Highlights
-
•
Mycorrhizal hairy roots as a dual resource of biological and biochemical products.
-
•
Thirty degree is optimum temperature for biocompatible extraction.
-
•
Low percentage of methanol and DMSO as most suitable biocompatible solvent.
Keywords: Biocompatible, Hairy roots, Mycorrhiza, Rhizophagus irregularis, Rosmarinic acid
Abstract
Mycorrhizal hairy roots of Ocimum basilicum produce high amount of rosmarinic acid and are also valuable resource of quality mycorrhizal spores. To utilize their potential as continuous resource of biological and biochemical products, an efficient separation method is required. Solvent based extraction methods have a negative impact on mycorrhizal spore viability and vitality. Accordingly, we developed a biocompatible extraction method where spore and root viability is maintained with efficient extraction of rosmarinic acid. We screened temperature- and sonication-assisted techniques in ethanol, methanol, dimethyl sulfoxide, ionic liquid and surfactants. An inverse relationship was found between an increase in temperature and mycorrhizal and root viability. Optimum temperature for extraction was 30 °C. Most suitable solvents were 10% methanol; 0.25 M ionic liquid and dimethyl sulfoxide. Ethanol, nonane, dodecane, Triton X-100 and Tween-20 were not found suitable. Thus, our study sets a platform for optimization studies with mycorrhizal roots of other medicinal plants.
1. Introduction
Mycorrhizal co-cultures developed between hairy roots of medicinal plants and arbuscular mycorrhizal fungi in an in vitro culture system are potential resource of quality arbuscular mycorrhizal propagules and secondary metabolites that have agricultural and pharmaceutical applications respectively. Recently, we have shown that mycorrhizal hairy roots of Ocimum basilicum [1] are reserves of mycorrhizal propagules (Rhizophagus irregularis), antioxidants and rosmarinic acid (RA). In detail, arbuscular mycorrhizae (AM) as a biofertilizer promote plant growth, improve drought and stress tolerance and crop yield and also act as soil aggregator which prevents soil erosion and facilitates phytoremediation [2]. Biologically, RA possesses antioxidant, antiproliferative, anti-inflammatory, neuroprotective, hypoglycemic like properties and is also an active ingredient in a number of commercial products such as Neurex, Persen and Aquarox [3]. For the utilization of mycorrhizal hairy roots of O. basilicum as a continuous dual resource of agri- (mycorrhizae) and pharma- (rosmarinic acid) specific products, an essential requirement is development of a separation method that retains both mycorrhizal and host (hairy root) viability after extraction of secondary metabolites.
To date no study has provided a method for the simultaneous preservation of host viability (such as roots) and its symbiotic partner coupled with metabolite extraction however, a number of earlier studies had advocated development of biocompatible extraction methods to retain plant cell viability after metabolite extraction. For example, Weathers et al. [4] reported in hairy roots of Beta vulgaris (beetroot) use of three basic steps for extraction of a number of bioactive compounds such as betacyanin, betanin, betaxanthins and betalamic acid along with maintenance of plant cell viability. Similarly, Shotipruk et al. [5] reported the use of ultrasonication for menthol extraction from Mentha piperata (mint). Furthermore, Wang et al. [6] also reported use of a low percentage of methanol and increased temperature as being effective for extraction of isoflavonoids from Glycine max (soybean) while maintaining seed viability. Similar to plant cells, algae such as Dunaliella salina has also been tested for suitability of continuous extraction of β-carotene with its viability intact through utilization of biocompatible solvents [7,8].
Organic solvents such as ethanol and methanol are widely used for the extraction of RA [[9], [10], [11]]. Alternatives to organic solvents include ionic liquids [12] and Dimethyl Sulfoxide; DMSO [13] as potential green solvents for extraction of RA from Rosmarinus officinalis (rosemary) and suspension cultures of Coleus blumei (coleus) respectively. Ionic liquids are a class of organic salts that have an organic cation (such as dialkyl-imidazolium) and inorganic anion (such as Cl−) and exist as liquids at a relatively low temperature (<100 °C). They have gained attention as an attractive alternative to organic solvents due to their non-flammable, chemically and thermally stable nature [14]. In addition to solvents, heat treatment (within a range that maintains viability of the plant cell) has also been used to facilitate release of metabolites from hairy root systems [4]. Thus, for development of a biocompatible extraction technique with mycorrhizal hairy roots, determination of optimal temperature is one of the essential requirements.
In the present study we aim to develop a biocompatible extraction method that exhibits maximum metabolite solubility and extraction ability along with minimum loss of root biomass and low toxicity while maintaining root colonization potential of mycorrhizal fungi. The present study reports development of a biocompatible extraction method with mycorrhizal hairy roots of O. basilicum [1] by screening temperature- and sonication-assisted extraction methodologies through four sequential stages: 1) material preparation and selection, 2) assessment of the pretreatment regime, 3) extraction by utilization of two different agitation techniques: a) temperature- and b) sonication-assisted extraction with different solvents and 4) post treatment determination of extracted metabolite concentration and viability of both arbuscular mycorrhizae and host root.
2. Materials and methods
2.1. Establishment of mycorrhizal hairy roots for material selection, pretreatment and treatment analyses
O. basilicum hairy root line 4 (HR 4) colonized with Rhizophagus irregularis [1] was used for the screening studies. To raise co-cultures for experimental set up, a 6 week old co-culture of HR 4 raised in a culture jar (300 cm3, containing 100 mL minimal [M] semisolid medium) was divided into six equal sections and each section was inoculated to a freshly prepared culture jar containing 100 mL of M medium. Similar process was repeated to initiate co-cultures for all experimental treatments. Inoculated jars were then incubated in the dark at 26 °C (Incubator model: ET-650-8, Lovibond, Dortmund, Germany) for 120 days (d). The jars were observed weekly for any contamination and to monitor growth of the symbionts. After 120 d, to each mycorrhizal co-culture jar of HR 4; 100 mL of 10 mM sodium citrate buffer [15] was added and kept on a shaker (Kuhner Shaker, Basel, Switzerland) at 25 ± 2 °C for 90 min at 140 rpm for deionization. The root material after deionizaton from all culture jars was passed through sterile 60 μM (BSS standard) sieve. Subsequently, the collected root material was washed with sterile distilled water twice. After washing, collected mycorrhizal roots were blot dried on a sterile filter paper (Whatman # 1) disc thrice to remove excess water. Blot dried roots were mixed and then divided into 1 ± 0.5 cm long segments for screening studies. To study the loss of rosmarinic acid (RA) into the deionization buffer, its concentration was quantified using HPLC as described in the post treatment analyses section.
2.2. Pretreatment analysis
To examine the effect of soaking on extraction of RA, harvested mycorrhizal root material (100 mg) was soaked in 1 mL of water for three time periods (6, 12 and 24 h) at room temperature on a rocker (Rockymax, Tarsons, New Delhi, India). Five replicates were taken for each treatment. The concentration of RA released in water was quantified by HPLC (High performance liquid chromatography) as described in the post treatment analyses Section (2.4).
2.3. Treatment analyses: temperature- and sonication-assisted extraction
All screening studies were carried out under aseptic conditions with fresh mycorrhizal roots (100 mg) of HR 4 in 10 mL of solvent in a 70 mL test tube (Borosil, New Delhi, India). Required percentages of all solvents used in the study were prepared in a total volume of 10 mL of autoclaved distilled water. Each extraction experiment was performed with five replicates. After every treatment, roots were separated from the extractant by forceps and the final volume of extractant was made to 10 mL in a volumetric flask with respective solvent. After separation, extractant, mycorrhizal propagules (spores and hyphal biomass) and roots were processed as described in the post treatment methodology (2.4).
2.3.1. Temperature assisted extraction (TAE): water and organic solvents (low percentage of methanol and ethanol) as an extractant
Four temperatures (25 °C, 30 °C, 40 °C and 50 °C) were studied. Test tubes with 10 mL water were maintained to required temperature in a water bath (Heto HMT 200, Heto Lab Equipment, Allerod, Denmark) before addition of fresh mycorrhizal roots segments. After addition of mycorrhizal roots (100 mg), the test tubes were incubated for 6 and 12 h in a shaker (Kuhner Shaker, Basel, Switzerland) at the required temperature and rotation (100 rpm). The treated roots were then processed as described in the post treatment methodology. Similar steps were followed for screening studies with 10, 20 and 30% (v/v) of methanol and ethanol in water at four different temperatures.
2.3.2. Sonication assisted extraction (SAE): solvents and surfactants
Sonication assisted extraction was performed using a Waterbath assisted sonicator (B3510E-DTH, Branson Ultrasonics, Danbury, USA) at 30 °C for 15 min. The solvents, methanol (from 10 to 100% v/v in water), ethanol (10–100% v/v in water) (Merck, Mumbai, India), DMSO (1, 0.5, 0.1%; v/v in water), biocompatible solvents (nonane and dedecane; 100%) and surfactants (Triton X-100 and Tween-20 1, 2 and 3% v/v in water) (all from Sigma Aldrich, Bangalore, India) were screened. Sterile distilled water was used as a control for each of the above treatments. Each extraction experiment was performed with five replicates. After every treatment, the extractant (with final volume made to 10 mL in a volumetric flask), mycorrhizal propagules and roots were processed as described in the post treatment Section (2.4).
2.3.3. Sonication assisted extraction (SAE): ionic liquid as a green extractant
The potential of the ionic liquid 3 methyl-imidazolium bromide (1 M, Sigma-Aldrich, Bangalore, India) for extraction of RA from mycorrhizal roots of O. basilicum was examined in comparison with water and methanol (100%) using lyophilized mycorrhizal roots (lyophilzation was performed as described by Srivastava et al. [1]). In detail, 100 mg of the dried mycorrhizal roots were first soaked in 1 mL of water, methanol and ionic liquid respectively for 2 h on a rocker. After soaking, roots were submersed in 9 mL of the solvent in a test tube and sonicated (B3510E-DTH, Branson Ultrasonics, Danbury, USA) for 15 min at 25 ± 2 °C. Following sonication, roots were separated using sterile forcep and 1 mL of the solution used for soaking was added to the 9 mL of sonicated solution to make total volume to 10 mL. The pooled extractant, mycorrhizal propagules and roots were processed as described in the post treatment methodology (2.4).
2.3.4. Comparison between low molarity ionic liquid
Three low molar (0.25, 0.50 and 0.75 M) solutions of 3 methyl-imidazolium bromide were prepared in ultrapure water. To 10 mL of each solution, 100 mg of fresh mycorrhizal roots was added and sonicated at 25 ± 2 °C for 15 min. After sonication, final volume was made to 10 mL. The ionic liquid, mycorrhizal propagules and roots were processed as described in the post treatment methodology (2.4).
2.4. Post treatment analyses: RA concentration, mycorrhizal spore and host root viability assessment
After treatment three major analyses were performed to examine the effect of individual treatments on the RA concentration (HPLC analysis), mycorrhizal propagules viability (hyphal growth and spore germination on M medium) and colonized root viability (root growth).
2.4.1. Rosmarinic acid concentration: HPLC analysis
The concentration of RA was determined using HPLC. Briefly, an HPLC system (Shimadzu, Kyoto, Japan), equipped with a quaternary pump (LC – 20AT), solvent degasser system (DGU – 20 A5), autosampler (SIL – 20A) and diode array detector (SPDM – 20A) was used. Inbuilt software (LC solution, Shimadzu) was used to control the HPLC pump and acquire data from the diode array detector. Separations were performed on a C18 Phenomenex column (Gemini- NX 250 mm × 4.6 mm ×5 μm). A gradient program with HPLC grade water + 0.1% (v/v) ortho Phosphoric Acid (OPA) in water (Mobile phase A) and Methanol (HPLC grade, Merck, Mumbai, India) + 0.1% OPA (v/v) (Mobile phase B) in water as mobile phase was used for quantification of RA. A flow rate of 1.0 mL/ min, injection volume of 20 μL and detection wavelength of 280 nm was used. Unknown samples were identified and quantified by comparison of the retention times with those of a commercial standard of RA (Sigma, Bangalore, India) prepared in the range of 20–100 mg/L [1,3].
2.4.2. Mycorrhizal spore viability assay
Viability stain MTT [3- (4, 5 Dimethylthiazol-2-yl) - 2, 5- diphenyltetrazolium bromide], Sigma, Bangalore, India) was prepared as a 0.01% (w/v) solution in water and the treated mycorrhizal roots were then soaked in 1 mL of MTT solution for 40 h at 28 °C. After incubation, the roots were observed under stereomicroscope (SZ16, Olympus, Japan) and viability percentage (viable spores/ total number of spores × 100) was calculated. Two hundred spores per sample were observed. Red, pink or purple colored spores were considered viable while colorless, green or black colored spores were considered nonviable.
2.4.3. In vitro mycorrhizal root viability studies
After treatment mycorrhizal roots were washed in sterile distil water thrice and then placed on M media plates and root tip positions were marked as a reference for root growth measurements. The plates were observed weekly for mycorrhizal (hyphal growth and spore germination) and root growth up to twelve weeks of age.
2.5. Statistical analysis
All statistical tests were performed using a commercial statistics software package (Prism 6.02, GraphPad Software Inc, La Jolla, USA). Prior to any statistical analyses, all data sets were tested for normality using the Shapiro–Wilk’s test and homogeneity of variance by Levene’s test. Mycorrhiza spore viability percentage data was arcsine square root transformed. All data are expressed in terms of mean ± SEM.
Pair-wise comparisons were made by the Holm’s Sidak method to identify the difference (p ≤ 0.05, 0.01) between i) 6 and 12 h samples of ethanol and methanol in TAE, ii) ethanol and methanol at different percentage in TAE and iii) soaking and soaking with sonication assisted extraction in low molar ionic liquids. One way analysis of variance using Tukey’s HSD (Honestly Significant Difference) at p ≤ 0.05 was used to determine significant differences in RA concentration between the different percentages used of ethanol, methanol, DMSO, ionic liquids Triton X-100 and Tween-20 after TAE and SAE.
Data obtained from the experiments on the effect of different treatments on mycorrhizal spore viability (viability percentage) were analyzed using Tukey’s HSD test of significance at p ≤ 0.05.
3. Results
3.1. Selection of starting material
No peak of RA (retention time, 15.38 min) was detected in the HPLC chromatogram of sodium citrate buffer obtained after deionization of solidified medium utilized for propagation of mycorrhizal hairy roots showing that deionization has no effect on RA extraction. However, other polyphenolics were detected at 280 nm (Fig. S1).
3.2. Pretreatment analysis: soaking in water
Soaking had no effect on the release of RA into water after 6, 12 and 24 h of incubation at room temperature. HPLC chromatograms obtained in pretreatment experiment at three different time periods were similar to that of sodium citrate buffer obtained after deionization of solidified medium used for propagation of mycorrhizal roots (Fig. S1).
3.3. Treatment analyses: temperature assisted extraction (TAE)
3.3.1. RA concentration in the extractant
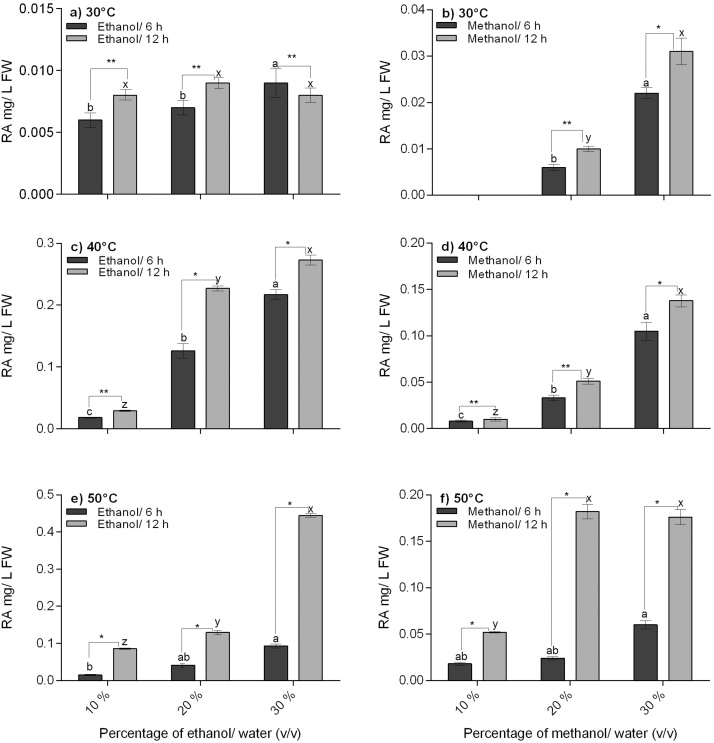
No RA was detected in any percentage of ethanol or methanol at 25 °C after 6 and 12 h of incubation (results not shown). At 30 °C, 30% methanol and 12 h was identified as the best solvent percentage and time combination for RA extraction (Fig. 1a, b). A significant difference in the concentration of RA extracted was found between 30% methanol and 30% ethanol after 6 and 12 h of incubation at 30 °C. At higher temperatures of 40 and 50 °C significant differences were found between 6 and 12 h samples at all percentages for both ethanol and methanol (Fig. 1c–f). A very low amount of RA (0.006 ± 0.001 mg/L) was detected in water after 12 h of incubation at 50 °C. On comparison between extraction with ethanol and methanol at 40 and 50 °C, higher concentration of RA was found in all ethanol treatments. Among the four temperature studies, highest yield of RA was obtained in 30% ethanol at 50 °C (0.44 mg/L). In conclusion, use of a higher percentage of solvent (methanol or ethanol) at a higher temperature (50 °C > 40 °C > 30 °C) for a prolonged time period (12 h > 6 h) enhanced RA yield.
Fig. 1.
Temperature assisted extraction of rosmarinic acid from mycorrhizal hairy roots of O. basilicum in low percentages of ethanol and methanol at two different incubation periods. RA (mg/L FW) extracted in different percentages of ethanol and methanol and comparison between 6 and 12 h of incubation: a and b) 30 °C, c and d) 40 °C and e and f) 50 °C. Data is represented as mean ± SEM of five replicates (n = 5). Bars topped by different letters indicate significant difference between 10, 20 and 30% of solvents for 6 (a, b) and12 (x, y, z) hours at p ≤ 0.05 by Tukey’s HSD. “*” and “**” Indicates pair-wise comparison made between 6 and 12 h samples at p ≤ 0.01 and p ≤ 0.001 by t-test based on Holm’s Sidak method.
3.3.2. Mycorrhizal spore viability
Red colored spores (intra-radical and extra-radical) were considered viable (Fig. 2a, b). Highest viability percentage was found in control for all the treatments. Temperature clearly affects mycorrhizal viability as a decrease in viability percentage was observed with increased temperature. Minimal or no viability was found at 50 °C. Decrease in mycorrhizal viability with increased percentage of solvent in the extraction medium and exposure time at all temperatures was also observed. 6 h treatment can be considered better than 12 h in terms of maintaining higher mycorrhizal viability percentage. Thus, solvent (10% methanol or ethanol), temperature (30 and 40 °C) and time (6 h) were identified as optimal conditions for maintaining mycorrhizal viability (Table 1).
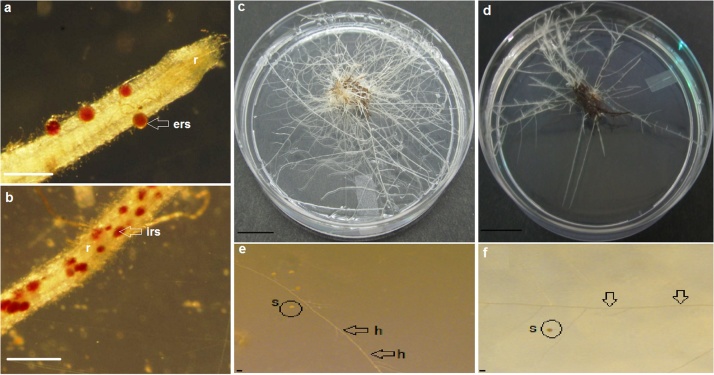
Fig. 2.
A representative figure showing post treatment results obtained for mycorrhizal propagules and roots. a and b) Viable (red colored) mycorrhizal spore on the colonised roots of O. basilicum after temperature assisted extraction. a) Extra-radical viable spore (ers), b) intra-radical viable spore (irs). “r” stands for colonised root. c and d) Root growth on M medium in a 90 mm Petri dish for control and treated samples after 1 month of incubation at 26 °C. c) Control roots after 6 h of treatment in water at 30 °C, d) roots treated in 10% methanol after 6 h of incubation at 30 °C. e and f) Hyphal growth in the control and treated samples after one month of growth. e) Control plate (6 h of treatment in water at 30 °C), showing growing hyphae (h; indicated by arrow) and spores (s; indicated in a circle), f) Treated plate (roots treated in 10% methanol after 6 h of incubation at 30 °C) showing hyphae (h) and a spore. Scale of a and b = 100 μm, c and d = 1.8 cm and e and f = 50 μm.
Table 1.
Viability percentage of mycorrhizal spores after temperature-assisted extraction in three different percentages of ethanol and methanol at four different temperatures and two time periods.z
 |
Data is represented as a mean ± SEM of each of five replicates (n = 5) in percentage. Same letters indicate no significant differences (p ≤ 0.05) between different percentages of solvent at one temperature according to Tukey’s HSD. Highlighted figures show percentage and temperature which can be selected for further investigation studies.
3.3.3. Root and mycorrhiza growth on M media
Root growth was observed for samples treated at 25 and 30 °C, while no root growth or retarded growth was obtained for samples treated at 40 and 50 °C after 6 and 12 h. Both root and hyphal growth was obtained only in 10% methanol at 25 and 30 °C after 6 and 12 h of treatment (Fig. 2c–f). 10% methanol at 30 °C was therefore found to be the optimal solvent and temperature combination for maintaining mycorrhizal and root growth.
3.4. Treatment analyses: sonication assisted extraction (SAE) in organic solvents
3.4.1. RA concentration in the extractant
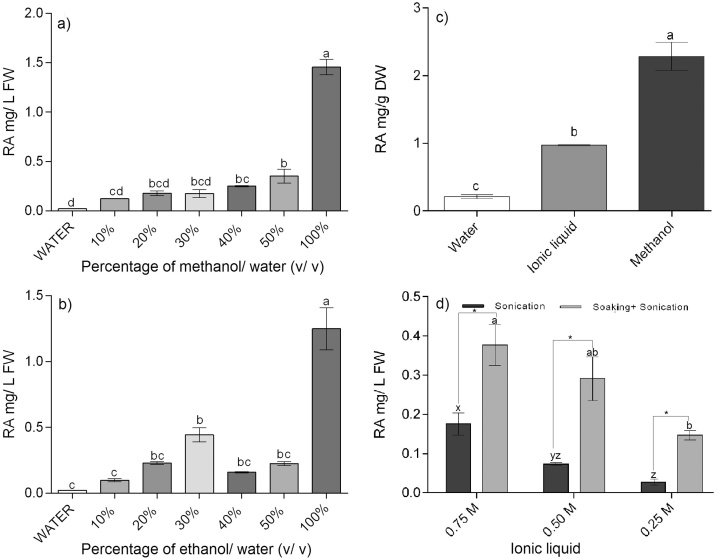
A low concentration (0.022 ± 0.001 mg/L) of RA was detected in water after sonication of mycorrhizal roots for 15 min. Significant differences were found between different percentages of all organic solvents used for SAE (Fig. 3a, b). At low percentages (10 and 20%) similar RA levels were found in methanol and ethanol while at 40 and 50%, a higher RA concentration was found in methanol than in ethanol.
Fig. 3.
Sonication assisted extraction of rosmarinic acid from mycorrhizal hairy roots of O. basilicum in organic solvents, ionic liquid (comparison study) and low molar ionic liquid. i) RA extracted (mg/L FW) in different percentage of methanol and ethanol (a and b). ii) Comparison studies for extraction of RA in water, ionic liquid and methanol (c) and low molar ionic liquid with sonication and soaking plus sonication (d). Data is represented as mean ± SEM of each of five replicates (n = 5) in all subfigures. Bars topped by same letters in all subfigures do not differ significantly (p ≤ 0.05) between i) percentages of organic solvents (methanol and ethanol) in a and b, ii) water, methanol and ionic liquid in c, and iii) low molar ionic liquids by Tukey’s HSD test. “*” In subfigure d shows pair-wise comparison made between sonication and soaking plus sonication by t-test based on Holm’s Sidak method.
3.4.2. Mycorrhizal spore viability
A lower spore viability percentage was found in ethanol than in methanol at all percentages (Table 2a). Higher mycorrhizal spore viability percentage was found in 10 and 20% methanol. Methanol was selected as the solvent system that retains the highest viability of mycorrhizal spores.
Table 2.
Viability percentage of mycorrhizal spores after sonication-assisted extraction in organic solvents, ionic liquid, dimethyl sulfoxide, biocompatible solvents and surfactants.z
 |
Data is represented as mean ± SEM of each of five replicates (n = 5) in percentage. In section (a) same letters indicate no significant differences (p ≤ 0.05) between different percentages of solvent according to Tukey’s HSD. Highlighted figures show percentage of solvent which can be selected for further investigation. In section (c), different letters indicate significant differences (p ≤ 0.05) between different molarity of the ionic liquid for one process used. Highlighted figure shows highest viability obtained with ionic liquid and sonication assisted extraction. In section (d) and (e) similar letters indicate no significant differences (p ≤ 0.05) between different percentages of DMSO, biocompatible solvents. “Nil” indicates that no red (viable) spores were observed in the samples.
3.4.3. Root and mycorrhiza growth on M media
Similar to the mycorrhizal viability results, no root and hyphal growth was obtained in samples sonicated in ethanol. Growth of both roots and hyphae similar to that observed in TAE was found for samples sonicated in 10% methanol (Fig. 2c–f).
3.5. Treatment analyses: sonication assisted extraction (SAE) in ionic liquid
3.5.1. Comparison between water, ionic liquid and methanol for extraction of RA and mycorrhizal spore viability
3 methyl-imidazolium bromide (1 M) was compared with water and methanol as an extractant of RA from freeze-dried mycorrhizal roots (Fig. 3c). 0.97 ± 0.01 mg/g DW of RA was found in ionic liquid extracts. The RA concentration (mg/g DW) found in the ionic liquid was 4.5 fold higher than water but lower than methanol (two-fold). Viability studies showed no viable spores (Table 2b) in 1 M ionic liquid (similar to methanol), hence it was concluded that low molar ionic liquid can be used for screening studies as an alternative for biocompatible extraction method development.
3.5.2. Low molar ionic liquid extraction
3.5.2.1. RA concentration
An increase in RA concentration was found with increasing molarity of ionic liquid with both types of processes used (sonication and soaking + sonication) (Fig. 3d). On comparison between soaking plus sonication and sonication at three different molarities, a higher RA concentration was found in the soaking plus sonication treatment. At 0.75 M, almost double the amount (0.37 mg/L) of RA was found in soaking plus sonication in comparison to sonication alone (0.17 mg/L). The lowest RA was found at 0.25 M in sonication-assisted extraction (0.02 mg/L).
3.5.2.2. Mycorrhizal spore viability
Viability of mycorrhizal spores was found to decrease with increase in molarity of the ionic liquid (Table 2c, b). Highest mycorrhizal viability (85%) was found in 0.25 M ionic liquid with sonication. On comparison between both the treatments 0.25 M of ionic liquid with sonication alone was selected as it showed the higher percentage of mycorrhizal viability.
3.5.2.3. Root and mycorrhizal growth on M media
No root or fungal growth was observed on M media for mycorrhizal roots treated with 0.50 and 0.75 M ionic liquid. Samples sonicated in 0.25 M ionic liquid showed slow root and hyphal growth on M media. Even though ionic liquid is a useful extractant it was not used in further studies due to its negative effect on mycorrhizal and root viability.
3.6. Treatment analyses: Sonication assited extraction (SAE) in the biocompatible solvents (Nonane, Dodecane), DMSO and surfactants
3.6.1. RA concentration, mycorrhizal and root viability
Nonane and Dodecane were not found suitable for biocompatible extraction method development in our study as no RA was detected in the extracted solution. Low levels of RA were found in 0.5% and 1.0% of DMSO (0.016 mg/L and 0.034 mg/L respectively). Further, no RA was detected in all perecentages of Triton X-100 and Tween-20.
Samples sonicated in biocompatible solvents and in three different percentages of DMSO showed 100% viable spores while no viability was observed with Triton X-100 and Tween 20 (Table 2d–f). Similar to viability results, root and hyphal growth was observed for samples treated in DMSO, showing its potential for selection as a solvent of choice.
4. Discussion
A range of options for the extraction of secondary metabolites by biocompatible extraction methods have been presented in the literature [7,8] but none have dealt with mycorrhizal roots and there are only four reports [[4], [5], [6],13] with protocols that simultaneously maintain viability of the plant samples after extraction. For the development of an efficient extraction protocol, selection of starting material, pretreatment, solvent type and its percentage, extraction methodology and determination technique play a key role [16,17]. In our study different organic and biocompatible solvents and surfactants were screened with two agitation techniques that used variation in temperature and sonication to assist extraction of rosmarinic acid from mycorrhizal hairy roots.
Selection of starting material and its pretreatment play a critical role in development of the extraction methodologies [16]. Conventionally, dried and powdered plant materials are used for the extraction of polyphenolics. Being free from excess water and of homogenous particle size makes these materials highly suitable for secondary metabolite extraction [9]. However, in our study where maintenance of mycorrhizal and root viability for continuous propagation was the primary requirement, we utilized fresh and intact mycorrhizal roots (non-homogenized). Soaking as a pretreatment at room temperature was not found suitable for the release of RA from mycorrhizal roots of O. basilicum. Pretreatment analysis clearly suggested that processes other than soaking in water at room temperature which have ability to degrade or disrupt plant cell walls and membranes in a controlled manner such as chemical destabilizers (ammonium sulfate; EDTA), surfactants (Tween-80), pH or cellulolytic (cellulases; hemicellulases) or pectinolytic (pectinases) like enzyme factors [4,[18], [19], [20]] can be explored in future as pretreatment alternatives with mycorrhizal roots for biocompatible extraction of RA.
We screened different percentages of aqueous ethanol and methanol with temperature (extended contact and slow release of secondary metabolites) and sonication (fast release of secondary metabolites) effect for the extraction of RA in a biocompatible way. Shaking ensured homogenous and continuous contact between the solvent and mycorrhized hairy roots for metabolite extraction. Ethanol was used because of commercial applicability, acceptability and GRAS (generally recognized as a safe) status in the food industry [10,21]. Polarity of a solvent affects extraction efficiency [17] hence; methanol was explored as an alternative to ethanol in our study. Increase in the concentration of RA with increased temperature, percentage of solvent and exposure time was recorded, however, negative effect of the same was found on mycorrhiza and root viability. Extraction efficiency improves due to increase in the diffusion rate of the solvent and also due to decrease in its viscosity and surface tension [22,23]. Water alone as an extractant was not found to be effective for RA extraction with TAE. 10% methanol at 30 °C and 6 h emerged as the most suitable treatment for root and mycorrhizal viability (Table 3). Through TAE analysis, 30 °C was identified as the optimum temperature for sonication assisted extraction.
Table 3.
Leads for future biocompatible extraction method development.
 |
“+” and its multiples shows the level of response obtained for viability studies and RA extracted in the current study. Highlighted figures shows best biocompatible method identified in the present study.
Many reports exist on the use of sonication assisted extraction for enhanced extraction of polyphenolics [10,20]. In contrast to TAE, RA was detected in water and sonication was found as a suitable technique for extraction of RA in lower percentages of organic solvents. Sonication was found to increase RA concentration with increased percentage of the organic solvent used. Although high concentration of RA was obtained in SAE treatment, it was found to have negative impact on viability of mycorrhizal roots. Ten percent methanol with fifteen minutes sonication was found as the best combination for biocompatible extraction in our study. A constant sonication frequency (45 KHz) was used in our study which indicates that increase in sonication frequency with continuous or periodic exposure can be an alternative for prospection in future. While we believe that we have analyzed the major aspects of a successful extraction regimes, clearly there are other possibilities such as higher sonication frequencies and refinement of the solid: liquid ratio (S/L), for further exploration that will provide further optimization of extraction conditions.
Ionic liquids with sonication are the most recently used green extraction solvents for polyphenolic extraction [12,22]. Ionic liquid was found to be effective for RA extraction from mycorrhizal roots however, an inverse relationship was found between molarity of ionic liquids and mycorrhizal viability. Low molar alkyl substituted ionic liquids such as 1-ethyl-3-methylimidazolium, 1-butyl-3-methylimidazolium, 1-hexyl-3-methylimidazolium, 1-octyl-3-methylimidazolium may provide alternative extraction opportunities for the maintenance of mycorrhizal viability along with metabolite extraction. No RA was found following extraction with the biocompatible solvents (nonane, dodecane) and this is likely due to their non-polar nature. Among all the solvents used DMSO showed highest mycorrhizal viability and root growth but very low amount of RA suggesting further exploration. Surfactants (Triton X-100 and Tween-20) at 1–3% were not found suitable for mycorrhizal viability hinting lowering of their percentage in future studies.
5. Conclusions
In conclusion, we explored several extraction alternatives that can both preserve and maintain the viability of the fungus and plant root with RA extraction and have opened up avenues for use of mycorrhizal roots as continuous resource of biological (mycorrhizal propagules) and biochemical (secondary metabolite) products. 10% methanol in water, higher percentages of DMSO and low molar ionic liquids with sonication are the leads for future exploration studies (Table 3).
While the extraction of RA used a suite of specifically optimised parameters, these are likely to vary with host plant and mycorrhiza species. The work presented here has been developed as a platform for the exploration of other protocols for biocompatible extraction of medicinally important compounds and also provides insights into the use of axenically developed mycorrhizal co-cultures for continuous mycorrhizae and metabolite production.
Author’s contribution
SS designed and carried out all the experiments, analyzed the results, prepared all the figures and tables and drafted the manuscript under supervision of AA and DC. DC contributed to the draft preparations, review and finalization of the manuscript. AA conceived the work, supervised design of the experiments and provided comments on all drafts of the manuscript.
Funding source
Deakin University, Australia provided a post graduate scholarship to SS.
Conflict of interest
Authors declare no conflict of interest.
Acknowledgements
Infrastructure support provided by TERI, India and Deakin University, Australia is duly acknowledged. Technical assistance provided by Ms Deep Rajni for HPLC analysis is highly acknowledged.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2019.e00325.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Srivastava S., Conlan X.A., Cahill D.M., Adholeya A. Rhizophagus irregularis as an elicitor of rosmarinic acid and antioxidant production by transformed roots of Ocimum basilicum in an in vitro co-culture system. Mycorrhiza. 2016;26(8):919–930. doi: 10.1007/s00572-016-0721-4. [DOI] [PubMed] [Google Scholar]
- 2.Gianinazzi S., Gollotte A., Binet M.N., Van Tuinen D., Redecker D., Wipf D. Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza. 2010;20(8):519–530. doi: 10.1007/s00572-010-0333-3. [DOI] [PubMed] [Google Scholar]
- 3.Srivastava S., Cahill D.M., Conlan X.A., Adholeya A. A novel in vitro whole plant system for analysis of polyphenolics and their antioxidant potential in cultivars of Ocimum basilicum. J. Agric. Food Chem. 2014;62(41):10064–10075. doi: 10.1021/jf502709e. [DOI] [PubMed] [Google Scholar]
- 4.P.J. Weathers, R.D. Cheetham, A. Diiorio, Process for extracting enhanced amounts of plant secondary metabolites with limited loss of plant viability (Worcester Polytechnic Institute). US5413928 A (1995).
- 5.Shotipruk A., Kaufman P.B., Wang H.Y. Feasibility study of repeated harvesting of menthol from biologically viable Mentha x piperata using ultrasonic extraction. Biotechnol. Prog. 2001;17(5):924–928. doi: 10.1021/bp010074u. [DOI] [PubMed] [Google Scholar]
- 6.Wang H.Y., Komolpis K., Kaufman P.B., Malakul P., Shotipruk A. Permeabilization of metabolites from biologically viable soybeans (Glycine max) Biotechnol. Prog. 2001;17(3):424–430. doi: 10.1021/bp0001664. [DOI] [PubMed] [Google Scholar]
- 7.Mojaat M., Foucault A., Pruvost J., Legrand J. Optimal selection of organic solvents for biocompatible extraction of β-carotene from Dunaliella salina. J. Biotechnol. 2008;133(4):433–441. doi: 10.1016/j.jbiotec.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Marchal L., Mojaat-Guemir M., Foucault A., Pruvost J. Centrifugal partition extraction of β-carotene from Dunaliella salina for efficient and biocompatible recovery of metabolites. Bioresour. Technol. 2013;134:396–400. doi: 10.1016/j.biortech.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Paniwnyk L., Cai H., Albu S., Mason T.J., Cole R. The enhancement and scale up of the extraction of anti-oxidants from Rosmarinus officinalis using ultrasound. Ultrason. Sonochem. 2009;16(2):287–292. doi: 10.1016/j.ultsonch.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Rodríguez-Rojo S., Visentin A., Maestri D., Cocero M.J. Assisted extraction of rosemary antioxidants with green solvents. J. Food Eng. 2012;109(1):98–103. [Google Scholar]
- 11.Dent M., Dragovic-Uzelac V., Penic M., Brncic M., Bosiljkov T., Levaj B. The effect of extraction solvents, temperature and time on the composition and mass fraction of polyphenols in Dalmatian wild sage (Salvia officinalis L.) extracts. Food Technol. Biotechnol. 2013;51:84–91. [Google Scholar]
- 12.Zu G., Zhang R., Yang L., Ma C., Zu Y., Wang W., Zhao C. Ultrasound-assisted extraction of carnosic acid and rosmarinic acid using ionic liquid solution from Rosmarinus officinalis. Int. J. Mol. Sci. 2012;13(9):11027–11043. doi: 10.3390/ijms130911027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park C.H., Martinez B.C. Enhanced release of rosmarinic acid from Coleus blumei permeabilized by dimethyl sulfoxide (DMSO) while preserving cell viability and growth. Biotechnol. Bioeng. 1992;40(4):459–464. doi: 10.1002/bit.260400403. [DOI] [PubMed] [Google Scholar]
- 14.Zhu S., Wu Y., Chen Q., Yu Z., Wang C., Jin S., Ding Y., Wu G. Dissolution of cellulose with ionic liquids and its application: a mini-review. Green Chem. 2006;8(4):325–327. [Google Scholar]
- 15.Doner L.W., Becard G. Solubilization of gellan gels by chelation of cations. Biotechnol. Tech. 1991;5(1):25–28. [Google Scholar]
- 16.Perino S., Chemat F. Green process intensification techniques for bio-refinery. Curr. Opin. Food Sci. 2019;25:8–13. [Google Scholar]
- 17.Oreopoulou A., Tsimogiannis D., Oreopoulou V. Extraction of polyphenols from aromatic and medicinal plants: an overview of the methods and the effect of extraction parameters. In: Watson R.R., editor. Polyphenols in Plants. second edition. Academic Press; London: 2019. pp. 243–259. [Google Scholar]
- 18.Puri M., Sharma D., Barrow C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012;30(1):37–44. doi: 10.1016/j.tibtech.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Thoo Y.Y., Ho S.K., Liang J.Y., Ho C.W., Tan C.P. Effects of binary solvent extraction system, extraction time and extraction temperature on phenolic antioxidants and antioxidant capacity from mengkudu (Morinda citrifolia) Food Chem. 2010;120(1):290–295. [Google Scholar]
- 20.Kelly N.P., Kelly A.L., O’Mahony J.A. Strategies for enrichment and purification of polyphenols from fruit-based materials. Trends Food Sci. Technol. 2018;83:248–258. [Google Scholar]
- 21.del Valle J.M., Martínb Á., Cocerob M.J., de la Fuentec J.C., de la Cruz-Quiroz R. Supercritical CO2 extraction of solids using aqueous ethanol as static modifier is a two-step mass transfer process. J. Supercrit. Fluids. 2018;143:179–190. [Google Scholar]
- 22.Liu T., Sui X., Zhang R., Yang L., Zu Y., Zhang L., Zhang Y., Zhang Z. Application of ionic liquids based microwave-assisted simultaneous extraction of carnosic acid, rosmarinic acid and essential oil from Rosmarinus officinalis. J. Chromatogr. A. 2011;1218(47):8480–8489. doi: 10.1016/j.chroma.2011.09.073. [DOI] [PubMed] [Google Scholar]
- 23.Hossain M.B., Brunton N.P., Patras A., Tiwari B., O’Donnell C.P., Martin-Diana A.B., Barry-Ryan C. Optimization of ultrasound assisted extraction of antioxidant compounds from marjoram (Origanum majorana L.) using response surface methodology. Ultrason. Sonochem. 2012;19(3):582–590. doi: 10.1016/j.ultsonch.2011.11.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.