Summary
LP-261 is a novel tubulin targeting anticancer agent that binds at the colchicine site on tubulin, inducing G2/M arrest. Screening in the NC160 cancer cell lines resulted in a mean G150 of approximately 100 nM. Here, we report the results of testing in multiple mouse xenograft models and angiogenesis assays, along with bioavailability studies. To determine the antiangiogenic activity of LP-261, both in vitro and ex vivo experiments were performed. Human Umbilical Vein Endothelial cells (HUVECs) were incubated with LP-261 at 50 nM to 10 μM. LP-261 was also tested in a rat aortic ring assay, from 20 nM to 10 μM. Multiple mouse xenograft studies were performed to assess in vivo antitumor activity. LP-261 was tested as a single agent in colon adenocarcinoma (SW620) and prostate cancer (LNCaP and PC3) xenografts, evaluating several different dosing schedules. LP-261 was also used in combination with bevacizumab in the SW620 xenograft model. LP-261 also exhibited high oral bioavailability and apparent lack of efflux by intestinal transporters such as ABCBI. LP-261 is a very potent inhibitor of angiogenesis, preventing microvessel outgrowth in the rat aortic ring assay and HUVEC cell proliferation at nanomolar concentrations. Complete inhibition of tumor growth was achieved in the PC3 xenograft model and shown to be schedule dependent. Excellent inhibition of tumor growth in the SW620 model was observed, comparable with paclitaxel. Combining oral, low dose LP-261 with bevacizumab led to significantly improved tumor inhibition. Oral LP-261 is very effective at inhibiting tumor growth in multiple mouse xenograft models and is well tolerated.
Keywords: Anticancer agent, Tubulin, In vitro, In vivo
Introduction
Tubulin has long been a popular target for anticancer agents, as ligand binding results in either stabilization or destabilization of microtubules, disrupting the cell cycle, leading to apoptosis [1]. Two classes of tubulin-binding compounds are currently approved for cancer therapy. The first, Vinca alkaloids, including vinblastine, vincristine and vinorelbine, bind to tubulin at one of the three known pharmacologically active binding sites, the Vinca binding domain, resulting in microtubule de-stabilization. Vincas and taxanes are frequently used in the treatment of lymphoma, along with solid tumors such as non-small cell lung cancer. The second class, taxanes (paclitaxel and docetaxel), bind to tubulin at the taxane binding domain, resulting in microtubule stabilization. These two drugs are widely used in the treatment of solid tumor malignancies, including ovarian, breast and lung cancers.
Though often initially effective, there are numerous drawbacks to treatment with these traditional chemotherapeutic agents. Not only is the oral bioavailability limited, necessitating intravenous administration, but severe toxicity is often observed. Furthermore, multidrug resistance often develops over the course of treatment and all five drugs are known to be substrates for ABCBI (P-glycoprotein). As such, the development of an orally active tubulin binding agent that is not transported by ABCBI is highly desirable.
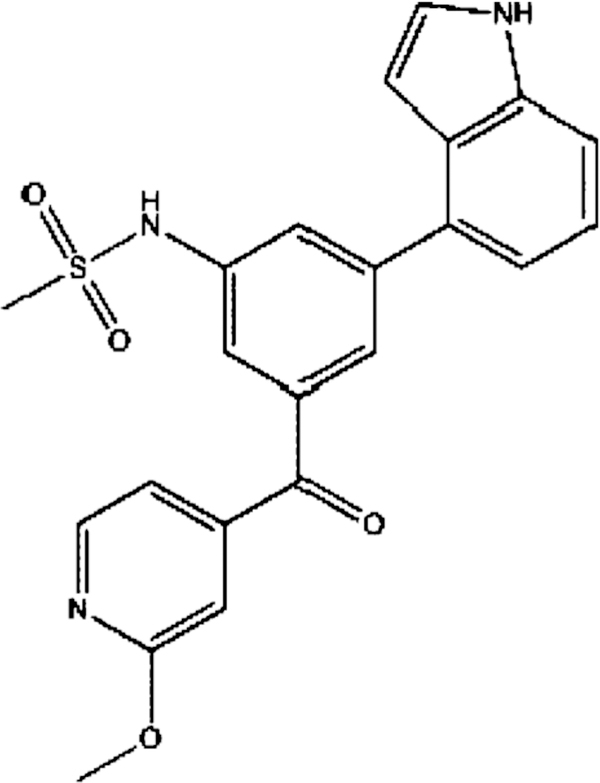
LP-261, N-(3-(lH-indol-4-yl)-5-(2 methoxyisonicotinoyl) phenyl)methanesulfonamide, as shown in Fig. 1, is a novel tubulin targeting anticancer agent, believed to bind at the third pharmacologically active site on tubulin, the colchicine binding domain, inducing G2/M arrest. It was rationally designed, based on the available crystal structures of several colchicine analogs or inhibitors complexed to tubulin [2].
Fig. 1.
Chemical structure of LP-261
We sought to determine whether LP-261 possesses antiangiogenic activity in multiple models, including HUVEC cell proliferation and the ex vivo rat aortic ring assay. Subsequently, the in vivo activity of LP-261 in solid tumor xenograft models both as a single agent and in combination with bevacizumab, a monoclonal antibody against VEGF, was evaluated. In addition, transport by ABCBI and oral bioavailability were assessed.
Materials and methods
Chemicals and reagents
LP-261 was supplied by Ansaris Pharmaceuticals (Blue Bell, PA). Bevacizumab (Avastin) was supplied by Genentech (South San Francisco, CA). DMSO was purchased from Sigma-Aldrich (St. Louis, MO). PBS was purchased from Gibco (Rockville, MD).
Rat aortic ring
Twelve-well tissue culture plates were coated with 250 μl of Matrigel (Becton-Dickinson, Bedford, MA) and allowed to gel for 30 min at 37°C and 5% CO2. Thoracic aortas were excised from 8- to 10-week-old male Sprague Dawley rats. After careful removal of fibroadipose tissue, the aortas were cut into 1-mm-long cross-sections, placed on Matrigel-coated wells, and covered with an additional 250 μl of Matrigel. After the second layer of Matrigel had set (30 min at 37°C and 5% CO2), 1 mL of EGM-2 was added and rings were incubated overnight at 37 °C and 5% CO2. EGM-2 consists of endothelial cell basal medium (EBM-2; Lonza, Walkersville, MD) plus endothelial cell growth factors provided as the EGM-2 Bulletkit (Lonza). The culture medium was subsequently changed to EBM-2 supplemented with 2% heat inactivated fetal bovine serum, 0.25 g/ml amphotericin B, and 10 g/ml gentamicin. Aortic rings were treated daily with either the vehicle (0.5% DMSO) or LP-261 (in 0.5% DMSO) for 4 days and photographed on the 5th day using a 2x objective. CAI, a known antiangiogenic agent, was used at higher than clinically achievable concentration as a positive control. Experiments were repeated four times using aortas from four different rats. The area of angiogenic sprouting was quantified using Adobe Photoshop.
HUVEC proliferation assay
HUVECs (Lonza) were maintained in EGM-2, supplemented with a Bullet-Kit (Lonza) at 37°C and 5% CO2. Cells were seeded onto 96-well plates at a density of 5,000 cells/well (50,000 cells/mL) and allowed to incubate for 48 h at 37°C and 5% CO2. The culture medium was then aspirated, and fresh culture medium containing either the vehicle (0.5% DMSO) or drug solution (in 0.5% DMSO) was added. After 24 h, 10 μI of CCK-8 (Dojindo Molecular Technologies, Gaithersburg, MD) was added to each well. Following 4 h incubation, absorbance was measured at 450 nm and cell counts were back-calculated from a standard curve.
NC160 cell line screen
LP-261 was tested at five concentrations, in the NC160 cell line screen, following standard methodology [3].
Caco-2 permeability
Caco-2 cells (ATCC, Manassas, VA) were grown as monolayers in the top chamber of 24-well Transwell plates. DMEM culture medium supplemented with 100 units/mL penicillin, 100 μg/mL streptomycin and 10% heat inactivated FBS (Invitrogen, Carlsbad, CA) was present in both the top and bottom chambers. LP-261 (50 μM in HEPES buffer containing 1% DMSO) was added to either the apical chamber or the basolateral chamber and allowed to equilibrate across the semi-permeable membrane and through the Caco-2 cell monolayer for 30, 60 and 90 min. At each time point, samples (n=3) were taken and LP-261 was measured in the top and bottom chambers by mass spectrometry. Transit of LP-261 across the cell monolayer in each direction was measured as appearance of LP-261 in the chamber opposite of its starting chamber. Apparent permeability (Papp) was calculated followed by calculation of the efflux ratio (as PappB>A/PappA>B for duplicate experiments.
Mouse xenograft studies
Three separate xenograft models were established. The colon adenocarcinoma xenograft was established by subcutaneous injection of 7.5 × 107 SW-620 cells (ATCC, Manassas, VA) into the right flank of nude mice (Athymic NCr-nu/nu obtained from Charles River Laboratories, Wilmington, MA). Prostate cancer xenografts were established following subcutaneous injection of 3 × 107 PC3 cells (ATCC) or 5 × 107 LNCaP cells (ATCC) into SCID mice (NOD.SCID/NCr, obtained from Charles River Laboratories). For all xenografts, treatment began following establishment of measurable tumors. All mice were allowed unlimited access to water and rodent chow prior to, and during the experiment. NCI-Bethesda and NCI-Frederick are accredited by AAA-LAC International and follow the Public Health Service Policy for the Care and Use of Laboratory Animals. Animal care was provided in accordance with the procedures outlined in the “Guide for Care and Use of Laboratory Animals” (National Research Council; 1996; National Academy Press; Washington, DC). The study design and protocol were approved by the NCI Animal Care and Use Committee (Bethesda, MD).
Drug was formulated in 75% PEG 400 with 25% 100 mM sodium citrate buffer, pH 3 and administered via oral gavage for all studies. This formulation was chosen to maximize solubility and tolerability without significantly hindering bioavailability. Each study included a control group of animals that received vehicle only. Five separate experiments were performed, with the following LP-261 drug administration doses and schedules: Study l, 50 mg/kg, BID, 7 days/week in SW-620 xenograft model or 15 mg/kg paclitaxel; Study 2, 50 mg/kg, BID, 5 days/week in PC3 xenograft model; Study 3, 50 mg/kg BID, 5 days/week in LNCaP xenograft model; Study 4, 100 mg/kg BID, 3 days on, 4 days off in PC3 xenograft model; Study 5, 12.5 mg/kgqd, 5 days/week plus bevacizumab (5 mg/kg, ip, twice weekly), or single agents alone, in SW-620 xenograft model.
Tumor and weight measurements
Mice were weighed and tumors measured using digital calipers twice weekly. Animals weighed 19–25 g at the start of each experiment. Tumor volume was calculated using the formula π/6×a×b2, where a is the longest dimension of the tumor and b is the width.
Pharmacokinetics and bioavailability
Male Sprague Dawley rats (6 to 8 week old, 250 to 300 g, Charles River Laboratories, Wilmington, MA) were used for the study, 4 animals per group. All animals were surgically implanted with a catheter in a jugular vein using polyurethane tubing, and housed individually. Animals were fasted overnight prior to dosing and given standard veterinary diet 4 h post-dosing. Tap water was available ad libitum to each animal via an automatic watering device. LP-261 (l mg/mL formulated as for efficacy studies) was administered as a bolus injection into a lateral tail vein (IV, dose volume: 2 mL/kg,) to one group of animals and as an oral gavage (PO, dose volume: 4 mL/kg) to another group of animals.
Use of rats, as opposed to mice, allowed for serial blood samples to be collected from individual animals and individual concentration-time profiles constructed. Blood samples were collected from each of three animals in each treatment group at 0 (predose), 0.083, 0.25, 0.5, l, 2, 4, and 8 h for IV dosing and 0 (predose), 0.25, 0.5, l, 1.5, 2, 4, and 8 h for PO dosing. Just before each sampling time point, blood was withdrawn (a volume slightly larger than the catheter tubing void volume) and discarded. A blood sample (approximately 0.25 mL) was obtained from the jugular vein catheter using a syringe with blunted needle. The blood sample was then transferred into plastic tubes containing 10 μL of a 1000 IU/mL sodium heparin solution. Following collection of the 0.25 mL blood sample, the catheter was flushed with approximately 0.25 mL of 50 IU/mL sodium heparin in sterile saline. Plasma was separated by centrifugation, withdrawn and stored at −20 °C or below.
LP-261 calibration standards were prepared using naïve Sprague Dawley male rat plasma treated with sodium heparin (Bioreclamation, Inc., East Meadow, NY). The LP-261 calibration standards were prepared by first spiking naïve rat plasma with a defined amount of the saved LP-261 formulation. Then, a series of calibration standards with LP-261 concentration ranging from 0.005 to 10 μg/mL was generated by serial dilution in naïve rat plasma. A portion (60 μL) of each calibration standard and experimental sample was mixed with two volumes (120 μL) of acetonitrile containing an analytical internal standard (LOC-OI 1039, a related chemical compound with differentiable chromatographic and mass profiles), which was included to provide a basis for normalization of the LC/MS/MS peak areas. The mixture was centrifuged at 2000×g at 4°C for 30 min. The supernatants containing the organic component from each sample were used for analysis. Using this protein precipitation method, recovery of LP-261 was greater than 85%, and the lower limit of quantitation was 5 ng/mL.
Plasma concentrations of LP-261 were determined by LC/MS/MS analysis. Liquid chromatography was performed using an Alliance 2695 (Waters, Milford, MA) system with a C18 column (Xterra 100×4.6 mm, particle size 3.5 μM, Waters, Milford, MA). The mobile phases consisted of 0.1% formic acid in water (A) and 0.1% formic acid in methanol (B). Isolation of LP-261 was achieved using a gradient from 20% to 98% B over 8 min, followed by 3 min of isocratic elution at 20% B. The multiple reaction transition of LP-261 was monitored by Quattro LC (Waters, Milford, MA) using an ElectroSpray Ionization method with helium as the collision gas. The transitions of parent molecular ion, m/z (mass to charge ratio) 422.3 to daughters, m/z 343.2 and m/z 136.1 were monitored in positive ion mode with a source temperature of 150°C and a desolvation temperature of 350°C. The peak area of baseline corrected m/z intensity for each calibration standard, experimental sample and internal standard was determined by automated integration. A calibration equation was constructed by fitting a quadratic curve without weighting to the normalized peak area (ratio of LP-261 peak area to internal standard peak area) as a function of the logarithm of LP-261 concentration in each calibration standard. The concentration of LP-261 in each experimental sample was calculated by substituting the normalized peak area into the calibration equation.
Non-compartmental pharmacokinetic parameters such as area under the curve (AUC), elimination half life (T1/2), total systemic clearance (CL), and steady state volume of distribution (Vss) were calculated using the software program WinNonlin 4.0 (Pharsight, Mountain View, CA). Oral bioavailability (% F) was calculated as: %F = 100×(AUCOral×DoseIV) / (AUCIV×DosePO) based on the assumption that the pharmacokinetics of LP-261 are linear from 2 to 4 mg/kg.
Statistics
For studies comparing drug treatment with control group, a Student’s t-test was used to assess the difference at study completion. For studies of 3 or more groups, a one-way ANOVA was used. P-values of less than 0.05 were considered to be significant.
Results
In vitro and ex vivo efficacy
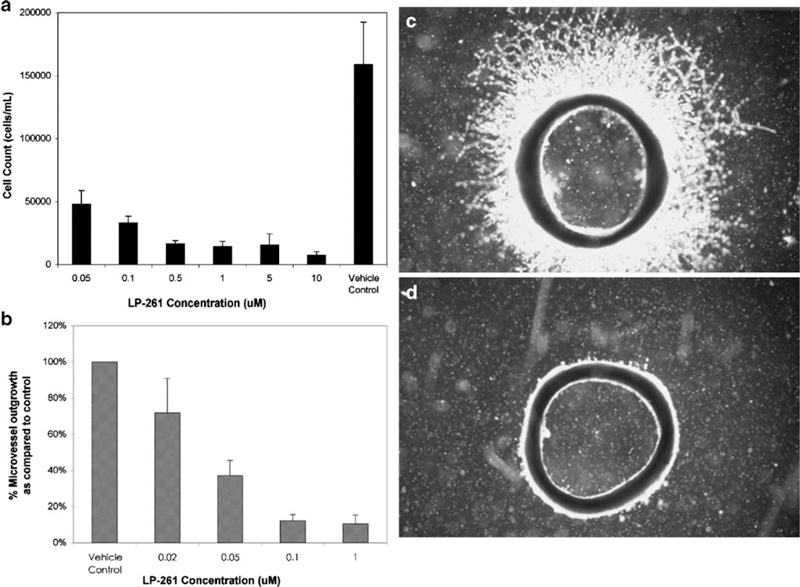
LP-261 was found to inhibit HUVEC proliferation in a dose-dependent manner within the tested concentration range of 100 nM to 10 μM, Fig. 2a. It was similarly shown to significantly inhibit microvessel outgrowth in the rat aortic ring, with greater than 50% growth inhibition at 50 nM, Fig. 2b, c and d depict aortic ring sections treated with vehicle control and 100 nM of LP-261, respectively. In repeat experiments in the NC160 cell line screen, LP-261 was found to inhibit cell growth with a mean G150 of approximately 100 nM, with a number of lines exhibiting a G150 of less than 10 nM (Supplementary Data).
Fig. 2.
Antiangiogenic activity of LP-261. a HUVEC cell proliferation assay, following 24 h of treatment with either LP-261 or vehicle (0.5% DMSO) in EGM-2. Bars depict mean cell count per mL ±S.D., n=8; b Ex vivo rat aortic ring assay. Bars depict mean microvessel outgrowth, as a percentage of vehicle control ±S.D., n=4; c Rat aortic ring treated with vehicle only; d Rat aortic ring treated with 100 nM LP-261
Caco-2 permeability
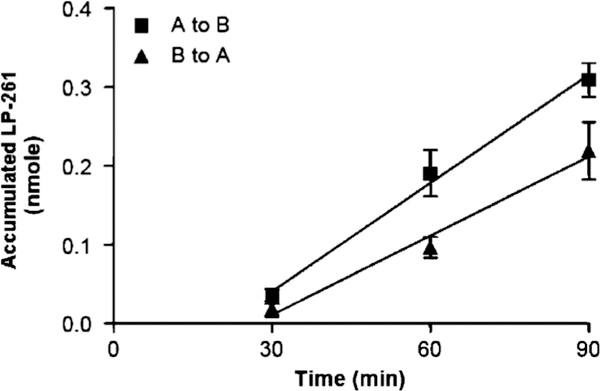
As LP-261 is intended for oral administration, permeability experiments were performed in Caco-2 cells, as shown in Fig. 3. LP-261 is transported at a higher rate from the apical (A) to basolateral (B) side than from the basolateral to apical side of Caco-2 monolayers. The apical to basal apparent permeability ranged from 6.60 ± 0.42 × 10−6 cm/s to 9.0 ± 1.99 × 10−6 cm/s. The basal to apical apparent permeability ranged from 4.59 ± 2.19 × 10−6 cm/s to 5.15 ± 1.96 × 10−6 cm/s. The overall efflux ratio ranged from 0.58 to 0.70, demonstrating lower B to A movement than A to B movement and is suggestive of the possible involvement of a carrier mediated transport process. These results suggest minimal transporter-mediated efflux is occurring.
Fig. 3.
LP-261 accumulation after addition of 50 μM LP-261 to either the apical (a) or basolateral (b) side of Caco-2 cells in 24-well Transwell culture plates. Each marker represents 6 measurements (3 measurements per experiment, for two separate experiments
Pharmacokinetics and bioavailability
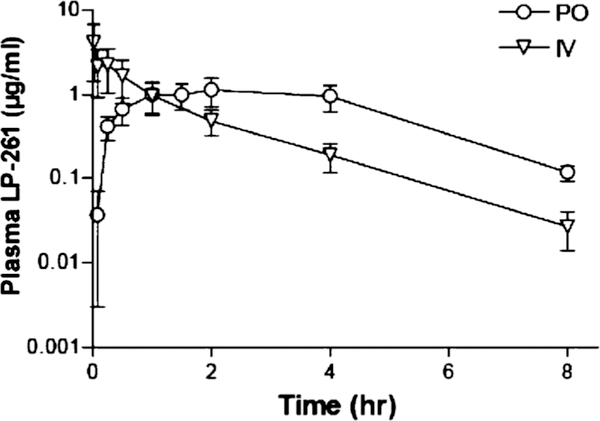
LP-261 plasma concentration-time profile following oral and IV administration in rats is shown in Fig. 4. Oral administration resulted in rapid absorption, with the maximal plasma concentration (Tmax) occurring after 2 h. The terminal half-life (t1/2) was relatively short (1.4±0.2 h), as calculated after IV administration. Overall, the oral availability of LP-261 was determined to be 80% in rats.
Fig. 4.
LP-261 plasma concentration-time profile (mean ± SD, n=4 mice per timepoint, per route) following IV (2 mg/kg) or oral (4 mg/kg) dosing
In vivo efficacy
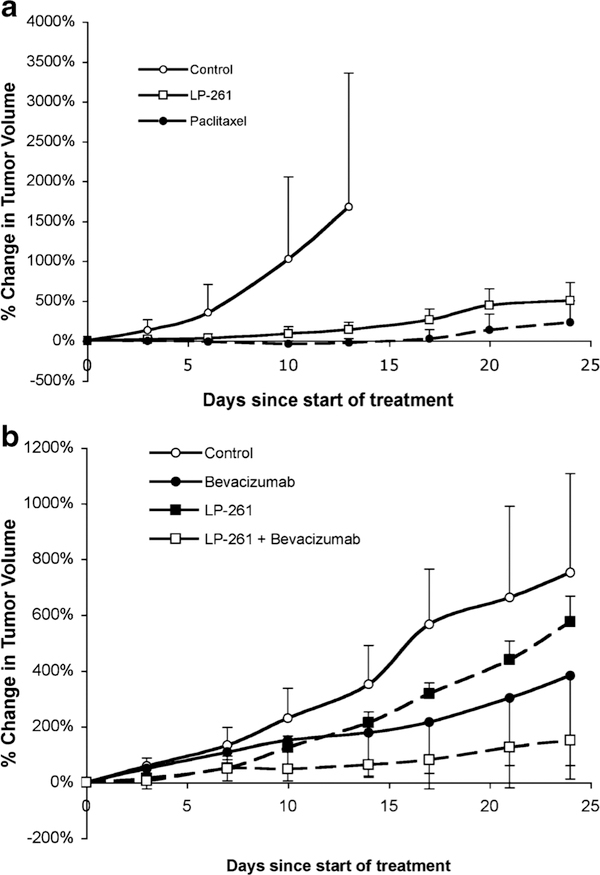
LP-261 was relatively well tolerated at the doses and schedules tested, and animals did not lose greater than 10% of their body weight during treatment. The initial in vivo study evaluated LP-261 at 50 mg/kg dosed orally, twice daily, in mice with SW620 colon adenocarcinoma xenografts. This dosing frequency was chosen based on the short plasma half-life observed in the pharmacokinetic studies. The results are shown in Fig. 5a. Control mice were euthanized after 14 days of treatment, due to tumor volume. LP-261 significantly inhibited tumor growth, as compared to control at day 14 (P=0.009). The activity was comparable to that of paclitaxel dosed at 15 mg/kg once weekly. Paclitaxel was chosen as the control for this experiment since it is a commonly-used anti-tubulin agent known to be highly active in the SW620 xenograft model.
Fig. 5.
a SW620 xenograft tumor growth. Open circles represent animals treated with vehicle control only (n=10). Open squares represent animals treated with 50 mg/kg LP-261, po, BID, daily (n=6). Filled circles represent animals treated with 15 mg/kg paclitaxel, IV, once weekly (n=6). Animals were treated for 24 days except for control animals sacrificed at day 14. b) Change in SW620 xenograft tumor volume with vehicle control (open circles), 12.5 mg/kg LP-261 po daily, 5×/week (filled squares), 5 mg/kg bevacizumab ip twice weekly (filled circles) or the combination (open squares).All animals were treated for 24 days. Each marker represents the mean (n≥6)±S.D. percentage change in tumor volume since the start of treatment
Mice with prostate cancer xenografts (PC3 or LNCaP) were treated at the same dose (50 mg/kg), administered twice daily via oral gavage, five times per week (total dose per week=500 mg/kg). However, this dose and schedule proved ineffective in these tumor types, with no difference in tumor size between vehicle control and treatment groups (data not shown).
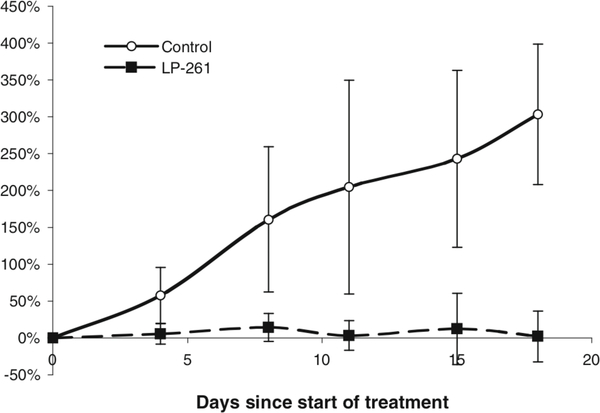
Subsequently, mice bearing PC3 xenografts were treated with 100 mg/kg, twice daily via oral gavage, for 3 days, followed by 4 days without treatment (total dose per week=600 mg/kg). As shown in Fig. 6, this modification in schedule and dose resulted in significant tumor growth inhibition as compared to vehicle control treated animals (P=0.007, comparison of change in tumor volume on last day of treatment). Due to the doubling of the dose, the 3 days on-4 days off schedule was necessary to avoid unnecessary toxicity or weight loss in the mice.
Fig. 6.
Change in PC3 xenograft tumor volume with vehicle control (open circles) or 100 mg/kg LP-261, po, BID for 3 days, followed by 4 days off (filled squares). Animals (n=6) were treated for 18 days. Each marker represents the mean±S.D. percentage change in tumor volume since the start of treatment
Low dose LP-261 (12.5 mg/kg once daily, 5 days per week) was tested as a single agent and in combination with bevacizumab, in the SW620 xenograft model. As shown in Fig. 5b, treatment with low dose LP-261 did not result in tumor growth inhibition. However, combination of LP-261 with bevacizumab resulted in significant tumor growth inhibition as compared to vehicle control (P=0.005) and was superior to either agent given alone (combination versus bevacizumab alone, P=0.004; combination versus LP-261 alone, P=0.02).
Discussion
There has been sustained interest in the development of an orally active anti-tubulin agent, to simplify drug administration, and to reduce the toxicity associated with the excipients used in the formulation of the currently approved drugs. For example, paclitaxel is highly insoluble and is therefore formulated in a 50:50 mixture of ethanol and Cremophor EL, a polyethoxylated castor oil. The Cremophor EL, as opposed to the active pharmacologic agent, is considered to be dose-limiting, requires a lengthy infusion, and pre-treatment with corticosteroids and antihistamines is necessary to avoid hypersensitivity reactions [4].
Numerous studies have aimed to improve oral bioavailability of the currently approved therapies, often through inhibition of ABCBI. Vinblastine, vincristine, vinorelbine, paclitaxel and docetaxel are all known to be substrates for ABCB I, or P-glycoprotein, which acts as an efflux transporter in the intestine, severely limiting oral bioavailability. In addition, ABCBI limits distribution to other organs, such as the brain due to high expression in the cells forming the blood brain barrier. The Caco-2 permeability experiments presented here, designed to simulate conditions in the intestinal epithelium, suggest that LP-261 would not be significantly effluxed in the intestine by efflux transporters such as ABCB I or ABCG2. Additionally, the calculated efflux ratio of less than I suggests that the transport of LP-261 may be mediated by an influx transporter. These properties should theoretically improve the oral absorption ofLP-261, and this is reflected in the 80% bioavailability observed in rats. As such, oral dosing was employed for all in vivo efficacy studies.
If LP-261 is indeed not a substrate for efflux transporters, as suggested by the Caco-2 experiments, it may be able to overcome multidrug resistance, a major hurdle to effective cancer therapy. ABCBI has been shown to be upregulated in many tumors, due to rapid efflux of substrate drugs out of the tumor, resulting in minimal tumor exposure to the anticancer agent. This can result in treatment failure both with the agent of interest and other substrates of the transporter.
The significant activity noted in the HUVEC proliferation experiments and the rat aortic ring assay suggest that LP-261 possesses antiangiogenic properties, in addition to the impressive cytotoxic activity observed in the NC160 cancer cell line screen. The use of other tubulin targeting agents at lower, more frequent doses (metronomic therapy) to achieve an antiangiogenic effect, appears to be a promising clinical strategy [5, 6].
LP-261 exhibited significant activity in the tested colon xenograft model when administered at 50 mg/kg, twice daily, 7 days per week (total drug per week=700 mg/kg). However, the same dose, given twice daily, 5 days per week (total drug per week=500 mg/kg) had no effect on either the androgen dependent LNCaP tumors or the androgen-independent PC3 tumors. Doubling of the dose, while reducing the number of days administered to three sequential days per week (total drug per week=600 mg/kg) resulted in excellent activity in the PC3 xenograft model. This data suggests that the activity is highly schedule and dose dependent and that there may be an exposure threshold required for LP-261 activity.
Promising anticancer activity has been observed preclinically and clinically with the combination of metronomic chemotherapy and antiangiogenic agents [7]. Combining low-dose LP-261 (12.5 mg/kg) with bevacizumab (Avastin), a monoclonal antibody against VEGF, led to improved efficacy as compared to low-dose LP-261 alone and this treatment regimen was well tolerated in mice. Though the studies were performed independently, the combination of low-dose LP-261 (12.5 mg/kg, daily) with bevacizumab appears also to be superior to the activity of high-dose LP-261 (50 mg/kg). Bevacizumab has been clinically tested in combination with numerous other anticancer agents, including paclitaxel, docetaxel and vinorelbine [8–10]. In a large study of bevacizumab with paclitaxel in women with breast cancer, longer progression-free survival, but not overall survival was observed, as compared to paclitaxel alone [10].
LP-261 appears to be a promising orally active anticancer drug with several mechanisms of action that may be able to circumvent problems with multidrug resistance and warrants further investigation. It is currently in clinical development, and Phase I studies are underway.
Supplementary Material
Acknowledgements
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract NOI-CO-12400 and HHSN261200800001E. 1 The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
This work was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
E. R. Gardner
Contributor Information
Erin R. Gardner, Clinical Pharmacology Program, SAIC-Frederick, NCI-Frederick, Frederick, MD 21702, USA
Martha Kelly, Blue Bell, PA 19422, USA.
Eric Springman, Blue Bell, PA 19422, USA.
Kyoung-jin Lee, Blue Bell, PA 19422, USA.
Haiqing Li, Molecular Pharmacology Section, Medical Oncology Branch, National Cancer Institute, Bethesda, MD 20892, USA.
William Moore, Blue Bell, PA 19422, USA.
William D. Figg, Molecular Pharmacology Section, Medical Oncology Branch, National Cancer Institute, Bethesda, MD 20892, USA Medical Oncology Branch, National Cancer Institute, 9000 Rockville Pike, Building 10, Room 5A01, Bethesda, MD 20892, USA, wdfigg@helix.nih.gov.
References
- 1.Andreopoulou E, Muggia F (2008) Pharmacodynamics of tubulin and tubulin-binding agents: extending their potential beyond taxanes. Clin Breast Cancer 8(Suppl 2):S54–S60 [DOI] [PubMed] [Google Scholar]
- 2.Shetty R, Lee Y, Liu B, et al. (2010) Discovery of N-(3-(lH-indol-4-yl)-5-(2-methoxyisonicotinoyl)phenyl)methanesulfonamide (LP-261), a potent antimitotic agent: synthesis, structure activity relationship study, pharmacokinetics and in vivo antitumor activity evaluation. J Med Chem (in press) [DOI] [PubMed] [Google Scholar]
- 3.Shoemaker RH (2006) The NC160 human tumour cell line anticancer drug screen. Nat Rev Cancer 6:813–823. doi: 10.1038/nrc1951 [DOI] [PubMed] [Google Scholar]
- 4.Gelderblom H, Verweij J, Nooter K, Sparreboom A (2001) Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer 37: 1590–1598 [DOI] [PubMed] [Google Scholar]
- 5.Lennernas B, Albertsson P, Je D, Norrby K (2004) Antiangiogenic effect of metronomic paclitaxel treatment in prostate cancer and non-tumor tissue in the same animals: a quantitative study. APMIS 112:201–209. doi: 10.1111/j.1600-0463.2004.apm1120306.x [DOI] [PubMed] [Google Scholar]
- 6.Sl S, Mm C, Dowlati A et al. (2008) Phase I trial of docetaxel and thalidomide: a regimen based on metronomic therapeutic principles. Invest New Drugs 26:355–362. doi: 10.1007/s10637-008-9137-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rs K, Ba K (2004) The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer 4:423–436. doi: 10.1038/nrc1369 [DOI] [PubMed] [Google Scholar]
- 8.Hj B, Chen Yh, Lm P et al. (2008) VEGF as a marker for outcome among advanced breast cancer patients receiving anti-VEGF therapy with bevacizumab and vinorelbine chemotherapy. Clin Cancer Res 14:7871–7877. doi: 10.1158/1078-0432.CCR-08-0593 [DOI] [PubMed] [Google Scholar]
- 9.Di Lorenzo G, Wd F, Sd F et al. (2008) Combination of bevacizumab and docetaxel in docetaxel-pretreated hormone-refractory prostate cancer: a phase 2 study. Eur Urol 54:1089–1094. doi: 10.1016/j.eururo.2008.01.082 [DOI] [PubMed] [Google Scholar]
- 10.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Ea P, Shenkier T, Cella D, Ne D (2007) Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 357:2666–2676. 10.1056/NEJMoa072113 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.