Abstract
Syzygium cumini is used worldwide for the treatment of metabolic syndrome-associated outcomes. Previously, we described the antihypertriglyceridemic effect of the hydroethanolic extract of S. cumini leaf (HESc) in monosodium L-glutamate- (MSG-) induced obese rats. This study sought to investigate the molecular mechanisms underlying the antihypertriglyceridemic effect of HESc in MSG-obese rats. Newborn male Wistar rats were injected subcutaneously with MSG (4.0 g/kg/day, obese group) or saline 1.25% (1.0 mL/kg/day, lean group), from 2nd through 10th postnatal day. At 8 weeks old, obese rats started to be orally treated with HESc (0.5 or 1.0 g/kg/day, n = 7) or saline 0.9% (1 mL/kg/day, n = 7). Lean rats received saline solution (1 mL/kg/day, n = 7). Upon 8-week treatment, animals were euthanized for blood and tissue collection. Another set of adult nonobese Wistar rats was used for the assessment of HESc acute effects on Triton WR1339-induced hypertriglyceridemia. HESc reduced weight gain, as well as adipose tissue fat pads, without altering food intake of obese rats. HESc restored fasting serum glucose, triglycerides, total cholesterol, and free fatty acids, as well as insulin sensitivity, to levels similar to lean rats. Additionally, HESc halved the triglyceride content into very low-density lipoprotein particles, as well as healed liver steatosis, in obese rats. Hepatic protein expression of the endoplasmic reticulum chaperone GRP94 was decreased by HESc, which also downregulated the hepatic triglyceride secretion pathway by reducing the splicing of X-box binding protein 1 (XBP-1s), as well as protein disulfide isomerase (PDI) and microsomal triglyceride transfer protein (MTP) translational levels. This action was further corroborated by the acute inhibitory effect of HESc on triglyceride accumulation on Triton WR1339-treated rats. Our data support the downregulation of the XBP-1s/PDI/MTP axis in the liver of MSG-obese rats as a novel feasible mechanism for the antihypertriglyceridemic effect promoted by the polyphenolic phytocomplex present in S. cumini leaf.
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) is considered the main hepatic manifestation of metabolic syndrome (MetS) [1]. Under MetS, white adipose tissue hypertrophy causes local insulin resistance that, in turn, increases adipocyte lipolytic activity and decreases local free fatty acid (FFA) recycling, raising serum FFA levels. Increased FFA uptake by hepatocytes leads to ectopic fat accumulation and lipotoxicity due to the limited liver capacity to oxidize and/or export excess FFA [2]. Hyperinsulinemia additionally imposes de novo lipogenesis oversizing hepatic fat accumulation, an outcome partially compensated by increased triglyceride (TG) secretion via very low-density lipoprotein (VLDL) particles, that ultimately leads to hypertriglyceridemia [3]. Hypertriglyceridemia is an independent risk factor for cardiovascular diseases, which are the leading cause of morbimortality worldwide [4].
Despite the abovementioned evidences, the molecular mechanisms involved in NAFLD and hypertriglyceridemia onset remain incompletely defined. During the last decade, the endoplasmic reticulum (ER) stress has been proposed as a key player by its role in unfolded protein response (UPR) [5]. UPR occurs when the ER becomes overwhelmed and causes luminal misfolded protein accumulation, eliciting the phosphorylation of three ER transmembrane sensing proteins, namely, inositol-requiring enzyme-1α (IRE-1α), protein kinase RNA-like ER kinase (PERK), and activating transcription factor 6 (ATF6) [5]. IRE-1α subsequently splices the X-box binding protein 1 (XBP-1s) mRNA, a transcription factor importantly involved in the reestablishment of ER homeostasis [6] and hepatic lipogenesis regulation [7]. Studies conducted by us [8] and others [9–11] have demonstrated the importance of the IRE1α/XBP-1s pathway for hepatic lipid homeostasis, through either lipogenesis modulation or stimulation of TG secretion, a process mediated by upregulation of microsomal triglyceride transfer protein (MTP) and protein disulfide isomerase (PDI) expression in hepatocytes [8]. Henceforth, the XBP-1s/PDI/MTP axis has emerged as a potential therapeutic target for the treatment of lipid metabolism disorders, especially hypertriglyceridemia [12], despite the plethora of other regulatory targets.
Herbal medicines constitute an important source of bioactive and potentially therapeutic molecules enabled to fulfill a multiple-target strategy for MetS-related outcome treatment [13, 14]. These properties are specially related to their antioxidant capacity, although other mechanisms might feasibly be involved [15]. In this context, cardiometabolic potentialities of Syzygium cumini (L.) Skeels (syn: S. jambolanum D.C., Eugenia jambolana Lam.) have been highlighted [16]. S. cumini is an Indian native tree from the Myrtaceae family widely cultivated throughout the world and popularly known as jambolão, jambolan, java plum, or black plum [17]. It is traditionally used to treat a variety of illnesses, most of them related to MetS and its comorbidities [16, 18–21]. Moreover, its ethnopharmacological relevance has been recognized by the Brazilian Ministry of Health, which included S. cumini species in the National Index of Medicinal Plants of Interest to the Unified Public Health System, acronym RENISUS [22].
In a previous study, we showed that the hydroethanolic extract of S. cumini leaf (HESc) improved the metabolic profile of monosodium L-glutamate- (MSG-) induced obese rats, especially by reverting TG accumulation in both the liver and serum. These effects were associated with the improvement of peripheral insulin sensitivity and β-cell function and attributed to the polyphenolic profile—mainly composed by myricetin derivatives, as well as other flavonoids and tannins—identified in HESc [23]. More recently, we reported the HPLC-MS/MS phytochemical characterization of a polyphenol-rich extract (PESc) prepared from the aforementioned HESc, which allowed the identification of five main compounds as follows: gallic acid, myricetin, myricetin-3-α-arabinopyranoside, myricetin deoxyhexoside, and quercetin, with myricetin accounting for nearly 20% of PESc total polyphenol content [24]. Notwithstanding, PESc exhibited a strong antioxidant capacity against both biological and nonbiological oxidants, which enabled it to protect mice from an oxidative stress-induced diabetic state [24]. However, the molecular mechanisms responsible for the improvement of lipid metabolism promoted by S. cumini leaf remain poorly investigated.
Thus, taking into account our previous reports that hypertriglyceridemia in MSG-obese rats is associated with activation of the XBP-1s/PDI/MTP axis [8] and that HESc reverted their characteristic NAFLD and hypertriglyceridemia [23], in the present study, we sought to investigate the molecular mechanisms underlying the antihypertriglyceridemic activity of HESc in MSG-obese rats. The data presented herein endorse our hypothesis by presenting a novel feasible mechanism for the HESc antihypertriglyceridemic effect, which corroborates S. cumini leaf as a source of compounds for hypolipemiant purposes.
2. Materials and Methods
2.1. Plant Material
Leaves from Syzygium cumini (L.) Skeels, popularly known as jambolão in Brazil and java plum or black plum in English-spoken countries, were collected from specimens located at the Dom Delgado Campus (2°33′11.7″S 44°18′22.7″W) of the Federal University of Maranhão (UFMA; São Luís, Maranhão, Brazil). A voucher specimen was identified by Prof. Dr. Eduardo Bezerra Almeida Jr., a botanist at the Herbarium of Maranhão (MAR, Department of Biology, UFMA), and stored under the register number 4574. Furthermore, the species' name was confirmed in http://www.theplantlist.org on 08/15/2018.
2.2. Hydroethanolic Extract Preparation
After leaf collection, the hydroethanolic extract of S. cumini leaf (HESc) was obtained as previously described [23]. Upon lyophilization, HESc powder was stored at 4°C and freshly diluted in 0.9% NaCl at proper concentrations for oral administration to the animals. An aliquot of HESc was analyzed by HPLC-MS/MS to validate its authenticity. As shown in Supplementary Figure 1, HESc fingerprint corresponds to the same polyphenolic profile previously reported by us [23], whose main compounds are shown in Figure 1.
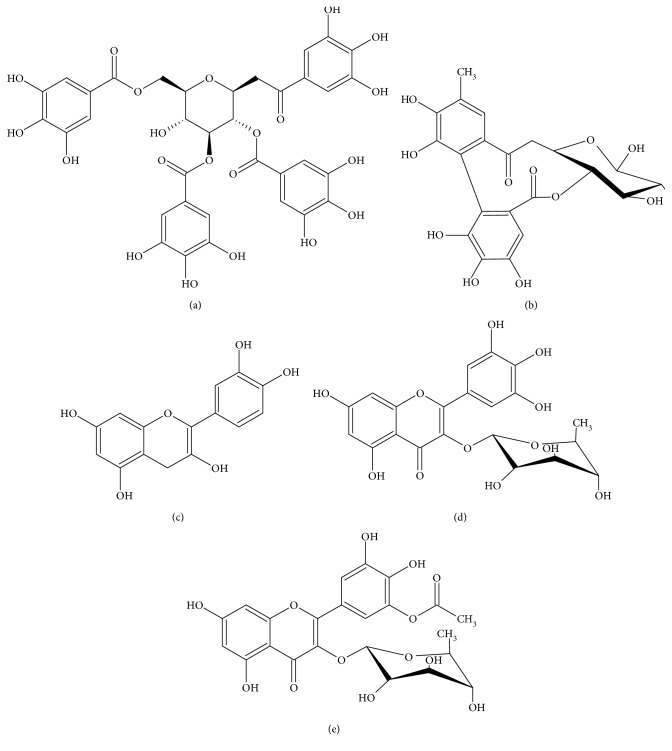
Figure 1.
Main polyphenolic compounds identified in the hydroethanolic extract of Syzygium cumini leaves (HESc). (a) Tetragalloylglucose, (b) hexahydroxydiphenoyl-glucose, (c) quercetin, (d) myricetin deoxyhexoside, and (e) acylated myricetin deoxyhexoside.
2.3. MSG Obesity Induction and Experimental Design
Newborn male Wistar rats were subcutaneously injected with the MSG solution (4.0 g/kg/day, Sigma-Aldrich, USA, Cat# G1626) or saline 1.25% (1.0 mL/kg/day), in accordance with our previous report [8]. From birth, all animals were kept under controlled conditions of temperature (23 ± 2°C) and light (12 h light/12 h dark) with filtered water and regular chow (CR-1 Nuvilab, Curitiba, Brazil) provided ad libitum. At 8 weeks of age, obesity development was assessed by calculating the Lee index (LI) (body weight (g)1/3/nasoanal length (cm) × 1000) [25]. MSG-obese rats and their appropriated lean controls were randomly divided into 4 groups and orally treated (gavage) as follows:
Lean: lean rats receiving 1.0 mL/kg/day saline 0.9% (n = 7)
Obese: MSG-obese rats receiving 1.0 mL/kg/day saline 0.9% (n = 7)
Obese+HESc 0.5: MSG-obese rats receiving 0.5 g/kg/day HESc (n = 7)
Obese+HESc 1.0: MSG-obese rats receiving 1.0 g/kg/day HESc (n = 7)
Body weight and food intake were measured twice a week throughout 8 weeks of treatment. At the end, the LI was again calculated to evaluate the effects of the treatment on body mass. Next, upon overnight fasting, animals were anesthetized (10 mg/kg xylazine+40 mg/kg ketamine, i.p.) for blood collection via abdominal aorta puncture and subsequent euthanasia by exsanguination. The liver and both retroperitoneal and periepididymal fat pads were collected, weighed, and appropriately stored for posterior assessments. All animal procedures were in accordance with the National Council for the Control of Animal Experimentation (CONCEA, Brazil) and approved by the Committee for Ethics and Welfare on Animal Use (CEUA) of UFMA under ruling number 23115.01983/2013-41.
2.4. Serum Biochemical Analysis and Assessment of Insulin Resistance
Glucose (GL), total cholesterol (TC), TG, FFA, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels were assessed in serum samples using spectrophotometric commercial kits according to the manufacturers' instructions (Labtest, MG, Brazil, and Wako, VA, USA). Insulin resistance was inferred by calculating the TyG index (TyG = natural logarithm [fasting TG (mg/dL) × fasting GL (mg/dL)/2]) [26].
2.5. Liver Histological Analysis
Liver slides were obtained through 6 μm thick transversal sections, stained with hematoxylin-eosin (HE), and assessed by 2 independent researchers in a double-blind way for the determination of the NAFLD activity score (NAS). This score is based on a semiquantitative analysis of the three definer criteria of NASH: steatosis (0-3), ballooning (0-3), and lobular inflammation (0-2). Total score is a value ranging from 0 to 8, which indicates a hepatic prognostic status. Scores > 6 indicate NASH; from 3 to 5, borderline; and from 0 to 2, it is not NASH [27].
2.6. Liver Lipid Profile
The hepatic lipid profile was assessed as previously described [28]. Briefly, a chloroform : methanol (2 : 1) solution was used to extract total fat from 500 mg liver samples, which were resuspended in a Triton-X100 : methanol (2 : 1) solution for the measurement of TG and TC levels as described in Section 2.4.
2.7. Chromatographic Analysis of Serum Lipoproteins
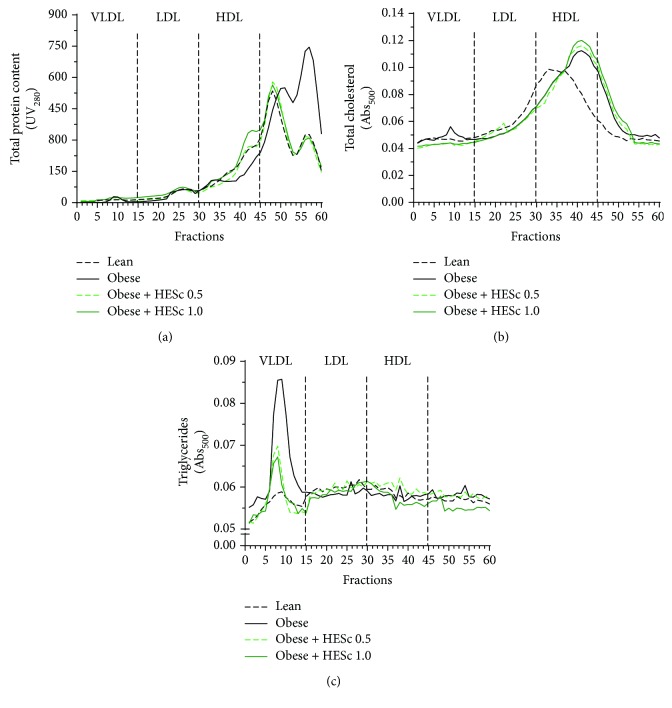
Serum lipoproteins were separated by fast protein liquid chromatography (FPLC) in a Superose 6 HR 10/30 column (Amersham Biosciences, Sweden) eluted with Tris buffer (pH 7.0; 10 mmol Tris, 150 mmol NaCl, 1 M EDTA, and 0.03% NaN3) at a rate of 0.5 mL/min, as previously described [29]. A total of 60 fractions were collected in a chronological order representing the density of each lipoprotein particle. Specifically, fractions 1-15 were labeled as VLDL, 16-30 were labeled as low-density lipoprotein (LDL), 31-45 were labeled as high-density lipoprotein (HDL), and 45-60 were labeled as other serum proteins. The TC and TG contents in each fraction were measured as described in Section 2.4. The total protein content was determined from absorbance at 280 nm.
2.8. Evaluation of Protein Expression by Western Blotting
Liver samples (n = 7) were homogenized by sonication with lysis buffer containing protease inhibitors (1 μg/mL aprotinin, 1 μg/mL leupeptin, and 10 mM PMSF). For each sample, 30 μg of total protein was diluted with sample buffer and loaded into a SDS-PAGE gel for protein separation, which was transferred to nitrocellulose membranes. For the detection of the proteins of interest, membranes were incubated with primary antibodies: anti-KDEL (Enzo Life Sciences, USA, Cat# ADI-SPA-827), anti-XBP1 (Enzo Life Sciences, USA, Cat# ADI-905-739), anti-PDI (Enzo Life Sciences, USA, Cat# ADI-SPA-891), and anti-MTP (Sigma-Aldrich, USA, Cat# AV43618), followed by incubation with peroxidase-conjugated secondary antibodies for chemiluminescent detection (peroxidase-H2O2-luminol). β-Actin (Sigma-Aldrich, USA, Cat# A5441) was used as protein loading control.
2.9. Induction of Acute Hypertriglyceridemia with Triton WR1339
Eight-week-old male nonobese Wistar rats were randomized and administered with a single oral dose of saline 0.9% (0.1 mL/100 g) or either 0.5 g/kg or 1.0 g/kg HESc. After 1 hour, HESc-treated rats were intraperitoneally injected with 0.3 g/kg Triton WR1339 (Sigma-Aldrich, USA, Cat# T8761) and referred as the +HESc 0.5 g/kg and +HESc 1.0 g/kg groups (n = 7 per group). Saline-treated rats were injected with either equal Triton WR1339 dose (Triton WR1339 group, n = 7) or saline 0.9% (0.1 mL/100 g; control group, n = 7). Fasting blood samples were collected from the tail vein before (0 h) as well as 24, 48, and 72 h after the administration of Triton WR1339 for the determination of TG levels as described in Section 2.4 [30].
2.10. Statistical Analysis
Results were expressed as the mean ± standard error of the mean (SEM). The Shapiro-Wilk test was applied for normality assuring and groups compared by one-way analysis of variance (ANOVA) followed by the Newman-Keuls as posttest with Prism 7.0 (GraphPad, USA). Statistically significant differences were set at 5% with p < 0.05.
3. Results
3.1. HESc Reduces Adipose Tissue Accumulation in Obese Rats
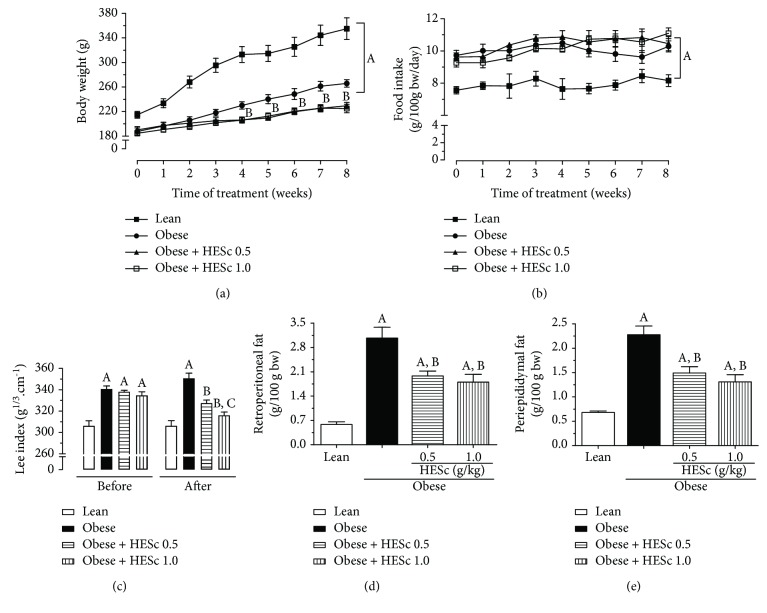
The lean group had higher mean body weight than the MSG-obese groups throughout the 8-week treatment period (Figure 2(a)), a peculiar feature of this animal model because of its shorter body length associated with deficient growth hormone (GH) secretion. However, MSG-obese rats presented a LI value 11% higher than the lean group (Figure 2(c)), accompanied by a 4-fold increase of retroperitoneal and periepididymal fat pads (Figures 2(d) and 2(e), respectively), denoting their obese condition. HESc treatment (0.5 and 1.0 g/kg/day) reduced the weight gain of obese rats by 15% regardless of the administered dose (Figure 2(a)), though no effect had been detected on food intake (Figure 2(b)). Besides, the LI was reduced by 7% and 10% in the obese+HESc 0.5 and obese+HESc 1.0 groups, respectively (Figure 2(c)). Weight loss was followed by a strong decrease of white adipose tissue accumulation, since retroperitoneal and periepididymal fat pads were, respectively, reduced by nearly 47% and 40% at both doses (Figures 2(d) and 2(e)).
Figure 2.
Administration of hydroethanolic extract of Syzygium cumini leaves (HESc) reduces fat accumulation in obese rats. (a) Evolution of body weight. (b) Daily food intake. (c) Lee index before and after 8 weeks of treatment. (d) Retroperitoneal fat. (e) Periepididymal fat. Lean: control group. Obese: obese group. Obese+HESc 0.5: obese animals treated with 0.5 g/kg of HESc. Obese+HESc 1.0: obese animals treated with 1.0 g/kg of HESc. Results are expressed as mean ± SEM (n = 7 per group). Letters indicate differences (p < 0.05; ANOVA; Newman-Keuls) as compared to alean, bobese, and cobese+HESc 0.5.
3.2. HESc Improves Serum Glycolipid Profile in Obese Rats
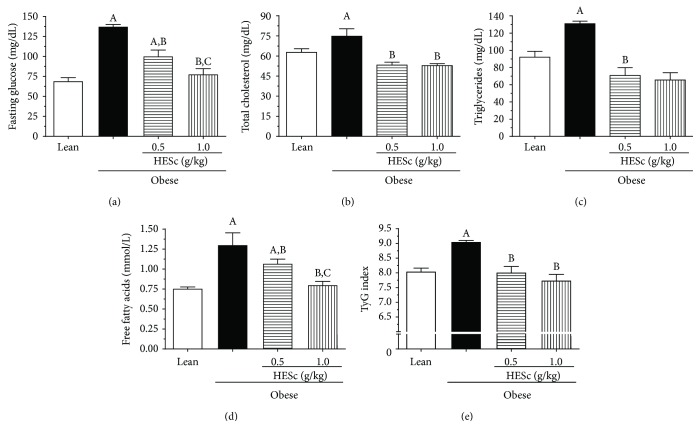
At the end of treatment, obese rats presented serum fasting glucose levels 2-fold higher than the lean ones, which were dose-dependently reduced by 27% and 43% in the obese+HESc 0.5 and obese+HESc 1.0 groups, respectively (Figure 3(a)). The TC and TG levels of the HESc-treated groups were completely restored at both doses in relation to lean (Figures 3(b) and 3(c)). Circulating FFA levels on the HESc-treated groups were also reduced in a dose-dependent manner by 23% and 49% at doses of 0.5 and 1.0 g/kg, respectively, when compared to the MSG-obese group (Figure 3(d)). TyG index was used as a surrogate method for insulin sensitivity assessment. The data in Figure 3(e) indicates impaired insulin sensitivity on the MSG-obese group in relation to the lean group, which was precluded on HESc-treated obese animals.
Figure 3.
Administration of hydroethanolic extract of Syzygium cumini leaves (HESc) improves glucose homeostasis and serum lipid profile in obese rats. (a) Fasting glucose. (b) Total cholesterol. (c) Triglycerides. (d) Free fatty acids. (e) TyG index. Lean: control group. Obese: obese group. Obese+HESc 0.5: obese animals treated with 0.5 g/kg of HESc. Obese+HESc 1.0: obese animals treated with 1.0 g/kg of HESc. Results are expressed as mean ± SEM (n = 7 per group). Letters indicate differences (p < 0.05; ANOVA; Newman-Keuls) with respect to alean; bobese, and cobese+HESc 0.5.
3.3. HESc Reverts NAFLD and Improves Liver Function in Obese Rats
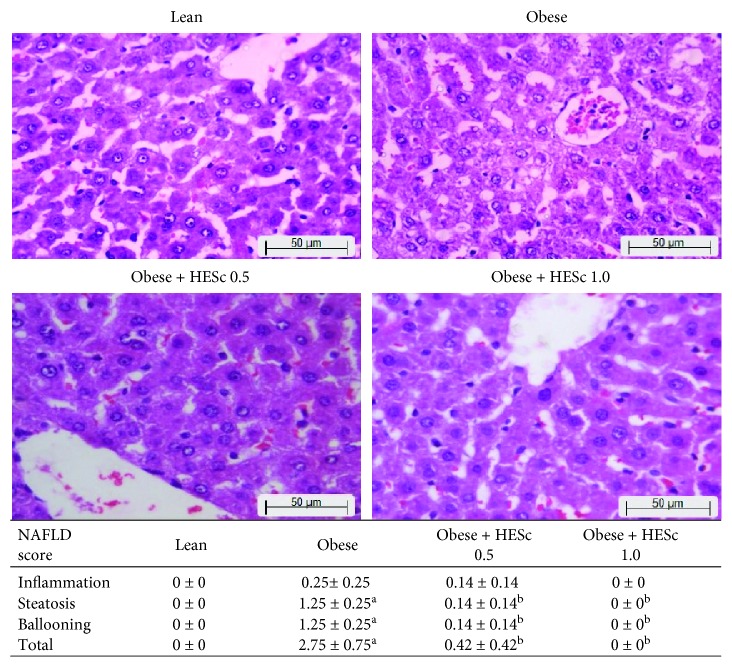
As shown in Figure 4, the NAS score for obese animals supported the presence of hepatic steatosis and cell ballooning and suggested low-grade inflammation, as compared to lean rats, putting those animals in an intermediate way toward nonalcoholic steatohepatitis (NASH). However, the administration of HESc reverted these features in both groups. Noteworthy, obese+HESc 1.0 animals did not score in any assessed parameters. Histological analysis was corroborated by data from the liver lipid profile. Even though no difference had been seen in the livers' relative weights (Figure 5(a)), the total fat content was increased by nearly 50% in the obese group, as compared to lean. Again, HESc reduced such fat accumulation by 13% in obese+HESc 0.5 and 27% in obese+HESc 1.0, as compared to the obese group (Figure 5(b)). Alike, the TG levels were increased by 76% in obese animals as compared to the lean group but reduced by 44% and 57% in the obese+HESc 0.5 and obese+HESc 1.0 groups, respectively (Figure 5(d)). No difference was observed in the liver cholesterol content among the groups (Figure 5(c)). To evaluate to which extent this fat accumulation impaired liver function, the activities of AST and ALT were assessed. As Figure 5(e) shows, there was a 60% increase in AST activity on obese rats compared to the lean group, whereas HESc reduced its activity by nearly 35% at both doses. On the other hand, there was no change in ALT activity among the groups (Figure 5(f)).
Figure 4.
Administration of hydroethanolic extract of Syzygium cumini leaves (HESc) reverses nonalcoholic fatty liver disease (NAFLD) in obese rats. Analysis of liver histology with hematoxylin and eosin [27]. Lean: control group. Obese: obese group. Obese+HESc 0.5: obese animals treated with 0.5 g/kg of HESc. Obese+HESc 1.0: obese animals treated with 1.0 g/kg of HESc. Results are expressed as the mean ± SEM (n = 7 per group). Letters indicate differences (p < 0.05; ANOVA; Newman-Keuls) with respect to alean and bobese.
Figure 5.
Administration of hydroethanolic extract of Syzygium cumini leaves (HESc) improves lipid profile and liver function of obese rats. (a) Liver weight. (b) Total liver fat. (c) Hepatic cholesterol. (d) Hepatic triglycerides. (e) Serum aspartate aminotransferase (AST). (f) Serum alanine aminotransferase (ALT). Lean: control group. Obese: obese group. Obese+HESc 0.5: obese animals treated with 0.5 g/kg of HESc. Obese+HESc 1.0: obese animals treated with 1.0 g/kg of HESc. Results are expressed as the mean ± SEM (n = 7 per group). Letters indicate differences (p < 0.05; ANOVA; Newman-Keuls) with respect to alean and bobese.
3.4. HESc Reduces the TG Content in VLDL Particles from Obese Rats
FPLC analysis of serum samples showed that the nonlipoprotein particle-bound protein content (fractions 45-60) of obese animals was 48% higher than in lean animals, which was fully restored in the animals treated with HESc (Figure 6(a)). In fractions corresponding to HDL particles (fractions 30-45), the protein content was 30% lower in obese animals as compared with lean animals, whereas treatment with HESc at 0.5 and 1.0 g/kg increased these levels by 30% and 72%, respectively, in comparison with the former (Figure 6(a)). Likewise, the cholesterol content in HDL fractions from the obese group exhibited 36% higher levels than the lean group. HESc treatment did not change the cholesterol content in HDL particles but restored it in VLDL particles (fractions 1-15; Figure 6(b)). Noteworthy, VLDL particles from obese rats contained 3-fold more TG than the lean group. HESc dropped down these levels by nearly 50% in both treated groups (Figure 6(c)).
Figure 6.
Administration of hydroethanolic extract of Syzygium cumini leaves (HESc) reduces the triglyceride content in VLDL particles of obese rats. (a) Protein content of high-density lipoprotein (HDL), low-density lipoprotein (LDL), and very low-density lipoprotein (VLDL). (b) Cholesterol content of lipoproteins. (c) Triglyceride content of lipoproteins. Lean: control group. Obese: obese group. Obese+HESc 0.5: obese animals treated with 0.5 g/kg of HESc. Obese+HESc 1.0: obese animals treated with 1.0 g/kg of HESc. Results are expressed as the mean absorbance of fast protein liquid chromatography (FPLC) (n = 7 per group).
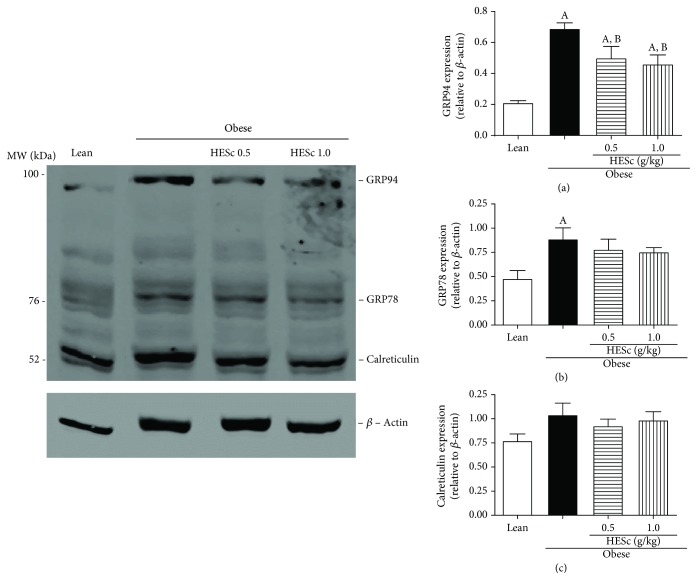
3.5. HESc Reduces ER Stress in the Liver of Obese Rats
Measurement of the translational levels of KDEL chaperones, which are involved in hepatic UPR, exhibited a 3-fold increase for GRP94 on obese rats as compared to lean. HESc treatment reduced this protein expression by 27% and 33% at doses of 0.5 and 1.0 g/kg, respectively. On the other hand, GRP78 expression was increased by 83% in the obese group, but only marginally reduced in HESc-treated animals, since the difference did not reach statistically significant values. Likewise, there was no difference in calreticulin expression among groups (Figure 7).
Figure 7.
Administration of hydroethanolic extract of Syzygium cumini leaves (HESc) attenuates endoplasmic reticulum stress in the livers of obese rats. Protein expression was determined by western blotting. (a) Glucose response protein 94 (GRP94). (b) Glucose response protein 78 (GRP78). (c) Calreticulin. Lean: control group. Obese: obese group. Obese+HESc 0.5: obese animals treated with 0.5 g/kg of HESc. Obese+HESc 1.0: obese animals treated with 1.0 g/kg of HESc. Densitometry results are expressed as the mean ± SEM (n = 4 per group). Letters indicate differences (p < 0.05; ANOVA; Newman-Keuls) with respect to alean and bobese.
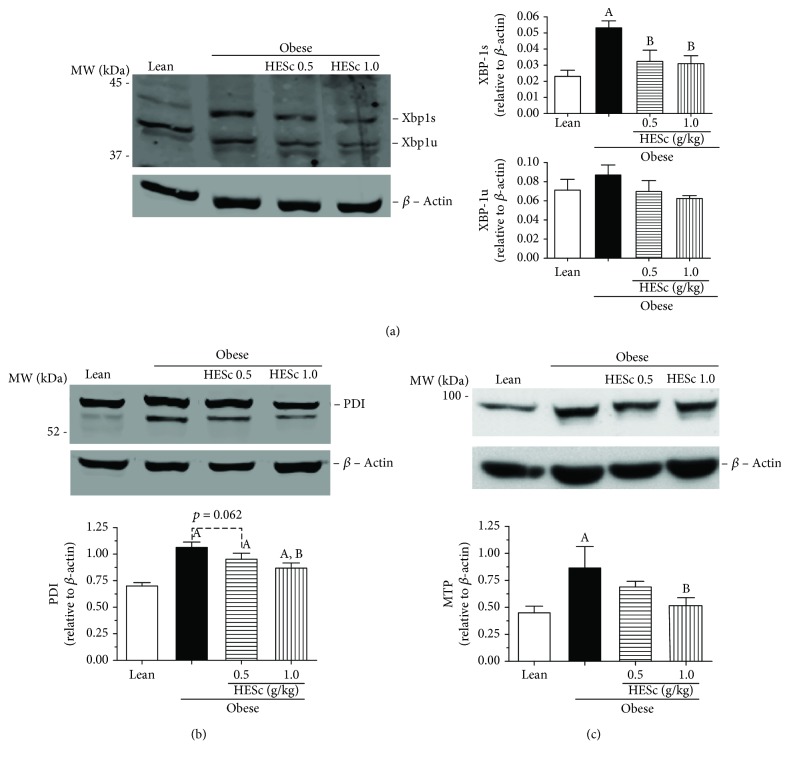
3.6. HESc Inhibits the XBP-1s/PDI/MTP Axis
Assessment of XBP-1 protein expression for both spliced (XBP-1s) and unspliced (XBP-1u) forms revealed a 2.5-fold higher splicing rates in MSG-obese rats than in the lean group. This increase was reduced to values similar to those of the lean group upon treatment with both doses of HESc (Figure 8(a)). PDI expression was increased by 52% on obese animals but brought back to intermediate levels in the obese+HESc 1.0 group, with a reduction of 21% (Figure 8(b)). Similarly, MTP expression was 2-fold higher on the obese group and reduced to lean-like levels in animals from the obese+HESc 1.0 group (Figure 8(c)). In order to verify the effect of HESc on MTP function, this enzyme's activity was assessed in nonobese rats acutely injected with Triton WR1339, which is a well-known model of MTP-mediated hypertriglyceridemia [30]. Oral administration of HESc, at the same abovementioned doses, 1-hour prior induction reduced serum TG accumulation within 24 h, as well as hastened its clearance in the following 48 h (Figure 9), which is in line with a lower rate of VLDL particles assembly and secretion from the liver.
Figure 8.
Administration of hydroethanolic extract of Syzygium cumini leaves (HESc) inhibits the XBP-1s/PDI/MTP pathway in the livers of obese rats. Protein expression was determined by western blotting. (a) X-box binding protein 1 (XBP-1). (b) Protein disulfide isomerase (PDI). (c) Microsomal triglyceride-transfer protein (MTP). Lean: control group. Obese 0: obese group. Obese+HESc 0.5: obese animals treated with 0.5 g/kg of HESc. Obese+HESc 1.0: obese animals treated with 1.0 g/kg of HESc. Densitometry results are expressed as the mean ± SEM (n = 4 per group). Letters indicate differences (p < 0.05; ANOVA; Newman-Keuls) with respect to alean and bobese.
Figure 9.
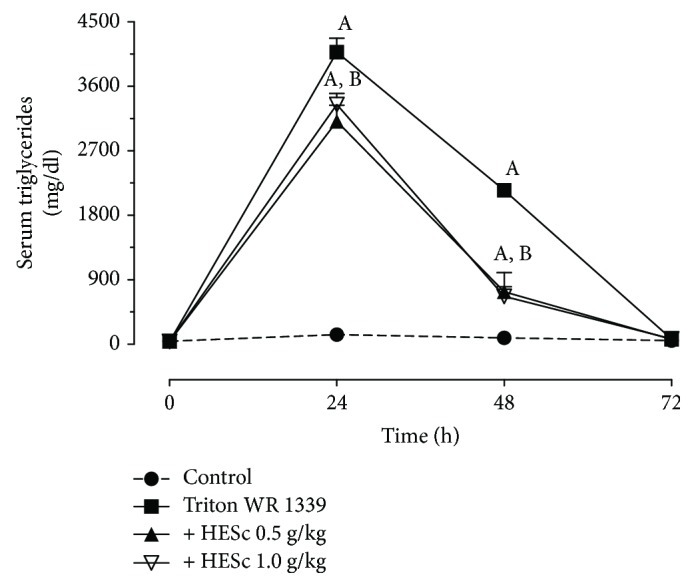
Administration of hydroethanolic extract of Syzygium cumini leaves (HESc) reduces the export of liver triglycerides in rats with Triton WR1339-induced acute hypertriglyceridemia. Acute hypertriglyceridemia was induced by an intraperitoneal injection of Triton WR1339 (0.3 g/kg) 1 hour after a single dose of HESc was administered, and the rats were monitored for 72 h. Control: normotriglyceridemic group. Triton WR1339: hypertriglyceridemic group. +HESc 0.5: hypertriglyceridemic group treated with 0.5 g/kg of HESc. +HESc 1.0: hypertriglyceridemic group treated with 1.0 g/kg of HESc. Results are expressed as the mean ± SEM (n = 7 per group). Letters indicate differences (p < 0.05; ANOVA; Newman-Keuls) with respect to alean and bobese.
4. Discussion
This study strengthens S. cumini pharmacological potentialities since it corroborates our previous report that HESc restores serum TG levels in hypertriglyceridemic MSG-obese rats [23]. The data presented herein expand these findings by showing that oral administration of HESc to MSG-obese rats for 8 weeks detains weight gain, improves fatty liver disease, and reverts hypertriglyceridemia, besides other metabolic outcomes typically found in this MetS rodent model. Specifically, this study shows that HESc inhibited both expression and activity of hepatic MTP by downregulation of the XBP-1s/PDI/MTP axis, reducing the incorporation of TG into VLDL particles and consequently lowering the circulating TG levels.
Neonatal administration of MSG damages hypothalamic nuclei, e.g., arcuate nucleus, leading to impaired GH secretion; therefore, adult animals are shorter and lighter than age-matched controls but present higher fat mass [31]. Furthermore, these animals also exhibit autonomic unbalance characterized by enhanced vagus nerve tonus, which imposes increased insulin secretion and consequent development of peripheral insulin resistance and elevation of fat stores [32]. In this study, treatment with HESc reduced body weight gain in obese rats but did not affect their food intake. Although an extract from S. cumini leaf had been shown to decrease food intake on nonobese rats [33], the lower weight gain displayed by our treated obese rats is most likely related to the lipolytic action of HESc, as we previously described [23]. HESc is particularly rich in tetragalloylglucose (Figure 1(a)), a gallotannin whose lipolytic effects have been attributed to the modulation of proliferative peroxisome-activated receptor gamma (PPARγ) [34], a mechanism shared by the compound vitalbosine A isolated from S. cumini seed [35].
Besides antiobesity effects, HESc also improved the serum lipid profile of obese rats. Particularly, it reverted the remarkable hypertriglyceridemia peculiarly displayed by MSG-obese rats [8], an effect further extensive to serum TC and FFA levels. In accordance, the TG content into VLDL particles from HESc-treated obese rats was halved in comparison to nontreated obese animals. HESc also reduced the excess ectopic liver fat in obese rats, an effect associated to TG but not to cholesterol content. This lipid-lowering effect of HESc seems to be responsible for the complete restoration of the hepatic histopathological pattern of obese rats, whose NAS score was brought back to values very similar to lean healthy animals. These effects might be related to improved hepatic insulin sensitivity promoted by HESc, which is supported by the reduced TyG index value found in treated obese animals. Importantly, the TyG index has been proposed as a biomarker of NAFLD initiation and progression even in asymptomatic subjects [36]. Studies carried out with S. cumini seed extract in HepG2 cells [37] and livers from streptozotocin-induced diabetic rats [38] have attributed its hypolipidemic effect to increased PPARγ activity and expression. Myricetin (Figures 1(d) and (e)), the most prevalent flavonoid in HESc [23], has been shown to improve insulin sensitivity [39] and promote hepatic lipid oxidation by increasing PPARα expression in the liver [40].
In addition to the extensive knowledge on S. cumini effects on peripheral insulin sensitivity, particularly on the PPARα/γ pathways, we hypothesize that HESc polyphenols might also interfere with the ER stress-sensing IRE1α/XBP-1s pathway, which has also been proposed as an important mechanism underlying the development of NAFLD and hypertriglyceridemia, as demonstrated in hepatocyte-specific IRE1α-null mice [11] and MSG-obese rats [8].
In the past decade, hepatic ER stress has been proposed as a main contributing factor for NAFLD onset and progression, as well as MetS-associated dyslipidemias [5, 41]. Initial UPR is characterized by elevated gene/protein expression of KDEL chaperones, namely, glucose-regulated protein 94 (GRP94), GRP78, and calreticulin, to mitigate protein misfolding and reestablish ER homeostasis within a negative feedback loop regulated by both the IRE1α and ATF6 pathways [41, 42]. Our obese rats showed a clear increase of hepatic GRP94 and GRP78 protein expressions, denoting active UPR, which was partially attenuated in HESc-treated obese rats. Many actions of HESc, such as improvement of insulin sensitivity and lower FFA circulating levels, might also be involved in this effect since it has been shown that increased serum FFA levels might induce hepatic ER stress [43], meanwhile polyphenols such as myricetin derivatives are able to attenuate it [44].
Studies have demonstrated that the IRE1α/XBP-1s pathway is the most conserved arm of UPR [45], which is importantly involved in the control of glucose homeostasis and lipid metabolism [9, 10]. Regardless of a recent discussion about its lipogenic [10] or antilipogenic role [46] in the liver, it is well established that XBP-1s acts as a hypertriglyceridemic factor [7, 46]. XBP-1s activates the expression of MTP and its active subunit PDI, which favors the incorporation of TG into nascent VLDL particles [11]. Noteworthy, the function of XBP-1 on hepatic lipogenesis is unrelated to its function in the UPR but nevertheless requires its splicing by IRE1α [7]. Here, HESc downregulated this pathway, since obese-treated animals presented lower splicing of XBP-1s along with lesser expression of both PDI and MTP, as compared to obese nontreated rats. The inhibitory effect of HESc on the XBP-1/PDI/MTP axis was further supported by its acute action on Triton WR1339-induced hypertriglyceridemic rats, which not only reduced total TG accumulation within 24 h but also hastened its clearance. Alike, a recent study has shown the inhibitory effect of polyphenols from Punica granatum flower on the IRE1α/XBP1s pathway [47]. To the best of our knowledge, this is the first study to describe a feasible molecular mechanism underlying the antihypertriglyceridemic properties of S. cumini.
There is consistent evidence about the effects of polyphenol-rich extracts or polyphenols per se on the ER stress pathways. Preincubation of polyphenol-rich extract of Vitis rotundifolia has been shown to inhibit thapsigargin-induced ER stress in human retinal endothelial cells [48]. Administration of polyphenol-rich extracts from pomegranate and green tea, for 20 weeks, to high-fat diet obese mice attenuated UPR activation in the skeletal muscle by reducing the gene expression of GRP78 and XBP1 splicing [49], an action also described for kaempferol in ischemic cardiomyocytes [50] and for apigenin in neuronal cells with ER stress induced by thapsigargin or brefeldin A [51]. In addition, myricetin and its derivatives have been shown to decrease protein expression of ER stress markers, such as GRP78 and IRE1α in the colon of mice with colitis-associated cancer [44]. Notwithstanding, since oxidative stress is enrolled as UPR inducer [52], polyphenols contained in HESc, particularly myricetin derivatives, would also have attenuated ER stress by reestablishing cellular redox balance in the liver of our treated MSG-obese rats, an obesity model whose oxidative stress has already been characterized [53, 54]. Thus, future studies must specifically address this issue as well as assess the effects of isolated compounds in order to pursue additional mechanisms and identify the molecules probably responsible for them.
5. Conclusions
In conclusion, the data presented herein reinforce the prominent metabolic properties of S. cumini leaf. The recovery of normal serum TG levels on HESc-treated obese rats discloses a novel feasible mechanism of action for the hypolipemiant effect traditionally ascribed to this plant species via inhibition of the hepatic XBP-1s/PDI/MTP axis. Hepatic MTP inhibitors have been considered important agents to treat familial dyslipidemia, as that seen in abetalipoproteinemia, but their clinical utility has been restricted by the increased risk of hepatic steatosis [55, 56]. In addition to its antihypertriglyceridemic effect, HESc also restored the hepatic fat accumulation of obese rats. This secondary, but not less important, property supports the possibility of synergism among the mechanism shown herein and other properties previously described for HESc, such as huge antioxidant capacity and improvement of peripheral insulin sensitivity [23, 24]. Finally, this dual action further corroborates the multitarget potentialities of the polyphenols contained into HESc as a potential phytocomplex against MetS-derived metabolic disturbances.
Acknowledgments
The authors are thankful to Dr. Valéria Sutti Nunes and Dr. Edna Regina Nakandakare, from the School of Medicine of the University of São Paulo, for allowing us to use the FPLC equipment, as well as to Dr. Cáritas de Jesus Mendonça, from the Department of Chemistry of the Federal University of Maranhão, for allowing us to use the HPLC equipment. The authors are specially grateful to LeFisio's staff for all technical support during the experimental procedures. This study was supported by the Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico, Tecnológico e Inovação do Estado do Maranhão (FAPEMA) (Universal 00280/12, PAEDT 00297/14, Universal 00643/15, and Universal 01571/16) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Finance code 001). CFFC, LNCF, and ILSS received fellowships from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Materials
Figure S1: HPLC fingerprint of the hydroethanolic extract of S. cumini leaf (HESc). Representative HPLC chromatogram with UV detection at 254 nm of HESc, which was performed in triplicate for the validation of its authenticity in accordance with the analysis previously reported (Sanches et al., [23]).
References
- 1.Younossi Z. M., Koenig A. B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Fabbrini E., Sullivan S., Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51(2):679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shulman G. I. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. The New England Journal of Medicine. 2014;371(12):1131–1141. doi: 10.1056/NEJMra1011035. [DOI] [PubMed] [Google Scholar]
- 4.Borén J., Matikainen N., Adiels M., Taskinen M.-R. Postprandial hypertriglyceridemia as a coronary risk factor. Clinica Chimica Acta. 2014;431:131–142. doi: 10.1016/j.cca.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X.-Q., Xu C. F., Yu C. H., Chen W. X., Li Y. M. Role of endoplasmic reticulum stress in the pathogenesis of nonalcoholic fatty liver disease. World Journal of Gastroenterology. 2014;20(7):1768–1776. doi: 10.3748/wjg.v20.i7.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshida H., Matsui T., Yamamoto A., Okada T., Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107(7):881–891. doi: 10.1016/S0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 7.Lee A.-H., Scapa E. F., Cohen D. E., Glimcher L. H. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320(5882):1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franca L. M., Freitas L. N. C., Chagas V. T., et al. Mechanisms underlying hypertriglyceridemia in rats with monosodium L-glutamate-induced obesity: evidence of XBP-1/PDI/MTP axis activation. Biochemical and Biophysical Research Communications. 2014;443(2):725–730. doi: 10.1016/j.bbrc.2013.12.042. [DOI] [PubMed] [Google Scholar]
- 9.Ning J., Hong T., Ward A., et al. Constitutive role for IRE1α-XBP1 signaling pathway in the insulin-mediated hepatic lipogenic program. Endocrinology. 2011;152(6):2247–2255. doi: 10.1210/en.2010-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.So J. S., Hur K. Y., Tarrio M., et al. Silencing of lipid metabolism genes through IRE1α-mediated mRNA decay lowers plasma lipids in mice. Cell Metabolism. 2012;16(4):487–499. doi: 10.1016/j.cmet.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S., Chen Z., Lam V., et al. IRE1α-XBP1s induces PDI expression to increase MTP activity for hepatic VLDL assembly and lipid homeostasis. Cell Metabolism. 2012;16(4):473–486. doi: 10.1016/j.cmet.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piperi C., Adamopoulos C., Papavassiliou A. G. XBP1: a pivotal transcriptional regulator of glucose and lipid metabolism. Trends in Endocrinology and Metabolism. 2016;27(3):119–122. doi: 10.1016/j.tem.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Vasudeva N., Yadav N., Sharma S. K. Natural products: a safest approach for obesity. Chinese Journal of Integrative Medicine. 2012;18(6):473–480. doi: 10.1007/s11655-012-1120-0. [DOI] [PubMed] [Google Scholar]
- 14.Prabhakar P. K., Doble M. A target based therapeutic approach towards diabetes mellitus using medicinal plants. Current Diabetes Reviews. 2008;4(4):291–308. doi: 10.2174/157339908786241124. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y. J., Gan R. Y., Li S., et al. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules. 2015;20(12):21138–21156. doi: 10.3390/molecules201219753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chagas V. T., França L. M., Malik S., Paes A. M. . A. Syzygium cumini (L.) skeels: a prominent source of bioactive molecules against cardiometabolic diseases. Frontiers in Pharmacology. 2015;6:p. 259. doi: 10.3389/fphar.2015.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayyanar M., Subash-Babu P. Syzygium cumini (L.) Skeels: a review of its phytochemical constituents and traditional uses. Asian Pacific Journal of Tropical Biomedicine. 2012;2(3):240–246. doi: 10.1016/S2221-1691(12)60050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helmstadter A. Syzygium cumini (L.) Skeels (Myrtaceae) against diabetes – 125 years of research. Pharmazie. 2008;63(2):91–101. doi: 10.1691/ph.2008.7335. [DOI] [PubMed] [Google Scholar]
- 19.Baldissera G., Sperotto N. D. M., Rosa H. T., et al. Effects of crude hydroalcoholic extract of Syzygium cumini (L.) Skeels leaves and continuous aerobic training in rats with diabetes induced by a high-fat diet and low doses of streptozotocin. Journal of Ethnopharmacology. 2016;194:1012–1021. doi: 10.1016/j.jep.2016.10.056. [DOI] [PubMed] [Google Scholar]
- 20.Cercato L. M., White P. A. S., Nampo F. K., Santos M. R. V., Camargo E. A. A systematic review of medicinal plants used for weight loss in Brazil: is there potential for obesity treatment? Journal of Ethnopharmacology. 2015;176:286–296. doi: 10.1016/j.jep.2015.10.038. [DOI] [PubMed] [Google Scholar]
- 21.Braboza Da Silva N., Regis A. C. D., Esquibel M. A., do Espírito Santo Santos J., de Almeida M. Z. Uso de plantas medicinais na comunidade quilombola da Barra II–Bahia, Brasil. Boletin Latinoamericano y del Caribe de Plantas Medicinales y Aromaticas. 2012;11(5):435–453. [Google Scholar]
- 22.BRASIL R. Relação Nacional de Plantas Medicinais de Interesse ao SUS. Portal. Saúde. 2009. July 2019, gov.br/portal/arquivos/pdf/RENISUS.pdf.
- 23.Sanches J. R., França L. M., Chagas V. T., et al. Polyphenol-rich extract of Syzygium cumini leaf dually improves peripheral insulin sensitivity and pancreatic islet function in monosodium L-glutamate-induced obese rats. Frontiers in Pharmacology. 2016;7:p. 48. doi: 10.3389/fphar.2016.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chagas V. T., Coelho R. M. R. . S., Gaspar R. S., et al. Protective effects of a polyphenol-rich extract from Syzygium cumini (L.) Skeels leaf on oxidative stress-induced diabetic rats. Oxidative Medicine and Cellular Longevity. 2018;2018:13. doi: 10.1155/2018/5386079.5386079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernardis L. L., Patterson B. D. Correlation between ‘Lee index’ and carcass fat content in weanling and adult female rats with hypothalamic lesions. The Journal of Endocrinology. 1968;40(4):527–528. doi: 10.1677/joe.0.0400527. [DOI] [PubMed] [Google Scholar]
- 26.Simental-Mendia L. E., Rodriguez-Moran M., Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metabolic Syndrome and Related Disorders. 2008;6(4):299–304. doi: 10.1089/met.2008.0034. [DOI] [PubMed] [Google Scholar]
- 27.Kleiner D. E., Brunt E. M., van Natta M., et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 28.Freedman B. D., Lee E. J., Park Y., Jameson J. L. A dominant negative peroxisome proliferator-activated receptor-γ knock-in mouse exhibits features of the metabolic syndrome. Journal of Biological Chemistry. 2005;280(17):17118–17125. doi: 10.1074/jbc.M407539200. [DOI] [PubMed] [Google Scholar]
- 29.Jericó M. M., De Chiquito F. C., Kajihara K., et al. Chromatographic analysis of lipid fractions in healthy dogs and dogs with obesity or hyperadrenocorticism. Journal of Veterinary Diagnostic Investigation. 2009;21(2):203–207. doi: 10.1177/104063870902100204. [DOI] [PubMed] [Google Scholar]
- 30.Silva R. M., Santos F., Maciel M., Pinto A., Rao V. Effect of trans-dehydrocrotonin, a 19-nor-clerodane diterpene from Croton cajucara on experimental hypertriglyceridaemia and hypercholesterolaemia induced by Triton WR 1339 (tyloxapol) in mice. Planta Medica. 2001;67(8):763–765. doi: 10.1055/s-2001-18360. [DOI] [PubMed] [Google Scholar]
- 31.Millard W. J., Martin J. B., Audet J., Sagar S. M., Martin J. B. Evidence that reduced growth hormone secretion observed in monosodium glutamate-treated rats is the result of a deficiency in growth hormone-releasing factor. Endocrinology. 1982;110(2):540–550. doi: 10.1210/endo-110-2-540. [DOI] [PubMed] [Google Scholar]
- 32.Balbo S. L., Grassiolli S., Ribeiro R. A., et al. Fat storage is partially dependent on vagal activity and insulin secretion of hypothalamic obese rat. Endocrine. 2007;31(2):142–148. doi: 10.1007/s12020-007-0021-z. [DOI] [PubMed] [Google Scholar]
- 33.Oliveira A. C. P., Endringer D. C., Amorim L. A. S., Brandão M. D. G. L., Coelho M. M. Effect of the extracts and fractions of Baccharis trimera and Syzygium cumini on glycaemia of diabetic and non-diabetic mice. Journal of Ethnopharmacology. 2005;102(3):465–469. doi: 10.1016/j.jep.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 34.Yang M. H., Vasquez Y., Ali Z., Khan I. A., Khan S. I. Constituents from Terminalia species increase PPARα and PPARγ levels and stimulate glucose uptake without enhancing adipocyte differentiation. Journal of Ethnopharmacology. 2013;149(2):490–498. doi: 10.1016/j.jep.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Thiyagarajan G., Muthukumaran P., Sarath Kumar B., Muthusamy V. S., Lakshmi B. S. Selective inhibition of PTP1B by Vitalboside a from Syzygium cumini enhances insulin sensitivity and attenuates lipid accumulation via partial agonism to PPARγ: in vitro and in silico investigation. Chemical Biology & Drug Design. 2016;88(2):302–312. doi: 10.1111/cbdd.12757. [DOI] [PubMed] [Google Scholar]
- 36.Simental-Mendia L. E., Simental-Mendía E., Rodríguez-Hernández H., Rodríguez-Morán M., Guerrero-Romero F. The product of triglycerides and glucose as biomarker for screening simple steatosis and NASH in asymptomatic women. Annals of Hepatology. 2016;15(5):715–720. doi: 10.5604/16652681.1212431. [DOI] [PubMed] [Google Scholar]
- 37.Sharma B., Balomajumder C., Roy P. Hypoglycemic and hypolipidemic effects of flavonoid rich extract from Eugenia jambolana seeds on streptozotocin induced diabetic rats. Food and Chemical Toxicology. 2008;46(7):2376–2383. doi: 10.1016/j.fct.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 38.Sharma A. K., Bharti S., Kumar R., et al. Syzygium cumini ameliorates insulin resistance and β-cell dysfunction via modulation of PPARγ, dyslipidemia, oxidative stress, and TNF-α in type 2 diabetic rats. Journal of Pharmacological Sciences. 2012;119(3):205–213. doi: 10.1254/jphs.11184FP. [DOI] [PubMed] [Google Scholar]
- 39.Choi H. N., Kang M. J., Lee S. J., Kim J. I. Ameliorative effect of myricetin on insulin resistance in mice fed a high-fat, high-sucrose diet. Nutrition Research and Practice. 2014;8(5):544–549. doi: 10.4162/nrp.2014.8.5.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang C. J., Tzeng T. F., Liou S. S., Chang Y. S., Liu I. M. Myricetin increases hepatic peroxisome proliferator-activated receptor α protein expression and decreases plasma lipids and adiposity in rats. Evidence-based Complementary and Alternative Medicine. 2012;2012:11. doi: 10.1155/2012/787152.787152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cnop M., Foufelle F., Velloso L. A. Endoplasmic reticulum stress, obesity and diabetes. Trends in Molecular Medicine. 2012;18(1):59–68. doi: 10.1016/j.molmed.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 42.Shoulders M. D., Ryno L. M., Genereux J. C., et al. Stress-independent activation of XBP1s and/or ATF6 reveals three functionally diverse ER proteostasis environments. Cell Reports. 2013;3(4):1279–1292. doi: 10.1016/j.celrep.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nivala A. M., Reese L., Frye M., Gentile C. L., Pagliassotti M. J. Fatty acid-mediated endoplasmic reticulum stress in vivo: differential response to the infusion of soybean and lard oil in rats. Metabolism. 2013;62(5):753–760. doi: 10.1016/j.metabol.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang F., Song Z. Y., Qu X. J., et al. M10, a novel derivative of myricetin, prevents ulcerative colitis and colorectal tumor through attenuating robust endoplasmic reticulum stress. Carcinogenesis. 2018;39(7):889–899. doi: 10.1093/carcin/bgy057. [DOI] [PubMed] [Google Scholar]
- 45.Cox J. S., Shamu C. E., Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73(6):1197–1206. doi: 10.1016/0092-8674(93)90648-A. [DOI] [PubMed] [Google Scholar]
- 46.Herrema H., Zhou Y., Zhang D., et al. XBP1s is an anti-lipogenic protein. The Journal of Biological Chemistry. 2016;291(33):17394–17404. doi: 10.1074/jbc.M116.728949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang D., Liu L., Ajiakber D., et al. Anti-diabetic effect of Punica granatum flower polyphenols extract in type 2 diabetic rats: activation of Akt/GSK-3β and inhibition of IRE1α-XBP1 pathways. Frontiers in Endocrinology. 2018;9:p. 586. doi: 10.3389/fendo.2018.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ha J. H., Shil P. K., Zhu P., Gu L., Li Q., Chung S. Ocular inflammation and endoplasmic reticulum stress are attenuated by supplementation with grape polyphenols in human retinal pigmented epithelium cells and in C57BL/6 mice. The Journal of Nutrition. 2014;144(6):799–806. doi: 10.3945/jn.113.186957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguez J., Gilson H., Jamart C., et al. Pomegranate and green tea extracts protect against ER stress induced by a high-fat diet in skeletal muscle of mice. European Journal of Nutrition. 2015;54(3):377–389. doi: 10.1007/s00394-014-0717-9. [DOI] [PubMed] [Google Scholar]
- 50.Kim D. S., Ha K. C., Kwon D. Y., et al. Kaempferol protects ischemia/reperfusion-induced cardiac damage through the regulation of endoplasmic reticulum stress. Immunopharmacology and Immunotoxicology. 2008;30(2):257–270. doi: 10.1080/08923970701812530. [DOI] [PubMed] [Google Scholar]
- 51.Choi A. Y., Choi J. H., Lee J. Y., et al. Apigenin protects HT22 murine hippocampal neuronal cells against endoplasmic reticulum stress-induced apoptosis. Neurochemistry International. 2010;57(2):143–152. doi: 10.1016/j.neuint.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 52.Bozaykut P., Sahin A., Karademir B., Ozer N. K. Endoplasmic reticulum stress related molecular mechanisms in nonalcoholic steatohepatitis. Mechanisms of Ageing and Development. 2016;157:17–29. doi: 10.1016/j.mad.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 53.Park C. H., Kim M. Y., Sok D. E., Kim J. H., Lee J. H., Kim M. R. Butterbur (Petasites japonicus Max.) extract improves lipid profiles and antioxidant activities in monosodium L-glutamate-challenged mice. Journal of Medicinal Food. 2010;13(5):1216–1223. doi: 10.1089/jmf.2009.1380. [DOI] [PubMed] [Google Scholar]
- 54.Seiva F. R., Chuffa L. G. A., Braga C. P., Amorim J. P. A., Fernandes A. A. H. Quercetin ameliorates glucose and lipid metabolism and improves antioxidant status in postnatally monosodium glutamate-induced metabolic alterations. Food and Chemical Toxicology. 2012;50(10):3556–3561. doi: 10.1016/j.fct.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 55.Wierzbicki A. S., Hardman T., Prince W. T. Future challenges for microsomal transport protein inhibitors. Current Vascular Pharmacology. 2009;7(3):277–286. doi: 10.2174/157016109788340703. [DOI] [PubMed] [Google Scholar]
- 56.Rizzo M., Wierzbicki A. S. New lipid modulating drugs: the role of microsomal transport protein inhibitors. Current Pharmaceutical Design. 2011;17(9):943–949. doi: 10.2174/138161211795428768. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: HPLC fingerprint of the hydroethanolic extract of S. cumini leaf (HESc). Representative HPLC chromatogram with UV detection at 254 nm of HESc, which was performed in triplicate for the validation of its authenticity in accordance with the analysis previously reported (Sanches et al., [23]).
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.