Abstract
In the military, explosive blasts are a significant cause of mild traumatic brain injuries (mTBIs). The symptoms associated with blast mTBIs causes significant economic burdens and a diminished quality of life for many service members. At present, the distinction of the injury mechanism (blast versus non-blast) may not influence TBI diagnosis. However, using noninvasive imaging, this study reveals significant distinctions between the blast and non-blast TBI mechanisms. A cortical whole-brain thickness analysis was performed using structural high-resolution T1-weighted MRI to identify the effects of blasts in persistent mTBI (pmTBI) subjects. A total of 41 blast pmTBI subjects were individually age- and gender-matched to 41 non-blast pmTBI subjects. Using FreeSurfer, cortical thickness was quantified for the blast group, relative to the non-blast group. Cortical thinning was identified within the blast mTBI group, in two clusters bilaterally. In the left hemisphere, the cluster overlapped with the lateral orbitofrontal, rostral middle frontal, medial orbitofrontal, superior frontal, rostral anterior cingulate and frontal pole cortices (p < 0.02, two-tailed, size = 1680 mm2). In the right hemisphere, the cluster overlapped with the lateral orbitofrontal, rostral middle frontal, medial orbitofrontal, pars orbitalis, pars triangularis and insula cortices (p < 0.002, two-tailed, cluster size = 2453 mm2).
Self-report assessments suggest significant differences in the Post-Traumatic Stress Disorder Checklist-Civilian Version (p < 0.05, Bonferroni-corrected) and the Neurobehavioral Symptom Inventory (p < 0.01, uncorrected) between the blast and non-blast mTBI groups.
These results suggest that blast may cause a unique injury pattern related to a reduction in cortical thickness within specific brain regions which could affect symptoms. No other study has found cortical thickness difference between blast and non-blast mTBI groups and further replication is needed to confirm these initial observations.
Keywords: Mild traumatic brain injury, Military, Cortical thickness, Blast, Post-traumatic stress disorder
Highlights
-
•
Non-invasive T1 weighted MRI was used to assess military service members.
-
•
Blast mTBI group showed lower cortical thickness compared to non-blast mTBI group.
-
•
Blast related cortical thickness differences were located in bilateral frontal lobes.
-
•
Patients with mTBI were scored using PTSD, neurobehavioral and other assessments.
1. Introduction
Traumatic brain injury (TBI) has been defined as an injury to the head, arising from blunt/penetrating trauma or from acceleration/deceleration forces, and resulting in a decreased level of consciousness and/or other adverse effects (Zasler et al., 2013). Approximately 1.7 million incidents of civilian TBI are reported annually in the United States (Faul and Coronado, 2015), most of which are diagnosed as mild TBI (mTBI; Cassidy et al., 2004). Mild TBI is also prominent among military personnel; according to the Defense and Veterans Brain Injury Center, mTBIs account for 82% of all TBIs reported between 2000 and 2015. (https://dvbic.dcoe.mil/files/tbi-numbers/worldwide-totals-2015_jun-21-2018_v1.0_2018-07-26_0.pdf). In accordance with McCrea et al. (2003), most mTBI subjects experience full recovery within three months post injury. However, in accordance with Cicerone and Kalmar (1995), some mTBI patients may suffer from persistent post-concussive symptoms (e.g., headaches, dizziness, and memory complaints), which may be present three to twelve months post injury. In accordance with previous literature, persistent post-concussive symptoms in mTBI patients (pmTBI) may be present during assessment at three to four months post injury (Hoge et al., 2008; Ruff, 2005) or six months post injury (Bohnen et al., 1992). For the purpose of this study, a pmTBI patient has had persistent mTBI symptoms for at least one year after injury. These persistent symptoms may result in a significant reduction in quality of life and represent an annual financial burden of care estimated between $25,572 and $30,730 per pmTBI-service member, exclusive of costs associated with loss of employment and productivity (Tanielian and Jaycox, 2008). A challenge associated with pmTBI is the prevalence of a variety of comorbid symptoms such as PTSD, depression, headaches, dizziness, mild cognitive impairment and sleep disturbances (Bryant, 2001; Brenner et al., 2009; Stein and McAllister, 2009; Lucas et al., 2016; Guskiewicz et al., 2005).
According to the Department of Defense, the mTBI diagnosis is characterized by a loss of consciousness (LOC) of <30 min, an alteration of consciousness (AOC) of <24 h, post-traumatic amnesia (PTA) for up to 1 day, and the absence of radiological findings in “routine” imaging (The Management of Concussion-mTBI Working Group, 2016).
In the field, TBI is often caused by non-blast mechanisms, such as physical violence, falls, or motor vehicle accidents, or by blast mechanisms such as improvised explosive devices (IEDs), grenades, or mines. According to previous studies, the incidence of a blast mechanism was >30% among U.S. service members deployed in support of Operation Iraqi Freedom (OIF) or Operation Enduring Freedom (OEF; Tanielian and Jaycox, 2008), and Improvised Explosive Devises (IEDs) were a major source. In industrialized countries the occurrence of blast-induced TBI is unique to the military population.
Research suggests that blast-induced brain injury may result from four main effects (DePalma et al., 2005): 1) Primary blast injury is caused by direct exposure to the explosive pressure wave, which causes rapid pressure changes within the skull. 2); the secondary blast effect is associated with blast projectiles that penetrate the skin (DePalma et al., 2005); 3) tertiary blast effects that may involve structural collapse or blast waves that propel the person into involuntary motion, slamming the person's head against a hard surface like a motor vehicle dashboard, or a fall and subsequent strike to the head (DePalma et al., 2005); 4) quaternary blast effects refer to indirect injuries associated with the primary blast, such as thermal exposure, air embolisms, and toxic gases (Bass et al., 2012; DePalma et al., 2005; Ling et al., 2009). Computer simulations have also been used to analyze primary blast injuries. These simulations predict that blast effects from the front, side, or rear result in three virtually identical 3-D profiles of deviatoric energy, maximized around the inferior frontal lobes, which may explain neuronal damage sustained at the microscopic level (Taylor et al., 2014). Although protective gear, such as helmets, may be able to protect the head from penetrating injuries, they do not furnish the brain with complete protection from the powerful forces that originate from the primary blast wave (Ganpule et al., 2012; Nyein et al., 2010). Even when an individual is wearing a helmet, simulations predict that the pressure wave propagates through the multitude of bone and tissue layers that compose the head, tunneling through various tissue densities and gaps filled with cerebral spinal fluid, such as the area between the brain and the skull. A significant pressure increase may occur within the nasion region, forehead, and frontal regions of the brain (Ganpule et al., 2012; Nyein et al., 2010). The direct pressure wave may also produce hyperemia (Ling et al., 2009).
An animal study (Cernak et al., 2001) indicates that, even when the pressure wave is focused solely and directly at the chest, it may cause neural injury by spreading upward, via the thorax, to the head. Other animal studies demonstrate that the blast mechanism may induce a unique type of shear injury (Sosa et al., 2013) and that cortical thinning may occur subsequent to head trauma (Gao and Chen, 2011).
Primary injury biomarkers facilitating distinctions between blast and non-blast TBI among living human subjects are not well known, at this time. However, biomarkers have been suggested to distinguish TBI from control subjects, including hemorrhagic lesions (microbleeds), total ventricle volume, parenchymal volume, focal loss of brain tissue (encephalomalacia), and diffuse axonal injury (Bigler et al., 2013; Bigler et al., 2016). Pursuant to findings in previous literature, microbleeds may decrease in size as the time passes following the injury, rendering it increasingly difficult to observe microbleeds in a persistent mTBI population (Lawrence et al., 2017). Further, mTBI may result in considerably fewer microbleeds, relative to moderate and severe TBI (Riedy et al., 2015), thereby further decreasing the number of opportunities to observe this phenomenon in a pmTBI population. Another potential indicator of TBI is contusion, which is largely prevalent in moderate and severe TBI, and typically located in the frontal and temporal lobes, independent of force angles and sites of force impacts to the head (Bigler, 2007), but largely absent from pmTBI subjects (Riedy et al., 2015).
Microbleeds, contusions, encephalomalacia, and the incidence of diffuse axonal injuries may be sparse among mTBI patients (Riedy et al., 2015) and are devoid in literature when it comes to separating an mTBI group from a control group. However, the effects of mTBI on cortical thinning 90 or more days following the injury in mTBI groups have been detected by comparison to control groups as reported in the frontal lobe (Michael et al., 2015; Tate et al., 2014; Meier et al., 2016) and in the temporal lobe (Michael et al., 2015; Tate et al., 2014; Wang et al., 2015) when comparing mTBI and control subjects. Analogous studies also found thinner motor (Meier et al., 2016) and insular (Michael et al., 2015) cortices in the mTBI group. In addition, Singh et al. (2014) found smaller hippocampal volume in the mTBI group compared to a control group and Dean et al. (2015) used voxel-based morphometry and found less gray matter in the parietal lobe and the precuneus in the mTBI group as compared to a control group. Using a model that relies on comparing a non-TBI trauma group (instead of a control group) to a group of mTBI patients 90 or more days following the injury, Govindarajan et al. (2016) found cortical thinning in the temporal lobe in the mTBI group and Wang et al. (2015) found reduced cortical thickness in the temporal lobe and increased cortical thickness in the frontal lobe and in the precuneus cortex in the mTBI group. The frontal and temporal lobe regions that were reported to have thinner cortices in the mTBI group also had less gray matter in studies comparing patients with moderate and severe TBI to control groups (Silver et al., 2005; Gale, 2005).
According to Taber et al. (2006), the effects from the primary blast injury is an active and controversial area of research. It has been found that the blast mechanism of mTBI significantly increases reported PTSD symptoms compared to non-blast mTBI mechanisms (Sayer et al., 2008; Clark et al., 2009). Furthermore, a novel postmortem study suggested that blast-related persistent TBI subjects may be afflicted by a microstructural deficit termed astroglial scarring in the subpial glial plate and gray-white matter junctions, whereas non-blast persistent-TBI subjects are devoid of scarring in these regions (Shively et al., 2016). Cortical thickness may be a most appropriate method to detect specific blast effects between blast and non-blast mTBI if such an effect exists.
Interestingly, two mTBI studies that found cortical thinning in the mTBI group compared to control groups included military subjects who had been exposed to the blast mechanism (Michael et al., 2015; Tate et al., 2014). No study has described cortical differences between blast and non-blast mTBI, but if these differences are found it may contribute to an objective, noninvasive method capable of enhancing the predictive accuracy of long-term diagnoses, which in turn could reduce costs and suffering (Bazarian et al., 2006; Belanger et al., 2007; Wilde et al., 2015). These findings could also reduce the dependence on subjective self-reports for information about injury-related effects. The goal of this study is to determine whether differences in cortical thicknesses between blast and non-blast pmTBI populations exist, which may improve the assessment and subsequent treatment of military service members with pmTBI-related symptoms.
2. Materials and methods
2.1. Participants
This study was approved by the Walter Reed National Military Medical Center Institutional Review Board, and written informed consent was obtained from each subject. Information relating to the history of each subject's mTBI and corresponding mechanisms (e.g., motor vehicle accident, blast, fall, etc.) were recorded. The most significant injury for each subject was identified by a physician or clinic's report, and subsequently the injury mechanism of the injury was identified from the study self-report screening or patient's medical record. Subjects with pmTBI symptoms originating from non-blast mechanism(s) and no clinical record of any blast injuries were classified as non-blast pmTBI subjects. All potential subjects were categorized as having mild, moderate or severe TBI in accordance with the Department of Defense (The Management of Concussion-mTBI Working Group, 2016).
The data used in this study is part of a larger ongoing study, and structural T1-weighted MRIs were acquired and manually quality assured (QA) from 1054 subjects with TBI at our institution before March 16, 2016. The imaging examination QA process involved the exclusion of subjects based on failure of skull stripping or segmentation procedures, and inspection for incorrectly included or excluded cortical tissue segmentation. The following subjects were excluded: 190 that failed the QA, 37 females to control for gender, 63 with moderate or severe TBI, 174 not considered pmTBI due to imaging less than one year post injury. After the foregoing exclusions, 306 subjects were categorized as having a most significant injury from a blast and 42 as non-blast, leaving 242 subjects. Each of these 242 subjects had blast events that did not fulfill the “most significant injury was blast” requirements and, at the same time, had a record of at least a minor blast event, excluding them from the non-blast group and this study. Furthermore, subjects where the most significant injury was caused by a blast mechanism, referred to as the blast group (n = 306), were compared to subjects in the non-blast group (n = 42). In addition, this study individually age-matched subjects across the two groups and excluded all subjects that failed to meet the age-matching criteria across groups.
A total of 41 subjects from the non-blast group were individually age-matched to 41 subjects from the blast group. When the individual age-matched subjects from the intermediary classes across the two groups were selected, selection bias was avoided by using scan date to choose subjects. Selection bias could occur if each individually age-matched pair across groups had three or more subjects of the same age to choose from. However, since only the earliest blast subject and earliest non-blast subject, according to scan dates, were chosen to form a pair across groups, no manual selection was required during this process. The remaining 266 subjects from the two groups who were unable to be age-matched because of different age distributions were excluded from this study. In total, this study used 82 pmTBI subjects divided into blast and non-blast pmTBI groups with demographics shown in Table 1.
Table 1.
Means or percentages of TBI related variables and demographics, including SD, n and significances.
| Demographic | Non-blast mean (SD, n) | Blast mean (SD, n) | p-value |
|---|---|---|---|
| Age | 37.4 (8.5, 41) | 37.4 (8.5, 41) | >0.1 |
| Days post injury | 2.0 K (1.5 K, 41) | 1.7 K (1.1 K, 41) | >0.1 |
| LOC | 71% (N/A, 41) | 71% (N/A, 41) | >0.1 |
| PTA | 37% (N/A, 41) | 17% (N/A, 41) | <0.05 |
| AOC | 54% (N/A, 41) | 51% (N/A, 41) | >0.1 |
| Education | 14.0 (2.2, 37) | 14.0 (2.3, 26) | >0.1 |
| n | 41 (N/A) | 41 (N/A) | N/A |
Reported numbers of means of demographics or percentages of TBI related variables, having within parentheses the standard deviations and number of subjects. The subjects that were noted to have experienced a loss of consciousness (LOC), post traumatic amnesia (PTA), and/or alteration of consciousness (AOC) post injury are listed in percent of n. The p-values were calculated by the two-sample t-test, except from LOC, PTA and AOC that were tested using the chi-squared test.
Legend: SD, standard deviation; n, number of subjects; K, 1000; N/A, not applicable; Education, years of education from elementary school through post-graduate level, with 12 representing the conclusion of 12th grade.
2.2. Self-report assessments
All study participants completed a set of self-report questionnaires consisting of the PTSD Checklist—Civilian version (PCLC; Weathers et al., 1994), the Neurobehavioral Symptom Inventory (NSI; Cicerone and Kalmar, 1995), the RAND Corporation 36-item health survey (RAND-36) consisting of eight scaled scores (Hays et al., 1993), and the Satisfaction With Life Scale (SWLS; Diener et al. 1985). Incomplete questionnaires were excluded. Differences in assessment scores between groups were compared using Wilcoxon rank-sum tests and the SPSS software package (IBM Inc., Armonk, NY), and finally a Bonferroni correction was performed, correcting for 18 tests, on the p-value noted pFWE.
For the neuropsychological results, the Cohen's d effect size was calculated from the Wilcoxon rank-sum test, assuming normality, using Eq. (1) (Grissom, 1994; Ruscio, 2008),
| (1) |
where ϕ−1 is the inverse of the cumulative normal distribution function, U is the Mann-Whitney test statistic, and n1*n2 is of the number of non-blast and blast subjects.
2.3. Image processing
All subjects were scanned, using a GE 3 T Discovery 750 MRI (General Electric, Milwaukee, WI) with a 32-channel phased array head coil (MR Instruments, Inc., Minnetonka, MN). Anatomical T1-weighted brain images were collected with a 3D BRAVO sequence, with an inversion time (TI) = 450 ms, repetition time (TR) = 6.7 ms, echo time (TE) = 2.5 ms, flip angle = 12°, reconstructed image size = 512 × 512 × 312, and resolution = 0.47 × 0.47 × 0.6 mm3. The FreeSurfer software package, version 5.3.0 (64-bit LINUX), was used to calculate the cortical thickness for this study (Fischl, 2012; Fischl and Dale, 2000; Dale et al., 1999). The T1-weighted anatomical images were resampled to 1 × 1 × 1 mm3, spatially normalized, intensity normalized, skull stripped, anatomically segmented, tessellated, surface smoothed, and spherically inflated for hemispherical registration. Spatial localizations were determined via the Desikan-Killiany atlas (Desikan et al., 2006).
Microbleeds and contusions were counted manually by radiologists analogously with Riedy et al. (2015).
For each subject, a whole brain cortical thickness value was averaged from the MeanThickness values in the lh.aparc.stats and rh.aparc.stats files, resulting from FreeSurfer.
2.4. Statistical analysis
Query-Design-Estimate-Contrast (QDEC) version 1.4, which facilitates whole-brain analyses, included in FreeSurfer, was used to identify clusters with cortical thickness differences between groups, across the entire cortical surface. The QDEC two-sample and multiple-comparisons-corrected cluster-based analysis used the Monte Carlo Null-Z Simulation test, with a two-tailed (abs) option and a suprathreshold of p < 0.05. To conduct a visualization of the results, all clusters were displayed on the common brain space named “fsaverage” (Fischl, 2012; Fischl and Dale, 2000; Dale et al., 1999).
The QDEC tool used the 15 mm full width at half maximum Gaussian kernel smoothing, similar to prior cortical thickness analyses (Tate et al., 2014; Michael et al., 2015).
In accordance with previous literature, our study did not normalize the cortical thickness measures with any other individual body part such as the intracranial volume (Westman et al., 2013; Barnes et al., 2010).
3. Results
3.1. Imaging results
In accordance with Table 1 it was noticed that PTA was significantly different between blast and non-blast groups and may interfere with our model, which was only supposed to test the blast effects. This was solved by adding the PTA variable as a nuisance factor as a part of the QDEC model. This removed any bias that PTA could have introduced.
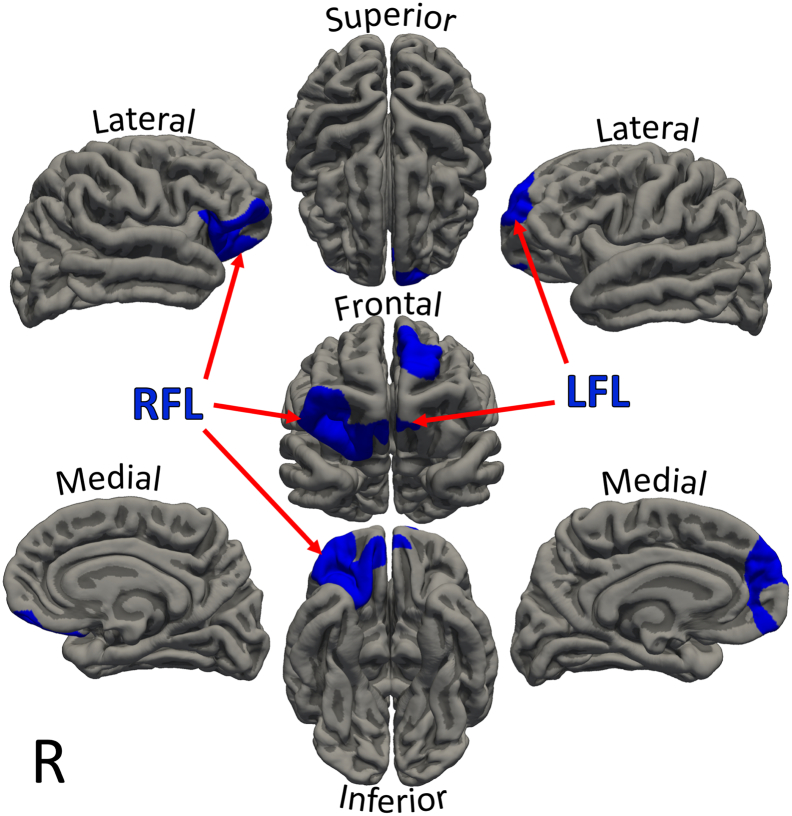
The two-sided test resulting from QDEC found significantly thinner cortices in the blast group, relative to the non-blast group in two clusters, which were positioned bilaterally in the frontal lobes of both hemispheres. First, the cluster in the left frontal lobe (LFL) overlapped with the lateral orbitofrontal, rostral middle frontal, medial orbitofrontal, superior frontal, rostral anterior cingulate and frontal pole cortices (p < 0.02, cluster size = 1680 mm2). Second, the cluster in the right frontal lobe (RFL) overlapped with the lateral orbitofrontal, rostral middle frontal, medial orbitofrontal, pars orbitalis, pars triangularis and insula cortices (p < 0.002, size = 2453 mm2). No cluster with a thicker cortex in the blast group was found, even at trend level. The clusters with cortical deficits in the blast group are depicted in Fig. 1.
Fig. 1.
Depicting two clusters in different hemispheres, in blue, with thinner cortices in the blast pmTBI group compared to the non-blast pmTBI group. The significant (two-sided) cluster in the left frontal lobe overlapped with the lateral orbitofrontal, rostral middle frontal, medial orbitofrontal, superior frontal, rostral anterior cingulate and frontal pole cortices (LFL, p < 0.02). In the right frontal lobe the cluster overlapped with the lateral orbitofrontal, rostral middle frontal, medial orbitofrontal, pars orbitalis, pars triangularis and insula cortices (RFL, p < 0.002). Statistics were calculated with FreeSurfer (Fischl, 2012; Fischl and Dale, 2000; Dale et al., 1999). Legend: R, right hemisphere. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Microbleeds and contusions have been shown to be prevalent for moderate and severe TBI populations, but not for the mTBI population (Riedy et al., 2015). In agreement with Riedy et al. (2015), our pmTBI blast group did not differ from the non-blast group for microbleeds or contusions seen in Table 2.
Table 2.
Means of neural measures possibly related with TBI observed for sample, including SD, n and significances.
| Neural measures | Non-blast mean (SD, n) | Blast mean (SD, n) | p-value |
|---|---|---|---|
| Subjects with contusions | 0% (N/A, 37) | 0% (N/A, 39) | >0.1 |
| Subjects with microbleeds | 2.7% (N/A, 37) | 2.6% (N/A, 39) | >0.1 |
| Microbleeds per subject | 0.1 (0.8, 37) | 0.03 (0.2, 39) | >0.1 |
| Whole brain cortical thickness | 2.46 (0.08, 41) | 2.43 (0.09, 41) | =0.08 |
Neural measures apart from the QDEC analysis were contusions, microbleeds, and whole brain cortical thickness. The p-value column used the chi-squared test for subjects with contusions and subjects with microbleeds (seen in first two rows) that were percentages of n. The remaining two significances were calculated using the two-sample t-test. From our pmTBI population the whole brain cortical thickness difference between the blast and non-blast groups trended toward significance. The remaining effects were less significant. The row with “microbleeds per subject” represented the average number of microbleeds per subject. The microbleeds and contusions were counted by radiologists. The standard deviations and number of subjects are in parentheses.
Legend: SD, standard deviation; n, number of subjects; N/A, not applicable.
The whole brain cortical thickness difference between the blast and the non-blast groups was trending toward significance seen in Table 2 (p = 0.08).
3.2. Self-report results
All the self-reported assessments (Table 3) were compared between the non-blast and blast groups using the two-sided Wilcoxon rank-sum test and corrected using the Bonferroni correction.
Table 3.
Self-report results including mean, standard deviation, number of subjects, significance, and effect size.
| Self-report | Non-blast mean (SD, n) | Blast mean (SD, n) | p | pFWE | d |
|---|---|---|---|---|---|
| Post-traumatic stress disorder checklist-civilian version | |||||
| Total score | 38 (14, 35) | 54 (22, 35) | 0.0007 | <0.05 | 0.89 |
| B (re-experiencing traumatic events) | 7.8 (4.1, 35) | 12 (6.1, 35) | 0.0023 | <0.05 | 0.78 |
| C (avoidance scores) | 17 (6.7, 35) | 24 (11, 35) | 0.0012 | <0.05 | 0.84 |
| D (hyperarousal scores) | 13 (4.5, 35) | 18 (6.4, 35) | 0.0006 | <0.05 | 0.90 |
| Neurobehavioral symptom inventory | |||||
| Total score | 29 (15, 34) | 42 (17, 34) | 0.0059 | =0.1 | 0.72 |
| Somatosensory | 6.8 (5.0, 34) | 10 (5.7, 34) | 0.0315 | >0.1 | 0.55 |
| Affective | 10 (4.8, 34) | 14 (5.9, 34) | 0.0157 | >0.1 | 0.62 |
| Cognitive | 7.4 (3.7, 34) | 10 (4.3, 34) | 0.0187 | >0.1 | 0.60 |
| Vestibular | 2.2 (2.4, 34) | 3.8 (2.7, 34) | 0.0366 | >0.1 | 0.53 |
| RAND corporation 36-item health survey | |||||
| Physical functioning | 75 (27, 36) | 72 (24, 35) | >0.1 | >0.1 | 0.20 |
| Role-physical | 47 (43, 36) | 30 (35, 35) | >0.1 | >0.1 | 0.25 |
| Bodily pain | 61 (24, 36) | 55 (24, 35) | >0.1 | >0.1 | 0.27 |
| General health | 61 (23, 36) | 54 (22, 35) | >0.1 | >0.1 | 0.36 |
| Vitality | 36 (19, 36) | 27 (23, 35) | 0.0188 | >0.1 | 0.59 |
| Social functioning | 53 (29, 36) | 53 (31, 35) | >0.1 | >0.1 | 0.04 |
| Role-emotional | 51 (46, 36) | 44 (46, 35) | >0.1 | >0.1 | 0.16 |
| Mental health | 60 (20, 36) | 50 (24, 35) | 0.0829 | >0.1 | 0.43 |
| Satisfaction with life scale | |||||
| Total | 23 (7.2, 37) | 22 (8.0, 33) | >0.1 | >0.1 | 0.12 |
The averages for each group are shown, and within parentheses the standard deviation and number of subjects who completed self-report assessments are listed. Using the Wilcoxon rank-sum tests, the significances between the non-blast and blast groups were calculated as an uncorrected p-value under p column. The pFWE is the Bonferroni corrected p value for all 18 tests. The final column shows the Cohen's d effect size between the non-blast and blast groups. Of all successfully imaged subjects, only those who accurately submitted and fully completed their self-report assessments were included in this table.
Legend: SD, standard deviation; n, number of subjects; FWE, family-wise error; d, Cohen's d effect size.
The total PCL score for blast group and the non-blast group, respectively, were significantly (pFWE < 0.05) different from each other. The PCLC subscores for subgroup B (re-experiencing traumatic events symptoms), subgroup C (avoidance symptoms), and subgroup D (hyperarousal symptoms) were also significantly (pFWE < 0.05) distinct the between blast and non-blast groups. Further, the symptom scores were higher in the blast group, relative to the non-blast group across all PCLC scores, indicating that there may be a higher incidence of PTSD-related symptoms in the blast group.
The total NSI score was only significantly higher (p < 0.01) in the blast group relative to the non-blast group at an uncorrected level (trending to significance with multiple comparisons correction). Analogously, the somatosensory, affective, cognitive and vestibular NSI subscores were significantly higher (p < 0.05) in the blast group, relative to the non-blast group at an uncorrected level.
The difference between the blast and non-blast groups for the RAND-36, the vitality subscore was significant at an uncorrected level and the mental health subscore trended toward significance (p = 0.08). The non-blast group generally attained higher scores than the blast group. The remaining subscores (physical functioning, role-physical, bodily pain, general health, social functioning, and role-emotional) did not reflect any statistically significant differences between the blast and non-blast groups.
The final self-report, the SWLS, was not significantly different between the blast and non-blast groups.
4. Discussion
One of the most important findings of this study is the reduced cortical thicknesses bilaterally around the anterior frontal lobes for the blast-related pmTBI subjects relative to non-blast-related pmTBI subjects. To our knowledge, this may be the first published report to indicate that pmTBI injury mechanisms by which blast and non-blast injuries produce distinctive effects on cortical thickness. This cortical thinning may be related to the findings cited by Shively et al. (2016), which included the observation of microstructural astroglial scarring in blast TBI populations that does not exist in non-blast TBI populations. If astroglial scarring, which appears at the intersection of white and gray matter at the microstructural (microscopic) level (Shively et al., 2016), indirectly degrades the neural cortex, then it may also contribute to the decreased cortical thickness that occurs in a gradual time-delayed process with time post injury.
The thinner cortices in the frontal lobes of blast pmTBI subjects, relative to non-blast pmTBI subjects may derive from three possible sources: 1) effects from the primary blast wave behind the nasion (Ganpule et al., 2012), which is believed to have minimal neural protection and could lead to direct cortical lesions; 2) the blast mechanism may target the frontal lobe more homogenously than non-blast mTBI mechanisms do, leading to the difference within the imaging results; and 3) a TBI injury resulting in white matter lesions, which indirectly degrade connected cortical tissue.
Our pmTBI groups imaged at least one year post injury did not show any significant difference of microbleeds or contusions between the blast and non-blast groups. The presence of radiological findings such as microbleeds, contusions, encephalomalacia and diffuse axonal injuries are pronounced in moderate and severe TBI (Riedy et al., 2015). In addition, the presence of microbleeds may decrease in post-acute TBI populations compared to acute populations (Lawrence et al., 2017). The results from this study suggest that cortical thinning may be the most sensitive measure to detect the blast effect in pmTBI populations compared to any other neuroimaging biomarker.
Other mTBI studies have also reported the presence of cortical thinning in the frontal lobe in mTBI subjects (Tate et al., 2014; Michael et al., 2015; Meier et al., 2016). Apart from structural cortical abnormalities, other abnormal findings in the frontal lobe have been reported in mTBI subjects for functional MRI and diffusion tensor imaging (Eierud et al., 2014). Table 2 notes that the whole brain cortical thickness differed between the blast and non-blast groups at the trend level. As the thicknesses of the LFL and RFL regions are part of the whole brain cortical thickness, the whole brain cortical thickness may depend on the LFL and RFL regions. It is possible the LFL and RFL regions represent locations with maximum difference between the groups at the same time other regions are thinner in the blast group compared to the non-blast group. The root constituent underlying the specific frontal lobe cortical thickness difference between the blast pmTBI and non-blast pmTBI groups may not be completely known at this point. However, other volume analyses (e.g., those of ventricular volume or gray and white matter volumes) may be related with this cortical thickness difference. Future volume analyses exploring these variables may discover important information related to this cortical thickness difference.
Cortical abnormalities in the prefrontal area may affect executive function and decision-making (Powell and Voeller, 2004). This relationship may be related to the increased reporting of symptoms on the self-report scores in the blast pmTBI group. It has also been shown that subjects with post-concussive syndrome (analogous to pmTBI) have lower executive function relative to control subjects (Bohnen et al., 1992). Other literature has reported that pmTBI may be associated with cognitive and emotional symptoms (Ruff, 2005).
The characteristic PTSD symptom increase in the blast group, relative to the non-blast group (Table 3) is in agreement with previously reported findings (Sayer et al., 2008; Clark et al., 2009), confirming that our blast and non-blast groups shared common characteristics previously reported in the literature. Even though the NSI subscores were significantly higher in the blast group, relative to the non-blast group only at an uncorrected level testing, they were similar to previously reported results that tested mTBI subjects from non-clinical military populations (Soble et al., 2014). These findings highlight the heterogeneity of mTBI-related symptoms and the complicated nature of mTBI. However, it is possible that an analysis modeling the cluster shapes optimally, for specific symptomatic measures, may complement this study, warranting further clinical testing related to cortical symptomatic effects.
The results from this study suggest potentially valuable areas of subsequent research in blast injuried patients, including astroglial scarring and cortical thickness differences, aimed at the development of more precise and sensitive tools for blast TBI detection.
Limitations of the current study are as follows. First, FreeSurfer does not support cortical thickness analysis of the cerebellum, though injury to the cerebellum has been identified in blast TBI (Taber et al., 2006). A voxel-based morphometry analysis could be a solution that would enable the detection of cortical differences in the cerebellum and subcortical structures, which could also be considered for use in future analyses. A second limitation involves the absence of female participants and the potential effects of gender on our results. Female subjects made up <3% of our total blast pmTBI population, yielding too few subjects for an adequately powered statistical analysis. Third, medications could have influenced the results; however, definite indicators of medications on cortical thinning are not currently known and warrant further research. Finally, the use of patient reports to determine injury histories is susceptible to bias and more objective assessments are needed.
5. Conclusion
This study demonstrates significantly reduced cortical thickness in blast-induced pmTBI compared to non-blast pmTBI, and that this reduction in thickness occurs in two bilateral clusters. The clusters in both left and right hemispheres overlapped with the lateral orbitofrontal, rostral middle frontal, and medial orbitofrontal cortices. These results have potential value in the development of biomarkers and more sensitive diagnostic techniques that could adequately detect cortical changes caused by microscopic neuronal damage that may not be detected using conventional clinical tools. However, further replication with a larger sample size is warranted.
Disclaimers
The identification of specific products or scientific instrumentation is considered an integral part of the scientific endeavor and does not constitute endorsement or implied endorsement on the part of the author, DoD, or any component agency. The views expressed in this manuscript are those of the authors and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or U.S. Government.
Acknowledgements
This publication would not be possible without valuable help from Julie Kim, Adam Scott, Cheng Koay, Jaquel Barnes, Elyssa Sham, Ping-Hong Yeh, Theresa Teslovich, Siamak Ardekani, Mark Varvaris, Alex Kubli, Christopher Aura, Patrick Regan, Jennifer Mehki, Anmarie Widener and Justin Senseney. Finally, we thank Arnold Fisher and the Intrepid Fallen Heroes Fund for founding the National Intrepid Center of Excellence. This work was supported by the Congressionally Directed Medical Research Programs (grant number DM130132).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101793.
Appendix A. Supplementary data
Supplementary material
References
- Barnes J., Ridgway G.R., Bartlett J., Henley S.M., Lehmann M., Hobbs N., Clarkson M.J., Macmanus D.G., Ourselin S., Fox N.C. Head size, age and gender adjustment in MRI studies: a necessary nuisance? NeuroImage. 2010;53:1244–1255. doi: 10.1016/j.neuroimage.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Bass C.R., Panzer M.B., Rafaels K.A., Wood G., Shridharani J., Capehart B. Brain injuries from blast. Ann. Biomed. Eng. 2012;40:185–202. doi: 10.1007/s10439-011-0424-0. [DOI] [PubMed] [Google Scholar]
- Bazarian J.J., Blyth B., Cimpello L. Bench to bedside: evidence for brain injury after concussion-looking beyond the computed tomography scan. Acad. Emerg. Med. 2006;13:199–214. doi: 10.1197/j.aem.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Belanger H.G., Vanderploeg R.D., Curtiss G., Warden D.L. Recent neuroimaging techniques in mild traumatic brain injury. J. Neuropsychiatr. Clin. Neurosci. 2007;19:5–20. doi: 10.1176/jnp.2007.19.1.5. [DOI] [PubMed] [Google Scholar]
- Bigler E.D. Anterior and middle cranial fossa in traumatic brain injury: relevant neuroanatomy and neuropathology in the study of neuropsychological outcome. Neuropsychology. 2007;21:515–531. doi: 10.1037/0894-4105.21.5.515. [DOI] [PubMed] [Google Scholar]
- Bigler E.D., Abildskov T.J., Petrie J., Farrer T.J., Dennis M., Simic N., Taylor H.G., Rubin K.H., Vannatta K., Gerhardt C.A., Stancin T., Yeates K.O. Heterogeneity of brain lesions in pediatric traumatic brain injury. Neuropsychology. 2013;27:438–451. doi: 10.1037/a0032837. [DOI] [PubMed] [Google Scholar]
- Bigler E.D., Zielinski B.A., Goodrich-Hunsaker N., Black G.M., Huff B.S.T., Christiansen Z., Wood D.-M., Abildskov T.J., Dennis M., Taylor H.G., Rubin K., Vannatta K., Gerhardt C.A., Stancin T., Yeates K.O. The relation of focal lesions to cortical thickness in pediatric traumatic brain injury. J. Child Neurol. 2016;31:1302–1311. doi: 10.1177/0883073816654143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen N., Jolles J., Twijnstra A. Neuropsychological deficits in patients with persistent symptoms six months after mild head injury. Neurosurgery. 1992;30:692–696. [PubMed] [Google Scholar]
- Brenner L.A., Vanderploeg R.D., Terrio H. Assessment and diagnosis of mild traumatic brain injury, posttraumatic stress disorder, and other polytrauma conditions: burden of adversity hypothesis. Rehab. Psychol. 2009;54:239–246. doi: 10.1037/a0016908. [DOI] [PubMed] [Google Scholar]
- Bryant R.A. Posttraumatic stress disorder and mild brain injury: controversies, causes and consequences. J. Clin. Exp. Neuropsychol. 2001;23:718–728. doi: 10.1076/jcen.23.6.718.1024. [DOI] [PubMed] [Google Scholar]
- Cassidy J.D., Carroll L., Peloso P., Borg J.F., Holst H.V., Holm L., Kraus J., Coronado V. Incidence, risk factors and prevention of mild traumatic brain injury: results of the who collaborating centre task force on mild traumatic brain injury. J. Rehabil. Med. 2004;36:28–60. doi: 10.1080/16501960410023732. [DOI] [PubMed] [Google Scholar]
- Cernak I., Wang Z., Jiang J., Bian X., Savic J. Ultrastructural and functional characteristics of blast injury-induced neurotrauma. J. Trauma. 2001;50:695–706. doi: 10.1097/00005373-200104000-00017. [DOI] [PubMed] [Google Scholar]
- Cicerone K.D., Kalmar K. Persistent postconcussion syndrome. J. Head Trauma Rehab. 1995;10:1–17. [Google Scholar]
- Clark M.E., Walker R.L., Gironda R.J., Scholten J.D. Comparison of pain and emotional symptoms in soldiers with polytrauma: unique aspects of blast exposure. Pain Med. 2009;10:447–455. doi: 10.1111/j.1526-4637.2009.00590.x. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dean P.J.A., Sato J.R., Vieira G., Mcnamara A., Sterr A. Long-term structural changes after mTBI and their relation to post-concussion symptoms. Brain Inj. 2015;29:1211–1218. doi: 10.3109/02699052.2015.1035334. [DOI] [PubMed] [Google Scholar]
- Depalma R.G., Burris D.G., Champion H.R., Hodgson M.J. Blast injuries. N. Engl. J. Med. 2005;352:1335–1342. doi: 10.1056/NEJMra042083. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Diener, E., Emmons, R.A., Larsen, R.J., scale. J. Pers. Assess. 49, 71–75. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed]
- Eierud C., Craddock R.C., Fletcher S., Aulakh M., King-Casas B., Kuehl D., Laconte S.M. Neuroimaging after mild traumatic brain injury: review and meta-analysis. NeuroImage. 2014;4:283–294. doi: 10.1016/j.nicl.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul M., Coronado V. Epidemiology of traumatic brain injury. Handb. Clin. Neurol. 2015;127:3–13. doi: 10.1016/B978-0-444-52892-6.00001-5. [DOI] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. NeuroImage. 2012;62:774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale S.D. Traumatic brain injury and grey matter concentration: a preliminary voxel based morphometry study. J. Neurol. Neurosurg. Psychiatr. 2005;76:984–988. doi: 10.1136/jnnp.2004.036210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganpule S., Gu L., Alai A., Chandra N. Role of helmet in the mechanics of shock wave propagation under blast loading conditions. Comp. Methods Biomech. Biomed. Eng. 2012;15:1233–1244. doi: 10.1080/10255842.2011.597353. [DOI] [PubMed] [Google Scholar]
- Gao X., Chen J. Mild traumatic brain injury results in extensive neuronal degeneration in the cerebral cortex. J. Neuropathol. 2011;70:183–191. doi: 10.1097/NEN.0b013e31820c6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan K.A., Narayana P.A., Hasan K.M., Wilde E.A., Levin H.S., Hunter J.V., Miller E.R., Patel V.K.S., Robertson C.S., Mccarthy J.J. Cortical thickness in mild traumatic brain injury. J. Neurotrauma. 2016;33:1809–1817. doi: 10.1089/neu.2015.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom R.J. Probability of the superior outcome of one treatment over another. J. Appl. Psychol. 1994;79:314–316. [Google Scholar]
- Guskiewicz K.M., Marshall S.W., Bailes J., Mccrea M., Cantu R.C., Randolph C., Jordan B.D. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57:719–726. doi: 10.1093/neurosurgery/57.4.719. [DOI] [PubMed] [Google Scholar]
- Hays R.D., Sherbourne C.D., Mazel R.M. The rand 36-item health survey 1.0. Health Econ. 1993;2:217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- Hoge C.W., Mcgurk D., Thomas J.L., Cox A.L., Engel C.C., Castro C.A. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N. Engl. J. Med. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Lawrence T.P., Pretorius P.M., Ezra M., Cadoux-Hudson T., Voets N.L. Early detection of cerebral microbleeds following traumatic brain injury using MRI in the hyper-acute phase. Neurosci. Lett. 2017;655:143–150. doi: 10.1016/j.neulet.2017.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling G., Bandak F., Armonda R., Grant G., Ecklund J. Explosive blast neurotrauma. J. Neurotrauma. 2009;26:815–825. doi: 10.1089/neu.2007.0484. [DOI] [PubMed] [Google Scholar]
- Lucas S., Smith B.M., Temkin N., Bell K.R., Dikmen S., Hoffman J.M. Comorbidity of headache and depression after mild traumatic brain injury. Headache. 2016;56:323–330. doi: 10.1111/head.12762. [DOI] [PubMed] [Google Scholar]
- Mccrea M., Guskiewicz K.M., Marshall S.W., Barr W., Randolph C., Cantu R.C., Onate J.A., Yang J., Kelly J.P. Acute effects and recovery time following concussion in collegiate football players. Jama. 2003;290:2556. doi: 10.1001/jama.290.19.2556. [DOI] [PubMed] [Google Scholar]
- Meier T.B., Bellgowan P.S., Bergamino M., Ling J.M., Mayer A.R. Thinner cortex in collegiate football players with, but not without, a self-reported history of concussion. J. Neurotrauma. 2016;33:330–338. doi: 10.1089/neu.2015.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael A.P., Stout J., Roskos P.T., Bolzenius J., Gfeller J., Mogul D., Bucholz R. Evaluation of cortical thickness after traumatic brain injury in military veterans. J. Neurotrauma. 2015;32:1751–1758. doi: 10.1089/neu.2015.3918. [DOI] [PubMed] [Google Scholar]
- Nyein M.K., Jason A.M., Yu L., Pita C.M., Joannopoulos J.D., Moore D.F., Radovitzky R.A. 2010. In Silico Investigation of Intracranial Blast Mitigation with Relevance to Military Traumatic Brain Injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K.B., Voeller K.K.S. Prefrontal executive function syndromes in children. J. Child Neurol. 2004;19:785–797. doi: 10.1177/08830738040190100801. [DOI] [PubMed] [Google Scholar]
- Riedy G., Senseney J.S., Liu W., Ollinger J., Sham E., Krapiva P., Patel J.B., Smith A., Yeh P.-H., Graner J., Nathan D., Caban J., French L.M., Harper J., Eskay V., Morissette J., Oakes T.R. Findings from structural MR imaging in military traumatic brain injury. Radiology. 2015;279:207–215. doi: 10.1148/radiol.2015150438. [DOI] [PubMed] [Google Scholar]
- Ruff R. Two decades of advances in understanding of mild traumatic brain injury. J. Head Trauma Rehab. 2005;20:5–18. doi: 10.1097/00001199-200501000-00003. [DOI] [PubMed] [Google Scholar]
- Ruscio J. A probability-based measure of effect size: robustness to base rates and other factors. Psychol. Methods. 2008;13:19–30. doi: 10.1037/1082-989X.13.1.19. [DOI] [PubMed] [Google Scholar]
- Sayer N.A., Chiros C.E., Sigford B., Scott S., Clothier B., Pickett T., Lew H.L. Characteristics and rehabilitation outcomes among patients with blast and other injuries sustained during the global war on terror. Arch. Phys. Med. Rehabil. 2008;89:163–170. doi: 10.1016/j.apmr.2007.05.025. [DOI] [PubMed] [Google Scholar]
- Shively S.B., Horkayne-Szakaly I., Jones R.V., Kelly J.P., Armstrong R.C., Perl D.P. Characterisation of interface astroglial scarring in the human brain after blast exposure: a post-mortem case series. Lancet Neurol. 2016;15:944–953. doi: 10.1016/S1474-4422(16)30057-6. [DOI] [PubMed] [Google Scholar]
- Silver J.M., Yudofsky S.C., McAllister T.W. American Psychiatric Pub; Washington, DC: 2005. Textbook of Traumatic Brain Injury. [Google Scholar]
- Singh R., Meier T.B., Kuplicki R., Savitz J., Mukai I., Cavanagh L., Allen T., Teague T.K., Nerio C., Polanski D., Bellgowan P.S.F. Relationship of collegiate football experience and concussion with hippocampal volume and cognitive outcomes. Jama. 2014;311:1883. doi: 10.1001/jama.2014.3313. [DOI] [PubMed] [Google Scholar]
- Soble J.R., Silva M.A., Vanderploeg R.D., Curtiss G., Belanger H.G., Donnell A.J., Scott S.G. Normative data for the neurobehavioral symptom inventory (NSI) and post-concussion symptom profiles among TBI, PTSD, and nonclinical samples. Clin. Neuropsychol. 2014;28:614–632. doi: 10.1080/13854046.2014.894576. [DOI] [PubMed] [Google Scholar]
- Sosa M.A., Gasperi R.D., Paulino A.J., Pricop P.E., Shaughness M.C., Maudlin-Jeronimo E., Hall A.A., Janssen W.G.M., Yuk F.J., Dorr N.P., Dickstein D.L., Mccarron R.M., Chavko M., Hof P.R., Ahlers S.T., Elder G.A. Blast overpressure induces shear-related injuries in the brain of rats exposed to a mild traumatic brain injury. Acta Neuropathol. Commun. 2013;1 doi: 10.1186/2051-5960-1-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M.B., Mcallister T.W. Exploring the convergence of posttraumatic stress disorder and mild traumatic brain injury. Am. J. Psychiatr. 2009;166:768–776. doi: 10.1176/appi.ajp.2009.08101604. [DOI] [PubMed] [Google Scholar]
- Taber K.H., Warden D.L., Hurley R.A. Blast-related traumatic brain injury: what is known? J. Neuropsychiat. 2006;18:141–145. doi: 10.1176/jnp.2006.18.2.141. [DOI] [PubMed] [Google Scholar]
- Tanielian T., Jaycox L.H. RAND; Santa Monica, CA: 2008. Invisible Wounds of War Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. [Google Scholar]
- Tate D.F., York G.E., Reid M.W., Cooper D.B., Jones L., Robin D.A., Kennedy J.E., Lewis J. Preliminary findings of cortical thickness abnormalities in blast injured service members and their relationship to clinical findings. Brain Imaging Behav. 2014;8:102–109. doi: 10.1007/s11682-013-9257-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P.A., Ludwigsen J.S., Ford C.C. Investigation of blast-induced traumatic brain injury. Brain Inj. 2014;28:879–895. doi: 10.3109/02699052.2014.888478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Management of Concussion-mTBI Working Group . 2nd ed. Department of Veterans Affairs; Washington, DC: 2016. VA/DoD Clinical Practice Guideline for the Management of Concussion-Mild Traumatic Brain Injury. [Google Scholar]
- Wang X., Xie H., Cotton A.S., Tamburrino M.B., Brickman K.R., Lewis T.J., Mclean S.A., Liberzon I. Early cortical thickness change after mild traumatic brain injury following motor vehicle collision. J. Neurotrauma. 2015;32:455–463. doi: 10.1089/neu.2014.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers F.W., Litz B.T., Herman D., Huska J., Keane T. National Center for PTSD; Boston, MA: 1994. The PTSD Checklist—Civilian Version (PCL-C) [Google Scholar]
- Westman E., Aguilar C., Muehlboeck J.S., Simmons A. Regional magnetic resonance imaging measures for multivariate analysis in Alzheimer's disease and mild cognitive impairment. Brain Topogr. 2013;26:9–23. doi: 10.1007/s10548-012-0246-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde E.A., Bouix S., Tate D.F., Lin A.P., Newsome M.R., Taylor B.A., Stone J.R., Montier J., Gandy S.E., Biekman B., Shenton M.E., York G. Advanced neuroimaging applied to veterans and service personnel with traumatic brain injury: state of the art and potential benefits. Brain Imaging Behav. 2015;9:367–402. doi: 10.1007/s11682-015-9444-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasler N.D., Katz D.I., Zafonte R.D. 2nd ed. Demos Medical; New York, NY: 2013. Brain injury medicine: principles and practice. (ISBN-10 1936287277) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material