Abstract
Benign metastasizing leiomyoma (BML) is a rare benign smooth muscle neoplasm that originates in the uterus and metastasizes to distant sites—most commonly the lungs. BMLs are often found incidentally in patients with a history of uterine leiomyoma(s) and tend to be indolent. Occasionally they may be symptomatic and rarely follow an aggressive clinical course.
We report an unusual case of BML presenting in a 46-year-old woman as a miliary nodular pattern bilaterally in the lungs and progressive respiratory failure. Her past medical history was significant for uterine “leiomyomas” of at least 9 years' duration. Post mortem histologic evaluation of the uterine and lung lesions revealed benign smooth muscle neoplasms (leiomyomas and BMLs, respectively), and molecular analyses demonstrated identical clonal MED12 mutation, though with greater mutant allelic frequency in the uterus. We document a rare aggressive clinical course in a patient with BML, which presented as a miliary radiologic pattern mimicking an infectious etiology or interstitial lung disease.
Keywords: Benign metastasizing leiomyoma, Miliary, MED 12
1. Introduction
Benign metastasizing leiomyoma (BML) is a rare condition that was first described in 1939 by Steiner [1] as “metastasizing fibroleiomyomas” comprised of benign-appearing smooth muscle cells in the uterus and metastatic sites. Typically BML is found incidentally in the lungs in a patient with a history of hysterectomy for leiomyomas, but there are reports of associated cough, chest pain, dyspnea, hemoptysis, hemothorax and pneumothorax [2]. Though pulmonary manifestation is most common, BML may occur in the pelvis, lymph nodes [3], breast [4], bones and heart [5,6], amongst other sites [2]. BML(s) may manifest as a solitary pulmonary nodule, but the pattern of involvement is bilateral (macro-) nodules in most instances. We illustrate a rapidly fatal presentation of BML with a miliary pattern, which to the best of our knowledge has not been previously reported and also describe the results of next generation sequencing of the uterine and lung lesions.
2. Case report
A 46-year-old woman with a past medical history significant for hypertension presented to the emergency department (ED) with three days of fatigue, chills, decreased appetite, dry cough and progressive dyspnea. Review of systems was notable for intermittent fatigue and dyspnea on exertion for the preceding three months. Upon initial evaluation, she was in multi-system organ failure characterized by hypoxemic respiratory failure, shock and severe lactic acidosis. Following endotracheal intubation and volume resuscitation, her cardiopulmonary status initially stabilized; low-dose vasopressors and broad spectrum antimicrobials were initiated. Shortly thereafter, she suffered cardiopulmonary arrest with pulseless electrical activity. Following return of spontaneous circulation, gas exchange and hemodynamics deteriorated rapidly, and required high-levels of mechanical ventilatory support, vasopressors and inotropic agents, renal replacement therapy and emergent cannulation onto venoarterial extracorporeal membrane oxygenation. Despite these measures, she progressed to catastrophic multi-organ failure (severe acute respiratory distress syndrome, refractory shock and cardiac failure, and disseminated intravascular coagulation) and passed approximately 12 hours following presentation to the ED.
Approximately 5 years prior, she had presented to the ED with menorrhagia without anemia. Her gynecological/obstetrical history (G4P4; mode of delivery—vaginal vs C-section—unavailable) and exam were significant for a palpable anterior “fibroid” and 10 week size uterus. A transvaginal ultrasound was notable for 3 intramuscular “fibroids” and 1 cm thick endometrial stripe. Both ovaries were within normal limits and no free fluid was seen.
Imaging reports as part of 2nd/3rd trimester fetal evaluations 9 years earlier describe 2 suspected leiomyomata measuring up 3.5 cm in greatest dimension with a follow-up scan one year later showing increase to 3.7 cm.
3. Imaging
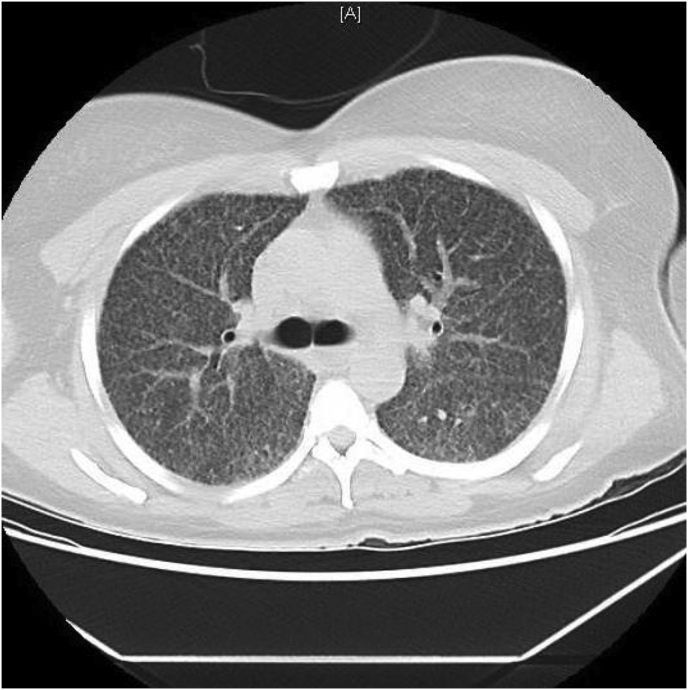
A chest x-ray taken two days prior to presentation to the ED showed a diffuse granular pattern without consolidation bilaterally suggestive of infection (e.g., viral pneumonia, pneumocystis). At presentation to the ED, the chest computed tomography (CT) scan demonstrated diffuse, randomly-distributed, innumerable punctate nodules mostly ≤ 5 mm. Additionally, mild interstitial septal thickening, mildly heterogeneous diffuse ground-glass opacification of the lungs with lower lobe predominance and mediastinal lymphadenopathy were noted (Fig. 1). No honeycombing, significant confluent consolidation or mass(es) were seen. The differential included infectious/inflammatory conditions such as atypical pneumonia (e.g., military tuberculosis, fungus) and acute pneumonitis (e.g., hypersensitivity pneumonitis). Interstitial lung disease and sarcoidosis were less likely considerations in light of the acute presentation.
Fig. 1.
CT scan showing innumerable nodules and ground glass opacity.
4. External & gross examination
Consent for an autopsy, excluding the head, was granted. Serosanguineous fluid was noted in the pericardial sac (75 cc), pleural cavities (50 cc and 75 cc in the right and left lungs, respectively) and peritoneal cavity (300 cc). Vague nodularity was noted on the surface of the lungs; the left and right lungs weighed 1000 g and 1190 g, respectively. The heart weighted 390 g; the right ventricle was grossly dilated with the wall measuring 0.3 cm.
Gross sectioning of the lungs demonstrated diffuse consolidation and numerous whitish nodules bilaterally spanning between 0.5 cm and 0.8 cm without predilection for any specific region (Fig. 2). Mediastinal lymphadenopathy, with lymph nodes ranging from 1.0 to 1.5 cm, was also noted. There was dilatation of the right heart and left ventricular hypertrophy (1.3 cm thickness).
Fig. 2.
Multiple small nodules in the lung on cut sections.
The uterus weighed 860 g and measured 13.5 × 9.0 × 6.0 cm. Myometrial sectioning revealed multiple 0.5 cm–4 cm white, well-circumscribed and firm nodules with a whorled appearance resembling leiomyomata in the subserosal, intramuscular and intramucosal locations. The endometrial lining, ovaries and fallopian tubes were unremarkable.
Other than hepatomegaly (2040 g), gross examination of the other sites, including breast, gastrointestinal organs, pancreas, spleen, adrenal, kidneys, bladder and bone was unremarkable and without any additional masses.
5. Methods
Routine histologic evaluation was performed. Immunohistochemistry [cytokeratin (CK), estrogen receptor (ER), progesterone receptor (PR), Ki-67, HMB45, Melan A, Tyrosinase, c-Kit, DOG-1, CD34, CD31, D2-40, desmin and human herpesvirus-8 (HHV-8)] was performed on paraffin-embedded tissue.
Molecular analysis of the neoplasms in the lung and uterus were performed to assess for a clonal relationship, if any, between the two, and identify the mutation(s).
DNA sequencing of the coding regions from 467 cancer-associated genes was used for sequencing the tumors. Basically, a minimum of 50ng of sheared DNA, from paraffin-embedded tumor sections was subject to capture using Sure Select reagents from Agilent (Santa Clara, CA). Sequencing was performed on the Illumina HiSeq 2500 (San Diego, CA) NGS platform. Variant calling and interpretation was performed as previously reported [7].
6. Results
6.1. Histology
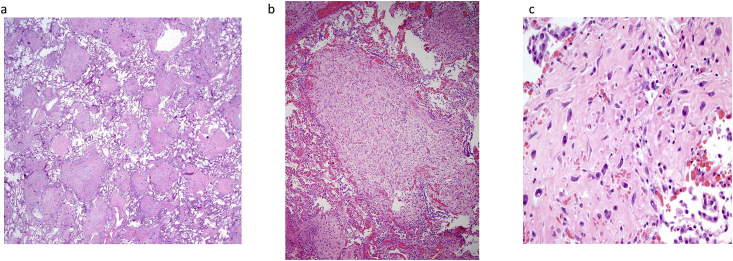
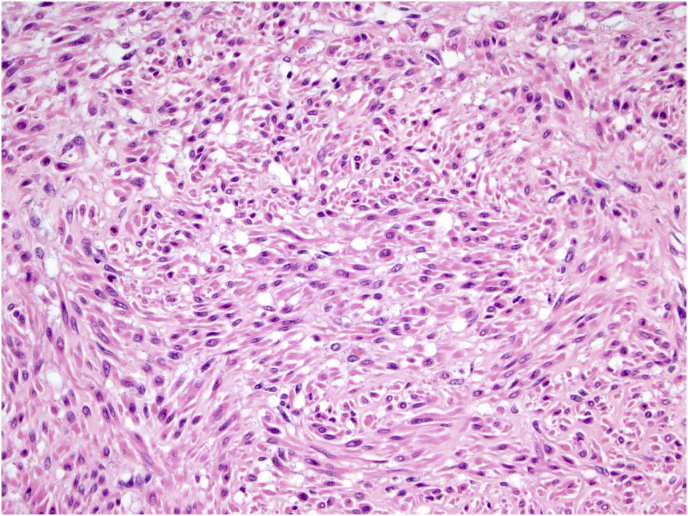
Sections of lung demonstrated >25% of the parenchyma comprised of numerous inter-anastomosing cords and nodules throughout all lobes (Fig. 3a and b). No specific anatomic distribution (e.g., centrilobular, interlobular septa, lymphatic, subpleural) was noted. The tissue cords and nodules were composed of spindle-shaped cells arranged mostly as whorls. On higher magnification, the cells were homogenously distributed without hypercellular areas. With the exception of rare isolated fields of slightly larger cells (Fig. 3c), no pleomorphism was seen. Additionally, no mitotic activity or necrosis were appreciated. Several uterine nodules were sampled and showed a smooth muscle proliferation, compatible with leiomyomata. No pleomorphism, necrosis, appreciable mitotic activity or hemorrhage were noted (Fig. 4). No histological evidence of acute lung injury, such as hyaline membranes or fibrin, was identified.
Fig. 3.
Lung parenchyma with (a) anastomosing bands and nodules (b) without mitoses and necrosis, and (c) focal pleomorphism.
Fig. 4.
Uterine leiomyoma without atypia, mitotic activity or necrosis.
6.2. Immunohistochemistry
The lesional cells in the lung stained with only desmin and PR (weak); Ki67 was present in <5% nuclei. All other markers, including HMB45, Melan A, tyrosinase, ER, HHV-8, c-Kit, Dog 1, CD34, CD31 and D2-40, were negative. The morphology and staining pattern were consistent with a smooth muscle neoplasm, with benign metastasizing leiomyomas (BMLs) leading the differential based on the histology. The uterine leiomyomata stained with ER and PR; Ki 67 was seen in <1% cells. Microscopic examination of the other organs and sampled lymph nodes was unremarkable.
6.3. Molecular analysis
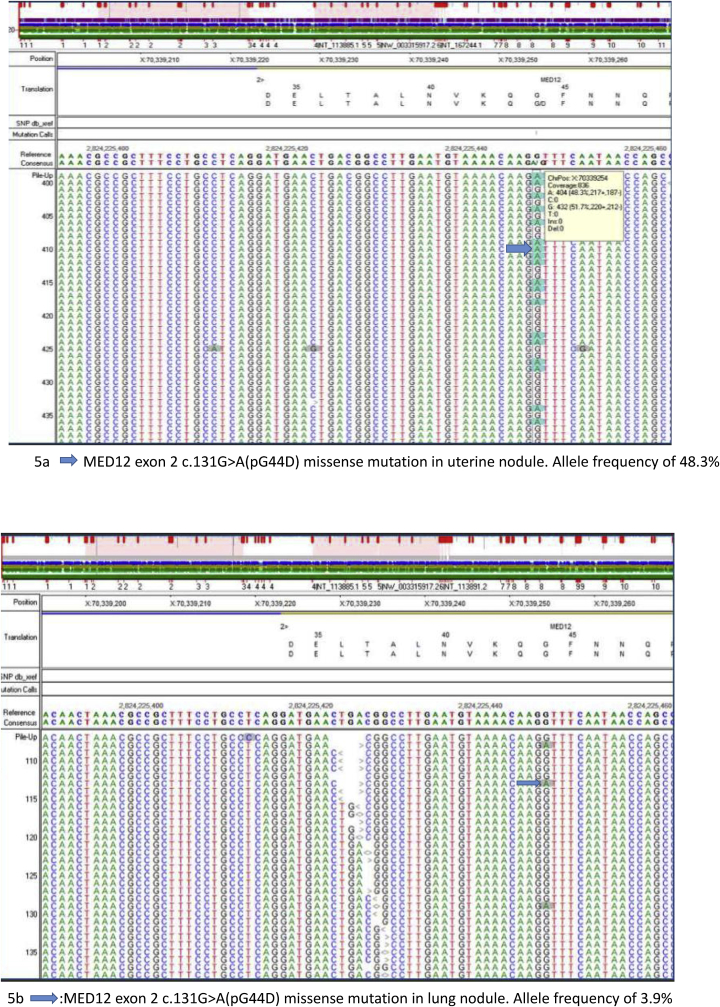
A missense mutation in the MED12 gene (c.131G>A (pG44D)) was detected in both the uterine and lung nodules with mutant allele frequency of 48.3% and 3.9% respectively (Fig. 5a and b). In addition, six missense variants of uncertain significance, including ARID1A (c.5770G>A, p E1924K), NOTCH2 (c.492C > A, pN164K), ETV6 (c.985G>A, p A329T), MSH2 (c.2260A > T, p T754S), and TOPBP1 (c.3616C > T, p R1206C) and splice site mutations in LMO1 (c.240-5G>T) and U2AF2 (c.186-4C>G) were identified in both lesions. The detection of same MED12 mutations in both lung and uterine nodules, is consistent with a common clonal origin for both lesions. The lower MED12 mutant allele fraction in the lung nodule was most likely due to the small size and reduced neoplastic cellularity in the tumor that was sequenced.
Fig. 5.
(a) MED12 exon 2 c.131G>A (pG44D) missense mutation in uterine nodule with allele frequency of 48.3% and (b) MED12 exon 2 c.131G>A (pG44D) missense mutation in lung nodule with allele frequency of 3.9%.
7. Discussion
Benign metastasizing leiomyomas (BMLs) represent a relatively uncommon entity morphologically similar to uterine leiomyomas. Since the initial publication, at least 161 cases have been described in the English literature, all in women [2]. Most affected women have a history of myomectomy/hysterectomy (at a mean age of 38.5 years; range 18–72 years) and subsequent diagnosis of BML (at a mean age 47.3 years; range 22–77 years) [2]. Though often noted incidentally and asymptomatic, some BMLs may cause respiratory symptoms. The presentation of BML(s) in the lung is variable with some occurring as a solitary pulmonary nodule and others as multiple bilateral nodules—it has been reported that in 70% of cases, there was a mean number of 6 nodules with an average size of 1.8 cm [8]. Rare pulmonary presentation of BML imaging include cystic/cavitary lesions [9] and a miliary pattern [10,11]. Therapeutic options (surgical resection, hormonal therapy, chemotherapy) hinge upon the extent of disease and symptomology. Depending on the pattern, the differential includes metastatic disease, infectious etiology (e.g., tuberculosis) and interstitial lung disease (e.g., sarcoidosis). Despite the “metastatic” designation, BMLs usually follow a stable/indolent course with progression reported only in isolated cases [8].
Akin to our case, a miliary pattern has been reported rarely [[10], [11], [12], [13]]. Lipton et al. illustrated a case of a 30-year-old woman with abnormal preoperative imaging for a hysterectomy for leiomyomas following metomenorrhagia [10]. Based on the miliary pattern, tuberculosis was a consideration; additional history revealed dyspnea on exertion but no cough or other systemic signs. A transbronchial biopsy showed multiple mitotically inactive BMLs, while the uterus was diagnosed with an atypical cellular leiomyoma. Orejola et al. noted 2–3 mm scattered nodules spread evenly and bilaterally in a 41-year-old woman following hysterectomy with leiomyoma; during the course of a limited follow-up, the patient was symptom free [11]. Another 32-year-old woman with labored breathing was treated for tuberculosis based on the miliary pattern prior to establishing a diagnosis of BML [12].
To the best of our knowledge, this represents the first case of BML presenting initially with a rapidly progressive abruptly fatal outcome. Most BMLs are indolent, but an aggressive clinical course resulting in death has been described [1]. In 1939, Steiner reported the effects of extensive pulmonary BMLs leading to death 18 months following presentation. Additionally, Jiang et al. described a case of a 48-year-old woman with a 4.9 cm BML in the right lung following a hysterectomy for leiomyoma status post hysterectomy. She received 6 cycles of chemotherapy for the BML. Two years later, she developed multiple additional metastases and eventually passed from cancer cachexia. Identical molecular signatures (mutations in MED12, BLMH, LRP2, SMAD2, UGT1A8) were noted in the uterine and BML with the exception of an additional PTEN mutation, a tumor suppressor, in the BML. It was theorized that this may accelerate the disease course and confer resistance to hormonal therapy [14].
Management of BML varies with the pattern of presentation and the extent of symptoms. In instances of isolated lesions, surgical resection may be treatment of choice. Cases with diffuse disease are more likely to benefit from a systemic approach. As these are considered to be hormonally-driven, agents such as tamoxifen may be more effective. Bilateral oophorectomy has also been proposed [15]. In our case, ER expression was lacking in two spatially separated contralateral lung BMLs. Though an artifact (e.g., fixation) cannot entirely be excluded, true loss of ER expression is likely. Based on this, it may be theorized that hormonal therapy may not be effective in such instances. Whether this loss contributed to the aggressiveness needs examination of additional cases. Given that some follow an unpredictable course, imaging surveillance may be indicated.
The etiology of pulmonary BML has been a topic of contention. One theory suggests that BMLs are primary pulmonary lesions that represent multifocal leiomyomatosis [14] while others contend that they represent metastases. Some have suggested hematogenous extension following uterine surgical procedures [16], but presence of BML in the absence of such invasive procedures, including in our case, makes this a less likely etiology. The possibility of BML representing a metastasis from a low grade uterine leiomyosarcoma has also been entertained, but the histology and the molecular studies in which distinct signatures are noted only for uterine leiomyomas and BMLs makes this unlikely. For instance, HMGA1 rearrangement [17] and 19q/22q deletions [18] are restricted to benign smooth muscle tumors and leiomyomas/BMLs and are not identified in leiomyosarcomas or other neoplasms, respectively.
In our case, a MED12 mutation, reported in 37%–85.5% uterine leiomyomas [18], was seen. Detection of same MED12 mutation and the same variant alleles in the lung and uterine nodules is consistent with derivation of the lesions from the same clone. MED12, which resides on the long arm of chromosome X (Xq12.1), is a component of the mediator complex, which regulates the initiation of transcription of RNA polymerase II [19]. MED12 mutation is the most frequent tumor specific alteration in uterine leiomyoma, with 49% of mutations occurring in codon 44 [20] and has been reported in 20% of leiomyosarcomas [21]. The MED12 gene G44D mutation is pathogenic and likely leads to loss of function of the protein [22] and has been frequently reported in uterine leiomyoma [23,24]. NGS also identified six variants of uncertain significance (VOUS) that were present in both tumors, some of which were reported to be associated with uterine leiomyomata. Of the VOUS that were present in both the uterine and lung tumors, upregulation of MSH2 gene expression, may be considered as a marker for early detection of uterine fibroids [25]. However, without a matched normal, to rule out germline variants, the significance of these variants is not clear.
The morphological differential diagnoses include primary and metastatic spindle cell/smooth muscle neoplasms (Table 1) [e g. lymphangioleiomyomatosis (LAM), metastatic leiomyosarcoma, gastrointestinal stromal tumor (GIST), malignant melanoma] and interstitial lung disease (sarcoidosis). Like BML, LAM is seen mostly in women of reproductive age and occurs either sporadically or in a setting of tuberous sclerosis. Dyspnea and pneumothorax in a setting of thin-walled cysts with nodules are among the common presentations. Like BML, smooth muscle markers, ER and PR are expressed in LAM; however, LAM is also positive for melanocytic markers (HMB-45 and Melan-A). Metastatic leiomyosarcoma is another smooth muscle tumor that may present in the lung as single or multiple nodules with a staining pattern similar to that of BML. Unlike their benign counterpart, metastatic uterine leiomyosarcomas tend to occur in slightly older women and have greater pleomorphism, mitiotic activity (high Ki67 indices) necrosis and/or hemorrhage. The clinical history and staining pattern are useful in excluding metastatic GIST (c-kit, DOG-1 positive) and amelanotic spindle cell melanoma (S100, HMB45, Melan-A and tyrosinase positive). Finally, the inter-anastomosing nodular pattern is concerning for sarcoidosis. Lesions of sarcoidosis tend to have be hypocellular, have greater hyalinization and most importantly, are associated with granulomas.
Table 1.
Differential diagnoses and clinicopathologic features.
| Diagnostic Entity | Presentation | Radiology | Histology | Immunohistochemistry |
|---|---|---|---|---|
| Lymphangioleiomyomatosis | Cough Dyspnea Pneumothorax Chylous effusion |
Multiple cystic and nodular lesions. | Spindle cell proliferation lining cystic spaces. | HMB-45, MelanA, Desmin, ER, PR, |
| Metastatic Uterine Leiomyosarcoma | Abnormal Uterine bleeding Cachexia Cough Dyspnea History of pelvic mass |
Discrete mass forming lesion(s) | Spindle cell lesion with significant atypia, coagulative necrosis and mitoses | Desmin, SMMS, SMA, ER, PR and increased Ki-67 |
| Gastrointestinal Stromal Tumor | Cough Dyspnea History of gastrointestinal primary tumor |
Discrete mass forming lesion(s) | Spindle cell proliferation. | c-Kit, DOG1, CD34 |
| Malignant Melanoma | Cough Dyspnea History of primary tumor |
Discrete mass lesion(s) | May show spindle cell proliferation in the spindle cell type. | Melanocytic markers (e.g., S100, HMB45, Melan A, tyrosinase) |
| Sarcoidosis | Cough Dyspnea Fever |
Hilar and mediastinal lymph- adenopathy; reticulonodular opacities | Non-caseating granuloma | Histiocytes (CD68 and CD 163) |
| Atypical pneumonia | Dyspnea Cough Fever |
Consolidation | Inflammatory infiltrate | Gram stain, GMS or AFB may be positive |
| Benign Metastasizing Leiomyoma | Usually incidental. Rarely dyspnea, hemoptysis, chest pain | Variable: single to few or sometimes multiple nodules ranging from a few millimeters to a few centimeters | Spindle cell lesion(s) with no significant atypia, coagulative necrosis and mitoses | ER, PR, Desmin, SMA, SMMS. Ki-67 not increased |
Legend: ER: estrogen receptor; PR: progesterone receptor; SMA: smooth muscle actin; SMMS: smooth muscle myosin.
In summary, we describe a case of BML with an unusual presentation clinically, radiographically and pathologically. This case highlights the potential for BML, what is typically an indolent disease, to have a fulminant clinical course either by itself or due to superimposed respiratory insults. Since the initial description 1939, a clonal relationship between uterine leiomyomas and BMLs has been established. However, additional studies to identify molecular signature(s) to separate BMLs with indolent and aggressive clinical course are necessary to determine appropriate management.
Conflicts of Interest and Source of Funding
None were declared.
Contributor Information
Kenneth Ofori, Email: ko2427@cumc.columbia.edu.
Helen Fernandes, Email: hf2340@cumc.columbia.edu.
Matthew Cummings, Email: mjc2244@cumc.columbia.edu.
Thomas Colby, Email: colby.thomas@mayo.edu.
Anjali Saqi, Email: aas177@cumc.columbia.edu.
References
- 1.Steiner P.E. Metastasizing fibroleiomyoma of the uterus: report of a case and review of the literature. Am. J. Pathol. 1939;15(1):89–110. 117. [PMC free article] [PubMed] [Google Scholar]
- 2.Barnas E., Ksiazek M., Ras R., Skret A., Skret-Magierlo J., Dmoch-Gajzlerska E. Benign metastasizing leiomyoma: a review of current literature in respect to the time and type of previous gynecological surgery. PLoS One. 2017;12(4) doi: 10.1371/journal.pone.0175875. e0175875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoon G., Kim T.J., Sung C.O. Benign metastasizing leiomyoma with multiple lymph node metastasis: a case report. Cancer Res. Treat. 2011;43(2):131–133. doi: 10.4143/crt.2011.43.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jo J.H., Lee J.H., Kim D.C. A case of benign metastasizing leiomyoma with multiple metastasis to the soft tissue, skeletal muscle, lung and breast. Korean J. Intern. Med. 2006;21(3):199–201. doi: 10.3904/kjim.2006.21.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Consamus E.N., Reardon M.J., Ayala A.G., Schwartz M.R., Ro J.Y. Metastasizing leiomyoma to heart. Methodist Debakey Cardiovasc. J. 2014;10(4):251–254. doi: 10.14797/mdcj-10-4-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meddeb M., Chow R.D., Whipps R., Haque R. The heart as a site of metastasis of benign metastasizing leiomyoma: case report and review of the literature. Case Rep. Cardiol. 2018;2018:7231326. doi: 10.1155/2018/7231326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sireci A.N., Aggarwal V.S., Turk A.T., Gindin T., Mansukhani M.M., Hsiao S.J. Clinical genomic profiling of a diverse array of oncology specimens at a large academic cancer center: identification of targetable variants and experience with reimbursement. J. Mol. Diagn. 2017;19(2):277–287. doi: 10.1016/j.jmoldx.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Miller J., Shoni M., Siegert C., Lebenthal A., Godleski J., McNamee C. Benign metastasizing leiomyomas to the lungs: an institutional case series and a review of the recent literature. Ann. Thorac. Surg. 2016;101(1):253–258. doi: 10.1016/j.athoracsur.2015.05.107. [DOI] [PubMed] [Google Scholar]
- 9.Choe Y.H., Jeon S.Y., Lee Y.C. Benign metastasizing leiomyoma presenting as multiple cystic pulmonary nodules: a case report. BMC Women's Health. 2017;17(1):81. doi: 10.1186/s12905-017-0435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipton J.H., Fong T.C., Burgess K.R. Miliary pattern as presentation of leiomyomatosis of the lung. Chest. 1987;91(5):781–782. doi: 10.1378/chest.91.5.781. [DOI] [PubMed] [Google Scholar]
- 11.Orejola W.C., Vaidya A.P., Elmann E.M. Benign metastasizing leiomyomatosis of the lungs presenting a miliary pattern. Ann. Thorac. Surg. 2014;98(5):e113–114. doi: 10.1016/j.athoracsur.2014.07.057. [DOI] [PubMed] [Google Scholar]
- 12.Chen S., Liu R.M., Li T. Pulmonary benign metastasizing leiomyoma: a case report and literature review. J. Thorac. Dis. 2014;6(6):E92–E98. doi: 10.3978/j.issn.2072-1439.2014.04.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivera J.A., Christopoulos S., Small D., Trifiro M. Hormonal manipulation of benign metastasizing leiomyomas: report of two cases and review of the literature. J. Clin. Endocrinol. Metab. 2004;89(7):3183–3188. doi: 10.1210/jc.2003-032021. [DOI] [PubMed] [Google Scholar]
- 14.Jiang J., He M., Hu X., Ni C., Yang L. Deep sequencing reveals the molecular pathology characteristics between primary uterine leiomyoma and pulmonary benign metastasizing leiomyoma. Clin. Transl. Oncol. 2018;20(8):1080–1086. doi: 10.1007/s12094-018-1847-y. [DOI] [PubMed] [Google Scholar]
- 15.Abu-Rustum N.R., Curtin J.P., Burt M., Jones W.B. Regression of uterine low-grade smooth-muscle tumors metastatic to the lung after oophorectomy. Obstet. Gynecol. 1997;89(5 Pt 2):850–852. doi: 10.1016/s0029-7844(97)00033-1. [DOI] [PubMed] [Google Scholar]
- 16.Takemura G., Takatsu Y., Kaitani K. Metastasizing uterine leiomyoma. A case with cardiac and pulmonary metastasis. Pathol. Res. Pract. 1996;192(6):622–629. doi: 10.1016/S0344-0338(96)80116-6. discussion 630-623. [DOI] [PubMed] [Google Scholar]
- 17.Bowen J.M., Cates J.M., Kash S. Genomic imbalances in benign metastasizing leiomyoma: characterization by conventional karyotypic, fluorescence in situ hybridization, and whole genome SNP array analysis. Cancer Genet. 2012;205(5):249–254. doi: 10.1016/j.cancergen.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nucci M.R., Drapkin R., Dal Cin P., Fletcher C.D., Fletcher J.A. Distinctive cytogenetic profile in benign metastasizing leiomyoma: pathogenetic implications. Am. J. Surg. Pathol. 2007;31(5):737–743. doi: 10.1097/01.pas.0000213414.15633.4e. [DOI] [PubMed] [Google Scholar]
- 19.Thompson C.M., Koleske A.J., Chao D.M., Young R.A. A multisubunit complex associated with the RNA polymerase II CTD and TATA-binding protein in yeast. Cell. 1993;73(7):1361–1375. doi: 10.1016/0092-8674(93)90362-t. [DOI] [PubMed] [Google Scholar]
- 20.Makinen N., Mehine M., Tolvanen J. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science. 2011;334(6053):252–255. doi: 10.1126/science.1208930. [DOI] [PubMed] [Google Scholar]
- 21.Perot G., Croce S., Ribeiro A. MED12 alterations in both human benign and malignant uterine soft tissue tumors. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0040015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakravarty D., Gao J., Phillips S.M. OncoKB: a precision oncology knowledge base. JCO Precis Oncol. 2017;2017 doi: 10.1200/PO.17.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makinen N., Heinonen H.R., Moore S., Tomlinson I.P., van der Spuy Z.M., Aaltonen L.A. MED12 exon 2 mutations are common in uterine leiomyomas from South African patients. Oncotarget. 2011;2(12):966–969. doi: 10.18632/oncotarget.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGuire M.M., Yatsenko A., Hoffner L., Jones M., Surti U., Rajkovic A. Whole exome sequencing in a random sample of North American women with leiomyomas identifies MED12 mutations in majority of uterine leiomyomas. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0033251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Q., Laknaur A., Elam L. Identification of polycomb group protein EZH2-mediated DNA mismatch repair gene MSH2 in human uterine fibroids. Reprod. Sci. 2016;23(10):1314–1325. doi: 10.1177/1933719116638186. [DOI] [PMC free article] [PubMed] [Google Scholar]