Introduction
Schnitzler syndrome (SS) is a rare autoinflammatory disease with unclear etiopathogenesis. The disease is associated with cytokine dysregulation involving interleukin (IL)-1β and the inflammasome pathway and manifests with major clinical criteria, namely IgM paraprotein and urticarial rash, and minor criteria, including fever, lymphadenopathy, musculoskeletal pain, and thrombosis.1
Anakinra is a recombinant version of human IL-1 receptor (IL-1R) antagonist and binds to IL-1R, inhibiting IL-1α and IL-1β signaling. SS typically responds dramatically to IL-1R blockade.2 No recurrent genetic alterations have been identified in SS to date. Interestingly, a case series examining 21 SS patients sequenced 32 autoinflammatory genes without identifying a single genetic alteration in the cohorts.3
SS carries a 15% to 20% risk of an associated lymphoproliferative disorder, similar to that of other conditions with IgM paraproteinemia, including lymphoplasmacytic lymphoma (LPL)/Waldenström macroglobulinemia (WM), and systemic amyloidosis. The precise relationship amongst hematologic disorders is unknown.
WM is an IgM-secreting LPL with clinical features including cytopenias and lymphadenopathy and paraprotein-related manifestations. MYD88 is a gene involved in nuclear factor κ light chain enhancer of activated B cells (NF-κB) signaling. Mutations in MYD88 (eg, L265P) are the molecular hallmark of WM and have been identified in more than 90% of WM cases.4 This mutation was not included in previous molecular panels investigating SS.3
Here we present the first case, to our knowledge, of SS with MYD88 L265P mutation and explore the proposed role of this alteration in the pathogenesis of SS. Consent was obtained in accordance with University of California San Diego Institutional Review Board guidelines.
Case
A 79-year-old man presented with a history of right inguinal lymphadenopathy for 7 years. Initial past workup found an IgM paraprotein, without malignancy, associated with κ light chain restriction in the bone marrow (CD5-/CD10- B cells) (Table I).
Table I.
Pathology specimens (lymph nodes, bone marrow, skin), morphology, flow cytometry, cytogenetics, and genomic profiling/biomarker analysis
| Specimen | Morphology | Flow cytometry | Cytogenetics |
|---|---|---|---|
| Right inguinal node | Reactive follicular hyperplasia, clusters of plasma cells with mature morphology | κ-preponderant (κ:λ ratio of 3:1) B cells (43%); T cells exhibit increased CD4:CD8 ratio at 9:1 | 3 copies of MALT1 |
| Bone marrow | No morphologic evidence of lymphoma | Monotypic κ-restricted B-cell population identified CD19+, CD20+, CD22+, CD23+, CD25+, FMC7+ CD5−, CD10−, CD11c−, CD103− |
N/A |
| ∗Left inguinal node | Follicular hyperplasia with marked polyclonal plasmacytosis | Monotypic κ-restricted B cells, CD5+, CD19+, CD20+, CD22+, partial CD23+, partial FMC7+, CD10−, CD38−, EBER−, ISH−, HHV8−, Ki67 high | N/A |
| Bone marrow | IgM(+) plasma cell neoplasm involving hypercellular marrow with trilineage hematopoiesis. No B-lineage cells present. | No definitive B cells and plasma cells; 8.4% small mature T cells with CD4:CD8 ratio of 2.8:1; 1.1% NK cells. | Normal male karyotype |
| Skin | Interstitial neutrophilic infiltrate with epitheliotropism. No vasculitis. | N/A | N/A |
| Comprehensive genomic profiling and biomarker analysis | |
|---|---|
| Test | Findings |
| Tissue NGS of lymph node∗† |
MAP2K1 P124S MYD88 L265P ARID1A D1810fs∗† DNMT3A R736C |
| Blood cfDNA‡ |
TP53 L145P (0.2% cfDNA) TERT promoter SNV (7.7% cfDNA) |
| TMB† | 1 mutation/mb |
| PD-1/L1 IHC of lymph node tumor cells§ | PD-1 expression: low positive PD-L1 expression: Negative |
cfDNA, cell free DNA; EBER, Epstein Barr virus–encoded small RNAs; HHV8, human herpes virus 8; IHC, immunohistochemistry; ISH, in-situ hybridization; NGS, next-generation sequencing; NK, natural killer; PD, programmed death receptor; TMB, tumor mutational burden.
Tissue NGS done on left inguinal lymph node as noted.
Performed by Foundation One Heme panel (406 gene panel).
Performed by Guardant 360 (73 gene panel).
Performed by Pathline emerge using clone NAT105 for PD-1 staining and clone SP142 for PD-L1 staining.
Over the next 7 years, he suffered from arthralgias, bone pain, and an episodic urticarial eruption. He had a persistent leukocytosis, anemia, and elevated erythrocyte sedimentation rate (ESR). A lymph node biopsy was performed, which found monotypic CD5-/CD10- B cells again without malignancy (Table I). Positron emission tomography–computed tomography found mild fluorodeoxyglucose-avid bilateral axillary and inguinal lymph nodes, so he was empirically treated with rituximab monotherapy without improvement.
The patient eventually presented to our clinic with persistent fatigue, chronic anemia, arthralgias, and urticarial rash (Fig 1). Repeat bone marrow biopsy found an IgM+ κ-restricted plasmacytosis with hypercellular marrow. No B cells were identified, which excluded LPL/WM. Cytogenetic tests found a normal male karyotype. MYD88 L265P mutation was identified on next-generation sequencing (Table I). A skin biopsy found interstitial neutrophilic infiltrates compatible with neutrophilic urticarial dermatosis related to SS (Fig 2). Anakinra, 100-mg subcutaneous daily injections, were started, which within days led to near-complete resolution of rash and arthralgias, and hemoglobin plus ancillary inflammatory markers (thrombocytosis, ESR, and C-reactive protein) normalized (Fig 3). He remains symptom free in clinical remission on anakinra, 100 mg/d, 1 year into treatment.
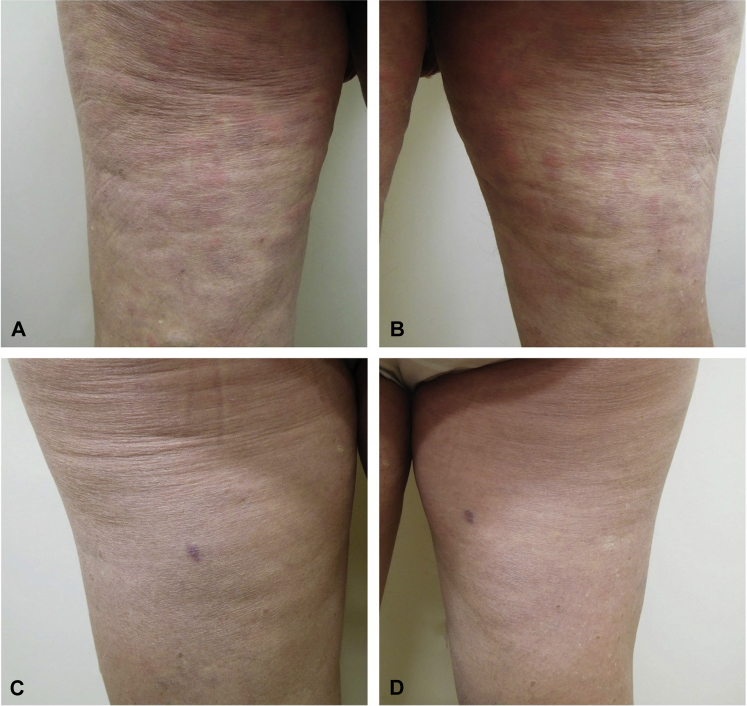
Fig 1.
Erythematous urticarial plaques most prominent on the left (A) and right (B) posterior thighs. Follow-up examination on day 9 after daily subcutaneous anakinra, 100 mg, shows complete resolution of skin lesions. Focal scar and dyspigmentation on the left (C) and right (D) thighs represent biopsy sites.
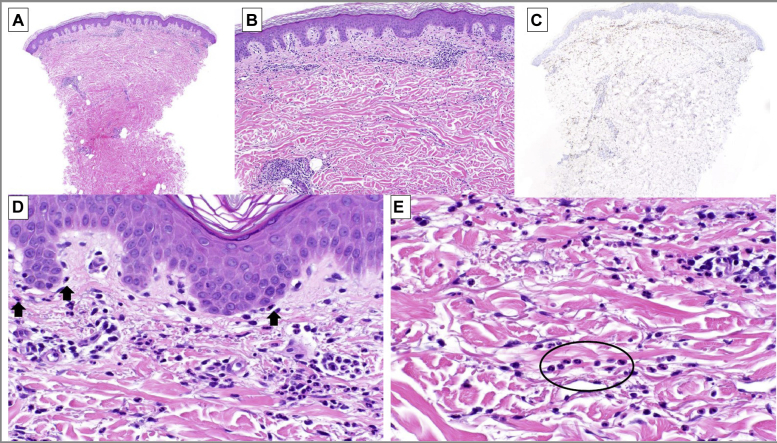
Fig 2.
Histopathologic examination found sparse mononuclear cells around the papillary and mid-dermal vascular plexus (A). The mononuclear cells exhibit a slight interstitial and perijunctional pattern (B). Myeloperoxidase labels the infiltrate confirming myeloid lineage (C). Neutrophils are positioned along the dermoepidermal junction. Neutrophilic epitheliotropism (arrows) and superficial vessels are patent without fibrinoid necrosis (D). Some neutrophils extend in single-file configuration between collagen bundles (circled) (E). (A, B, D, and E, Hematoxylin-eosin stain; original magnifications: A, ×40; B, ×100; D and E, ×200; C, myeloperoxidase immunoperoxidase; original magnification: ×40).
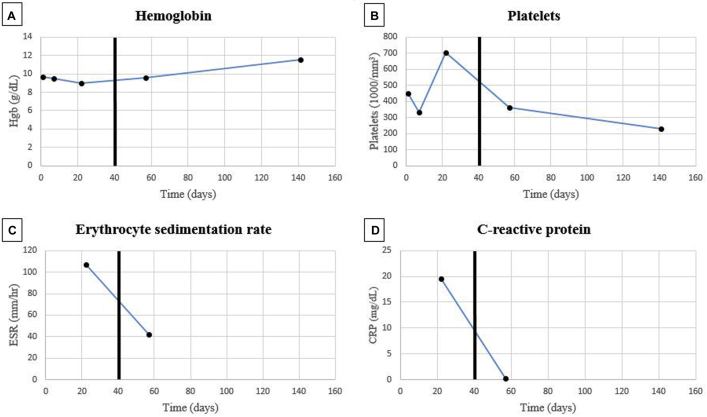
Fig 3.
The trends in hemoglobin (A), platelets (B), ESR (C), and CRP (D) are shown before and after treatment with 100-mg daily subcutaneous anakinra. The dark black lines represent the start date of anakinra.
Discussion
We show a common alteration in LPL/WM that also represents a novel finding in SS. MYD88 plays an important role in IL-1 signaling, mediating the association between the IL-1R and its IL-1 receptor–associated kinase (IRAK). MYD88 L265P gain-of-function mutation promotes cell survival by forming a protein complex of IRAK1 and IRAK4, contributing to increased expression of pro-inflammatory pathways.5 Given the apparent specificity of the L265P mutation for LPL and role in promoting survival, SS and LPL appear connected and likely represent a disease continuum. Although not previously reported, this mutation may exist in a subset of SS patients who may be at the highest risk of lymphoproliferative disorder development. Therapeutically, Bruton tyrosine kinase inhibitor ibrutinib has shown significant activity in MYD88-mutated LPL; this agent also deserves evaluation in SS.6 Further study of the genomics of SS is required with assessment of the prevalence of L265P mutation in a larger cohort.
Footnotes
Drs Cohen and Li contributed equally to this article.
Funding sources: Funded in part by National Cancer Institute grant P30 CA023100 (RK) and the Joan and Irwin Jacobs Fund philanthropic fund.
Conflicts of interest: Dr Goodman receives speaking fees from Seattle Genetics and consulting fees from Jazz Pharmaceuticals. Dr Kurzrock receives research funding from Genentech, Merck, Serono, Pfizer, Sequenom, Foundation Medicine, and Guardant. Dr Kurzrock receives consultant fees from X Biotech, Loxo, Neomed, and Actuate Therapeutics and speaker fees from Roche. Dr Kurzrock has stocks in IDbyDNA and has an ownership interest in Curematch Inc. The rest of the authors have no conflicts to disclose.
References
- 1.Simon A., Asli B., Braun-Falco M. Schnitzler's syndrome: diagnosis, treatment, and follow-up. Allergy. 2013;68(5):562–568. doi: 10.1111/all.12129. [DOI] [PubMed] [Google Scholar]
- 2.de Koning H.D. Schnitzler's syndrome: lessons from 281 cases. Clin Transl Allergy. 2014;4(1):41. doi: 10.1186/2045-7022-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rowczenio D.M., Pathak S., Arostegui J.I. Molecular genetic investigation, clinical features, and response to treatment in 21 patients with Schnitzler syndrome. Blood. 2018;131(9):974–981. doi: 10.1182/blood-2017-10-810366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Treon S.P., Xu L., Yang G. MYD88 L265P Somatic mutation in Waldenström's macroglobulinemia. N Engl J Med. 2012;367(9):826–833. doi: 10.1056/NEJMoa1200710. [DOI] [PubMed] [Google Scholar]
- 5.Ngo V.N., Young R.M., Schmitz R. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470(7332):115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Treon S.P., Tripsas C.K., Meid K. Ibrutinib in previously treated Waldenström's macroglobulinemia. N Engl J Med. 2015;372(15):1430–1440. doi: 10.1056/NEJMoa1501548. [DOI] [PubMed] [Google Scholar]