Abstract
Background
Experimental CCR5 antagonism with maraviroc in atherosclerosis-prone mice and preliminary data in humans suggest an anti-atherosclerotic effect of the drug. We assessed the impact of maraviroc treatment in persons living with HIV on subclinical indicators of atherosclerosis.
Methods
Persons living with HIV on effective antiretroviral therapy (ART) including only protease inhibitors were recruited if they had a Framingham risk score >20% and brachial flow-mediated dilation (bFMD) <4%, as indices of high cardiovascular risk. Maraviroc (300 mg per os for 24 weeks) was administered, in addition to ongoing ART, to all patients using a crossover design. Brachial FMD, carotid-femoral pulse wave velocity (cfPWV), and carotid intima-media thickness (cIMT) were measured as markers of atherosclerosis. Vascular competence—as expressed by the ratio of circulating endothelial microparticles (EMPs) to endothelial progenitor cells (EPCs)—and markers of systemic inflammation and monocyte and platelet activation were assessed.
Results
Maraviroc treatment significantly improved bFMD, cfPWV, and cIMT by 66%, 11%, and 13%, respectively (P = .002, P = .022, P = .038, respectively). We also found a beneficial effect of maraviroc on the EMP/EPC ratio (P < .001) and platelet/leucocyte aggregates (P = .013). No significant changes in markers of systemic inflammation, monocyte activation, and microbial translocation were observed.
Conclusions
Maraviroc led to significant improvements in several markers for cardiovascular risk, endothelial dysfunction, arterial stiffness, and early carotid atherosclerosis, which was accompanied by an increase of vascular competence, without seeming to affect systemic inflammation. Our data support the need for larger studies to test for any effects of maraviroc on preventing atherosclerosis-driven pathologies.
Keywords: HIV, atherosclerosis, cardiovascular risk, maraviroc
Atherosclerosis is an inflammatory disease in which immune mechanisms interact with metabolic risk factors to initiate, propagate, and activate lesion formation and progression [1]. In people living with HIV (PLWH), a residual chronic immune-inflammatory derangement typically persists despite complete viral suppression with ART [2, 3]. In addition, exposure to traditional cardiovascular (CV) risk factors does not completely explain the increased CV risk [4, 5], suggesting that additional conditions, such as chronic inflammation and immune activation, might play roles in promoting CV diseases (CVD) in such patients [6]. Indeed, markers of inflammation, such as high-sensitivity C-reactive protein (hsCRP) and interleukin-6 (IL-6), can predict coronary events, stroke, and CVD mortality in individuals with or without HIV infection [7, 8].
The chemokine (C-C motif) ligand 5 (CCL5)/C-C chemokine receptor type 5 (CCR5) axis plays a pivotal role in the development and progression of atherosclerotic inflammatory disease [9]. Specifically, CCR5, a G-protein-coupled receptor of the CCL3/MIP-1α, CCL4/MIP-1β, and CCL5/Regulated on Activation Normal T Cell Expressed and Secreted (RANTES) chemokines regulates the migration of monocytes, natural killer cells, and T helper-1 (Th1) cells into inflammation sites, including atherosclerotic plaques, thus promoting atherosclerosis progression [10]. CCR5 is also the co-receptor for macrophage tropic (R5) HIV-1 [11] and is the target of the CCR5 antagonist maraviroc (MVC), which is used for treating HIV infection [12]. A polymorphism in CCR5, a 32-nucleotide deletion known as CCR5 Δ32, has been observed in subjects who remain uninfected despite extensive exposure to HIV-1 [13] and has also been associated with reduced risk for severe coronary artery disease (CAD) [14] and myocardial infarction in different clinical settings [15]. The CCR5 ligand CCL5 has been detected in atherosclerotic plaques and is mostly increased in late-stage atherosclerotic lesions [16], where it correlates to an unstable phenotype [17]. Moreover, CCL5 is released by activated degranulating platelets and can trigger shear-resistant monocyte arrest on the inflamed endothelium, therein favoring the early stages of atherosclerosis and its progression [18].
We have previously reported that MVC was able to reduce atherosclerotic progression in 2 different murine models by interfering with the recruitment of inflammatory cells. Moreover, in a ritonavir-treated experimental model, MVC modulated both the inflammatory plaque profile and systemic inflammation [19].
In light of such findings and considering that the safety profile of MVC has been deemed similar to placebo in trials on PLWH, we undertook a pilot study to evaluate the anti-atherosclerotic effectiveness of MVC intensification in suppressed patients at high CVD risk. Early atherosclerosis was investigated by measuring brachial flow-mediated dilation (bFMD), carotid-femoral pulse wave velocity (cfPWV), and carotid intima-media thickness (cIMT); in addition, markers of vascular competence, soluble markers of systemic inflammation, monocyte and platelet activation, and microbial translocation were measured as putative mediators of the anti-atherosclerotic effect of MVC.
METHODS
Patients
Patients were consecutively recruited at the Infectious Disease Clinic of Santa Maria della Misericordia Teaching Hospital, Department of Medicine, University of Perugia, from January 15, 2016, to July 15, 2017. Inclusion criteria were age ≥50 years, on treatment over the past 12 months with an effective protease inhibitor ART regimen (HIV RNA < 50 copies/mL), CD4 T-cell counts >300/ mm3 over the past 6 months, a Framingham risk score >20%, and a bFMD <4%. Exclusion criteria included age >70 years, life expectancy <12 months, previously recorded platelet functional defects, or any history of chronic alcohol abuse. The study was approved by the Umbria Region Ethics Committee (CEAS registry No. 2166/14; Clinical Trial Registration, NCT 03402815). All patients provided written informed consent.
Study Design
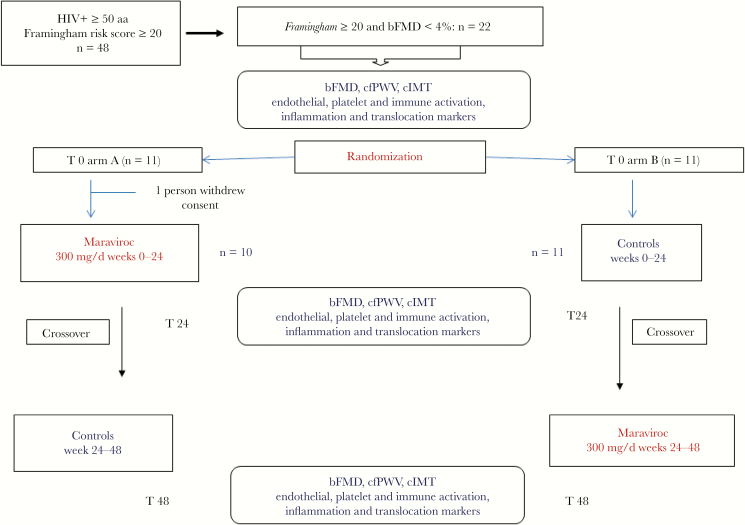
We performed a 48-week randomized (1:1) crossover study. After giving their informed consent, all patients with the above-mentioned characteristics were randomly allocated with an AB/BA crossover design to either maraviroc 300 mg/d plus ongoing ART for 24 weeks (A) or ongoing ART alone for 24 weeks (B) (Figure 1). At the end of the first 24-week period, all patients were switched to the alternative arm. A washout period was not performed because of the short half-life of MVC relative to the length of the MVC intervention. At time 0 and every 12 weeks, a clinical check-up was performed for all patients, whereas noninvasive examinations of the studied markers for preclinical atherosclerosis were carried out every 24 weeks. The primary outcome with respect to treatment effect was the change in bFMD after 24 weeks. Secondary outcomes with respect to treatment were cIMT and cfPWV. Additional analyses were performed for endothelial progenitor cells (EPCs), endothelial microparticles (EMPs), soluble markers of systemic inflammation, monocyte and platelet activation, and microbial translocation.
Figure 1.
Crossover study. The diagram shows screening eligibility, randomization, treatment interventions, and specific laboratory assay evaluations. Abbreviations: bFMD, brachial flow-mediated dilation; cfPWV, carotid-femoral pulse wave velocity; cIMT, carotid intima-media thickness.
Treating physicians and nurses were not blinded to either study week or treatment assignment, whereas bFMD, cIMT, and cfPWV operators were blinded to study week and treatment assignment.
At the end of the study (48th week), after a preliminary evaluation of any observed efficacy, MVC was again added to only arm A. At the 72nd week, an evaluation of only bFMD was carried out on both arms.
Clinical and Demographic Characteristics
All data are detailed in the Supplementary Data.
Blood Sampling and Laboratory Assays
Plasma HIV-RNA, CD4+ and CD8+ T cells, hsCRP, D-Dimer, IL-6, sDC14, sCD163, MCP-1, sVCAM, LBP, EPCs, EMPs, angiogenic T cells (Tangs), platelet microparticles (PMPs), and platelet-leukocyte complexes were evaluated in peripheral blood.
Details of blood samples and laboratory assays are provided in the Supplementary Data.
Noninvasive Markers of Preclinical Atherosclerosis
Brachial FMD on the nondominant arm cfPWV and cIMT was evaluated as previously described [20–22].
Details on bFMD, cfPWV, and cIMT measurement are provided in the Supplementary Data.
Statistical Analysis
We planned a 2-arm crossover study, with restricted randomization (random permuted blocks and phone central randomization), with brachial FMD (primary outcome measure) as a continuous response variable. Random allocation sequence, enrollment of participants, and assignment of treatment were done by different study personnel.
Sample size was estimated assuming a baseline SD for bFMD of 2% and a post-treatment bFMD increase of 2%. A minimum sample size of 18 participants was required to detect statistically significant differences in bFMD with a power of 80% and an α error of 5%. With a withdrawal/nonevaluable subject rate of 10%, a total of 20 subjects needed to be recruited.
We used the SPSS statistical package, release 17.0 (SPSS Inc, Chicago, IL), for all statistical analyses, with data analyzed anonymously and according to the originally assigned groups. Values are expressed as the mean and SD or the median and interquartile range, as appropriate. Base 10 logarithmic (log) transformation was performed for skewed variables, and log-values were used. An independent-sample t test and the Mann-Whitney U test were used to compare changes in the variables between the treatment orders (AB vs BA). Carryover was assessed by comparing the sum of the variable responses (response/1 + response/2) between the treatment orders (AB vs BA). Correlation analyses were performed using the Pearson’s and Spearman’s coefficients of correlation.
RESULTS
Patient Population
A total of 48 patients with Framingham risk scores ≥ 20% were screened for bFMD, and 22 underwent randomization. One patient withdrew his consent before MVC intensification and was excluded from the study. Patient demographic and clinical characteristics at baseline are reported in Table 1. At enrollment, patients had a long history of HIV infection (mean, 18 years), a mean CD4 T-cell nadir of 149/mm3, a median length of undetectable viral load of 5 years, a good control of HIV replication (with 90% of patients having <20 copies/mL), and a good immunological status (76% of patients had >500 CD4 T cell/mm3, a mean CD4/CD8 ratio of 0.847). Twelve patients were taking boosted darunavir, 8 boosted atazanavir, and 1 boosted lopinavir. Three patients were taking abacavir too. Sixty-two percent were current smokers, 33% had a history of diabetes, and 43% were hypertensive (33% on antihypertensive therapy). Patients had, on average, borderline-high fasting glucose and triglyceride levels. Total and low-density lipoprotein cholesterol and creatinine clearance levels were in range.
Table 1.
Demographic and Clinical Baseline Characteristics
| Baseline Characterisics | Total Patients (n = 21) |
|---|---|
| Male, No. (%) | 20 (95) |
| Mean age (SD), y | 61 (9) |
| Median BMI (IQR), kg/m2 | 26.87 (24.28–28.17) |
| Current smoking, No. (%) | 13 (62) |
| Diabetes, No. (%) | 7 (33) |
| Hypertension, No. (%) | 9 (43) |
| History of CVD, No. (%) | 5 (24) |
| Mean systolic blood pressure (SD), mmHg | 127 (14) |
| Mean diastolic blood pressure (SD), mmHg | 78 (9) |
| Mean blood glucose (SD), mg/dL | 104 (34) |
| Mean total cholesterol (SD), mg/dL | 197 (40) |
| Mean HDL cholesterol (SD), mg/dL | 43 (10) |
| Mean LDL cholesterol (SD), mg/dL | 108 (41) |
| Median triglycerides (IQR), mg/dL | 172 (144–232) |
| Mean clearance creatinine (SD), mL/min | 90 (17) |
| Mean known HIV (SD), y | 17.81 (7.903) |
| Median nadir CD4 cell count, cells/mm3 (IQR) | 120 (81–260) |
| Median CD4 T-cell count, cells/mm3 (IQR) | 642 (497–852) |
| HIV-RNA<20 copies/mL, No. (%) | 19 (90) |
| Boosted darunavir, No. | 12 |
| Boosted atazanavir, No. | 8 |
| Boosted lopinavir, No. | 1 |
| Abacavir, No. | 3 |
| Statin use, No. (%) | 6 (28.5) |
| Acetylsalicylic acid treatment, No. (%) | 5 (24) |
| Antihypertensive treatment, No. (%) | 7 (33.3) |
Abbreviations: BMI, body mass index; CVD, cardiovascular disease; HDL, high-density lipoprotein cholesterol; IQR, interquartile range; LDL, low-density lipoprotein cholesterol.
Overall, baseline functional and structural vascular parameters, vascular competence markers, inflammation, platelet and monocyte activation indices, and microbial translocation parameters are reported in Table 2.
Table 2.
Baseline Markers of Inflammation, Platelet and Monocyte Activation, Microbial Translocation, Vascular Homeostasis, and Preclinical Atherosclerosis
| Variables | Baseline Median Value (IQR) |
|---|---|
| hsCRP, mg/L | 1.81 (1.02–3.56) |
| IL6, pg/mL | 3.8 (2.6–6.0) |
| D-Dimer, ng/mL | 116.0 (74.0–156.0) |
| sCD14, ng/mL | 5815 (5024–6723) |
| sCD163, ng/mL | 2009 (1166–2287) |
| MCP-1, pg/mL | 173.4 (147.3–286.2) |
| LBP, ng/mL | 18 544 (13 458–22 353) |
| sVCAM, ng/mL | 936.6 (653.1–1474.0) |
| Platelets/leukocyte aggregates, % | 15.2 (11.0–20.8) |
| Platelet-derived microparticles, n/µL | 60 481 (28 686–88 547) |
| EMP, n/µL | 362.0 (310.0–606.5) |
| EPC, n/mL | 130.6 (75.4–215.3) |
| Tang, n/mL | 597.7 (402.6–934.4) |
| Brachial artery diameter, mm | 4.73 (4.21–4.95) |
| bFMD, % | 3.1 (1.7–4.9) |
| cfPWV, m/s | 9.0 (7.1–9.5) |
| cIMT max, mm | 0.690 (0.605–0.755) |
Abbreviations: bFMD, brachial flow-mediated dilation; cfPWV, carotid-femoral pulse wave velocity; cIMT max, maximum carotid intima-media thickness; EMP, endothelial microparticle; EPC, endothelial microparticle; hsCRP, high-sensitivity C-reactive protein; IL6, interleukin-6; IQR, interquartile range; LBP, lipopolysaccharide binding protein; MCP, monocyte chemotactic protein 1; sVCAM, soluble vascular cell adhesion molecule-1; Tang, angiogenic T cell.
Effect of Maraviroc on Outcome Measures
Clinical and biochemical parameters before and after 24 weeks in both the treatment and control groups are reported in Supplementary Table 1. No changes were observed in body mass index, current smoking habits, glucose, cholesterol, or triglyceride levels or in renal function within and between groups.
Noninvasive Markers of Preclinical Atherosclerosis
The treatment effect of MVC treatment weighted for control treatment is reported in Table 3. Noninvasive markers of atherosclerosis significantly improved with MVC treatment. Specifically, a 2.6% increase in bFMD was observed with MVC treatment (P = .002). Of note, the median brachial artery diameter at 24 and 48 weeks did not change over 5% compared with baseline values, showing the reproducibility assessment of bFMD (at 24 weeks, 4.6; interquartile range [IQR], 4.20–4.93; at 48 weeks, 4.56; IQR, 4.11–4.9). A 1.0 m/s reduction in cfPWV was observed (P = .022). A significant reduction in cIMT max of about 13% was observed in the between-group evaluation (–90 µm; P = .038). The carryover effects were not significant (P > .1) for the above investigated markers.
Table 3.
Treatment Effect of Maraviroc, Weighted for Control Treatment, on Markers of Inflammation, Platelet and Monocyte Activation, Microbial Translocation, Vascular Homeostasis, and Preclinical Atherosclerosis
| Variables | Mean Change, SD | P |
|---|---|---|
| hsCRP, mg/L | –6.39 ± 29 | .888 |
| IL6, pg/mL | –5.0 ± 24 | .324 |
| D-Dimer, ng/mL | 1.3 ± 32 | .525 |
| sCD14, ng/mL | 786 ± 3924 | .944 |
| sCD163, ng/mL | 63.0 ± 624 | .231 |
| MCP-1, pg/mL | –20.6 ± 102 | .101 |
| LBP, ng/mL | –1577 ± 7445 | .573 |
| sVCAM, ng/mL | 90.3 ± 389 | .491 |
| Platelets/leukocyte aggregates, % | –3.2 ± 4.6 | .013 |
| Platelet-derived microparticles, n/µL | –14765 ± 35573 | .132 |
| EMP, n/µL | –311 ± 472 | <.001 |
| EPC, n/mL | 40 ± 192 | .014 |
| EMP/EPC ratio | –0.22 | <.001 |
| Tang, n/mL | 13 ± 474 | .833 |
| Brachial artery diameter, mm | 0.02 ± 0.22 | .600 |
| bFMD, % | 2.6 ± 3.0 | .002 |
| cfPWV, m/s | –1.0 ± 1.6 | .022 |
| cIMT, mm | –0.09 ± 0.18 | .038 |
Change was measured as the difference of the variable responses in the treatment orders as follows: (response/2 – response/1) in the AB order and (response/1 – response/2) in the BA order.
Abbreviations: bFMD, brachial flow-mediated dilation; cfPWV, carotid-femoral pulse wave velocity; cIMT, carotid intima-media thickness; EMP, endothelial microparticle; EPC, endothelial microparticle; HsCRP, high-sensitivity C-reactive protein; IL6, interleukin-6; LBP, lipopolysaccharide binding protein; MCP, monocyte chemotactic protein 1; sVCAM, soluble vascular cell adhesion molecule-1; Tang, angiogenic T cell.
Extending the evaluation of FMD, we observed a consistent bFMD improvement for arm A, which had resumed the drug therapy from weeks 48 to 72, whereas a worsening was observed for arm B, which had discontinued it at the 48th week (Supplementary Figure 1).
Markers of Inflammation, Microbial Translocation, Endothelial Homeostasis, and Platelet and Monocyte Activation
A significant decrease in the EMP/EPC ratio was observed (–0.22, P < .001). No modifications in Tang counts were seen. Also, significant reductions in platelet/leukocytes aggregates (–3.2%, P < .001) were observed. No significant changes, either for the maraviroc group or the cumulative analysis, were observed for the other investigated inflammatory markers and immune activation parameters.
Discussion
We had previously reported that MVC reduced the atherosclerosis burden by modulating the inflammatory plaque recruitment in 2 ApoE knockout mice models with either early ritonavir-induced atherogenesis or late spontaneous atherosclerotic progression. Moreover, MVC reversed ritonavir-induced systemic inflammation in the former but not in the latter model [19]. In light of these results, a comprehensive evaluation of any potential anti-atherosclerotic effect associated with MVC in patients at high CV risk was warranted.
In this study, we observed significant improvements for all the surrogate noninvasive markers of early atherosclerosis in the maraviroc-treated patients, without any reductions of plasma cholesterol levels or any modifications for markers of systemic inflammation and immune activation.
Virally suppressed PLWH at high CV risk (Framingham risk score ≥ 20%) with evidence of impaired bFMD at baseline and taking only protease inhibitor–boosted regimens, thus allowing once-daily maraviroc administration, were enrolled. Indeed, maraviroc was not administered to control viral replication but to evaluate anti-atherosclerotic effectiveness. Moreover, in real life, once-daily MVC with boosted protease inhibitors (PIs) has been often administered, although the approved dosage was 150 mg twice daily in people taking PIs.
Sixty-three percent of these were current smokers, and 43% and 33% had a history of hypertension and diabetes, respectively. Moreover, 47.6% had plasma hsCRP levels ≥2 mg/L, and 61.9% had an IL-6 >3.01 pg/mL; the latter was associated with a CV risk over 12% at 48 months according to the SMART study results [8]. In our study population, we investigated for any impact from MVC use on several markers of early atherosclerosis (ie, endothelial dysfunction, aortic stiffness, cIMT). Additionally, markers of systemic inflammation (hsCRP, IL-6, MCP-1, LBP, sCD163, and sCD14), endothelial homeostasis (circulating EMPs, EPCs, and VCAM levels), platelet reactivity (PMPs and platelet-leukocyte aggregates), and microbial translocation were also investigated.
We found that adding MVC to the ART regimens of patients with viral suppression improved bFMD, cfPWV, and cIMT by 66%, 11%, and 13%, respectively. Moreover, the EMP/EPC ratio, which is considered a marker of vascular competence [22, 23], and platelet/leucocyte aggregates were significantly reduced.
Throughout the study, neither changes in lifestyle, in particular, current smoking, nor reductions in lipid levels were observed. After MVC intensification, hsCRP, IL-6, d-Dimer, sCD14, sCD163, and MCP-1 did not change, whereas microbial translocation was blunted without reaching statistical significance.
To date, evidence regarding the impact of MVC intensification on surrogate markers of atherosclerosis has been limited [24, 25]. Krikke et al. [24] have reported a mild improvement in endothelial function following MVC intensification in patients with HIV on abacavir. This improvement persisted after MVC discontinuation. However, the duration of this crossover study (16 weeks) was shorter than ours. Piconi et al. [25] have reported that MVC improved cfPWV, cIMT, IL-6, microbial translocation, and VCAM levels in 6 PLWH with a Framingham risk score of 10%–20%. However, the small sample size and the retrospective enrollment of control subjects might have biased the results. Conversely, a preliminary report by Hsue et al. did not provide evidence of a clinically relevant effect of MVC intensification on bFMD in persons living with HIV on treatment [26]; however, the characteristics of the recruited population were not fully described [26].
From a pathophysiological perspective, the beneficial effects of 24-week MVC intensification that we observed on bFMD, cfPWV, and cIMT are supported by several lines of evidence.
First, brachial FMD is a reliable indicator of endothelial function and a significant predictor of CV risk [27]. Endothelial fragmentation into microparticles and a reduced number of circulating EPCs have both been associated with impaired bFMD and increased CV risk [28–30]. Hence, the observed improvement of bFMD following MVC intensification was related to its positive impact on the balance between endothelial injury and repair. Given that nitric oxide (NO) is involved in the regulation of endothelial function and has been associated with both reduced endothelial cell injury and higher circulating EPC counts [23, 30], it is plausible that increased NO bioavailability could have mediated our observed MVC-induced improvements in endothelial function and overall vascular structural integrity.
Second, cfPWV has been reported to be influenced by NO bioavailability [31] and by endothelial function as well. Despite elastic fiber degeneration and a reduced elastin/collagen ratio being key factors in inducing arterial stiffening [32], endothelial dysfunction and reduced NO bioavailability may promote arterial stiffening by modulation of vascular smooth muscle cell relaxation and arterial tone [31, 33]. As elastic fiber degeneration and the elastin/collagen ratio are rather difficult to revert [32], the rapid improvement observed in our study in cfPWV is more likely attributable to an endothelium-dependent NO-mediated effect of MVC on vascular tone.
Third, several studies have reported a time-dependent cIMT progression in persons living with HIV [34] similar to what we observed in our control subjects; a 20-µm progression of cIMT was seen over a 24-week period. Notably, in our study, cIMT was reduced by 60 µm with MVC intensification. This result is in agreement with a study exploring the effect of CCR5 inhibition by MVC in persons with HIV/HCV coinfection [35]. In that study, a 48-week intensification with maraviroc was associated with a reduction in atherosclerotic plaque progression. Improvements in endothelial function and vascular competence might mediate the beneficial effects of maraviroc on cIMT, as both reduced bFMD and increased EMP/EPC ratio have been associated with early atherosclerosis in the carotid arteries [22, 36].
Several hypotheses may further explain the observed maraviroc-induced efficacy on major markers of early atherosclerosis. First, an endothelium-protective impact from CCR5 inhibition might be involved. This is suggested by a recent in vitro study reporting that MVC incubation with coronary artery endothelium resulted in inhibiting vasoconstriction and stimulation of intimal hyperplasia [37]. Moreover, CCR5 antagonism can downregulate inflammatory cell recruitment into vascular walls, leading to a further improvement in major markers of atherosclerosis. This local anti-inflammatory effect with MVC was also observed in our previous study, where we reported a 50% reduction in plaque monocyte/macrophage CCR5+ infiltration following MVC treatment in animal models [19]. Hence, we hypothesize that there is a bidirectional causative association between endothelial injury and arterial wall inflammation [38]; if so, MVC treatment might play a key role in interfering with this pro-atherogenic loop.
Strengths of our study include (1) its homogeneous population of persons living with HIV and near-complete ART-induced viral suppression; (2) its design, which measured several recognized major early indicators for atherosclerotic risk and putative biochemical and cellular mediators within the atherosclerotic process; (3) its observation period of up to 72 weeks; and (4) the widely documented safety of MVC in HIV-infected patients.
We recognize several limitations. First, the sample size was small, although sufficiently powered to reach statistical significance, qualifying this as a pilot, hypothesis-generating study. Second, our crossover design did not include a washout period. This decision was made based on the following: MVC’s half-life is about 16 hours, and the study treatment period lasted 24 weeks. Of note, a carryover effect was evaluated and found not to be significant. Third, we did not perform a direct evaluation of vascular wall inflammation or a direct measure of NO bioavailability, thus limiting our ability to fully interpret the results. Fourth, we did not evaluate any markers of oxidative stress, which may have contributed to the observed anti-atherosclerotic effects of MVC. Fifth, we did not investigate endothelial progenitor cell homing. In fact, the observed trend toward an increased EPC count following MVC intensification does not necessarily imply active participation of these cells in endothelial repair. It has been reported that CCR5 expressed by EPCs facilitates EPC recruitment and exerts anti-atherosclerotic effects in ApoE-/- mice [39]. Hence, maraviroc-induced CCR5 inhibition might contribute to an increased circulating availability of EPCs due to reduced vascular homing. Finally, only PI-treated individuals were included in our study; thus these results cannot be generalized to PLWH treated with non-PI-containing regimens.
In conclusion, in this pilot study of persons living with HIV, treated with PIs, and having complete viral suppression, but at a high risk of CVD, MVC intensification was associated with significant and consistent improvements in several major indicators of increased CV risk, namely EMP/EPC ratio, endothelial dysfunction, arterial stiffness, and cIMT.
The nondocumented interference of MVC in systemic inflammation and its widely documented tolerability may support its use as an off-label anti-atherosclerotic therapy, as drugs targeting systemic inflammation can be burdened by higher incidence of serious fatal infections [40]. Further investigations with MVC are warranted in larger samples of patients treated also with other antiretroviral drugs or even individuals without HIV infection but at high risk of cardiovascular diseases.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We would like to thank Thomas Charles Kilcline for his important editorial assistance, Verena De Angelis for her expert advice on the statistical analysis, and both Patrizia Sforna and Sabrina Bastianelli for technical support.
Financial support. This work was supported by Fondazione Cassa di Risparmio di Perugia, Italy (Award No. 2014.0339011 to F.B.).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med 1999; 340:115–26. [DOI] [PubMed] [Google Scholar]
- 2. Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med 2011; 62:141–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wada NI, Jacobson LP, Margolick JB, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015; 29:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013; 173:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Post WS, Budoff M, Kingsley L, et al. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med 2014; 160:458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. J Infect Dis 2012; 205(Suppl 3):S375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352:1685–95. [DOI] [PubMed] [Google Scholar]
- 8. Duprez DA, Neuhaus J, Kuller LH, et al. ; INSIGHT SMART Study Group Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One 2012; 7:e44454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jones KL, Maguire JJ, Davenport AP. Chemokine receptor CCR5: from AIDS to atherosclerosis. Br J Pharmacol 2011; 162:1453–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 2006; 354:610–21. [DOI] [PubMed] [Google Scholar]
- 11. Dragic T, Litwin V, Allaway GP, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 1996; 381:667–73. [DOI] [PubMed] [Google Scholar]
- 12. Jones KL, Maguire JJ, Davenport AP. Chemokine receptor CCR5: from AIDS to atherosclerosis. Br J Pharmacol 2011; 162:1453–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang Y, Paxton WA, Wolinsky SM, et al. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med 1996; 2:1240–3. [DOI] [PubMed] [Google Scholar]
- 14. Szalai C, Duba J, Prohászka Z, et al. Involvement of polymorphisms in the chemokine system in the susceptibility for coronary artery disease (CAD). Coincidence of elevated Lp(a) and MCP-1 -2518 G/G genotype in CAD patients. Atherosclerosis 2001; 158:233–9. [DOI] [PubMed] [Google Scholar]
- 15. Rodríguez-Rodríguez L, González-Juanatey C, García-Bermúdez M, et al. CCR5Δ32 variant and cardiovascular disease in patients with rheumatoid arthritis: a cohort study. Arthritis Res Ther 2011; 13:R133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilcox JN, Nelken NA, Coughlin SR, et al. Local expression of inflammatory cytokines in human atherosclerotic plaques. J Atheroscler Thromb 1994; 1(Suppl 1):S10–3. [DOI] [PubMed] [Google Scholar]
- 17. Herder C, Peeters W, Illig T, et al. ; CARDIoGRAM Consortium RANTES/CCL5 and risk for coronary events: results from the MONICA/KORA Augsburg case-cohort, Athero-Express and CARDIoGRAM studies. PLoS One 2011; 6:e25734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. von Hundelshausen P, Weber KS, Huo Y, et al. RANTES deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation 2001; 103:1772–7. [DOI] [PubMed] [Google Scholar]
- 19. Cipriani S, Francisci D, Mencarelli A, et al. Efficacy of the CCR5 antagonist maraviroc in reducing early, ritonavir-induced atherogenesis and advanced plaque progression in mice. Circulation 2013; 127:2114–24. [DOI] [PubMed] [Google Scholar]
- 20. Pirro M, Schillaci G, Romagno PF, et al. Influence of short-term rosuvastatin therapy on endothelial progenitor cells and endothelial function. J Cardiovasc Pharmacol Ther 2009; 14:14–21. [DOI] [PubMed] [Google Scholar]
- 21. Pirro M, Schillaci G, Mannarino MR, et al. Circulating immature osteoprogenitor cells and arterial stiffening in postmenopausal osteoporosis. Nutr Metab Cardiovasc Dis 2011; 21:636–42. [DOI] [PubMed] [Google Scholar]
- 22. Pirro M, Stingeni L, Vaudo G, et al. Systemic inflammation and imbalance between endothelial injury and repair in patients with psoriasis are associated with preclinical atherosclerosis. Eur J Prev Cardiol 2015; 22:1027–35. [DOI] [PubMed] [Google Scholar]
- 23. Sabatier F, Camoin-Jau L, Anfosso F, et al. Circulating endothelial cells, microparticles and progenitors: key players towards the definition of vascular competence. J Cell Mol Med 2009; 13:454–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krikke M, Tesselaar K, Arends JE, et al. Maraviroc intensification improves endothelial function in abacavir-treated patients, an open-label randomized cross-over pilot study. Infect Dis Ther 2016; 5:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Piconi S, Pocaterra D, Rainone V, et al. Maraviroc reduces arterial stiffness in PI-treated HIV-infected patients. Sci Rep 2016; 6:28853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hsue P, Scherzer R, Gilman L, et al. Effect of maraviroc intensification on endothelial function in treated HIV infection. Abstract 123. In: 19th Conference on Retroviruses and Opportunistic Infections; March 5–8,2012; Seattle, WA. [Google Scholar]
- 27. Ghiadoni L, Salvetti M, Muiesan ML, Taddei S. Evaluation of endothelial function by flow mediated dilation: methodological issues and clinical importance. High Blood Press Cardiovasc Prev 2015; 22:17–22. [DOI] [PubMed] [Google Scholar]
- 28. Sinning JM, Losch J, Walenta K, et al. Circulating CD31+/Annexin V+ microparticles correlate with cardiovascular outcomes. Eur Heart J 2011; 32:2034–41. [DOI] [PubMed] [Google Scholar]
- 29. Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 2005; 353:999–1007. [DOI] [PubMed] [Google Scholar]
- 30. Mannarino E, Pirro M. Endothelial injury and repair: a novel theory for atherosclerosis. Angiology 2008; 59:69S–72S. [DOI] [PubMed] [Google Scholar]
- 31. Bellien J, Favre J, Iacob M, et al. Arterial stiffness is regulated by nitric oxide and endothelium-derived hyperpolarizing factor during changes in blood flow in humans. Hypertension 2010; 55:674–80. [DOI] [PubMed] [Google Scholar]
- 32. Wagenseil JE, Mecham RP. Elastin in large artery stiffness and hypertension. J Cardiovasc Transl Res 2012; 5:264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kinlay S, Creager MA, Fukumoto M, et al. Endothelium-derived nitric oxide regulates arterial elasticity in human arteries in vivo. Hypertension 2001; 38:1049–53. [DOI] [PubMed] [Google Scholar]
- 34. Longenecker CT, Sattar A, Gilkeson R, McComsey GA. Rosuvastatin slows progression of subclinical atherosclerosis in patients with treated HIV infection. AIDS 2016; 30:2195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maggi P, Bruno G, Perilli F, et al. Effects of therapy with maraviroc on the carotid intima media thickness in HIV-1/HCV co-infected patients. In Vivo 2017; 31:125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Juonala M, Viikari JS, Laitinen T, et al. Interrelations between brachial endothelial function and carotid intima-media thickness in young adults: the cardiovascular risk in Young Finns Study. Circulation 2004; 110:2918–23. [DOI] [PubMed] [Google Scholar]
- 37. Maguire JJ, Jones KL, Kuc RE, et al. The CCR5 chemokine receptor mediates vasoconstriction and stimulates intimal hyperplasia in human vessels in vitro. Cardiovasc Res 2014; 101:513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. De Caterina R, Massaro M, Scoditti E, Annunziata Carluccio M. Pharmacological modulation of vascular inflammation in atherothrombosis. Ann N Y Acad Sci 2010; 1207:23–31. [DOI] [PubMed] [Google Scholar]
- 39. Zhang Z, Dong J, Lobe CG, et al. CCR5 facilitates endothelial progenitor cell recruitment and promotes the stabilization of atherosclerotic plaques in ApoE-/- mice. Stem Cell Res Ther 2015; 6:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ridker PM, Everett BM, Thuren T, et al. ; CANTOS Trial Group Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377:1119–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.