Abstract
Introduction
We examined the influence of common preanalytical factors on the measurement of Alzheimer's disease–specific biomarkers in human plasma.
Methods
Amyloid β peptides (Aβ[1-40], Aβ[1-42]) and total Tau plasma concentrations were quantified using fully automated Roche Elecsys assays.
Results
Aβ(1-40), Aβ(1-42), and total Tau plasma concentrations were not affected by up to three freeze/thaw cycles, up to five tube transfers, the collection tube material, or the size; circadian rhythm had a minor effect. All three biomarkers were influenced by the anticoagulant used, particularly total Tau. Aβ concentrations began decreasing 1 hour after blood draw/before centrifugation and decreased by up to 5% and 10% at 2 and 6 hours, respectively. For separated plasma, time to measurement influenced Aβ levels by up to 7% after 6 hours and 10% after 24 hours.
Discussion
Our findings provide guidance for standardizing blood sample collection, handling, and storage to ensure reliable analysis of Alzheimer's disease plasma biomarkers in routine practice and clinical trials.
Keywords: Alzheimer's disease-specific biomarkers, Elecsys immunoassay, Plasma, Sample handling, Amyloid, Tau, Preanalytics, Alzheimer's disease, AD, Biomarkers, Fully automated
Highlights
-
•
Blood-based Alzheimer's disease biomarkers were measured with fully automated and highly precise Roche Elecsys assays.
-
•
Preanalytical sample handling affected measured concentrations of biomarkers.
-
•
Time between sample collection and centrifugation, and between centrifugation and measurement, impacted the measured concentrations of Aβ peptides.
-
•
Type of anticoagulant substantially affected measured levels of total Tau.
-
•
Recommendations for standardized sample collection and processing are provided.
1. Introduction
Dementia is considered one of the biggest threats to the aging population and is also a major public health problem worldwide, with significant socioeconomic implications. The leading cause of dementia is Alzheimer's disease (AD), accounting for 50%–80% of all dementia cases [1]. At present, clinical diagnosis of AD is only correct in ∼77% of cases, with a significant number of patients diagnosed when their disease has already advanced [2], [3]. Poor diagnostic accuracy hampers appropriate and comprehensive management of AD. Given that novel disease-modifying therapies for AD will likely be most efficacious when administered in the early stages of the disease, there is a growing demand for tools that can accurately detect and diagnose AD at presymptomatic stages. A promising approach to improving the diagnostic certainty of AD is the application of disease-associated biomarkers that reflect the nature and severity of the neuropathologic processes in the AD brain. Numerous studies have confirmed the relevance of the cerebrospinal fluid (CSF) biomarkers amyloid β peptide 42 (Aβ[1-42]), total Tau (tTau), and 181Thr-phosphorylated Tau to the diagnosis of AD [4], [5], [6]. In combination, these biomarkers distinguish patients with AD from controls with sensitivity and specificity in the range of 85%–95% [7]. CSF biomarkers are already integrated into the National Institute on Aging/Alzheimer's Association Diagnostic Guidelines for AD and are being used routinely in clinical trials for participant selection and monitoring of drug response and disease progression [8], [9], [10]. However, in recent years, considerable research efforts have been devoted to identifying blood-based biomarkers that could eventually replace CSF and positron emission tomography biomarkers. The greatest advantage of blood-based biomarkers versus other types of biomarker is that collection of blood is less invasive and involves a more cost-effective and time-efficient procedure. As an intermediate step before replacing CSF and positron emission tomography biomarkers, blood-based biomarkers for AD could be considered optimal screening tools to identify individuals at risk who could subsequently be screened, and their condition confirmed, by positron emission tomography imaging or CSF analysis [11], [12]. Experience with CSF AD biomarkers, however, shows that preanalytical factors linked to sample handling and processing before measurement can greatly influence the measured concentration of protein biomarkers [13]. Lack of standardization across these preanalytical procedures inevitably leads to significant variability in the results, preventing the establishment of universal cutoff values and direct between-laboratory and between-study comparisons.
Given the rapid progress toward developing blood-based biomarkers for clinical use in AD [14], [15], [16], [17], the primary aim of the present study was to investigate the impact of common preanalytical variables (circadian rhythm, type of collection tube, type of anticoagulant, varying times to blood centrifugation and plasma separation, storage time and temperature of plasma samples before analysis, as well as the number of freeze/thaw cycles and tube transfers) on the measurable concentrations of Aβ(1-40), Aβ(1-42), and tTau in human plasma samples. In addition, results presented in this study utilized, for the first time, a highly precise, robust, fully automated, high-throughput analytical method (Elecsys technology) to recommend procedures for collection, handling, and storage of blood samples that will support accurate and reliable analysis of AD-specific plasma biomarkers in routine practice and clinical trials.
2. Methods
2.1. Plasma measurements
Plasma concentrations of Aβ(1-40), Aβ(1-42), and tTau were quantified using Roche Elecsys robust prototype assays on the fully automated cobas e 601 analyzer. The Roche Elecsys robust prototype assays used in this study were adaptations from the commercially available CSF assays using the same antibodies and buffer compositions but using plasma calibrators and controls [18]. The coefficient of variation (CV) for intraassay and interassay precision, respectively, are ≤1.4% and ≤1.1% for Aβ(1-40), ≤1.6% and ≤3.0% for Aβ(1-42), ≤1.3% and ≤2.1% for tTau. The lower limit of quantification of these noncommercial, robust prototype assays is 9 pg/mL, 6 pg/mL, and 1 pg/mL for plasma Aβ(1-40), Aβ(1-42), and tTau assay, respectively.
2.2. Sample collection and handling
Blood samples were obtained from healthy volunteers at Roche Medical Center (Penzberg, Germany). All participants gave written informed consent for their blood samples to be used for research. Ethical approval was received from the local ethics committee (Die Ethikkommission bei der Bayerischen Landesärztekammer) for use of blood samples in this study. Venipuncture was performed following standard operating procedures. Free flow of blood with mild aspiration was ensured to avoid hemolysis. Immediately after filling, the collection tube was inverted five times to ensure proper mixing of blood and anticoagulant, or other additive. The collected blood was kept in the collection tube at room temperature (RT) pending centrifugation. Unless otherwise stated, blood samples were centrifuged within 1 hour of collection for 10 minutes at 2000 g at RT in a swing bucket centrifuge (except for the experiment reported in Section 3.4). Separated plasma was transferred into a 15 mL polypropylene (PP) tube then, mixed by inversion several times before immediate aliquoting into 0.5 mL PP tubes. All samples were processed as described unless specified; details of all tubes are provided in Supplementary Table 1.
2.3. Sample collection/handling parameter assessments
We analyzed the effects of the following preanalytical variables on quantitative analysis of Aβ(1-40), Aβ(1-42), Aβ(1-42): Aβ(1-40) ratio, and tTau in human plasma: circadian rhythm, type of anticoagulant, material, size and filling height of a primary collection tube, time between blood draw and centrifugation, duration and temperature of storage of plasma before measurement, number of tube transfers, and number of freeze/thaw cycles at different freezing temperatures. Detailed information on blood collection and sample processing for individual analyses is provided in Supplementary Table 2.
2.4. Statistical analysis
For all analyses, the mean and CV of tested subjects were calculated. Sample recovery rates under test conditions were reported as mean percentage differences. Outliers were defined as any data points >1.5 interquartile ranges below the first quartile or above the third quartile. No formal statistical testing was conducted; all analyses are descriptive.
3. Results
A summary of the analyses conducted and results obtained is provided in Table 1.
Table 1.
Summary of the preanalytical variable assessments in this study
| Parameter | Factor | Outcome∗ |
|---|---|---|
| Circadian rhythm (Fig. 1A) |
|
|
| Anticoagulant (Fig. 1B) (EDTA used as reference) |
|
|
|
|
|
| Tube parameters (Fig. 1C; Fig. 1D) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Whole blood stability in EDTA tube (Fig. 2A) |
|
|
| ||
| ||
| EDTA plasma storage after thawing (Fig. 2B) |
|
|
|
|
|
| Fresh EDTA plasma storage (Fig. 2C) |
|
|
| Number of tube transfers (Fig. 3A) |
|
|
| Effect of the collection tube on stability during freezing (Fig. 3B) |
|
|
|
|
|
|
|
|
|
|
|
| Freeze/thaw cycles (–80°C) (Fig. 3C) |
|
|
| Freeze/thaw cycles (–25°C) (Fig. 3D) |
|
|
| Freeze/thaw cycles (–20°C) (Fig. 3D) |
|
|
Abbreviations: Aβ, amyloid β; EDTA, ethylenediaminetetraacetic acid; Li-heparin, lithium heparin; PET, polyethylene terephthalate; PP, polypropylene; RT, room temperature; tTau, total Tau.
Outcomes listed apply to all peptide measures unless otherwise indicated.
Only Aβ(1-40) and Aβ(1-42) and Aβ(1-42): Aβ(1-40) assessed; tTau not evaluated due to the high stability of tTau in thawed plasma.
3.1. Impact of circadian rhythm
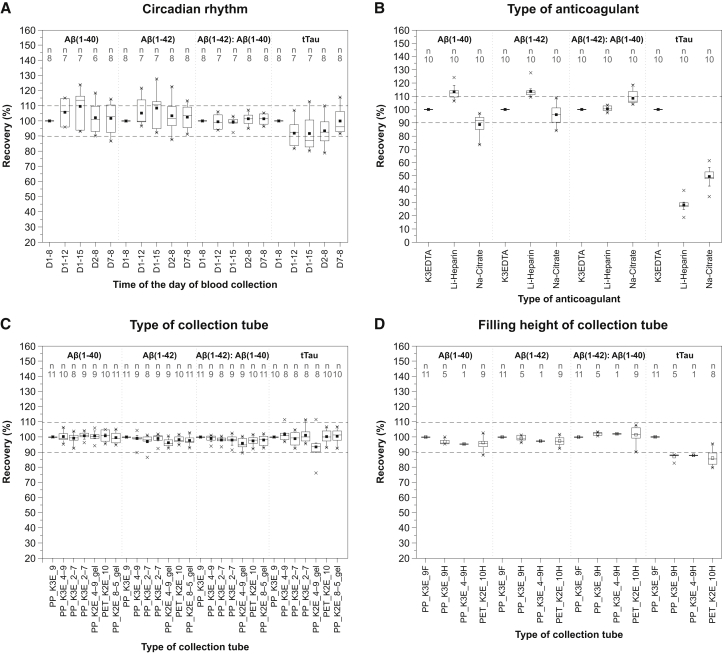
All three biomarkers showed a weak throughout-the-day circadian rhythmicity. Aβ(1-40) and Aβ(1-42) levels were a mean average of 5%–9% higher in samples collected in the afternoon versus morning, while the opposite trend was observed for tTau (Fig. 1A). Intrasubject variability (CV) over the 8-hour period was between 3% and 13% for plasma Aβ and tTau proteins. Within-day fluctuations in blood levels of Aβ(1-40) and Aβ(1-42) had no effect on the Aβ(1-42): Aβ(1-40) ratio. No marked difference in plasma concentrations of Aβ(1-40), Aβ(1-42), or tTau was observed between the 8:00 AM values on days 1, 2, and 7.
Fig. 1.
The effect of parameters related to blood sample collection on plasma levels of Aβ peptides and tTau. Circadian rhythm (A): plasma Aβ(1-40), Aβ(1-42), Aβ(1-42): Aβ(1-40) ratio, and tTau throughout the day (8:00 AM, 12:00 PM, and 3:00 PM) and across 1, 2, and 7 days (8:00 AM). Values for each time point are normalized to the baseline 8:00 AM values on day 1. D1–8 = day 1 at 8:00 AM; D1–12 = day 1 at 12:00 PM; D1–15 = day 1 at 3:00 PM; D2–8 = day 2 at 8:00 AM; D7–8 = day 7 at 8:00 AM. Type of anticoagulant (B): Aβ(1-40), Aβ(1-42), Aβ(1-42): Aβ(1-40) ratio, and tTau in K3-EDTA, Li-heparin, and Na-citrate plasma. Values for heparinized and citrated plasma were normalized to the values in K3-EDTA plasma. Type of collection tube (C): Aβ(1-40), Aβ(1-42), Aβ(1-42): Aβ(1-40) ratio, and tTau in plasma separated from blood collected into different primary collection tubes. Values for different tubes are normalized to the S-Monovette® K3-EDTA 9 mL tube. Filling height (D): Aβ(1-40), Aβ(1-42), Aβ(1-42): Aβ(1-40) ratio, and tTau in half-filled tubes. Values for different tubes are normalized to fully filled S-Monovette® K3-EDTA 9 mL tube. Horizontal lines and filled squares within each box represent the median and the mean of the sample, respectively. The bottom and the top of each box represent the first and the fourth quartiles. Error bars represent the standard deviation. Outliers are represented as small stars. Abbreviations: Aβ, amyloid β; gel, presence of gel separator in a tube; K2E, K2-EDTA; K3E, K3-EDTA; Li-heparin, lithium heparin; Na-citrate, sodium citrate; PET, polyethylene terephthalate; PP, polypropylene; tTau, total Tau, 9F, 9 mL tube filled 100%; 9H, 9 mL tube filled 50%; 4-9H, 4.9 mL tube filled 50%; 10H, 10 mL tube filled 50%. For tube type abbreviations, please see Supplementary Table 1.
3.2. Impact of choice of anticoagulant
Since ethylenediaminetetraacetic acid (EDTA) allows the best preservation of cellular components and morphology of blood cells, it is often used as the anticoagulant of choice for hematologic testing [19]. Aβ(1-40), Aβ(1-42), Aβ(1-42): Aβ(1-40) ratio, and tTau concentrations determined in heparinized and citrated plasma were normalized to the levels in K3-EDTA plasma. Both commonly used anticoagulants (lithium heparin [Li-heparin] and sodium citrate [Na-citrate]) had a significant impact on the measurement of all three AD biomarkers in plasma, and on tTau in particular (Fig. 1B). For tTau, use of Na-citrate or Li-heparin reduced tTau levels by up to 50% and 70%, respectively, versus K3-EDTA. Aβ(1-40) and Aβ(1-42) levels in plasma with Na-citrate were 10% and 5% lower, respectively, versus K3-EDTA. Samples collected in Li-heparin showed a 15% increase in Aβ(1-40) and Aβ(1-42) levels, compared with K3-EDTA samples. The presence of Na-citrate but not Li-heparin led to an 8% increase in the Aβ(1-42): Aβ(1-40) ratio, versus K3-EDTA.
3.3. Impact of size, material, and filling height of the primary collection tube
There was no marked difference in the levels of Aβ(1-40), Aβ(1-42), Aβ(1-42): Aβ(1-40) ratio, and tTau, between different materials (PP vs. polyethylene terephthalate) or tube sizes (Fig. 1C). There was no change in measured concentrations of any biomarker with use of different potassium salts (K2-EDTA vs. K3-EDTA) or the presence of a gel separator in the collection device. The mean values of intratube CV were 2.3%, 2.4%, 2.6%, and 4.6% for Aβ(1-40), Aβ(1-42), Aβ(1-42): Aβ(1-40) ratio, and tTau, respectively.
There was no marked difference in the recovery of Aβ(1-40), Aβ(1-42), and/or Aβ(1-42): Aβ(1-40) ratio between half-filled and fully filled tubes (Fig. 1D). A 12%–14% reduction in the measured concentration of tTau was observed for half-filled tubes versus fully filled tubes.
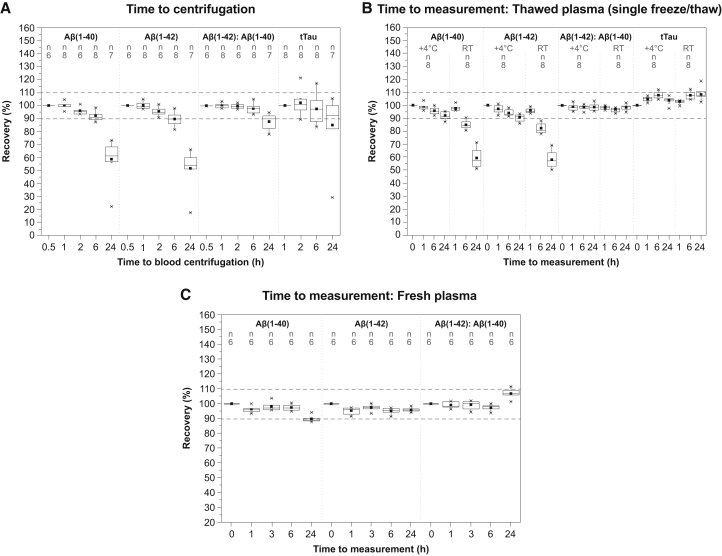
3.4. Stability of whole blood before plasma separation
Aβ(1-40) and Aβ(1-42) were unstable in whole blood; levels of both biomarkers started decreasing 1 hour after blood collection, leading to the reduction of Aβ peptides in plasma levels by 4%–5% and 8%–10% after 2 and 6 hours, respectively, compared with baseline (30 minutes after blood collection). Increasing the time between blood draw and centrifugation to 24 hours resulted in the loss of 50% of the measurable amount of Aβ(1-40) and Aβ(1-42) at baseline. The Aβ(1-42): Aβ(1-40) ratio had a higher tolerance with respect to time to plasma separation and remained unchanged for up to 6 hours after blood collection. tTau demonstrated very high stability in whole blood, for at least 6 hours.
3.5. Stability of previously frozen and thawed plasma
The stability of Aβ(1-40) and Aβ(1-42) was not affected by sample storage at +4°C or RT during the first hour after thawing (Fig. 2B). At later time points, the decrease in Aβ(1-40) and Aβ(1-42) levels was less if stored at +4°C, compared with RT: mean concentrations of Aβ(1-40) and Aβ(1-42) after 6 hours decreased by 5%–7% at +4°C compared with 15%–18% at RT; after 24 hours, Aβ(1-40) and Aβ(1-42) concentrations were decreased by 8%–10% versus 40% in plasma samples stored at +4°C and RT, respectively. Storage time and temperature did not impact the Aβ(1-42): Aβ(1-40) ratio or tTau concentrations, which remained unaffected for up to 24 hours at both +4°C and RT.
Fig. 2.
The effect of time to centrifugation and storage conditions before analysis on plasma levels of Aβ peptides and tTau. Time to centrifugation (A): Aβ(1-40), Aβ(1-42), Aβ(1-42): Aβ(1-40) ratio, and tTau in plasma separated after 0.5 (Aβ peptides only), 1, 2, 6, and 24 hours at RT after blood collection. Values for each time point were normalized to the baseline 0.5 hour and 1 hour for Aβ(1-40), Aβ(1-42), Aβ(1-42): Aβ(1-40) ratio, and tTau, respectively. Time to measurement in plasma samples that were frozen and thawed once (B): Aβ(1-40), Aβ(1-42), Aβ(1-42): Aβ(1-40) ratio, and tTau stored at +4°C or at RT for 1, 6, and 24 hours after thawing. Values were normalized to baseline levels in freshly thawed plasma. Time to measurement–fresh plasma (C): Aβ(1-40), Aβ(1-42), and Aβ(1-42): Aβ(1-40) ratio in freshly separated plasma after storage for 1, 3, 6, and 24 hours at +4°C. Values were normalized to baseline (time = 0) in freshly separated plasma. Horizontal lines and filled squares within each box represent the median and the mean of the sample, respectively. The bottom and the top of each box represent the first and the fourth quartiles. Error bars represent the standard deviation. Outliers are represented as small stars. Abbreviations: Aβ, amyloid β; RT, room temperature; tTau, total Tau.
3.6. Stability of freshly separated plasma
During the first 3 hours after centrifugation, there was no marked difference in the stability between Aβ(1-40) and Aβ(1-42) (Fig. 2C). However, after 6 hours, Aβ(1-40) and Aβ(1-42) levels decreased by 3% and 5%, respectively. After 24 hours, Aβ(1-40) levels were reduced by 10%, but Aβ(1-42) levels did not change further. This effect on Aβ(1-40) but not Aβ(1-42) negatively affected the Aβ(1-42): Aβ(1-40) ratio which increased by 10% after 24 hours.
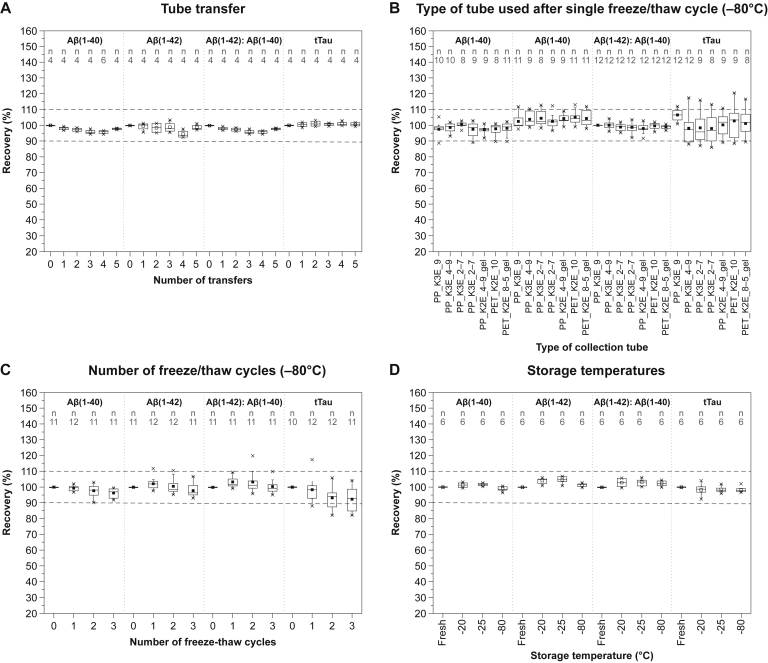
3.7. Impact of tube transfers
There was no marked effect of tube transfer on the measured levels of Aβ(1-40), Aβ(1-42), Aβ(1-42): Aβ(1-40) ratio, and tTau (mean difference in levels between no tube transfer and five tube transfers was 2%, 1%, 2%, and 1%, respectively [Fig. 3A]).
Fig. 3.
The effect of additional handling procedures on plasma levels of Aβ peptides and tTau. Tube transfer (A): Aβ1-40, Aβ1-42, Aβ42/40 ratio, and tTau in plasma after five consecutive transfers. Type of tube used after single freeze/thaw cycle (B): Aβ(1-40), Aβ(1-42), Aβ(1-42): Aβ(1-40) ratio, and tTau in plasma separated from blood collected into different primary tubes after one freeze/thaw cycle at –80°C. Values for different tubes were normalized to S-Monovette® K3-EDTA 9 mL tube. Number of freeze/thaw cycles (C): Aβ(1-40), Aβ(1-42), Aβ(1-42): Aβ(1-40) ratio, and tTau in plasma after one, two or three freeze/thaw cycles at –80°C. Values were normalized to baseline levels in freshly separated plasma before freezing. Storage temperatures (D): Aβ1-40, Aβ1-42, Aβ42/40 ratio, and tTau in plasma samples frozen and stored for 14 days at −20°C, −25°C, and −80°C. The values for each temperature were normalized to baseline levels in freshly separated plasma before freezing. Horizontal lines and filled squares within each box represent the median and the mean of the sample, respectively. The bottom and the top of each box represent the first and the fourth quartiles. Error bars represent the standard deviation. Outliers are represented as small stars. Abbreviations: Aβ, amyloid β; gel, presence of gel separator in a tube; K2E, K2-EDTA; K3E, K3-EDTA; PET, polyethylene terephthalate; PP, polypropylene; tTau, total Tau. For tube type abbreviations, please see Supplementary Table 1.
3.8. Impact of freeze/thaw cycles and freezing temperature
Before performing multiple freeze/thaw experiments, we first evaluated whether there was an association between the material and size of the primary blood collection tube and the recovery of Aβ(1-40), Aβ(1-42), Aβ(1-42): Aβ(1-40) ratio, and tTau after one freeze/thaw cycle. The recovery of Aβ(1-40), Aβ(1-42), Aβ(1-42): Aβ(1-40) ratio, and tTau after one freeze/thaw cycle was unaffected by the size and material of the collection tube (Fig. 3B). Between-tube variability was 1.4%–4.6%, 1.2%–2.6%, 0.5%–3.9%, and 1.2%–4.9% for Aβ(1-40), Aβ(1-42), Aβ(1-42): Aβ(1-40) ratio, and tTau, respectively. Therefore, we conducted a multiple freeze/thaw cycle experiment using only the S-Monovette® K3-EDTA 9 mL tube (Sarstedt, Nümbrecht, Germany). Aβ(1-40) and Aβ(1-42) levels were only minimally reduced (decrease did not exceed 4%) by repeated freeze/thaw cycles at –80°C. tTau levels decreased by 7% after the second freeze/thaw cycle but remained unchanged between the second and third freeze/thaw cycle (Fig. 3C). Alongside –80°C, freeze/thaw stability at −20°C and −25°C was also evaluated (data not shown). tTau levels slightly decreased after the third freeze/thaw cycle at −20°C and −25°C (by 3% and 2%, respectively). Aβ(1-40) levels were unchanged, but slight increases of 4% and 5% were seen for Aβ(1-42) after the first freeze/thaw cycle at −20°C and −25°C, respectively.
In addition, the storage stability of all three biomarkers at −20°C, −25°C, and –80°C for 14 days was tested. No marked differences in the measured concentrations of Aβ(1-40), Aβ(1-42), Aβ(1-42): Aβ(1-40) ratio, and tTau were observed between plasma samples frozen and stored at −20°C, −25°C and −80°C for 14 days (Fig. 3D).
4. Discussion
Preanalytical factors are responsible for up to 60% of errors in laboratory medicine [20]. Numerous studies have shown that even small differences in preanalytical variables, such as collection and handling of a specimen before analysis, have a direct influence on the quality of analytical results and their clinical reliability [13]. Differences in preanalytical procedures were also found to be the main source of variability in absolute concentrations of CSF AD biomarkers between different centers, even when using the same assay [21], [22], [23]. Given an increasing interest in blood-based biomarkers for AD, the understanding and characterization of preanalytical sample handling factors that may impact the quantification of AD-related proteins in plasma is critical to ensuring interlaboratory and intralaboratory consistency of analytical results.
As previously reported, circadian rhythm is involved in many physiologic processes, and several functional proteins have been shown to exhibit strong diurnal dynamics [24], [25]. Such time-of-day-dependent oscillations in blood levels of molecules could be a potential cause of variability in laboratory results, making sampling time an important consideration. Consistent with earlier reports in which the circadian rhythm of Aβ isoforms in blood was assessed using the INNO-BIA multiplex assay [26], we found minor within-subject differences in the concentrations of Aβ(1-40), Aβ(1-42), and tTau throughout the day, with no effect on the Aβ(1-42): Aβ(1-40) ratio.
Another important factor when testing Aβ(1-40), Aβ(1-42), and tTau is the primary tube used for blood collection. A considerable number of studies have demonstrated that AD biomarkers (Aβ peptides in particular) are very prone to nonspecific adsorption to non-PP surfaces [27] especially for CSF [27], [28], [29]. In contrast to CSF, no measurable loss of Aβ was previously found in plasma when glass tubes were used for blood collection instead of plastic [26]. Our study provides further evidence that the primary collection tube material has no impact on the measured levels of plasma Aβ(1-40), Aβ(1-42), and tTau. Moreover, we show that quantification of Aβ(1-40), Aβ(1-42), and tTau is not affected by tube size, form of potassium salt, or the presence of gel separators. However, a 15% reduction in the measured concentration of tTau, but not Aβ(1-40) and Aβ(1-42), was seen when a collection tube was filled to only 50% of its nominal volume, compared with being fully filled.
We evaluated the potential impact of the time interval between blood collection and plasma separation, as well as the interval and storage temperature after plasma separation but before measurement. Several prior studies have demonstrated that delayed centrifugation of whole blood impacts the analysis of biochemical components in plasma and serum samples [30]. Quantification of blood-based protein biomarkers may be severely impaired due to, for example, degradation by multiple proteolytic enzymes in the blood, or in the plasma or serum present in supernatant after complete blood coagulation [31], [32], [33]. Moreover, the quantitative measurement of AD biomarkers might be affected by aggregation-dependent depletion of the monomeric forms of Aβ(1-40) and Aβ(1-42). Both Aβ peptides, but Aβ(1-42) in particular, are well known for their intrinsic instability manifested by their high propensity to oligomerize and form β-sheet–rich amyloid aggregates [34], [35]. Therefore, the time between blood collection and plasma separation, and between plasma separation and measurement, should be thoroughly investigated when testing AD biomarkers. Our study revealed a maximum of 1-hour tolerance of Aβ(1-40) and Aβ(1-42) between blood collection and separating plasma to avoid any decrease in peptide levels, whereas tTau remained stable for up to 6 hours. However, the use of Aβ(1-42): Aβ(1-40) ratio can compensate for the decrease in absolute peptide levels, with ratio measures unaffected in samples centrifuged 2 hours after collection. While we think that the impact of these preanalytical factors can be largely attributed to the degradation of the peptides ex vivo, the influencing factors identified in this study most likely apply to other detection methods as well. However, to be certain, we recommend that these common preanalytical factors be assessed individually for other detection methods and each additional biomarker, for example, neurofilament light.
Since experimental design frequently necessitates the use of frozen samples for retrospective analysis, we investigated the effect of storage conditions on the stability of Aβ(1-40), Aβ(1-42), and tTau in fresh (unfrozen) and previously frozen/thawed plasma samples. For thawed samples (after single freeze/thaw), we observed a time-dependent decrease in the stability of plasma Aβ(1-40) and Aβ(1-42), but not tTau, irrespective of RT or +4°C storage temperature. The decrease of the Aβ peptide levels is strongly influenced by temperature, leading to a 40% decrease in Aβ(1-40) and Aβ(1-42) levels in samples stored at RT. Storage at +4°C is acceptable for up to 6 hours for measurement of plasma Aβ(1-40), Aβ(1-42), and up to 24 hours if reporting the Aβ(1-42): Aβ(1-40) ratio. In cases of measuring fresh, previously unfrozen plasma samples, they are stable for at least 6 hours at +4°C, after which they should be analyzed or frozen for long-term storage.
Repeated freezing and thawing, often performed as part of sample processing, is also a potential source of variability. For plasma biomarkers, one study reported that measurable levels of Aβ(1-40) and Aβ(1-42) were decreased by up to 20% after three freeze/thaw cycles [36], while two other studies reported plasma Aβ(1-40), Aβ(1-42), and tTau remained stable over ≥3 cycles [36], [37]. In our study, plasma levels of Aβ(1-40) and Aβ(1-42) were unaffected for up to three freeze/thaw cycles at various freezing temperatures, while a 7% decrease was observed for tTau after the second freeze/thaw cycle. We also showed that the measured concentrations of plasma Aβ(1-40), Aβ(1-42), and tTau were not influenced by up to five consecutive transfers between tubes.
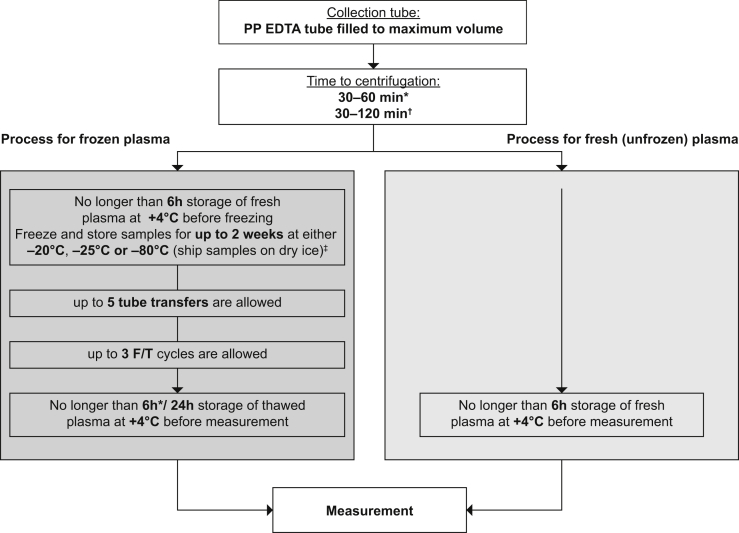
Based on our findings, we recommend the following guideline for the collection, handling, and storage of blood samples for the analysis of Aβ(1-40), Aβ(1-42), and tTau in plasma (Fig. 4): (1) collect blood in a PP tube containing EDTA as the anticoagulant; (2) fill the tube to its nominal volume; (3) separate the plasma from whole blood no later than 60 minutes after blood collection for Aβ(1-40) and Aβ(1-42) measurement, and no later than 120 minutes for the measurement of the Aβ(1-42): Aβ(1-40) ratio or tTau; (4) store plasma samples at +4°C before analysis irrespective of whether they are fresh (previously unfrozen) or previously frozen and thawed; (5) measure fresh plasma samples no later than 6 hours after plasma separation; (6) measure previously frozen plasma no later than 6 hours after plasma thawing for the measurement of Aβ(1-40) and Aβ(1-42) individually or 24 hours for the Aβ(1-42): Aβ(1-40) ratio; (7) if freezing, freeze freshly separated plasma at −20°C, −25°C, or −80°C no later than 6 hours after plasma separation; samples can be stored for up to 2 weeks at either −20°C, −25°C, or −80°C (ship samples on dry ice where necessary); (8) limit the number of freeze/thaw cycles to a maximum of three and the number of tube transfers to a maximum of five.
Fig. 4.
Recommendations for blood collection and sample handling for the analysis of AD-specific biomarkers. Abbreviations: EDTA, ethylenediaminetetraacetic acid; F/T, freeze/thaw. *If Aβ peptides are reported individually. †If Aβ(1-42): Aβ(1-40) ratio or tTau is reported. ‡Longer storage times have not been tested.
We believe that the recommendations outlined here will help greatly reduce the variability and enable the direct comparison of blood-based biomarker results across laboratories and studies. This will be an essential prerequisite for the integration of blood-based biomarkers into clinical trials and routine practice for supporting the diagnosis of AD.
Research in context.
-
1.
Systematic review: Alzheimer's disease (AD)–specific blood-based biomarkers could provide a less invasive, more cost- and time-efficient alternative to cerebrospinal fluid biomarkers in the diagnosis of AD. Experience with cerebrospinal fluid biomarkers suggests that preanalytical sample handling substantially impacts the measured concentration of protein biomarkers.
-
2.
Interpretation: Plasma concentrations of amyloid β (Aβ) peptide 1-40 (Aβ1-40), Aβ(1-42), and total Tau (tTau) were quantified using Roche Elecsys assays. Time between sample collection and centrifugation, and time between centrifugation and measurement, appeared to be an important source of variability for measuring Aβ concentrations. tTau levels varied substantially depending on the anticoagulant used.
-
3.
Future directions: Based on our findings, we provide recommendations for a standardized process for preanalytical sample handling. Establishing a standardized, robust process for sample collection and preanalytical sample handling is crucial for the assessment of blood-based biomarkers for AD to become a part of clinical trial methodology and routine clinical practice.
Acknowledgments
The authors would like to thank the research volunteers who participated in this study. The authors also gratefully acknowledge Lisa Protzer (Roche Diagnostics) for her technical support. Editorial assistance in the preparation of this manuscript was provided by Rachel Johnson, PhD, funded by F. Hoffman La Roche Ltd. Funding: This work was conducted by Roche Diagnostics.
Footnotes
Conflicts of interest: M Rózga is an employee of Roche Diagnostics GmbH. T. Bittner is an employee of F. Hoffmann-La Roche Ltd. Richard Batrla is an employee of Roche Diagnostics International Ltd. J Karl was an employee of Roche Diagnostics GmbH at the time of this study.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.dadm.2019.02.002.
Supplementary Data
Sample collection for individual analyses
Sample tubes used in this study
References
- 1.Waldemar G., Phung K.T., Burns A., Georges J., Hansen F.R., Iliffe S. Access to diagnostic evaluation and treatment for dementia in Europe. Int J Geriatr Psychiatry. 2007;22:47–54. doi: 10.1002/gps.1652. [DOI] [PubMed] [Google Scholar]
- 2.Beach T.G., Monsell S.E., Phillips L.E., Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005-2010. J Neuropathol Exp Neurol. 2012;71:266–273. doi: 10.1097/NEN.0b013e31824b211b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sabbagh M.N., Lue L.F., Fayard D., Shi J. Increasing precision of clinical diagnosis of Alzheimer's disease using a combined algorithm incorporating clinical and novel biomarker data. Neurol Ther. 2017;6:83–95. doi: 10.1007/s40120-017-0069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonsen A.H., Herukka S.K., Andreasen N., Baldeiras I., Bjerke M., Blennow K. Recommendations for CSF AD biomarkers in the diagnostic evaluation of dementia. Alzheimers Dement. 2017;13:274–284. doi: 10.1016/j.jalz.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Blennow K., Zetterberg H. Cerebrospinal fluid biomarkers for Alzheimer's disease. J Alzheimers Dis. 2009;18:413–417. doi: 10.3233/JAD-2009-1177. [DOI] [PubMed] [Google Scholar]
- 6.Holtzman D.M. CSF biomarkers for Alzheimer's disease: current utility and potential future use. Neurobiol Aging. 2011;32:S4–S9. doi: 10.1016/j.neurobiolaging.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engelborghs S., De Vreese K., Van de Casteele T., Vanderstichele H., Van Everbroeck B., Cras P. Diagnostic performance of a CSF-biomarker panel in autopsy-confirmed dementia. Neurobiol Aging. 2008;29:1143–1159. doi: 10.1016/j.neurobiolaging.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 8.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubois B., Feldman H.H., Jacova C., Hampel H., Molinuevo J.L., Blennow K. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 10.Jack C.R., Jr., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B. NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hampel H., O'Bryant S.E., Molinuevo J.L., Zetterberg H., Masters C.L., Lista S. Blood-based biomarkers for Alzheimer disease: mapping the road to the clinic. Nat Rev Neurol. 2018;14:639–652. doi: 10.1038/s41582-018-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Bryant S.E., Mielke M.M., Rissman R.A., Lista S., Vanderstichele H., Zetterberg H. Blood-based biomarkers in Alzheimer disease: Current state of the science and a novel collaborative paradigm for advancing from discovery to clinic. Alzheimers Dement. 2017;13:45–58. doi: 10.1016/j.jalz.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansson O., Mikulskis A., Fagan A.M., Teunissen C., Zetterberg H., Vanderstichele H. The impact of preanalytical variables on measuring cerebrospinal fluid biomarkers for Alzheimer's disease diagnosis: A review. Alzheimers Dement. 2018;14:1313–1333. doi: 10.1016/j.jalz.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Ovod V., Ramsey K.N., Mawuenyega K.G., Bollinger J.G., Hicks T., Schneider T. Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement. 2017;13:841–849. doi: 10.1016/j.jalz.2017.06.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura A., Kaneko N., Villemagne V.L., Kato T., Doecke J., Doré V. High performance plasma amyloid-β biomarkers for Alzheimer's disease. Nature. 2018;554:249–254. doi: 10.1038/nature25456. [DOI] [PubMed] [Google Scholar]
- 16.Verberk I.M.W., Slot R.E., Verfaillie S.C.J., Heijst H., Prins N.D., van Berckel B.N.M. Plasma amyloid as prescreener for the earliest Alzheimer pathological changes. Ann Neurol. 2018;84:648–658. doi: 10.1002/ana.25334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teunissen C.E., Chiu M.J., Yang C.C., Yang S.Y., Scheltens P., Zetterberg H. Plasma amyloid-β (Aβ42) correlates with cerebrospinal fluid Aβ42 in Alzheimer's disease. J Alzheimers Dis. 2018;62:1857–1863. doi: 10.3233/JAD-170784. [DOI] [PubMed] [Google Scholar]
- 18.Bittner T., Zetterberg H., Teunissen C.E., Ostlund R.E., Jr., Militello M., Andreasson U. Technical performance of a novel, fully automated electrochemiluminescence immunoassay for the quantitation of β-amyloid (1-42) in human cerebrospinal fluid. Alzheimers Dement. 2016;12:517–526. doi: 10.1016/j.jalz.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 19.England J.M., Rowan R.M., van Assendelft O.W., Bull B.S., Coulter W., Fujimoto K. Recommendations of the International Council for Standardization in Haematology for Ethylenediaminetetraacetic Acid Anticoagulation of Blood for Blood Cell Counting and Sizing. International Council for Standardization in Haematology: Expert Panel on Cytometry. Am J Clin Pathol. 1993;100:371–372. doi: 10.1093/ajcp/100.4.371. [DOI] [PubMed] [Google Scholar]
- 20.Lippi G., Guidi G.C., Mattiuzzi C., Plebani M. Preanalytical variability: the dark side of the moon in laboratory testing. Clin Chem Lab Med. 2006;44:358–365. doi: 10.1515/CCLM.2006.073. [DOI] [PubMed] [Google Scholar]
- 21.Bjerke M., Portelius E., Minthon L., Wallin A., Anckarsäter H., Anckarsäter R. Confounding factors influencing amyloid beta concentration in cerebrospinal fluid. Int J Alzheimers Dis. 2010;2010 doi: 10.4061/2010/986310. pii:986310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fourier A., Portelius E., Zetterberg H., Blennow K., Quadrio I., Perret-Liaudet A. Pre-analytical and analytical factors influencing Alzheimer's disease cerebrospinal fluid biomarker variability. Clin Chim Acta. 2015;449:9–15. doi: 10.1016/j.cca.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 23.Vanderstichele H., Bibl M., Engelborghs S., Le Bastard N., Lewczuk P., Molinuevo J.L. Standardization of preanalytical aspects of cerebrospinal fluid biomarker testing for Alzheimer's disease diagnosis: a consensus paper from the Alzheimer's Biomarkers Standardization Initiative. Alzheimers Dement. 2012;8:65–73. doi: 10.1016/j.jalz.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Rivera-Coll A., Fuentes-Arderiu X., Díez-Noguera A. Circadian rhythms of serum concentrations of 12 enzymes of clinical interest. Chronobiol Int. 1993;10:190–200. doi: 10.3109/07420529309073887. [DOI] [PubMed] [Google Scholar]
- 25.Fournier S., Iten L., Marques-Vidal P., Boulat O., Bardy D., Beggah A. Circadian rhythm of blood cardiac troponin T concentration. Clin Res Cardiol. 2017;106:1026–1032. doi: 10.1007/s00392-017-1152-8. [DOI] [PubMed] [Google Scholar]
- 26.Lachno D.R., Vanderstichele H., De Groote G., Kostanjevecki V., De Meyer G., Siemers E.R. The influence of matrix type, diurnal rhythm and sample collection and processing on the measurement of plasma beta-amyloid isoforms using the INNO-BIA plasma Abeta forms multiplex assay. J Nutr Health Aging. 2009;13:220–225. doi: 10.1007/s12603-009-0062-5. [DOI] [PubMed] [Google Scholar]
- 27.Lewczuk P., Beck G., Esselmann H., Bruckmoser R., Zimmermann R., Fiszer M. Effect of sample collection tubes on cerebrospinal fluid concentrations of tau proteins and amyloid beta peptides. Clin Chem. 2006;52:332–334. doi: 10.1373/clinchem.2005.058776. [DOI] [PubMed] [Google Scholar]
- 28.Pica-Mendez A.M., Tanen M., Dallob A., Tanaka W., Laterza O.F. Nonspecific binding of Aβ42 to polypropylene tubes and the effect of Tween-20. Clin Chim Acta. 2010;411:1833. doi: 10.1016/j.cca.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 29.Perret-Liaudet A., Pelpel M., Tholance Y., Dumont B., Vanderstichele H., Zorzi W. Risk of Alzheimer's disease biological misdiagnosis linked to cerebrospinal collection tubes. J Alzheimers Dis. 2012;31:13–20. doi: 10.3233/JAD-2012-120361. [DOI] [PubMed] [Google Scholar]
- 30.Clark S., Youngman L.D., Palmer A., Parish S., Peto R., Collins R. Stability of plasma analytes after delayed separation of whole blood: implications for epidemiological studies. Int J Epidemiol. 2003;32:125–130. doi: 10.1093/ije/dyg023. [DOI] [PubMed] [Google Scholar]
- 31.Böttger R., Hoffmann R., Knappe D. Differential stability of therapeutic peptides with different proteolytic cleavage sites in blood, plasma and serum. PLoS One. 2017;12:e0178943. doi: 10.1371/journal.pone.0178943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dufresne J., Florentinus-Mefailoski A., Ajambo J., Ferwa A., Bowden P., Marshall J. The proteins cleaved by endogenous tryptic proteases in normal EDTA plasma by C18 collection of peptides for liquid chromatography micro electrospray ionization and tandem mass spectrometry. Clin Proteomics. 2017;14:39. doi: 10.1186/s12014-017-9174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaisar M., van Dullemen L.F.A., Thézénas M.L., Zeeshan Akhtar M., Huang H., Rendel S. Plasma degradome affected by variable storage of human blood. Clin Proteomics. 2016;13:26. doi: 10.1186/s12014-016-9126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finder V.H., Glockshuber R. Amyloid-beta aggregation. Neurodegener Dis. 2007;4:13–27. doi: 10.1159/000100355. [DOI] [PubMed] [Google Scholar]
- 35.Tiiman A., Krishtal J., Palumaa P., Tõugu V. In vitro fibrillization of Alzheimer's amyloid-β peptide (1-42) AIP Adv. 2015;5:092401. [Google Scholar]
- 36.Lachno D.R., Emerson J.K., Vanderstichele H., Gonzales C., Martényi F., Konrad R.J. Validation of a multiplex assay for simultaneous quantification of amyloid-β peptide species in human plasma with utility for measurements in studies of Alzheimer's disease therapeutics. J Alzheimers Dis. 2012;32:905–918. doi: 10.3233/JAD-2012-121075. [DOI] [PubMed] [Google Scholar]
- 37.Keshavan A., Heslegrave A., Zetterberg H., Schott J.M. Stability of blood-based biomarkers of Alzheimer's disease over multiple freeze-thaw cycles. Alzheimers Dement (Amst) 2018;10:448–451. doi: 10.1016/j.dadm.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sample collection for individual analyses
Sample tubes used in this study