Abstract
Background
A/H5N1 influenza viruses have high pandemic potential; consequently, vaccines need to be produced rapidly. MF59® adjuvant reduces the antigen required per dose, allowing for dose sparing and more rapid vaccine availability.
Methods
Two multicenter, phase II trials were conducted to evaluate the safety and immunogenicity of an MF59-adjuvanted, cell culture–derived, A/H5N1 vaccine (aH5N1c) among 979 adult (18–64 years old) and 1393 elderly (≥65 years old) subjects. Participants were equally randomized to receive 2 full-dose (7.5 μg of hemagglutinin antigen per dose) or 2 half-dose aH5N1c vaccinations 3 weeks apart. Outcomes were based on Center for Biologics Evaluation Research and Review (CBER) and Committee for Medicinal Products for Human Use (CHMP) licensure criteria (titers ≥1:40 and seroconversions on day 43). Solicited reactions and adverse events were assessed (www.clinicaltrials.gov: NCT01776541 and NCT01766921).
Results
CBER and CHMP criteria were met by both age groups. CBER criteria for hemagglutination titers were met for the full-dose formulation. Solicited reaction frequencies tended to be higher in the full-dose group and were of mild to moderate intensity. No vaccine-related serious adverse events occurred.
Conclusions
In adult and elderly participants, the full-dose aH5N1c vaccine formulation was well tolerated and met US and European licensure criteria for pandemic vaccines.
Keywords: cell culture–derived vaccine, H5N1 subunit vaccine, influenza, MF59 adjuvant; pandemic influenza, phase II
Periodic influenza pandemics pose serious threats to global health and economies [1]. The highly pathogenic avian influenza virus H5N1 causes severe human disease and death [1] and has a higher case fatality rate than seasonal influenza infections [1, 2]. Of the 694 H5N1 cases reported to the World Health Organization between 2003 and 2015, 402 (~58%) were fatal [3]. Sporadic cases of human H5N1 virus infection have been associated with close contact with infected poultry. Moreover, circulation of H5N1 virus in bird flocks allows for potential mutations that can facilitate bird-to-human and human-to-human viral transmission [1]. Overall, the H5N1 virus retains pandemic potential because it has spread to most continents, and most humans are unlikely to be immune to the virus [1].
Rapid and efficient production of pandemic influenza vaccines is essential to meet the anticipated global demand [4, 5]. New cell culture–based production methods can eliminate dependency on egg supply and poultry flocks, which are also vulnerable to H5N1 infection. Cell culture–based vaccine manufacturing techniques shorten production times and increase production capacity [4, 6].
Manufacturing capacity may also benefit from the use of a vaccine adjuvant to enhance immunogenicity. MF59® (Novartis International AG, Basel, Switzerland) is a proprietary oil-in-water emulsion adjuvant that has been used in several registered pandemic and seasonal influenza vaccines since 1997. It has a well-established safety profile [7], allows for reduced antigen content per dose (7.5 μg of hemagglutinin [HA] in pandemic formulations vs 15 μg of HA per strain in seasonal formulations [6, 8–10]), promotes the production of cross-reactive antibodies that may provide heterologous immunity against antigenically divergent strains [8, 11, 12], and improves vaccine efficacy in elderly adults and children, who are particularly vulnerable to influenza infection [13].
Based on a previous phase I dose-ranging study of an adjuvanted, cell culture–derived, H5N1 subunit influenza virus vaccine, 2 antigen-sparing formulations were evaluated in phase II studies [14]. We present safety, tolerability, and immunogenicity data from healthy adult and elderly subjects to establish the optimal vaccine formulation for these age groups. Here we present primary and secondary outcome data from these 13-month studies, up to study day 43.
METHODS
Study Design
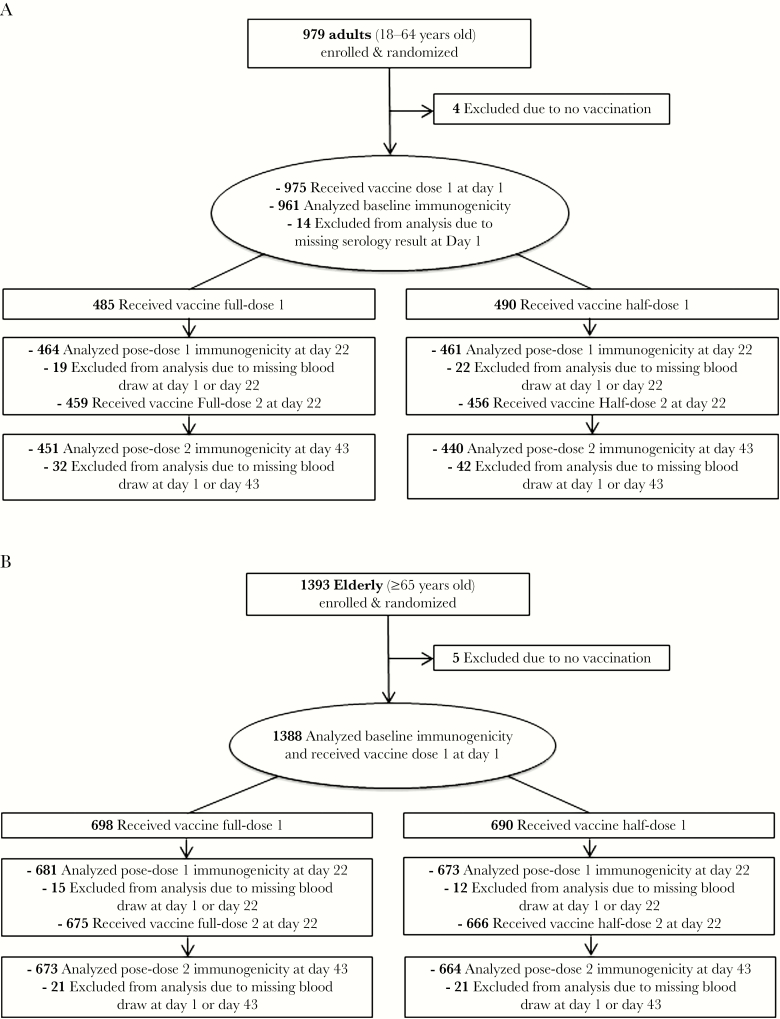
The adult (NCT01776541) and elderly (NCT01766921) trials were phase II, randomized, observer-blind, multicenter studies, conducted in Australia, New Zealand, the United States, and Thailand. The design, objectives, and endpoints were identical for both studies. The study protocols were approved by the Ethics Review Committees of the participating centers, and the studies were conducted in compliance with Good Clinical Practices guidelines and the Declaration of Helsinki. Written informed consent was obtained from subjects before enrollment. Subjects were randomized at a 1:1 ratio to receive 2 vaccinations with either full-dose or half-dose MF59-adjuvant, cell culture–derived, H5N1 vaccine (aH5N1c) given 3 weeks apart (Figure 1). The primary immunogenicity outcomes evaluated hemagglutination inhibition (HI) assay antibody responses in terms of the percentages of subjects achieving seroconversion and HI titers ≥1:40 on day 43. Each subject was followed for 12 months (day 387) after the second vaccine dose to assess safety and immunogenicity.
Figure 1.
Study design and subject disposition for adult (A; NCT01776541) and elderly (B; NCT01766921) clinical trials.
Study Participants
In the respective studies, 979 adult (aged 18–64 years) and 1393 elderly subjects (aged ≥65 years) were enrolled. The main exclusion criteria were presence of serious chronic or progressive disease, pregnancy or breastfeeding, prior receipt of any H5N1 vaccine, receipt of any other influenza vaccines within 60 days before enrollment, body temperature ≥38.0°C and/or any acute illness within 3 days of receiving study vaccines, and a body mass index ≥35 kg/m2 (see ClinicalTrials.gov for all exclusion criteria).
Vaccines
Both the adult and elderly studies used MF59-adjuvanted, cell culture–derived, monovalent, inactivated, subunit, H5N1 vaccines containing A/turkey/Turkey/1/05 (H5N1)-like strain (NIBRG-23) antigen (Seqirus Inc., Holly Springs, NC; f/k/a Novartis Influenza Vaccines GmbH, Marburg, Germany). One 0.5 mL dose (full-dose formulation) contained 7.5 μg of HA antigen with 0.25 mL of MF59. One 0.25 mL dose (half-dose formulation) contained 3.75 μg of HA with 0.125 mL of MF59. Vaccines were administered on day 1 and day 22 as single intramuscular injections in the nondominant arm.
Immunogenicity Assessments
Sera samples were obtained for immunogenicity analyses before each vaccination (day 1 and day 22) and on day 43 and day 387 (stored at –18°C). Immunogenicity was assessed by HI assay against the H5N1 vaccine strain according to standard methods [15] and expressed as the percentage of subjects achieving seroconversion, the percentage of subjects with an HI titer ≥1:40, geometric mean HI titers (GMTs), and the geometric mean ratios (GMRs) of HI titers. Seroconversion was defined as HI titers ≥1:40 for subjects who were seronegative at baseline (HI titer <1:10 on day 1) or a minimum 4-fold increase in HI titer for subjects who had detectable baseline HI titers (≥1:10). For both studies, the primary immunogenicity outcomes were assessed on day 43 and were based on the licensure criteria for pandemic influenza vaccines established by the Center for Biologics Evaluation Research and Review (CBER) [16]. For the adult population, the following CBER criteria were applied: (1) the lower limit (LL) of the 2-sided 97.5% confidence interval (CI) for the percentage of subjects achieving seroconversion for HI antibody responses should be ≥40%; (2) the LL of the 2-sided 97.5% CI for the percentage of subjects achieving an HI antibody titer of ≥1:40 should be ≥70%. For the elderly population, the values for the criteria described above were (1) ≥30% and (2) ≥60%, respectively. The secondary immunogenicity outcomes were also assessed on day 43 and were based on the licensure criteria for pandemic influenza vaccines established by the Committee for Medicinal Products for Human Use (CHMP) [17]. For the adult population, the following CHMP criteria applied: 1) the LL of the 2-sided 97.5% CI for the percentage of subjects achieving seroconversion for HI antibody responses should be ≥40%; 2) the LL of the 2-sided 97.5% CI for the percentage of subjects achieving an HI antibody titer of ≥1:40 should be ≥70%; 3) GMR should be >2.5. For the elderly population, the values for the criteria described above were (1) ≥30%, (2) ≥60%, and (3) >2.0, respectively.
Safety Assessments
After each vaccination, subjects were observed for 30 minutes to monitor for immediate reactions. Solicited local and systemic reactions were recorded by the subjects on diary cards for 7 days after each vaccination. Solicited local reactions included injection site induration, erythema, ecchymosis, and pain. Solicited systemic reactions included nausea, generalized myalgia, generalized arthralgia, headache, fatigue, loss of appetite, malaise, and fever (body temperature ≥38°C). The severity of solicited reactions was categorized as mild (transient with no limitation in normal daily activity), moderate (some limitation in normal daily activity), or severe (unable to perform normal daily activity). All unsolicited adverse events (AEs) were collected for 21 days after each vaccination. Serious adverse events (SAEs), the new onset of chronic diseases, medically attended AEs, AEs of special interest, AEs leading to study withdrawal, and the administration of concomitant medications associated with these events were recorded throughout the study. The causal relationships of AEs to the study vaccines were assessed by the investigators as being either nonrelated, possibly related, or probably related.
Statistical Analyses
Sample sizes for the adult and elderly studies were planned as 486 and 624 evaluable subjects per group (assuming a study dropout rate of 10%), respectively. The percentages of subjects achieving seroconversion and the percentages of subjects with HI titers ≥1:40, along with the associated 97.5% Clopper-Pearson CIs, were calculated as log10-transformed values using analysis of covariance (ANCOVA) with factors for dose, group, baseline titer, and study center. GMTs, GMRs, and the associated 2-sided adjusted 95% CIs were calculated using ANCOVA with factors for race, gender, and study center. Although the CBER criteria involve the lower limits of 95% CIs, a 97.5% CI was calculated because there were 2 vaccine formulations tested in the study, and the 0.05 alpha was distributed across tests. The immunogenicity analyses were performed on full analysis set (FAS) data, which included all subjects who received at least 1 dose of study vaccine and provided at least 1 serum sample at both prevaccination and postvaccination time points. Analyses of vaccine reactogenicity and safety were performed on data from subjects who had received at least 1 study vaccination and provided either postvaccination AE or reactogenicity data (safety data set). All safety analyses were descriptive.
RESULTS
Of the enrolled 979 adult and 1393 elderly subjects, 975 (>99%) and 1388 (>99%) received at least 1 dose of study vaccine, respectively (Figure 1). Overall, 91% of adult subjects (451 of 488 in the full-dose group and 440 of 491 in the half-dose group) and 96% of elderly subjects (673 of 700 in the full-dose group and 664 of 693 in the half-dose group) remained in the study and provided sera for immunogenicity analyses on day 43 (FAS data) (Figure 1). Approximately 9% of adult subjects and 4% of elderly subjects were excluded from the immunogenicity FAS data due to the absence of sera data. Subject demographics and baseline characteristics were well balanced between groups within the respective study populations (Table 1). In both studies, more females were enrolled than males (56% vs 44% in the adult study and 59% vs 41% in the elderly study, respectively).
Table 1.
Study Population Demographics
| Adult Subjects | Elderly Subjects | |||
|---|---|---|---|---|
| (Age 18–64 y, n = 979) | (Age ≥65 y, n = 1393) | |||
| Full-Dose | Half-Dose | Full-Dose | Half-Dose | |
| (n = 488) | (n = 491) | (n = 700) | (n = 693) | |
| Age, mean ± SD, y | 39.0 ± 13.7 | 38.4 ± 14.2 | 71.2 ± 5.1 | 70.7 ± 4.7 |
| Male, No. (%) | 203 (42) | 232 (47) | 293 (42) | 275 (40) |
| Weight, mean ± SD, kg | 74.1 ± 15.1 | 73.5 ± 16.3 | 71.0 ± 15.9 | 71.4 ± 16.2 |
| Height, mean ± SD, cm | 167.8 ± 10.5 | 168.5 ± 11.0 | 164.1 ± 10.7 | 163.5 ± 10.9 |
| BMI, mean ± SD, kg/m2 | 26.2 ± 4.3 | 25.7 ± 4.3 | 26.1 ± 4.0 | 26.5 ± 4.2 |
| Previous influenza vaccination, No. (%) | 118 (24) | 119 (24) | 429 (61) | 419 (60) |
| Influenza vaccination ≤12 mo,a No. (%) | 98 (20) | 95 (19) | 146 (21) | 137 (20) |
| White, No. (%) | 291 (60) | 290 (59) | 445 (64) | 444 (64) |
| Asian, No. (%) | 93 (19) | 96 (20) | 240 (34) | 237 (34) |
| Black/African American, No. (%) | 97 (20) | 99 (20) | 10 (1) | 10 (1) |
| American Indian/Alaska Native, No. (%) | 2 (<1) | 1 (<1) | 2 (<1) | 0 |
| Native Hawaiian/Pacific Islander, No. (%) | 0 | 1 (<1) | 0 | 0 |
Full-dose: 7.5 µg of aH5N1c antigen + 0.25 mL of MF59 adjuvant per dose; Half-dose: 3.75 µg of aH5N1c antigen + 0.125 mL of MF59 adjuvant per dose.
Abbreviation: BMI, body mass index; SD, standard deviation.
aTwelve months before enrollment in the study.
Immunogenicity
At day 43, 83% (97.5% CI, 78%–87%) and 61% (97.5% CI, 56%–66%) of adult subjects in the full-dose and half-dose groups, respectively, achieved seroconversion; the CBER and CHMP criteria for seroconversion were met for both treatment groups (Table 2) [16, 17]. In the elderly population, 74% (97.5% CI, 70%–77%) and 52% (97.5% CI, 48%–56%) of subjects in the full-dose and half-dose groups achieved seroconversion at day 43, respectively; the CBER and CHMP criteria for seroconversion were met for both treatment groups.
Table 2.
Percentage of Subjects With HI Titers ≥1:40 at Day 1 and Day 43, GMRs Day 43/Day 1, and Percentages of Subjects Achieving Seroconversion or Significant Increases in HI Titers at Day 43
| Adult Subjects | Elderly Subjects | |||
|---|---|---|---|---|
| (Age 18–64 y, n = 891) | (Age ≥65 y, n = 1337) | |||
| Full-Dose | Half-Dose | Full-Dose | Half-Dose | |
| (n = 451) | (n = 440) | (n = 673) | (n = 664) | |
| Day 1: HI titers ≥1:40 (97.5% CI), % | 4.0 (2.0–7.0) | 4.0 (2.0–6.0) | 12 (10–15) | 10 (8.0–13) |
| Day 43: HI titers ≥1:40 (97.5% CI), % | 85 (81–88)a,b | 63 (58–68) | 81 (77–84)a,b | 63 (58–67) |
| Day 43/day 1: GMRs (95% CI),c | 41 (34–49)b | 11 (8.7–13)b | 16 (14–18)b | 5.7 (5.0–6.6)b |
| Day 43: Positivity conversion (97.5% CI), % | 83 (78–87) | 61 (55–67) | 76 (71–80) | 56 (51–61) |
| Day 43: Significant increase (97.5% CI), % | 83 (69–92) | 61 (43–77) | 66 (57–74) | 38 (29–47) |
| Day 43: Seroconversion (97.5% CI), % | 83 (78–87)a,b | 61 (56–66)a,b | 74 (70–77)a,b | 52 (48–56)a,b |
Abbreviations: CI, confidence interval; GMR, geometric mean ratio; HI, hemagglutination inhibition; seroconversion, defined as positivity or significant increase.
aOutcome met CBER criterion [16] for age group.
bOutcome met CHMP criterion [17] for age group.
cGMR was a secondary end point in the adult study and was not adjusted for multiplicity; therefore, GMR (97.5% CI) data are not available. Positivity conversion was defined as postvaccination HI titer ≥1:40 for subjects negative (titer < 1:10) at baseline or a minimum 4-fold increase in HI titer for subjects seropositive (titer ≥ 1:10) at baseline; significant increase in antibody titer was defined as a minimum 4-fold increase in HI titer for subjects seropositive (titer ≥ 1:10) at baseline.
At day 43, 85% (97.5% CI, 81%–88%) and 63% (97.5% CI, 58%–68%) of adult subjects in the full-dose and half-dose groups achieved HI titers ≥1:40, respectively (Table 2). The LL of the 2-sided 97.5% CI for the percentage of subjects achieving an HI antibody titer ≥1:40 exceeded the CBER and CHMP requirements of 70% in the full-dose group, but not in the half-dose group. In the elderly population, 81% (97.5% CI, 77%–84%) of subjects in full-dose group and 63% (97.5% CI, 58%–67%) of subjects in the half-dose group achieved HI antibody titers ≥1:40 at day 43. The LL of the 2-sided 97.5% CI exceeded the CBER and CHMP criterion of 60% in the full-dose group, but not in the half-dose group.
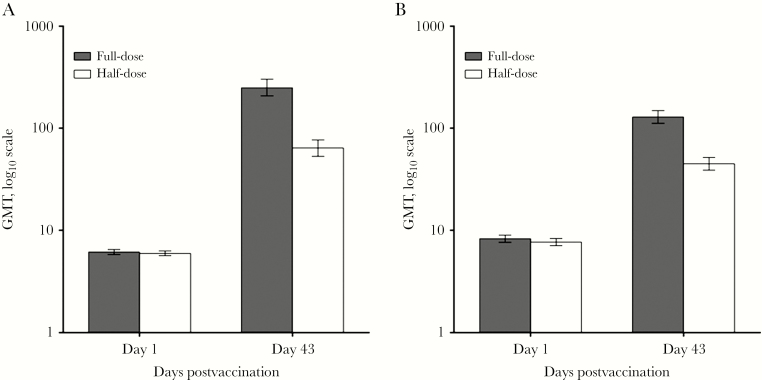
At baseline, GMTs within each study population were comparably low between the respective vaccine groups. Among adult subjects, 3 weeks after 2 doses of aH5N1c (day 43), GMTs rose to 250 (95% CI, 208%–302%) and 64 (95% CI, 53%–77%) in the full-dose and half-dose groups, respectively (Figure 2A). The day 43 to day 1 GMRs for the full-dose and half-dose groups were 41 (95% CI, 34%–49%) and 11 (95% CI, 9%–13%), respectively, and exceeded the CHMP GMR criterion of 2.5. Among elderly subjects, GMTs rose to 129 (95% CI, 112%–149%) and 45 (95% CI, 39%–52%) by day 43 in the full-dose and half-dose groups, respectively (Figure 2B). The day 43 to day 1 GMRs for the elderly full-dose and half-dose groups were 16 (95% CI, 14%–18%) and 5.7 (95% CI, 5%–7%), respectively, exceeding the CHMP criterion of 2.0 (Table 2).
Figure 2.
Geometric mean hemagglutination inhibition antibody titers (95% confidence interval) on day 1 and day 43 in adult (A; age 18–64 years) and elderly (B; age ≥65 years) subjects.
Safety
The rates of any solicited reactions within 30 minutes of administration of the first vaccine dose were low and comparable between the vaccine groups in each age population, with no increase in reactogenicity after administration of the second dose (adults 7% vs 5% following first and second doses; elderly 8% vs 7% following first and second doses, respectively). In both age populations, the frequencies of solicited local reactions reported within 7 days of each vaccination were higher for the full-dose groups compared with the respective half-dose groups, with no increase in reactogenicity after administration of the second doses (Table 3). For both age populations, injection site pain was the most frequently reported solicited local reaction. Within each age population, frequencies of individual solicited systemic reactions reported within 7 days of each vaccination were comparable between the respective full-dose and half-dose groups (Table 3). Among adults, after each vaccination, the most commonly reported systemic reactions were headache and fatigue, followed by malaise, with no difference between the full-dose and half-dose groups. Among elderly subjects, the most commonly reported systemic reactions after each vaccination were fatigue and malaise, with no difference between the full-dose and half-dose groups. After the second vaccination, frequencies of solicited systemic reactions were lower.
Table 3.
Overview of Solicited Adverse Events and Other Indicators of Reactogenicity During a 7-Day Period After Vaccination
| Adult Subjects | Elderly Subjects | |||||||
|---|---|---|---|---|---|---|---|---|
| (Age 18–64 y, n = 944) | (Age ≥65 y, n = 1376) | |||||||
| First Vaccination | Second Vaccination | First Vaccination | Second Vaccination | |||||
| Full-Dose | Half-Dose | Full-Dose | Half-Dose | Full-Dose | Half-Dose | Full-Dose | Half-Dose | |
| (n = 468) | (n = 469) | (n = 450) | (n = 443) | (n = 692) | (n = 681) | (n = 676) | (n = 665) | |
| Any reaction, % | 72 | 56 | 53 | 43 | 52 | 39 | 39 | 28 |
| Local reactions, No. of subjects | 460–462 | 464–466 | 445–448 | 439–442 | 677–685 | 667–673 | 672–674 | 660–662 |
| Local reactions, % | 63 | 43 | 48 | 34 | 38 | 22 | 29 | 17 |
| Pain (% severe), % | 63 (<1) | 43 (1) | 48 (0) | 34 (<1) | 38 (0) | 21 (0) | 29 (<1) | 16 (<1) |
| Ecchymosis (% severe), % | 1 (0) | <1 (0) | 1 (0) | 1 (0) | <1 (0) | 1 (0) | 1 (0) | 1 (0) |
| Erythema (% severe), % | 1 (0) | 0 | 0 | 0 | 2 (0) | <1 (0) | 1 (0) | <1 (0) |
| Induration (% severe), % | 3 (<1) | 1 (0) | 1 (0) | <1 (0) | 2 (0) | 1 (0) | 2 (0) | <1 (0) |
| Systemic reactions, No. of subjects | 452–457 | 452–460 | 439–446 | 432–438 | 668–688 | 656–677 | 663–676 | 652–664 |
| Systemic reactions, % | 43 | 38 | 27 | 23 | 28 | 26 | 19 | 17 |
| Fatigue (% severe), % | 23 (1) | 19 (1) | 13 (<1) | 11 (0) | 12 (<1) | 11 (<1) | 9 (<1) | 9 (<1) |
| Nausea (% severe), % | 7 (1) | 8 (<1) | 6 (<1) | 4 (0) | 4 (<1) | 4 (<1) | 3 (0) | 3 (<1) |
| Malaise (% severe), % | 21 (2) | 16 (1) | 12 (<1) | 9 (0) | 12 (<1) | 12 (<1) | 8 (<1) | 8 (<1) |
| Myalgia (% severe), % | 19 (1) | 14 (<1) | 10 (0) | 8 (0) | 10 (0) | 10 (0) | 7 (<1) | 5 (0) |
| Arthralgia (% severe), % | 12 (1) | 9 (1) | 6 (0) | 5 (0) | 6 (<1) | 8 (<1) | 5 (<1) | 4 (<1) |
| Headache (% severe), % | 21 (1) | 20 (<1) | 14 (<1) | 11 (0) | 10 (<1) | 10 (<1) | 6 (<1) | 6 (0) |
| Loss of appetite (% severe), % | 8 (<1) | 7 (<1) | 5 (<1) | 3 (0) | 4 (0) | 5 (<1) | 4 (0) | 2 (0) |
| Temperature, % ≥38°C (% ≥40°C) | 2 (<1) | 2 (0) | 1 (0) | <1 (0) | 2 (0) | <1 (0) | 1 (0) | <1 (0) |
| Other reactions, No. of subjects | 458–465 | 456–462 | 434–449 | 429–438 | 678–688 | 671–677 | 673–676 | 657–664 |
| Prevention of pain/fever (% severe), % | 3 (1) | 8 (2) | 4 (1) | 6 (1) | 10 (1) | 18 (3) | 11 (2) | 8 (1) |
| Treatment of pain/fever (% severe), % | 34 (7) | 24 (5) | 15 (3) | 7 (2) | 36 (5) | 29 (4) | 23 (3) | 19 (3) |
Between day 1 and day 43, unsolicited AEs were reported by up to 29% of subjects in the adult population and up to 32% of subjects in the elderly population, with comparable frequencies between the vaccine groups in each study. Following first or second vaccinations, 10% and 12% of subjects in the adult and elderly populations reported an unsolicited AE that was judged to be possibly or probably related to the study vaccine, respectively. Injection site bruising was the most commonly reported unsolicited AE in both age groups. At least 1 SAE was reported by 3 (<1%) subjects (appendicitis, pyelonephritis, and nerve compression) in the adult population, and by 10 (1%) subjects (atrial fibrillation, adenocarcinoma of left lung, transient ischemic attack, cholecystitis, benign positional vertigo, right inguinal hernia, acute kidney injury, infected wound of left leg, fractured ribs, and syncope) in the elderly population; none of the reported SAEs were considered to be possibly or probably vaccine-related. One elderly subject (<1%) withdrew prematurely from the study due to an unsolicited AE. Three (<1%) subjects in the adult population and 24 (2%) subjects in the elderly population were diagnosed with the new onset of chronic diseases up to day 43. Only 1 SAE had a fatal outcome (non-vaccine-related, elderly half-dose group; lung adenocarcinoma; occurred on day 155).
Discussion
We evaluated the immunogenicity and safety of an MF59-adjuvanted, cell culture–derived, H5N1 subunit influenza virus vaccine in 2 phase II studies; vaccine was administered as either a full- or half-dose formulation to healthy adult or elderly subjects. For both age populations, all CBER criteria (seroconversion and HI titer ≥1:40) were met for the full-dose group, whereas the half-dose group only met the criteria for seroconversion. Similarly, for both age groups, all CHMP criteria (seroconversion, HI titer ≥1:40, and GMR) were met for the full-dose group, whereas the half-dose group met 2 of the 3 criteria (seroconversion and GMR). Although both vaccine formulations were immunogenic and well tolerated in each age population, the full-dose (7.5 μg of H5N1 HA antigen with 0.25 mL of MF59) aH5N1c vaccine appears preferable—in terms of higher immunogenicity—for future clinical development. Overall, postvaccination immunogenicity outcomes (seroconversion, percentages of subjects with HI titers ≥1:40, and GMTs) were higher among adults compared with elderly subjects, regardless of vaccine formulation. The generally lower postvaccination antibody responses observed in the elderly population may partly be attributed to immunosenescence.
In both studies, the proportions of subjects who reported solicited reactions trended higher for the full-dose group compared with the half-dose group (mainly due to incidence of pain at the site of injection). Subjects ≥65 years of age appeared to report fewer solicited local and systemic reactions in each vaccine group compared with adult subjects. Irrespective of vaccine formulation, and for each age group, the majority of reported reactions were mild to moderate in severity, with no evidence of increased frequency of reactions following a second dose. In both age groups, there were no appreciable differences in unsolicited AEs between the full-dose and half-dose groups, in terms of both frequencies and specific conditions.
The immunogenicity data for the full-dose formulation are consistent with those of previous studies conducted in healthy adult and elderly individuals, in which subjects received 2 doses of MF59-adjuvanted H5N1 vaccine (7.5 μg of H5N1 HA per dose) [8, 11, 14, 18, 19]. Whereas in the present study the half-dose formulation (containing 3.75 μg of H5N1 antigen and a lower-than-standard quantity of MF59 per dose) did not meet all the licensure criteria evaluated, many studies conducted to assess the immunogenicity of 2 half-doses (3.75 μg of antigen with a reduced quantity of MF59) administered 3 weeks apart have consistently demonstrated that either 1 or 2 half-doses were sufficiently immunogenic to meet the CBER and/or CHMP licensure criteria; these studies included subjects of all ages and ethnicities, cell- and egg-derived vaccines, and A/H5N1 [14], A/H3N2 [20], and A/H1N1 [6, 21–28] strains. Overall, the reactogenicity and safety data presented here are similar to and are in agreement with the results of previous studies of MF59-adjuvanted H5N1 vaccine when administered to healthy adult and elderly subjects [8, 19].
There were some limitations to the study. First, because a non-H5N1 control group was not included, interpretation of the safety data is somewhat limited. However, the safety profile of the full-dose formulation observed in this study is consistent with that reported from a recent placebo-controlled trial of an egg-based, MF59-adjuvanted, H5N1 vaccine (Aflunov, Seqirus Vaccines Ltd., Liverpool, UK [f/k/a Novartis Influenza Vaccines Ltd., Liverpool, UK]; 7.5 μg of H5N1 HA per dose) conducted in adult and elderly subjects [19]. Second, the study assessed only short-term antibody responses; analyses of mid-term (6-month) responses and long-term antibody persistence are either ongoing or planned. Third, the relatively short follow-up period does not allow for the possible detection of rare, delayed AEs. Nonetheless, in a separate study of a cell culture–derived, MF59-adjuvanted H5N1 vaccine in adults, no vaccine-related AEs were reported throughout the study period [14].
In conclusion, two 7.5-μg doses of a cell culture–derived, MF59-adjuvanted H5N1 vaccine administered 3 weeks apart were well tolerated and highly immunogenic, raised no safety concerns, and induced robust antibody responses in adult and elderly subjects that met all the immunogenicity criteria required for pandemic vaccine licensure by both the US and European regulatory authorities. These results support the further development of the full-dose (7.5 μg), MF59-adjuvant, cell culture–derived H5N1 vaccine formulation for adult (age ≥18 years) pandemic preparedness programs.
Acknowledgments
We thank Eric Sheldon, MD, Yupin Suputtamongkol, MD, Khuanchai Supparatpinyo, MD, Sirakarn Tejavanich, MD, Peter Levins, MD, Laurence Chu, MD, David Seiden, MD, Suchet Patel, MD, Richard Egelhof, MD, James Todd Peterson, MD, Thomas Klein, MD, Shane Christensen, MD, Katie Ann Julien, MD, Gregg Lucksinger, MD, Janakan Krishnarajah, MD, Marc Russo, MD, Julie Todhunter, MD, Simon Carson, MD, Jason Pryke, MD, and the clinical research staff at all the participating sites for their contributions in conducting the study. We acknowledge the contributions of David Hering, MBA, David Lee, MD, MBA, Ken Tack, MD, Robin Wallace, BSN, and Heather Clouting, MSc, for operational support and Jenny Beygo, MSc, for statistical assistance.
We also acknowledge Debaditya Das, PhD (Novartis Healthcare Pvt. Ltd., India), Nicola West, PhD (Parexel Ltd., Uxbridge, UK), Melanie Meister-Broekema, MSc (Parexel Ltd., Uxbridge, UK), and Jamie Stirling, PhD (OLC Bioscience Ltd., London, UK), for providing support in manuscript preparation, revision, and editing.
Author contributions. M.H. had full access to all study data and takes responsibility for data integrity and the accuracy of data analyses. M.H. and N.K-T. were responsible for study design, analysis, and interpretation of data. Statistical expertise was provided by C.K. and P.J. M.H., C.K., P.J., B.V., and Z.M. were responsible for data acquisition. Studies were conducted by investigators S.F., S.S., E.S., T.S., P.R., P.C., and T.T.
Financial support. This work was supported by Novartis Vaccines and Diagnostics Inc. (Novartis’ influenza vaccine business was acquired by the CSL Group on July 31, 2015, and is currently operating as Seqirus Inc.) and by Federal funds from the US Office of Public Health Emergency Preparedness, Office of Research and Development Coordination (Contract No. HHSO100200600012C).
Potential conflicts of interest. Authors C.K., P.J., Z.M., B.V., and N.K-T. were permanent employees of Novartis Group companies at the time the study was conducted (Novartis’ influenza vaccine business was acquired by the CSL Group on July 31, 2015, and is currently operating as Seqirus Inc.). M.H. is a permanent employee of Seqirus Inc. (f/k/a Novartis’ influenza vaccine business), Cambridge, MA. PR has served on advisory boards for GlaxoSmithKline, Sanofi, and Janssen and has received institutional funding to undertake multicenter vaccine trials from Novartis, GlaxoSmithKline, CSL, and Janssen. S.F., S.S., E.S., T.S., P.C., and T.T. do not have any conflicts of interest to declare. MF59 is a registered trademark of Novartis International AG, Basel, Switzerland. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Avian influenza fact sheet 2014. http://www.who.int/mediacentre/factsheets/avian_influenza/en/. Accessed November 2015.
- 2. Zaman M, Gasimov V, Oner AF, et al. Recognizing true H5N1 infections in humans during confirmed outbreaks. J Infect Dev Ctries 2014; 8:202–7. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization. Cumulative number of confirmed human cases for avian influenza A(H5N1) reported to WHO, 2003–2015 2015. http://www.who.int/influenza/human_animal_interface/EN_GIP_20150106 CumulativeNumberH5N1cases.pdf?ua=1. Accessed November 2015.
- 4. World Health Organization. Pandemic influenza risk management WHO interim guidance June 2013 2013. http://www.who.int/influenza/preparedness/pandemic/GIP_PandemicInfluenzaRiskManagementInterimGuidance_Jun2013.pdf?ua=1. Accessed November 2015.
- 5. Rappuoli R, Dormitzer PR. Influenza: options to improve pandemic preparation. Science 2012; 336:1531–3. [DOI] [PubMed] [Google Scholar]
- 6. Hatz C, Cramer JP, Vertruyen A, et al. A randomised, single-blind, dose-range study to assess the immunogenicity and safety of a cell-culture-derived A/H1N1 influenza vaccine in adult and elderly populations. Vaccine 2012; 30:4820–7. [DOI] [PubMed] [Google Scholar]
- 7. Pellegrini M, Nicolay U, Lindert K, et al. MF59-adjuvanted versus non-adjuvanted influenza vaccines: integrated analysis from a large safety database. Vaccine 2009; 27:6959–65. [DOI] [PubMed] [Google Scholar]
- 8. Banzhoff A, Gasparini R, Laghi-Pasini F, et al. MF59-adjuvanted H5N1 vaccine induces immunologic memory and heterotypic antibody responses in non-elderly and elderly adults. PLoS One 2009; 4:e4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bernstein DI, Edwards KM, Dekker CL, et al. Effects of adjuvants on the safety and immunogenicity of an avian influenza H5N1 vaccine in adults. J Infect Dis 2008; 197:667–75. [DOI] [PubMed] [Google Scholar]
- 10. Leroux-Roels I, Borkowski A, Vanwolleghem T, et al. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet 2007; 370:580–9. [DOI] [PubMed] [Google Scholar]
- 11. Galli G, Hancock K, Hoschler K, et al. Fast rise of broadly cross-reactive antibodies after boosting long-lived human memory B cells primed by an MF59 adjuvanted prepandemic vaccine. Proc Natl Acad Sci U S A 2009; 106:7962–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stephenson I, Nicholson KG, Hoschler K, et al. Antigenically distinct MF59-adjuvanted vaccine to boost immunity to H5N1. N Engl J Med 2008; 359:1631–3. [DOI] [PubMed] [Google Scholar]
- 13. Van Buynder PG, Konrad S, Van Buynder JL, et al. The comparative effectiveness of adjuvanted and unadjuvanted trivalent inactivated influenza vaccine (TIV) in the elderly. Vaccine 2013; 31:6122–8. [DOI] [PubMed] [Google Scholar]
- 14. Keitel W, Groth N, Lattanzi M, et al. Dose ranging of adjuvant and antigen in a cell culture H5N1 influenza vaccine: safety and immunogenicity of a phase ½ clinical trial. Vaccine 2010; 28:840–8. [DOI] [PubMed] [Google Scholar]
- 15. Belshe RB, Frey SE, Graham IL, et al. Immunogenicity of avian influenza A/Anhui/01/2005(H5N1) vaccine with MF59 adjuvant: a randomized clinical trial. JAMA 2014; 312:1420–8. [DOI] [PubMed] [Google Scholar]
- 16. Food and Drug Administration, Center for Biologics Evaluation and Research. Guidance for industry: clinical data needed to support the licensure of pandemic influenza vaccines 2007. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm074786.htm. Accessed November 2015.
- 17. European Committee for Proprietary Medicinal Products. Note for guidance on harmonisation of requirements for influenza vaccines (CPMP/BWP/214/96) 1997. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003945.pdf. Accessed November 2015.
- 18. Czajka H, Unal S, Ulusoy S, et al. A phase II, randomised clinical trial to demonstrate the non-inferiority of low-dose MF59-adjuvanted pre-pandemic A/H5N1 influenza vaccine in adult and elderly subjects. J Prev Med Hyg 2012; 53:136–42. [PubMed] [Google Scholar]
- 19. Vesikari T, Forstén A, Herbinger KH, et al. Safety and immunogenicity of an MF59(®)-adjuvanted A/H5N1 pre-pandemic influenza vaccine in adults and the elderly. Vaccine 2012; 30:1388–96. [DOI] [PubMed] [Google Scholar]
- 20. Johnson C, Hohenboken M, Poling T, et al. Safety and immunogenicity of cell culture-derived A/H3N2 variant influenza vaccines: a phase I randomized, observer-blind, dose-ranging study. J Infect Dis 2015; 212:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fukase H, Furuie H, Yasuda Y, et al. Assessment of the immunogenicity and safety of varying doses of an MF59®-adjuvanted cell culture-derived A/H1N1 pandemic influenza vaccine in Japanese paediatric, adult and elderly subjects. Vaccine 2012; 30:5030–7. [DOI] [PubMed] [Google Scholar]
- 22. Hatz C, von Sonnenburg F, Casula D, et al. A randomized clinical trial to identify the optimal antigen and MF59(®) adjuvant dose of a monovalent A/H1N1 pandemic influenza vaccine in healthy adult and elderly subjects. Vaccine 2012; 30:3470–7. [DOI] [PubMed] [Google Scholar]
- 23. Knuf M, Leroux-Roels G, Rümke H, et al. Immunogenicity and safety of cell-derived MF59®-adjuvanted A/H1N1 influenza vaccine for children. Hum Vaccin Immunother 2015; 11:358–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Knuf M, Leroux-Roels G, Rümke HC, et al. Immunogenicity and tolerability of an MF59-adjuvanted, egg-derived, A/H1N1 pandemic influenza vaccine in children 6-35 months of age. Pediatr Infect Dis J 2014; 33:e320–9. [DOI] [PubMed] [Google Scholar]
- 25. Knuf M, Leroux-Roels G, Rümke HC, et al. Safety and immunogenicity of an MF59-adjuvanted A/H1N1 pandemic influenza vaccine in children from three to seventeen years of age. Vaccine 2015; 33:174–81. [DOI] [PubMed] [Google Scholar]
- 26. Nassim C, Christensen S, Henry D, et al. Identification of antigen and adjuvant doses resulting in optimal immunogenicity and antibody persistence up to 1 year after immunization with a pandemic A/H1N1 influenza vaccine in children 3 to < 9 years of age. Pediatr Infect Dis J 2012; 31:e59–65. [DOI] [PubMed] [Google Scholar]
- 27. Reisinger KS, Holmes SJ, Pedotti P, et al. A dose-ranging study of MF59(®)-adjuvanted and non-adjuvanted A/H1N1 pandemic influenza vaccine in young to middle-aged and older adult populations to assess safety, immunogenicity, and antibody persistence one year after vaccination. Hum Vaccin Immunother 2014; 10:2395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yasuda Y, Komatsu R, Matsushita K, et al. Comparison of half and full doses of an MF59-adjuvanted cell culture-derived A/H1N1v vaccine in Japanese children. Adv Ther 2010; 27:444–57. [DOI] [PubMed] [Google Scholar]