase in alcohol consumption (Fig. 3a; Genotype effect: F(1,33) = 7.70, p = 0.002; Genotype × Concentration: p = 0.16), without effects on water intake (data not shown; Genotype effect: p = 0.24). The elevated alcohol intake of GRM5AA/AA mice extended to alcohol reinforcement as indicated by greater average alcohol intake (Fig. 3b; Genotype effect: F(1,16)) = 4.53, p = 0.04; Genotype × Concentration: p = 0.08), and a greater number of alcohol reinforcers earned (Fig. 3c; Genotype effect: F(1,16) = 6.77, p = 0.02; Genotype × Lever: F(1,48) = 5.17, p = 0.04; all other p values >0.05), by GRM5AA/AA versus GRM5TS/TS mice when tested under operant-conditioning procedures. These data provide initial evidence that ERK-dependent phosphorylation of mGlu5(T1123/S1126) normally functions to curb alcohol intake.
Figure 3.
Alcohol drinking phenotype of GRM5AA/AA mice. a, Relative to wild-type controls (TS/TS), the dose–response function for the average alcohol intake under continuous-access conditions in the home cage was shifted upward in a genotype-dependent manner in male and female mice heterozygous (TS/AA) or homozygous (AA/AA) for point mutations at T1123 and S1126 on mGlu5. b, When required to lever-press for delivery of alcohol under an FR5 reinforcement schedule, male AA/AA mice exhibited higher alcohol intake than TS/TS controls. c, AA/AA mice consistently earned a greater number of alcohol reinforcers than TS/TS controls, regardless of the dose of alcohol available. Sample sizes are indicated in parentheses. *p < 0.05 versus TS/TS.
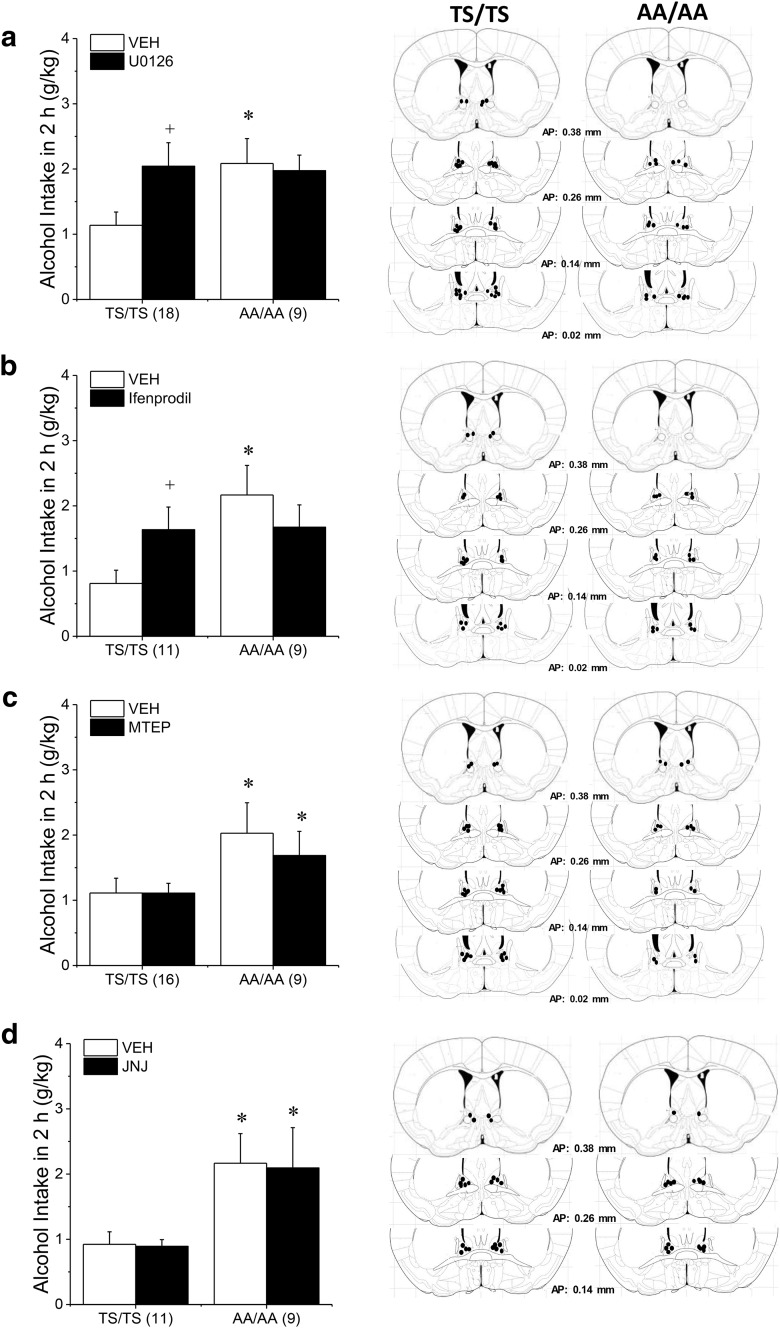
We next determined whether the BNST as a neural locus of underpinning the excessive alcohol-drinking of GRM5AA/AA mice, we performed a series of neuropharmacological experiments under DID procedures. Consistent with their B6.129 hybrid genetic background, GRM5TS/TS mice consumed less alcohol under single-bottle DID procedures than did isogenic B6 mice (Figs. 4a–d vs 1e). When sampled in a subset of GRM5TS/TS and GRM5AA/AA mice, BACs were positively correlated with intake (r = 0.77, p < 0.0001; N = 16); however, the BACs attained were well below the 80 mg% criterion for binge-drinking (GRM5TS/TS: 15.9 ± 2.7 mg%, n = 7; GRM5AA/AA: 54.7 ± 18.1 mg%, n = 9). However, vehicle-infused GRM5AA/AA mice consistently exhibited higher alcohol intake under DID procedures than their GRM5TS/TS counterparts (Fig. 4a–d), indicating that the higher propensity of GRM5AA/AA mice to drink alcohol (Fig. 3a,b) extends across models. Intra-BNST infusion of the ERK inhibitor U0126 potentiated alcohol intake in GRM5TS/TS but not GRM5AA/AA mice (Fig. 4a; Genotype × Infusion: F(1,27) = 4.41, p = 0.04; post hoc t tests, for TS/TS: t(17) = 2.85, p = 0.01; for AA/AA: p > 0.25). ERK is typically activated by NMDA receptor signaling (cf. Zamora-Martinez and Edwards, 2014), which is implicated in alcohol-induced neuroplasticity within BNST (Kash, 2012; Wills et al., 2012; Zamora-Martinez and Edwards 2014; Lovinger and Kash, 2015). BNST infusion of GluN2B selective NMDAR antagonist ifenprodil also increased alcohol consumption in GRM5TS/TS, but not GRM5AA/AA mice (Fig. 4b; Genotype × Infusion: F(1,18) = 5.46, p = 0.03; post hoc t tests, for TS/TS: t(10) = 2.26, p = 0.05; for AA/AA: p > 0.25). In contrast, an intra-BNST infusion of 30 μg/side of the mGlu5-negative allosteric modulator MTEP (Fig. 4c; Genotype effect: F(1,23) = 4.63, p = 0.04; no Infusion effect or interaction, p values > 0.40) or of 30 pg/side of the mGlu1-negative modular JNJ 16259685 (Fig. 4d; Genotype effect: F(1,18) = 6.77, p = 0.02; no Infusion effect or interaction, p > 0.10) were without effect in either genotype.
Figure 4.
The GRM5AA/AA mutation occludes the effects of intra-BNST ERK and GluN2b inhibition upon binge-drinking. a, Vehicle (VEH)-infused AA/AA mice exhibited higher alcohol intake under single-bottle DID procedures than TS/TS controls and were insensitive to the increase in alcohol intake produced by an intra-BNST infusion of 9.5 ng/side of the ERK inhibitor U-0126. b, A similar genotypic difference was also noted regarding the potentiation of drinking produced by an intra-BNST infusion of 11 ng/side of the GluN2B-containing NMDAR antagonist ifenprodil. c, In contrast, an intra-BNST infusion of 30 μg/side of the mGlu5-negative allosteric modulator, MTEP, did not influence alcohol drinking in either genotype nor did (d) an intra-BNST infusion of 30 pg/side of the mGlu1-negative modular, JNJ 16259685. Diagrams summarizing the locations of the microinjector tips for each experiment are presented in the right column and sample sizes are indicated in parentheses along the x-axis. *p < 0.05 versus TS/TS, +p < 0.05 versus VEH.
The insensitivity to inhibitors of ERK and GluN2B-containing NMDA receptors exhibited by GRM5AA/AA mice supports the notion that the effects of ERK and NMDAR signaling are mediated by phosphorylation of mGlu5, such that ERK antagonist effects are already maximized in GRM5AA/AA mice and therefore “occluded”. An alternative explanation is that GRM5AA/AA mice simply cannot consume more alcohol in response to antagonists (i.e., a “ceiling effect”); however, this is unlikely as B6.129 mice are reported to consume upward of 4 g/kg alcohol within a 2 h session (Cozzoli et al., 2012, 2014, 2016; Lum et al., 2014), whereas consumption in these neuropharmacological studies was much <3.0 g/kg.
Constitutive disruption of mGlu5 phosphorylation or Homer1 scaffolding increases alcohol intoxication
An increased capacity of GRM5AA/AA mice to consume alcohol might reflect an effect of the mGlu5 mutation upon alcohol pharmacokinetics. However, when injected intraperitoneally with 3 g/kg alcohol, no genotypic difference was apparent in BACs for up to 30 min postinjection (Fig. 5a; Time effect: F(2,28) = 87.66, p < 0.0001; no Genotype effect or interaction, p values >0.40). Thus, the mGlu5 mutation does not appear to impact alcohol metabolism to account for its marked effects on alcohol intake.
Figure 5.
GRM5AA/AA and Homer1−/− mice are supersensitive to the sedative-hypnotic effects of alcohol. a, When injected with 3 g/kg alcohol, no genotypic difference was apparent in blood alcohol concentrations for up to 30 min postinjection, indicating no obvious genotypic differences in alcohol metabolism. b, Acute alcohol injection (1 or 2 g/kg; 30 min prior) dose-dependently impaired rotarod performance, but GRM5AA/AA (AA/AA) mice were more sensitive to this effect than their wild-type controls (TS/TS) at the 2 g/kg dose. When injected acutely with 5 g/kg alcohol, both GRM5AA/AA (c) and Homer1−/− (d) mice exhibited greater sleep time than their wild-type controls. e, Intra-BNST infusion of U0126 did not alter the latency of B6 mice to fall from the rotarod when injected with 2 g/kg alcohol. f, U0126 infusion also did not alter the latency taken by B6 mice to regain their righting reflex following injection with 5 g/kg alcohol. g, Diagram of the microinjector placements within the BNST of U0126-infused mice. h, When injected acutely with alcohol, the dose–response function for locomotor activity was shifted upward in GRM5AA/AA versus GRM5TS/TS mice, although this effect appeared to be driven primarily by genotypic differences at the 2 g/kg dose. i, No genotypic difference was observed for the dose–response function of PCP-induced locomotion. *p < 0.05 versus TS/TS or Homer1+/+ (t tests). Samples sizes are indicated in parentheses.
The propensity to drink alcohol excessively can be inversely related to sensitivity to alcohol's sedative-hypnotic effects (Krystal et al., 2003; Trimm et al., 2009). However, AAV-mediated disruption of Homer2 scaffolding within the BNST promoted both alcohol-induced sedation and consumption (Fig. 1). This latter finding prompted us to compare GRM5TS/TS and GRM5AA/AA mice for differences in alcohol intoxication using a rotarod assay. Acute alcohol injection (1 or 2 g/kg; 30 min prior) dose-dependently impaired rotarod performance in both genotypes; however, GRM5AA/AA mice were more sensitive to this effect (Fig. 5b; Dose effect: F(1,45) = 59.55, p < 0.0001; Genotype × Dose: F(1,45) = 16.06, p < 0.0001), most notably at the 2 g/kg dose (t tests: 1 g/kg, p = 0.19; 2 g/kg, t(22) = 4.79, p < 0.0001). Thus, GRM5AA/AA mice exhibit increased sensitivity to alcohol intoxication.
One model that rationalizes this finding and those following intra-BNST shRNA-H2b infusion is that alcohol consumption normally induces a rapid increase of ERK activity, which increases mGlu5 phosphorylation and Homer crosslinking to reduce sensitivity to alcohol's motor-impairing effects. Consistent with this model, Homer2−/− mice are supersensitive to alcohol's sedative-hypnotic effects (Szumlinski et al., 2005). To further probe this model, we compared GRM5TS/TS versus GRM5AA/AA mice, as well as Homer1+/+ versus Homer1−/− mice, for their ability to right following injection with a sedative alcohol dose (5 mg/kg). As per Homer2−/− mice (Szumlinski et al., 2005), GRM5AA/AA mice (Fig. 5c; t(26) = 9.83, p < 0.0001) and Homer1−/− mice (Fig. 5d; t(13) = 4.21, p = 0.001) exhibited a significantly longer latency to right than their wild-type controls. However, the local inhibition of ERK activity within the BNST failed to alter the intoxicating effect of 2 g/kg alcohol on the rotarod (Fig. 5e; F(1,23) = 0.94, p = 0.41) or the time taken by B6 mice to regain their righting reflex, following injection with 5 g/kg alcohol (Fig. 5f; F(1,23) = 0.42, p = 0.66), despite microinjector localization within the BNST (Fig. 5g). Thus, although the genotypic differences in the sedative-hypnotic effects of alcohol further the idea that ERK-dependent mGlu5 phosphorylation and subsequent Homer crosslinking are important negative regulators of alcohol behavioral sensitivity, the neuropharmacological data argue against the BNST as the neural locus of this regulation.
Interestingly, while GRM5AA/AA mice clearly exhibit greater sensitivity to the sedative-hypnotic properties of alcohol (Fig. 5b,c), an examination of the dose–response function for the distance traveled early postinjection indicated greater locomotor stimulation overall in GRM5AA/AA versus GRM5TS/TS mice when treated acutely with alcohol (Fig. 5h; Genotype effect: F(1,57) = 5.70, p = 0.02; Genotype × Dose: p = 0.12). However, we failed to detect any genotypic differences in the change in alcohol-induced locomotor stimulation during repeated alcohol treatment (data not shown; Dose × Injection: F(14,364) = 2.55, p = 0.002; Genotype × Injection: p = 0.58, Genotype × Dose × Injection: p = 0.14). These data for acute alcohol-induced locomotion are consistent with, in part, with the Differentiator model, which poses that individuals at risk for, or suffering from, alcohol use disorder, tend to be more sensitive to the stimulating effects of low-dose alcohol (Morean et al., 2015).
To determine whether the effects of disrupting mGlu5 phosphorylation on alcohol-induced locomotor activity extended to another nonselective, and addictive, NMDA receptor antagonist, the locomotor response to increasing doses of PCP were compared between GRM5TS/TS and GRM5AA/AA mice. PCP dose-dependently increased locomotor hyperactivity; however, there were no genotypic differences observed in this regard (Fig. 5i; PCP Dose: F(3,75) = 5.92, p = 0.001; Genotype effect and interaction, p values >0.40). These data argue further that the potentiation of behavioral sensitivity to alcohol produced by disruption of mGlu5 phosphorylation is selective for alcohol.
Interrupting mGluR5 phosphorylation reduces the aversive properties of high-dose alcohol, but not cocaine
To relate the differences in alcohol intake/reinforcement and behavioral sensitivity observed between GRM5TS/TS and GRM5AA/AA mice to their perception of alcohol's interoceptive effects, GRM5TS/TS and GRM5AA/AA mice were tested for conditioned approach/avoidance using alcohol place-conditioning procedures. The dose–response function for alcohol-conditioning exhibited by GRM5AA/AA mice was shifted markedly to the right of GRMTS/TS controls (Fig. 6a; Genotype × Dose × Side interaction: F(2,52) = 10.96, p < 0.0001). At the 1 g/kg dose, the conditioned response exhibited by GRM5AA/AA mice was less than controls (Genotype × Side: F(1,19) = 5.64, p = 0.03), with only GRM5TS/TS mice exhibiting a significant place-preference (Paired vs Unpaired, for GRM5TS/TS: t(10) = 17.65, p = 0.002; for GRM5AA/AA: p = 0.52). A genotypic difference in place-conditioning was also observed at the 3 g/kg dose (Genotype × Side: F(1,17) = 18.81, p < 0.0001), which reflected a place-aversion in GRM5TS/TS mice (Paired vs Unpaired, t(8) = 5.97, p = 0.04), but a place-preference in GRM5AA/AA animals (t(9) = 14.67, p = 0.004). A priori comparisons of side differences at the 2 g/kg dose, indicated a place-preference in GRM5AA/AA mice only (for TS/TS, p > 0.35; for AA/AA, t(8) = 15.92, p = 0.004). These data indicate that GRM5AA/AA mice are less sensitive to both low-dose alcohol reward and high-dose alcohol aversion. To probe whether or not the BNST is a neural locus in which ERK-dependent phosphorylation gates the motivational valence of high-dose alcohol, we examined the effects of an intra-BNST infusion of 9.5 ng/side U0126 upon the expression of alcohol-induced place-aversion in B6 mice. Akin to the data for GRM5AA/AA mice (Fig. 6a), inhibiting ERK activity within the BNST completely reversed the place-aversion induced by conditioning with 3 g/kg alcohol (Fig. 6b; Dose × Side: F(1,12) = 12.56, p = 0.004), without affecting the distance traveled during the after conditioning test (data not shown; t(14) = 0.04, p = 0.85). Thus, ERK phosphorylation of mGlu5(T1123/S1126) functions to gate the motivational valence of the interoceptive effects of alcohol and its disruption by mutation of the receptor or pharmacological inhibition of kinase activity within the BNST, renders animals less sensitive to the aversive properties of high-dose alcohol.
Figure 6.
GRM5AA/AA mice are insensitive to the aversive properties of high-dose alcohol, but not cocaine. a, The dose–response function for alcohol-induced place-conditioning was shifted markedly to the right in GRM5AA/AA mice (AA/AA), relative to their GRM5TS/TS controls (TS/TS). b, An intra-BNST infusion of 9.5 ng/side U0126 mimicked the effect of the GRM5AA/AA mutation upon the conditioned response to 3 g/kg alcohol. c, In contrast, the dose–response function for cocaine-induced place-conditioning was shifted to the left in both GRM5AA/AA and GRM5AA/TS mice, relative to GRM5TS/TS controls. d, Diagram depicting the placements of the microinjectors within the BNST of the mice in b. *p < 0.05 versus TS/TS (t tests or one-way ANOVAs, followed by LSD post hoc tests), **p < 0.05 both genotypes versus TS/TS, +p < 0.05, Paired versus Unpaired (a priori t tests), ++p < 0.05 both genotypes versus Paired. Samples sizes are indicated in parentheses.
Cocaine also increases ERK activation throughout the extended amygdala and interconnected reward circuitry (Valjent et al., 2004) and GRM5AA/AA mice exhibit blunted cocaine-induced locomotor sensitization (Park et al., 2013). Thus, we determined whether disrupting mGlu5 phosphorylation might also alter the perception of cocaine's interoceptive effects. Opposite alcohol (Fig. 6a), the dose–response function for cocaine-induced place-conditioning was shifted to the left of wild-type controls in mice homozygous and heterozygous for the mGlu5 mutation versus wild-type controls (Fig. 6c; Genotype × Dose × Side: F(4,158) = 4.66, p = 0.001). A priori comparisons of the time spent on the cocaine-paired versus -unpaired compartment indicated place-ambivalence in GRM5TS/TS mice conditioned with 3 mg/kg cocaine (t test, p = 0.07), but a significant place-preference was detected in both GRM5TS/AA (t(17) = 6.88, p < 0.0001) and GRM5AA/AA animals (t(16) = 4.24, p = 0.001). At 10 mg/kg and 30 mg/kg cocaine, marked genotypic differences in cocaine-conditioning were also apparent (for 10 mg/kg, Genotype × Side: F(2,48) = 6.08, p = 0.004; for 30 mg/kg, Genotype × Side: F(2,61) = 4.87, p = 0.01), with GRM5TS/TS mice exhibiting a place-preference at both cocaine doses (for 10 mg/kg, t(16) = 2.95, p = 0.009; for 30 mg/kg, t(23) = 12.23, p < 0.0001). In contrast, the conditioning exhibited by both GRM5TS/AA and GRM5AA/AA mice trended toward aversion at 10 mg/kg cocaine (for both genotypes, t tests: p values >0.15) and both mutant genotypes exhibited a significant place-aversion at the 30 mg/kg cocaine dose (GRM5TS/AA: t(11) = 3.02, p = 0.01; GRM5AA/AA: t(27) = 2.26, p = 0.03). Combined, these cocaine-conditioning data suggest an important role for intact mGlu5(pT1123/S1126) in the development of tolerance to cocaine's aversive properties with repeated cocaine experience, a finding in line with a prior report of blunted cocaine-induced neuroplasticity in GRM5AA/AA mice (Park et al., 2013). Importantly, these cocaine data indicate that the insensitivity to alcohol-aversion exhibited by GRM5AA/AA mice does not generalize across addictive substances.
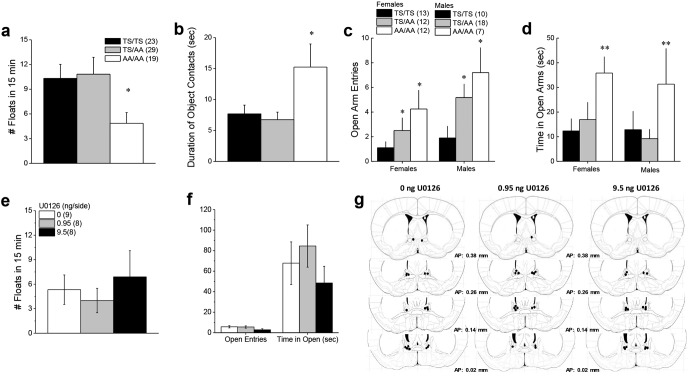
Interrupting mGlu5 phosphorylation reduces anxiety and increases novelty-seeking
Alcohol is an anxiolytic and this property can contribute to its abuse liability, particularly in hyper-anxious individuals (Becker, 2017; Blaine and Sinha, 2017). Thus, we tested the hypothesis that the excessive drinking phenotype exhibited by GRM5AA/AA mice might relate to a hyper-anxious state. However, when screened in a variety of tests for emotionality, GRM5AA/AA mice exhibited less negative affect than GRM5TS/TS controls. This was evidenced by reduced floating behavior in a forced swim test (Fig. 7a; F(2,71) = 3.51, p = 0.04) and a doubling of the time spent in contact with a novel object in a reactivity test (Fig. 7b; F(2,70) = 5.41, p = 0.007). Overall, females made fewer open arm entries than males when tested in the elevated plus maze (Sex effect: F(1,72) = 4.26, p = 0.04). Despite this sex difference, we observed a gene dose-dependent increase in the number of open arm entries by mutant mice (Fig. 7c; Genotype effect: F(2,72) = 4.93, p = 0.01; Sex × Genotype interaction: p > 0.25). Likewise, GRM5AA/AA mice also spent more time in the open arm of an elevated plus maze than GRM5TS/TS and GRM5TS/AA mice (Fig. 7d; Genotype effect: F(2,72) = 5.37, p = 0.007). These observations indicate that the excessive alcohol-drinking phenotype of GRM5AA/AA mice is not attributable to increased anxiety or depressive endophenotypes. However, enhanced novelty-seeking is potentially relevant to excessive drinking behavior as this endophenotype is highly correlated with the propensity to consume drug (Lee et al., 2014).
Figure 7.
Emotionality in GRM5AA/AA mice. a, An examination of the average number of floats exhibited by male and female mice during a 15 min forced swim test revealed less floating behavior in GRM5AA/AA (AA/AA) mice versus the other two genotypes. b, Likewise, GRM5AA/AA mice spent more time interacting with a novel object during a 2 min test. c, Although females made fewer open arm entries than males when tested in the elevated plus maze, the number of open arm entries was gene dose-dependent. d, GRM5AA/AA mice also spent more time in the open arm of an elevated plus maze than GRM5TS/TS (TS/TS) and GRM5TS/AA (TS/AA) mice. e, In contrast, an intra-BNST infusion of U0126 did not alter floating behavior in the forced swim test. f, Likewise, no effect of U0126 infusion was observed in the elevated plus maze. g, Cartoon depicting the locations of the microinjector tips within the BNST of mice treated with the different doses of U0126. Sample sizes are indicated in parentheses. *p < 0.05 versus TS/TS (t tests or LSD post hoc tests); **p < 0.05 versus TS/TS and TS/AA.
In contrast to the GRM5AA/AA mutation, the local inhibition of ERK signaling within the BNST did not alter behavior in the novel object encounter (data not shown; for all variables, one-way ANOVAs, p values > 0.40) or the forced swim tests (Fig. 7e; one-way ANOVA, p = 0.67). Although mice infused with 9.5 ng/side U0126 tended to make fewer entries into, and spend less time in, the open arm of the elevated plus maze, relative to the other doses (Fig. 7f), no significant U0126 effect was observed in this assay (one-way ANOVAs for time in open arm, time in closed arm and time spent in open arm, all p values > 0.11). From these data, it would not appear that the BNST is the neural locus mediating the anxiolytic effects of disrupting ERK-dependent phosphorylation of mGlu5.
Discussion
The present data support a model in which alcohol-induced changes in mGlu5 scaffolding and signaling within the BNST act to limit alcohol consumption. Alcohol augments Homer2 expression, which presumably increases Homer-crosslinking of mGlu5. This appears to be an adaptation that normally limits alcohol consumption as interrupting Homer2-crosslinking in BNST increases alcohol consumption and reinforcement. Binge-drinking also increases BNST ERK activity and ERK phosphorylates mGlu5 at the Homer binding site to facilitate Homer-crosslinking through increased Homer-EVH1 binding affinity (Beneken et al., 2000; Orlando et al., 2009; Hu et al., 2012; Park et al., 2013). Increases in BNST ERK activity appear to be a further adaptation that normally limits alcohol consumption because local ERK inhibition increases alcohol consumption in wild-type mice. The effect of ERK upregulation on mGluR5 is selectively disrupted in GRM5AA/AA mice (Park et al., 2013), which also show increased alcohol consumption. Moreover, local ERK inhibition does not increase alcohol consumption in GRM5AA/AA mice, suggesting that mGlu5 is the important site of action of ERK within the BNST to limit alcohol consumption.
Alternatively, the mGlu5(T1123A/S1126A) mutation may reduce the Homer-EVH1 binding affinity, independent of phosphorylation. However, prior studies of mice with an mGlu5(F1128R) mutation revealed either normal or reduced alcohol intake (Cozzoli et al., 2009, 2012); the mGlu5(F1129R) mutation markedly reduces the Homer-EVH1 binding affinity, but does not disrupt phosphorylation of mGlu5(T1123/S1126) or the consequent increases in Homer EVH1 binding affinity (Park et al., 2013). Accordingly, our data suggest that it is the alcohol-induced increase of Homer-mGlu5 crosslinking in the BNST that is most relevant to limiting alcohol consumption. Notably, although constitutive deletion of Homer2 does not result in excessive alcohol drinking (Szumlinski et al., 2005; Cozzoli et al., 2009, 2012), Homer2 knockdown within the NAsh and CeA, which are both highly interconnected with the BNST, blunts alcohol intake in mice (Szumlinski et al., 2008b; Cozzoli et al., 2012, 2014) indicating regionally localized roles of Homer2 in regulation of alcohol consumption.
The ability to dynamically regulate mGlu5-Homer crosslinking may be important to change the nature of signaling between the basal versus alcohol-intoxicated state. Homer2-crosslinking could increase mGlu5-coupling to multiple targets, including IP3 or ryanodine receptors to enhance glutamate-induced release of intracellular Ca2+, TRPC channels to increase Ca2+ influx, or Shank to increase association with postsynaptic density proteins, including NMDA receptors. Homer-crosslinking can also reduce coupling to membrane K+ channels (Kammermeier et al., 2000). Reciprocally, interrupting Homer-crosslinking increases agonist-independent mGlu5 activity and this process underlies homeostatic scaling-down of synaptic strength consequent to increased activity that induces Homer1a (Hu et al., 2010). Which of these various intracellular outputs is most critical for control of alcohol consumption remains a compelling question.
Alcohol-induced synaptic plasticity within the BNST
Alcohol-induced glutamatergic plasticity within the BNST is purported to regulate affective states to promote excessive drinking (McElligott and Winder, 2009; Kash, 2012; Wills et al., 2012; Lovinger and Kash, 2015; Vranjkovic et al., 2017). Although binge-drinking history did not alter the total glutamate receptor expression herein, chronic, intermittent alcohol can increase BNST glutamate receptor expression, notably that of the alcohol-sensitive GluN2B-containing NMDA receptor (Kash et al., 2008, 2009; Wills et al., 2012; Wills and Winder, 2013) as well as, GluN2B-dependent long-term potentiation within BNST (Wills et al., 2012). The robust upregulation of BNST Homer2 expression in binge-drinking mice form a possible molecular substrate for NMDA receptor-dependent synaptic plasticity as Homer2 targets GluN2B-containing NMDA receptors to synapses (Shiraishi et al., 2003) and is required for intact NMDA receptor function (Smothers et al., 2016).
The BNST also exhibits a postsynaptically mediated, mGlu5-dependent, long-term depression (LTD) of excitatory transmission that requires ERK activation (Grueter et al., 2006, 2008). In cocaine-experienced animals, this form of LTD increases excitatory drive within BNST-mesolimbic projections to promote cocaine-seeking/taking behavior (Grueter et al., 2006, 2008). The precise mechanism(s) through which ERK activation leads to mGlu5-dependent LTD within BNST, and its disruption by cocaine, are not clear. Nor is it known whether alcohol experience promotes or prevents this form of synaptic plasticity. However, increased mGluR5-Homer-crosslinking facilitates the cytosolic retention of mGlu5- a mechanism implicated in cocaine's ability to disrupt mGlu5-dependent LTD within the NAsh (Fourgeaud et al., 2004). ERK-mediated phosphorylation of mGlu5(T1223/S1126) also facilitates slow inward currents through NMDA receptors (Park et al., 2013), the activation of which is reported to inhibit mGlu5-dependent LTD within the NAsh via the activation of Ca2+-dependent signaling pathways and Homer phosphorylation (Huang and Hsu, 2012).
The induction and maintenance mechanisms for mGlu5-dependent LTD can vary across brain regions (cf. Grueter et al., 2007) and different environmental stimuli (McElligott et al., 2010). Indeed, the glutamatergic consequences of binge-drinking are not only brain region-specific (Cozzoli et al., 2012, 2014), but quite distinct from those observed in cocaine-experienced animals (Ary and Szumlinski, 2007; Knackstedt et al., 2010; Ghasemzadeh et al., 2011). Further studies are required to assess how ERK-dependent changes in mGlu5 scaffolding/signaling may contribute to alcohol-induced synaptic plasticity within the BNST to limit alcohol intake.
ERK-mGlu5 interactions in the affective/motivational properties of high-dose alcohol
The increase in alcohol intake, reinforcement, and conditioned reward produced by inhibiting ERK signaling/mGlu5 scaffolding within the BNST coincide with either no change in, or increased sensitivity to, the stimulant properties of low-dose alcohol, as well as the sedative-hypnotic properties of higher alcohol doses. The observation that GRM5AA/AA mice and B6 mice infused intra-BNST with U0126 perceive the interoceptive effects of high-dose alcohol as appetitive, rather than aversive, aligns well with the results of human and animal studies consistent with the differentiator model of alcoholism vulnerability (Newlin and Thomson, 1990, 1999; Holdstock et al., 2000; King et al., 2002; Fritz et al., 2013; Morean et al., 2015). This model posits that predisposition to excessive drinking reflects accentuated sensitivity to alcohol's psychomotor effects during the ascending limb of the blood alcohol curve, combined with attenuated sensitivity to the aversive negative affective state produced during the descending limb of the blood alcohol curve. The perception of high-dose alcohol's effects as appetitive by GRM5AA/AA mice does not reflect an inability to perceive interoceptive drug effects as aversive, as clearly indicated by the results of our cocaine place-conditioning studies. The observation that pharmacological inhibition of ERK within BNST mimicked the effects of global disruption of ERK-dependent mGlu5 phosphorylation points to this brain region as a neural locus contributing to the motivational valence of high-dose alcohol.
An alternative explanation that the excessive alcohol consumption and heightened reward/reinforcement produced by disrupting ERK signaling/mGlu5 scaffolding reflects an increase in the drug's negative reinforcing (i.e., anxiolytic) properties (Conger, 1956) is not supported by the following observations: (1) GRM5AA/AA mice were hypo-anxious on several behavioral measures, (2) intra-BNST cDNA-H1a infusion produced a transient increase in saccharin consumption, and (3) pharmacological inhibition of ERK signaling within the BNST did not significantly impact behavioral measures of anxiety- or depressive-like behavior. Thus, we propose that abhorrent ERK-mGlu5 interactions within BNST may contribute to individual differences in the affective/motivational valence of high-dose alcohol that bears directly upon the capacity to drink excessively.
BNST-related circuitry inhibiting the propensity to binge-drink
The BNST is neuroanatomically and cytoarchitecturally complex (Ju and Swanson, 1989; Bota and Swanson, 2010). The majority of BNST neurons are GABAergic (Cullinan et al., 1993; Esclapez et al., 1993), with modest expression of glutamate cells in the posterior, dorsomedial, and fusiform nuclei (Poulin et al., 2009). BNST efferents project to many mesolimbic structures, including the ventral tegmental area (VTA; Jennings et al., 2013; Kim et al., 2013), the latter of which receives dense GABAergic and more sparse glutamatergic efferents from the more ventral aspects of the BNST, as well as the anterior-dorsal BNST targeted herein (Jennings et al., 2013). BNST efferents to the VTA have been the major foci of investigation regarding the neurocircuitry of addiction because of their role in regulating motivated behavior (cf. Vranjkovic et al., 2017). Stimulation of both GABAergic and glutamatergic efferents from the BNST excite VTA neurons (Georges and Aston-Jones, 2001; Jennings et al., 2013) to drive drug/alcohol-conditioned reward and drug/alcohol-seeking behavior (Sartor and Aston-Jones, 2012; Jennings et al., 2013; Pina et al., 2015; Pina and Cunningham, 2017; Vranjkovic et al., 2017). Further, chemogenetically inhibiting BNST-VTA projections that contain corticotropin-releasing factor (CRF) attenuates binge-alcohol intake (Pleil et al., 2015; Rinker et al., 2017). The opposite effects of chemogenetic inhibition of CRF BNST-VTA projections (Pina et al., 2015; Pleil et al., 2015; Pina and Cunningham, 2017; Rinker et al., 2017) versus the local inhibition of mGlu5 crosslinking and ERK signaling within BNST (present study) upon alcohol reward and reinforcement argues a neurocircuitry model in which alcohol induces dynamic changes in mGlu5 scaffolding/signaling either within non-CRF BNST-VTA projections or within other BNST efferents to counteract the drive to drink/alcohol-seek exerted by alcohol's activation of the CRF projections to the VTA (Pleil et al., 2015; Rinker et al., 2017).
With respect to the latter, the GABAergic BNST projection to the magnocellular region of the periventricular nucleus of the hypothalamus (PVN) is a particularly intriguing candidate. The PVN is comprised of both oxytocinergic and vasopressinergic cells (Sawchenko and Swanson, 1983) that exert opposing effects upon the manifestation of negative affect, the propensity to drink, and the sedative-hypnotic effects of alcohol (Bowen et al., 2015; MacFadyen et al., 2016; King et al., 2017; Ryan et al., 2017); endophenotypes all affected herein by disrupting ERK-dependent mGlu5 crosslinking. It remains to be determined whether a binge-drinking history impacts neuroplasticity within BNST-PVN projections (or any other major target region, for that matter) and how this neuroplasticity influences neurohormone release to regulate alcoholism-related behaviors and affective states.
Conclusions
The present data support a model in which alcohol-induced changes in mGlu5 scaffolding and signaling within the BNST act to limit alcohol consumption and appear to do so by gating the motivational valence of the interoceptive effects of high-dose alcohol, rather than by affecting other factors typically associated with problem drinking. Additional study is necessary to understand more precisely how ERK-dependent mGlu5 scaffolding and signaling impacts alcohol-induced synaptic plasticity within the BNST to influence neuronal activity within its diverse projections. Nevertheless, the present study provides novel insight into the role for ERK-dependent phosphorylation of mGlu5, and for ERK signaling within the BNST, in regulating excessive alcohol consumption of relevance to our neurobiological understanding of alcohol abuse and alcoholism.
Footnotes
This work was supported by Grants from the National Institute on Alcohol and Alcoholism (K.K.S.) and the National Institute on Drug Abuse (K.K.S., P.F.W., T.E.K.), as well as by funds from the Australian Research Council (M.K.). We thank Andrew Lang and Rachel Turner for their assistance with the behavioral studies, Mackayla Class for her assistance with BACs, the National Institute on Drug Abuse for their generous donation of cocaine, and Drs. Schwartz and Seeburg (University of Heidelberg, Germany) for their assistance in creating the Homer1 KO mouse.
The authors declare no competing financial interests.
References
- Ary AW, Szumlinski KK (2007) Regional differences in the effects of withdrawal from repeated cocaine upon homer and glutamate receptor expression: a two-species comparison. Brain Res 1184:295–305. 10.1016/j.brainres.2007.09.035 [DOI] [PubMed] [Google Scholar]
- Ary AW, Lominac KD, Wroten MG, Williams AR, Campbell RR, Ben-Shahar O, von Jonquieres G, Klugmann M, Szumlinski KK (2013) Imbalances in prefrontal cortex CC-Homer1 versus CC-Homer2 expression promote cocaine preference. J Neurosci 33:8101–8113. 10.1523/JNEUROSCI.1727-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC. (2017) Influence of stress associated with chronic alcohol exposure on drinking. Neuropharmacology 122:115–126. 10.1016/j.neuropharm.2017.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneken J, Tu JC, Xiao B, Nuriya M, Yuan JP, Worley PF, Leahy DJ (2000) Structure of the homer EVH1 domain-peptide complex reveals a new twist in polyproline recognition. Neuron 26:143–154. 10.1016/S0896-6273(00)81145-9 [DOI] [PubMed] [Google Scholar]
- Blaine SK, Sinha R (2017) Alcohol, stress, and glucocorticoids: from risk to dependence and relapse in alcohol use disorders. Neuropharmacology 122:136–147. 10.1016/j.neuropharm.2017.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bota M, Swanson LW (2010) Collating and curating neuroanatomical nomenclatures: principles and use of the brain architecture knowledge management system (BAMS). Front Neuroinform 4:3. 10.3389/fninf.2010.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen MT, Peters ST, Absalom N, Chebib M, Neumann ID, McGregor IS (2015) Oxytocin prevents ethanol actions at δ subunit-containing GABAA receptors and attenuates ethanol-induced motor impairment in rats. Proc Natl Acad Sci U S A 112:3104–3109. 10.1073/pnas.1416900112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger JJ. (1956) Alcoholism: theory, problem and challenge: II. Reinforcement theory and the dynamics of alcoholism. Q J Stud Alcohol 17:296–305. [PubMed] [Google Scholar]
- Constantin B. (2016) Role of scaffolding proteins in the regulation of TRPC-dependent calcium entry. Adv Exp Med Biol 898:379–403. 10.1007/978-3-319-26974-0_16 [DOI] [PubMed] [Google Scholar]
- Cozzoli DK, Goulding SP, Zhang PW, Xiao B, Hu JH, Ary AW, Obara I, Rahn A, Abou-Ziab H, Tyrrel B, Marini C, Yoneyama N, Metten P, Snelling C, Dehoff MH, Crabbe JC, Finn DA, Klugmann M, Worley PF, Szumlinski KK (2009) Binge drinking up-regulates accumbens mGluR5-Homer2-PI3K signaling: functional implications for alcoholism. J Neurosci 29:8655–8668. 10.1523/JNEUROSCI.5900-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Caruana AL, Miller BW, Greentree DI, Thompson AB, Wroten MG, Zhang PW, Xiao B, Hu JH, Klugmann M, Metten P, Worley PF, Crabbe JC, Szumlinski KK (2012) Accumbens shell metabotropic glutamate receptor 5-associated signaling regulates binge alcohol drinking: evidence from drinking-in-the-dark studies. Alcohol Clin Exp Res 36:1623–1633. 10.1111/j.1530-0277.2012.01776.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Wroten MG, Greentree DI, Lum EN, Campbell RR, Thompson AB, Maliniak D, Worley PF, Jonquieres G, Klugmann M, Finn DA, Szumlinski KK (2014) Binge alcohol drinking by mice requires intact Group1 metabotropic glutamate receptor signaling within the central nucleus of the amygdala. Neuropsychopharmacology 39:435–444. 10.1038/npp.2013.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Rostock C, Campbell RR, Wroten MG, McGregor H, Caruana AL, Miller BW, Hu JH, Wu Zhang P, Xiao B, Worley PF, Crabbe JC, Finn DA, Szumlinski KK (2016) Extended amygdala protein kinase C epsilon signaling mediates binge alcohol consumption. Biol Psychiat 79:443–451. 10.1016/j.biopsych.2015.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Watson SJ (1993) Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J Comp Neurol 332:1–20. 10.1002/cne.903320102 [DOI] [PubMed] [Google Scholar]
- During MJ, Young D, Baer K, Lawlor P, Klugmann M (2003) Development and optimization of adeno-associated virus vector transfer into the central nervous system. In: Viral vectors for gene therapy: methods and protocols, Vol 76 (Machida CA, ed), pp 221–236. Totowa, Canada: Humana. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J (2001) Stress-induced relapse to drug seeking in the rat: role of the bed nucleus of the stria terminalis and amygdala. Stress 4:289–303. 10.3109/10253890109014753 [DOI] [PubMed] [Google Scholar]
- Esclapez M, Tillakaratne NJ, Tobin AJ, Houser CR (1993) Comparative localization of mRNAs encoding two forms of glutamic acid decarboxylase with nonradioactive in situ hybridization methods. J Comp Neurol 331:339–362. 10.1002/cne.903310305 [DOI] [PubMed] [Google Scholar]
- Faccidomo S, Besheer J, Stanford PC, Hodge CW (2009) Increased operant responding for ethanol in male C57BL/6J mice: specific regulation by the ERK1/2, but not JNK, MAP kinase pathway. Psychopharmacology 204:135–147. 10.1007/s00213-008-1444-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourgeaud L, Mato S, Bouchet D, Hémar A, Worley PF, Manzoni OJ (2004) A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J Neurosci 24:6939–6945. 10.1523/JNEUROSCI.0671-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franich NR, Fitzsimons HL, Fong DM, Klugmann M, During MJ, Young D (2008) AAV vector-mediated RNAi of mutant huntingtin expression is neuroprotective in a novel genetic rat model of Huntington's disease. Mol Ther 16:947–956. 10.1038/mt.2008.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz BM, Grahame NJ, Boehm SL 2nd (2013) Selection for high alcohol preference drinking in mice results in heightened sensitivity and rapid development of acute functional tolerance to alcohol's ataxic effects. Genes Brain Behav 12:78–86. 10.1111/j.1601-183X.2012.00830.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G (2001) Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. J Neurosci 21:RC160. 10.1523/JNEUROSCI.21-16-j0003.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Vasudevan P, Giles C, Purgianto A, Seubert C, Mantsch JR (2011) Glutamatergic plasticity in medial prefrontal cortex and ventral tegmental area following extended-access cocaine self-administration. Brain Res 1413:60–71. 10.1016/j.brainres.2011.06.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding SP, Obara I, Lominac KD, Gould AT, Miller BW, Klugmann M, Szumlinski KK (2011) Accumbens Homer2-mediated signaling: a factor contributing to mouse strain differences in alcohol drinking? Genes Brain Behav 10:111–126. 10.1111/j.1601-183X.2010.00647.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, Gosnell HB, Olsen CM, Schramm-Sapyta NL, Nekrasova T, Landreth GE, Winder DG (2006) Extracellular-signal regulated kinase 1-dependent metabotropic glutamate receptor 5-induced long-term depression in the bed nucleus of the stria terminalis is disrupted by cocaine administration. J Neurosci 26:3210–3219. 10.1523/JNEUROSCI.0170-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, McElligott ZA, Winder DG (2007) Group I mGluRs and long-term depression: potential roles in addiction? Mol Neurobiol 36:232–244. 10.1007/s12035-007-0037-7 [DOI] [PubMed] [Google Scholar]
- Grueter BA, McElligott ZA, Robison AJ, Mathews GC, Winder DG (2008) In vivo metabotropic glutamate receptor 5 (mGluR5) antagonism prevents cocaine-induced disruption of postsynaptically maintained mGluR5-dependent long-term depression. J Neurosci 28:9261–9270. 10.1523/JNEUROSCI.2886-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Molinaro G, Collins KA, Hays SA, Paylor R, Worley PF, Szumlinski KK, Huber KM (2016) Selective Disruption of Metabotropic Glutamate Receptor 5-Homer Interactions Mimics Phenotypes of Fragile X Syndrome in Mice. J Neurosci 36:2131–2147. 10.1523/jneurosci.2921-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider A, Woodward NC, Lominac KD, Sacramento AD, Klugmann M, Bell RL, Szumlinski KK (2015) Homer2 within the nucleus accumbens core bidirectionally regulates alcohol intake by both P and Wistar rats. Alcohol 49:533–542. 10.1016/j.alcohol.2015.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdstock L, King AC, de Wit H (2000) Subjective and objective responses to ethanol in moderate/heavy and light social drinkers. Alcohol Clin Exp Res 24:789–994. 10.1111/j.1530-0277.2000.tb02057.x [DOI] [PubMed] [Google Scholar]
- Hu JH, Park JM, Park S, Xiao B, Dehoff MH, Kim S, Hayashi T, Schwarz MK, Huganir RL, Seeburg PH, Linden DJ, Worley PF (2010) Homeostatic scaling requires group I mGluR activation mediated by Homer1a. Neuron 68:1128–1142. 10.1016/j.neuron.2010.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JH, Yang L, Kammermeier PJ, Moore CG, Brakeman PR, Tu J, Yu S, Petralia RS, Li Z, Zhang PW, Park JM, Dong X, Xiao B, Worley PF (2012) Preso1 dynamically regulates group I metabotropic glutamate receptors. Nat Neurosci 15:836–844. 10.1038/nn.3103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Hsu KS (2012) Activation of NMDA receptors reduces metabotropic glutamate receptor-induced long-term depression in the nucleus accumbens via a CaMKII-dependent mechanism. Neuropharmacology 63:1298–1307. 10.1016/j.neuropharm.2012.08.008 [DOI] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD (2013) Distinct extended amygdala circuits for divergent motivational states. Nature 496:224–228. 10.1038/nature12041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju G, Swanson LW (1989) Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: I. cytoarchitecture. J Comp Neurol 280:587–602. 10.1002/cne.902800409 [DOI] [PubMed] [Google Scholar]
- Kammermeier PJ, Xiao B, Tu JC, Worley PF, Ikeda SR (2000) Homer proteins regulate coupling of group I metabotropic glutamate receptors to N-type calcium and M-type potassium channels. J Neurosci 20:7238–7245. 10.1523/JNEUROSCI.20-19-07238.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL. (2012) The role of biogenic amine signaling in the bed nucleus of the stria terminals in alcohol abuse. Alcohol 46:303–308. 10.1016/j.alcohol.2011.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Nobis WP, Matthews RT, Winder DG (2008) Dopamine enhances fast excitatory synaptic transmission in the extended amygdala by a CRF-R1-dependent process. J Neurosci 28:13856–13865. 10.1523/JNEUROSCI.4715-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Baucum AJ 2nd, Conrad KL, Colbran RJ, Winder DG (2009) Alcohol exposure alters NMDAR function in the bed nucleus of the stria terminalis. Neuropsychopharmacology 34:2420–2429. 10.1038/npp.2009.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, Lo M, Pak S, Mattis J, Lim BK, Malenka RC, Warden MR, Neve R, Tye KM, Deisseroth K (2013) Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 496:219–223. 10.1038/nature12018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Houle T, de Wit H, Holdstock L, Schuster A (2002) Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res 26:827–835. 10.1111/j.1530-0277.2002.tb02611.x [DOI] [PubMed] [Google Scholar]
- King CE, Griffin WC, Luderman LN, Kates MM, McGinty JF, Becker HC (2017) Oxytocin reduces ethanol self-administration in mice. Alcohol Clin Exp Res 41:955–964. 10.1111/acer.13359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugmann M, Szumlinski KK (2008) Targeting homer genes using AAV: lessons learned from behavioural and neurochemical studies. Behav Pharmacol 19:485–500. 10.1097/FBP.0b013e32830c369f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugmann M, Symes CW, Leichtlein CB, Klaussner BK, Dunning J, Fong D, Young D, During MJ (2005) AAV-mediated hippocampal expression of short and long homer 1 proteins differentially affect cognition and seizure activity in adult rats. Mol Cell Neurosci 28:347–360. 10.1016/j.mcn.2004.10.002 [DOI] [PubMed] [Google Scholar]
- Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y (2009) Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology 56 Suppl 1:177–185. 10.1016/j.neuropharm.2008.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW (2010) Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci 30:7984–7992. 10.1523/JNEUROSCI.1244-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Krupitsky E, Schutz C, Trevisan L, D'Souza DC (2003) NMDA receptor antagonism and the ethanol intoxication signal: from alcoholism risk to pharmacotherapy. Ann N Y Acad Sci 1003:176–184. 10.1196/annals.1300.010 [DOI] [PubMed] [Google Scholar]
- Lee KM, Coehlo M, McGregor HA, Waltermire RS, Szumlinski KK (2015) Binge alcohol drinking elicits a persistent negative affective state in mice. Behav Brain Res 291:385–398. 10.1016/j.bbr.2015.05.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coelho MA, McGregor HA, Solton NR, Cohen M, Szumlinski KK (2016) Adolescent mice are resilient to alcohol withdrawal-induced anxiety and changes in indices of glutamate function within the nucleus accumbens. Front Cell Neurosci 10:265. 10.3389/fncel.2016.00265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Chen SL, Chang YH, Lu RB (2014) Variation of types of alcoholism: review and subtypes identified in han chinese. Prog Neuropsychopharmacol Biol Psychiatry 48:36–40. 10.1016/j.pnpbp.2013.09.013 [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Kash TL (2015) Mechanisms of neuroplasticity and ethanol's effects on plasticity in the striatum and bed nucleus of the stria terminalis. Alcohol Res 37:109–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum EN, Campbell RR, Rostock C, Szumlinski KK (2014) mGluR1 receptors within the nucleus accumbens regulate alcohol intake in mice under limited-access conditions. Neuropharmacology 79:679–687. 10.1016/j.neuropharm.2014.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFadyen K, Loveless R, DeLucca B, Wardley K, Deogan S, Thomas C, Peris J (2016) Peripheral oxytocin administration reduces ethanol consumption in rats. Pharmacol Biochem Behav 140:27–32. 10.1016/j.pbb.2015.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott ZA, Winder DG (2009) Modulation of glutamatergic synaptic transmission in the bed nucleus of the stria terminalis. Prog Neuropsychopharmacol Biol Psychiatry 33:1329–1335. 10.1016/j.pnpbp.2009.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott ZA, Klug JR, Nobis WP, Patel S, Grueter BA, Kash TL, Winder DG (2010) Distinct forms of Gq-receptor-dependent plasticity of excitatory transmission in the BNST are differentially affected by stress. Proc Natl Acad Sci U S A 107:2271–2276. 10.1073/pnas.0905568107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, Corbin WR, Treat TA (2015) Evaluating the accuracy of alcohol expectancies relative to subjective response to alcohol. Addict Behav 51:197–203. 10.1016/j.addbeh.2015.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB (1990) Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol Bull 108:383–402. 10.1037/0033-2909.108.3.383 [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB (1999) Chronic tolerance and sensitization to alcohol in sons of alcoholics: II. Replication and reanalysis. Exp Clin Psychopharmacol 7:234–243. 10.1037/1064-1297.7.3.234 [DOI] [PubMed] [Google Scholar]
- Orlando LR, Ayala R, Kett LR, Curley AA, Duffner J, Bragg DC, Tsai LH, Dunah AW, Young AB (2009) Phosphorylation of the homer binding domain of group I metabotropic glutamate receptors by cyclin-dependent kinase 5. J Neurochem 110:557–569. 10.1111/j.1471-4159.2009.06139.x [DOI] [PubMed] [Google Scholar]
- Park JM, Hu JH, Milshteyn A, Zhang PW, Moore CG, Park S, Datko MC, Domingo RD, Reyes CM, Wang XJ, Etzkorn FA, Xiao B, Szumlinski KK, Kern D, Linden DJ, Worley PF (2013) A prolyl-isomerase mediates dopamine-dependent plasticity and cocaine motor sensitization. Cell 154:637–650. 10.1016/j.cell.2013.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin K (2004) The Mouse Brain in Stereotaxic Coordinates (2nd ed.). San Diego, CA: Elsevier Academic Press. [Google Scholar]
- Pina MM, Cunningham CL (2017) Ethanol-seeking behavior is expressed directly through an extended amygdala to midbrain neural circuit. Neurobiol Learn Mem 137:83–91. 10.1016/j.nlm.2016.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pina MM, Young EA, Ryabinin AE, Cunningham CL (2015) The bed nucleus of the stria terminalis regulates ethanol-seeking behavior in mice. Neuropharmacology 99:627–638. 10.1016/j.neuropharm.2015.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleil KE, Rinker JA, Lowery-Gionta EG, Mazzone CM, McCall NM, Kendra AM, Olson DP, Lowell BB, Grant KA, Thiele TE, Kash TL (2015) NPY signaling inhibits extended amygdala CRF neurons to suppress binge alcohol drinking. Nat Neurosci 18:545–552. 10.1038/nn.3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Brossard G, Hautbois C, Roux S (2001) Rodent models of depression: forced swimming and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci, Chapter 8:Unit 8.10A. [DOI] [PubMed] [Google Scholar]
- Poulin JF, Arbour D, Laforest S, Drolet G (2009) Neuroanatomical characterization of endogenous opioids in the bed nucleus of the stria terminalis. Prog Neuropsychopharmacol Biol Psychiatry 33:1356–1365. 10.1016/j.pnpbp.2009.06.021 [DOI] [PubMed] [Google Scholar]
- Quadir SG, Guzelian E, Palmer MA, Martin DL, Kim J, Szumlinski KK (2017) Complex interactions between the subject factors of biological sex and prior histories of binge-drinking and unpredictable stress influence behavioral sensitivity to alcohol and alcohol intake. Physiol Behav. Advance online publication. Retrieved August 10, 2017. doi: 10.1016/j.physbeh.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadir SG, Santos JR, Campbell RR, Wroten MG, Singh N, Holloway JJ, Bal SK, Camarini R, Szumlinski KK (2016) Homer2 regulates alcohol and stress cross-sensitization. Addict Biol 21:613–633. 10.1111/adb.12252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinker JA, Marshall SA, Mazzone CM, Lowery-Gionta EG, Gulati V, Pleil KE, Kash TL, Navarro M, Thiele TE (2017) Extended amygdala to ventral tegmental area corticotropin releasing factor circuit controls binge ethanol intake. Biol Psychiatry 81:930–940. 10.1016/j.biopsych.2016.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan ML, Falk DE, Fertig JB, Rendenbach-Mueller B, Katz DA, Tracy KA, Strain EC, Dunn KE, Kampman K, Mahoney E, Ciraulo DA, Sickles-Colaneri L, Ait-Daoud N, Johnson BA, Ransom J, Scott C, Koob GF, Litten RZ (2017) A phase 2, double-blind, placebo-controlled randomized trial assessing the efficacy of ABT-436, a novel V1b receptor antagonist, for alcohol dependence. Neuropsychopharmacology 42:1012–1023. 10.1038/npp.2016.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor GC, Aston-Jones G (2012) Regulation of the ventral tegmental area by the bed nucleus of the stria terminalis is required for expression of cocaine preference. Eur J Neurosci 36:3549–3558. 10.1111/j.1460-9568.2012.08277.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW (1983) The organization of forebrain afferents to the paraventricular and supraoptic nuclei of the rat. J Comp Neurol 218:121–144. 10.1002/cne.902180202 [DOI] [PubMed] [Google Scholar]
- Shiraishi Y, Mizutani A, Mikoshiba K, Furuichi T (2003) Coincidence in dendritic clustering and synaptic targeting of homer proteins and NMDA receptor complex proteins NR2B and PSD95 during development of cultured hippocampal neurons. Mol Cell Neurosci 22:188–201. 10.1016/S1044-7431(03)00037-X [DOI] [PubMed] [Google Scholar]
- Shiraishi-Yamaguchi Y, Furuichi T (2007) The homer family proteins. Genome Biol 8:206–212. 10.1186/gb-2007-8-2-206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smothers CT, Szumlinski KK, Worley PF, Woodward JJ (2016) Altered NMDA receptor function in primary cultures of hippocampal neurons from mice lacking the Homer2 gene. Synapse 70:33–39. 10.1002/syn.21869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Oleson EB, Walker JK, Mason A, Dehoff MH, Klugman M, Cagle S, Welt K, During M, Worley PF, Middaugh LD, Kalivas PW (2005) Homer2 is necessary for ethanol-induced neuroplasticity. J Neurosci 25:7054–7061. 10.1523/JNEUROSCI.1529-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD (2008a) Homers regulate drug-induced neuroplasticity: implications for addiction. Biochem Pharmacol 75:112–133. 10.1016/j.bcp.2007.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD, Klugmann M, Kippin TE (2008b) Accumbens Homer2 over-expression facilitates alcohol-induced neuroplasticity in C57BL/6J mice. Neuropsychopharmacology 33:1365–1378. 10.1038/sj.npp.1301473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Dehoff MH, Kang SH, Frys KA, Lominac KD, Klugmann M, Rohrer J, Griffin W 3rd, Toda S, Champtiaux NP, Berry T, Tu JC, Shealy SE, During MJ, Middaugh LD, Worley PF, Kalivas PW (2004) Homer proteins regulate sensitivity to cocaine. Neuron 43:401–413. 10.1016/j.neuron.2004.07.019 [DOI] [PubMed] [Google Scholar]
- Trim RS, Schuckit MA, Smith TL (2009) The relationship of the level of response to alcohol and additional characteristics to alcohol use disorders across adulthood: a discrete-time survival analysis. Alcohol Clin Exp Res 33:1562–1570. 10.1111/j.1530-0277.2009.00984.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pagès C, Hervé D, Girault JA, Caboche J (2004) Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci 19:1826–1836. 10.1111/j.1460-9568.2004.03278.x [DOI] [PubMed] [Google Scholar]
- Vranjkovic O, Pina M, Kash TL, Winder DG (2017) The bed nucleus of the stria terminalis in drug-associated behavior and affect: a circuit-based perspective. Neuropharmacology 122:100–106. 10.1016/j.neuropharm.2017.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lanfranco MF, Gibb SL, Yowell QV, Carnicella S, Ron D (2010) Long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum play a crucial role in alcohol consumption and relapse. J Neurosci 30:10187–10198. 10.1523/jneurosci.2268-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Winder DG (2013) Ethanol effects on N-methyl-d-aspartate receptors in the bed nucleus of the stria terminalis. Cold Spring Harb Perspect Med 3:a012161. 10.1101/cshperspect.a012161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Klug JR, Silberman Y, Baucum AJ, Weitlauf C, Colbran RJ, Delpire E, Winder DG (2012) GluN2B subunit deletion reveals key role in acute and chronic ethanol sensitivity of glutamate synapses in bed nucleus of the stria terminalis. Proc Natl Acad Sci U S A 109:E278–E287. 10.1073/pnas.1113820109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, Dehoff MH, Schwarz MK, Seeburg PH, Muallem S, Worley PF (2003) Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell 114:777–789. 10.1016/s0092-8674(03)00716-5 [DOI] [PubMed] [Google Scholar]
- Zamora-Martinez ER, Edwards S (2014) Neuronal extracellular signal-regulated kinase (ERK) activity as marker and mediator of alcohol and opioid dependence. Front Integr Neurosci 8:24. 10.3389/fnint.2014.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Mi J, Wetsel WC, Davidson C, Xiong X, Chen Q, Ellinwood EH, Lee TH (2006) PI3 kinase is involved in cocaine behavioral sensitization and its reversal with brain area specificity. Biochem Biophys Res Commun 340:1144–1150. 10.1016/j.bbrc.2005.12.114 [DOI] [PubMed] [Google Scholar]