Abstract
Background: Total parathyroidectomy (tPTX) and total parathyroidectomy with autotransplantation (tPTX + AT) are effective and inexpensive treatments for secondary hyperparathyroidism (sHPT), but we do not know which one is the optimal approach. Therefore, we undertook a meta-analysis to compare the safety and efficacy of these two surgical procedures.
Methodology: Studies published in English on PubMed, Embase and the Cochrane Library from inception to 27 September 2016 were searched systematically. Eligible studies comparing tPTX with tPTX + AT for sHPT were included and Review Manager v5.3 was used.
Results: Eleven studies were included in this meta-analysis. Ten cohort studies and one randomized controlled trial (RCT) involving 1108 patients with sHPT were identified. There was no significant difference in the prevalence of surgical complications (relative risk [RR], 1.71; 95% confidence interval [CI], 0.77–3.79; p = .19), all-cause mortality (RR, 0.68; 95% CI, 0.33–1.39; p = .29), sHPT persistence (RR, 3.81; 95% CI, 0.56–25.95; p = .17) or symptomatic improvement (RR, 1.02; 95% CI, 0.91–1.13; p = .79). tPTX could reduce the risk of sHPT recurrence (RR, 0.19; 95% CI, 0.09–0.41; p < .0001) and reoperation because of recurrence or persistence of sHPT (RR, 0.46; 95% CI 0.24–0.86; p = .01) compared with tPTX + AT. Simultaneously, tPTX increased the risk of hypoparathyroidism (RR, 2.63; 95% CI, 1.06–6.51; p = .04).
Conclusions: We found tPTX and tPTX + AT to be useful methods for sHPT treatment. tPTX was superior for reducing the risk of sHPT recurrence and reoperation than tPTX + AT but, due to a lack of high statistical-power RCTs, comparative studies will be needed in the future.
Keywords: Secondary hyperparathyroidism, parathyroidectomy, chronic kidney disease, meta-analysis
Background
Chronic kidney disease (CKD) has become a worldwide public-health problem [1]. Secondary (renal) hyperparathyroidism (sHPT) is a prevalent complication of CKD [2]. Persistently elevated levels of parathyroid hormone (PTH) and parathyroid hyperplasia are features of sHPT. Hypocalcemia, hyperphosphatemia, and 1,25(OH)2D3 deficiency play important roles in sHTP [3]. The only way to cure sHPT is kidney transplantation but the number of available kidneys for transplantation is limited. Hence, medical and surgical treatments are the only ways to combat sHPT.
In the early stages, patients can be treated by a low-phosphate diet, phosphate binders, vitamin-D analogs, and calcimimetic agents, whereas more advanced stages often require surgical treatment [4]. The calcimimetic agent cinacalcet can achieve identical results to those elicited by surgery. In Germany, the annual cost for cinacalcet (60 mg/day) is ≈€5828.4, whereas the cost of surgery for one person is about €3755.38 [5]. The cost is one disadvantage of cinacalcet. Furthermore, it does not always work. Therefore, surgery is needed in the most advanced stages of sHPT and would be acceptable.
There are three surgical approaches for parathyroidectomy: subtotal parathyroidectomy (sPTX), total parathyroidectomy (tPTX) and total parathyroidectomy with autotransplantation (tPTX + AT). sPTX is resection of 3.5 parathyroid glands, leaving 40–80 mg of the most normal-appearing parathyroid gland in situ [6]. tPTX was first described in 1967, and involves identification and resection of all parathyroid glands. tPTX + AT comprises resection and AT. All parathyroid glands are resected, then the most normal-appearing glands are ‘minced’ into 10–201 mm3 pieces for AT. The sternocleidomastoid muscle and brachioradialis muscle of the non-dominant forearm as potential sites for AT. Guidelines set by the kidney disease outcomes quality initiative (K/DOQI) [7] and most experts recommend sPTX and tPTX + AT for sHPT, but a consensus on the best operative management is lacking.
K/DOQI [7] guidelines recommend that patients with severe hyperparathyroidism (persistent serum levels of intact PTH >800 pg/mL [88.0 pmol/L]), accompanied with hypercalcemia and/or hyperphosphatemia refractory to medical therapy should receive PTX. However, guidelines set by the Japanese society for dialysis therapy (JSDT) recommend that patients with severe hyperparathyroidism (persistent serum levels of intact PTH >500 pg/mL), accompanied with hypercalcemia (serum calcium >10.0 mg/dL) and/or hyperphosphatemia (serum phosphate >6.0 mg/dL) refractory to medical therapy should receive PTX [8].
Secondary hyperparathyroidism patients usually suffer from bone pain, pruritus, fractures, sleep disorders, restless leg syndrome and ‘shrinking man syndrome’. PTX can reduce the prevalence of all-cause mortality and cardiovascular mortality, and can improve the quality of life (QoL) simultaneously. Jia et al. [9] compared the two procedures up to December 2013, and new articles on this issue have been published since then. The aim of the present study was to compare tPTX with tPTX + AT by carrying out a systematic review and meta-analysis.
Methods
Data sources and search strategy
Two experienced reviewers designed and carried out the search strategy. We searched PubMed, Embase and Cochrane databases using the following keywords: ‘parathyroidectomy’, ‘hyperparathyroidism, secondary’, ‘hyperparathyroidism, renal’ and ‘kidney disease’. Searching was carried out from the inception of each database to 27 September 2016. In addition, references and cited articles were searched manually to identify other studies meeting the inclusion criteria.
Inclusion and exclusion criteria
Inclusion criteria were: (i) randomized controlled trials (RCTs), cohorts or case-control studies; (ii) comparison of short-term and long-term (follow-up duration ≥6 months) outcomes between tPTX and tPTX + AT in sHPT; (iii) outcomes included at least one of the following endpoints: all-cause mortality, surgical complications (bleeding and/or voice hoarseness), reoperation prevalence, persistent and/or recurrent hyperparathyroidism, hypoparathyroidism; (iv) results were published in English.
Exclusion criteria were: (i) enrolled patients with primary hyperparathyroidism or tertiary hyperparathyroidism; (ii) enrolled patients underwent repeated surgical procedures; (iii) articles were letters, case reports, reviews, comments, editorials or proceedings. In particular, if one surgical team or institution published more than one article, then repetitive publication was identified. If such duplicated articles could not be distinguished, then the article published most recently or the longest article was selected.
Data extraction and quality assessment
Two reviewers working independently examined and selected all potentially eligible articles. Disagreements were resolved by discussion with a third reviewer. The following information was extracted in standardized forms: name of the first author; year of publication; location of study; study type; population characteristics (age, sex, number); surgical indications and procedures; duration of follow-up; outcomes. The Newcastle–Ottawa scale (NOS) was used to assess the quality of non-randomized studies, whereas the Cochrane assessment tool was used to assess RCTs. The NOS consists of three quality parameters: ‘selection’, ‘comparability’ and ‘outcome’. ‘Quality’ is assessed using a star system: four in the selection domain, two in comparability and three in outcome, making a total of nine stars. A study graded with ≥5 stars is considered ‘high quality’, whereas that with <5 stars is considered ‘low quality’. Two reviewers assessed the quality of each study independently and disagreements were resolved by a third reviewer.
Statistical analyses
Data were analyzed using Review Manager v5.3.0 (Cochrane Collaboration). For dichotomous scales, data are expressed as relative risk (RR) along with a 95% confidence interval (CI). If there were continuous data of outcomes, then data are presented as the mean difference (MD). We used the χ2 test and I2 statistic to assess heterogeneity among studies. p < .1 [10] and I2 > 50% [11] indicated heterogeneity to be significant. Data that were not significantly heterogeneous (p > .1, I2 ≤ 50%) were calculated using a fixed-effects model, whereas heterogeneous data (p < .1, I2 > 50%) were calculated using a random-effects model. Publication bias was assessed using a funnel plot for standard error by effect size (log RR). p < .05 was considered significant.
Results
Literature search and characteristics
A flow chart of the selection process is shown in Figure 1. Finally, eleven studies [12–22] were included for studies comparing tPTX with tPTX + AT published between 1991 and 2016. A total of 275 patients were in the tPTX group and 833 patients were in the tPTX + AT group. The baseline characteristics of these included studies and NOS assessments are listed in Tables 1 and 2, respectively. For RCTs, the Cochrane assessment is listed in Table 3.
Figure 1.
Flow diagram of the identification process for eligible studies.
Table 1.
Detailed characteristics of studies included in the meta-analysis.
| Study | Year | Country | Study design | Study period | Follow up | Sample size | Age (years) | Sex (%, men) | Surgical indication |
|---|---|---|---|---|---|---|---|---|---|
| Higgins et al. | 1991 | UK | RCS | 1997–1990 | 62 m(4–152) m | tPTX: 9 tPTX + AT: 49 Other: 18 |
43.2 (26–67) | 50 | Yes |
| Nicholson et al. | 1996 | UK | RCS | 1982–1993 | 24 m | tPTX: 24 tPTX + AT: 13 Other: 11 |
33–62 | 50 | Yes |
| Coen et al. | 2001 | Italy | RCS | NA | 3.3 ± 2.3 y(1–10) y | tPTX: 10 tPTX + AT:10 Other: 25 |
56 ± 11 | 55.6 | No |
| Ockert et al. | 2002 | Germany | RCS | 1993–1999 | 23 m (1–49) m | tPTX: 11 tPTX + AT: 11 |
27–68 | 45 | Yes |
| Jofré et al. | 2003 | Argentina | RCS | 1990–2002 | 5.2 ± 3.9 y | tPTX: 19 tPTX + AT: 129 |
49 ± 14 | 48.6 | Yes |
| Shih et al. | 2009 | Taiwan China |
RCS | 1995–2006 | 43.1 m | tPTX: 44 tPTX + AT: 14 other:36 
|
50.5 46 |
48.9 | Yes |
| Conzo et al. | 2012 | Italy | PCS | 1999–2006 | 1 y | tPTX:20 tPTX + AT:20 |
52 (26–72) | 57.5 | Yes |
| Schneider et al. | 2012 | Germany | RCS | 1976–2009 | 57.6 ± 2.4 m | tPTX: 32 tPTX + AT: 504 Other: 70 |
48.5 ± 0.57 | 53.1 | Yes |
| He et al. | 2014 | China | RCS | 2008–2012 | 42 m | tPTX: 33 tPTX + AT: 14 |
46 (28–71) | 55.3 | Yes |
| Liang et al. | 2015 | China | PCS | 2010–2014 | 6 m | tPTX: 21 tPTX + AT: 21 Other: 21 |
53.2 ± 12.7 (28–72) | 60.3 | Yes |
| Schlosser et al. | 2016 | Germany | RCT | 2007–2010 | 36 m | tPTX: 52 tPTX + AT: 48 |
49.2 ± 15.6 | 65 | Yes |
RCS: retrospective cohort study; PCS: prospective cohort study; RCT: randomized controlled trial; NA: not applicable; tPTX: total parathyroidectomy; tPTX + AT: total parathyroidectomy with autotransplantation; Other: subtotal parathyroidectomy and/or incomplete parathyroidectomy.
Table 2.
Quality assessment of cohort studies.
| Studies | Year | Selection | Comparability | Outcome | Score |
|---|---|---|---|---|---|
| Higgins et al. | 1991 | ★★★★ | ⋆⋆ | ★⋆★ | 6 |
| Nicholson et al. | 1996 | ★★★★ | ★⋆ | ★★⋆ | 7 |
| Coen et al. | 2001 | ★★★★ | ⋆⋆ | ★★⋆ | 6 |
| Ockert et al. | 2002 | ★★★★ | ★★ | ★⋆★ | 8 |
| Jofré et al. | 2003 | ★★★★ | ⋆⋆ | ★★★ | 7 |
| Shih et al. | 2009 | ★★★★ | ★⋆ | ★★★ | 8 |
| Conzo et al. | 2012 | ★★★★ | ⋆⋆ | ★★★ | 7 |
| Schneider et al. | 2012 | ★★★★ | ⋆⋆ | ★★★ | 7 |
| He et al. | 2014 | ★★★★ | ★⋆ | ★★★ | 8 |
| Liang et al. | 2015 | ★★★★ | ★⋆ | ★★★ | 8 |
Table 3.
Risk of bias of randomized control trial.
| Study | Year | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| Schlosser et al. | 2016 | Low risk | High risk | Unclear | Low risk | Unclear | Unclear |
There were eight retrospective cohort studies, two prospective cohort studies and one RCT. The follow-up period was from 4 months to 13 years. The sample size of these included studies ranged from 20 to 585. Three studies were conducted in Germany, three in China, two in UK, two in Italy, one in Argentina. Ten studies [12–21] described the indications for PTX. Only one study [22] described the etiology of sHPT. The NOS score of 10 studies was >5 stars, which were considered high quality for admission.
Meta-analysis
Surgical complications
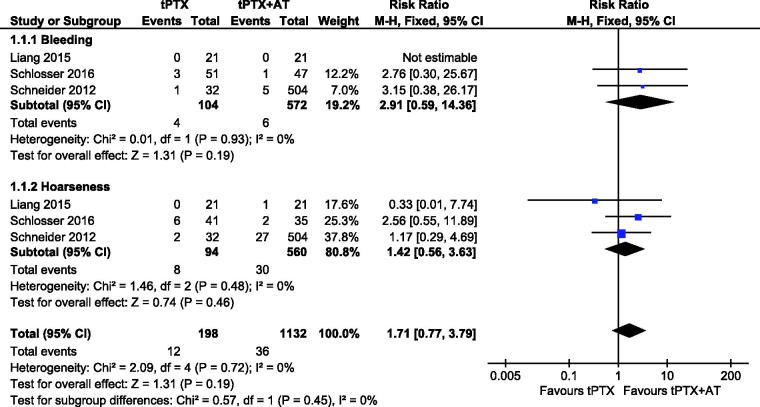
Three studies [13,14,17] reported data for bleeding, 4/104 (3.8%) for the tPTX group and 6/572 (1.0%) for the tPTX + AT group. There was no statistically significant difference between tPTX and tPTX + AT (RR, 2.91; 95% CI, 0.59–14.36; p = .19).The heterogeneity between the studies was not substantial (p = .93, I2 = 0%), so we used a fixed-effects model for analysis. Three studies [13,14,17] described voice hoarseness, 8/94 (8.5%) for the tPTX group and 30/560 (5.4%) for the tPTX + AT. There was no significant difference between the two groups (RR,1.42; 95% CI, 0.56–3.63; p = .46). There was no substantial heterogeneity (p = .48, I2 = 0%), so we used a fixed-effects model for it. These surgical complications between studies had no statistical difference (RR, 1.71; 95% CI, 0.77–3.79; p = .19) (Figure 2).
Figure 2.
Forest plot for surgical complications.
All-cause mortality
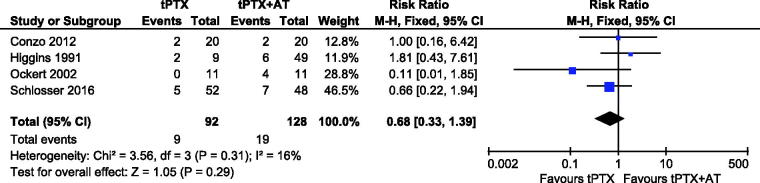
Data regarding to all-cause mortality were reported in four studies [14,15,19,21], nine patients in tPTX group and 19 patients in tPTX + AT group. Prevalence of mortality in the tPTX group and tPTX + AT group was 9.78% (9/92) and 14.84% (19/128), respectively. Although the all-cause mortality in the tPTX + AT group was higher than that of the tPTX group, meta-analysis showed no significant difference (RR, 0.68; 95% CI, 0.33–1.39; p = .29). There was no substantial heterogeneity between the studies (p = .31, I2 = 16%), so a fixed-effects model was used for the meta-analysis (Figure 3).
Figure 3.
Forest plot for all-cause mortality.
Reoperation because of persistence or recurrence of sHPT
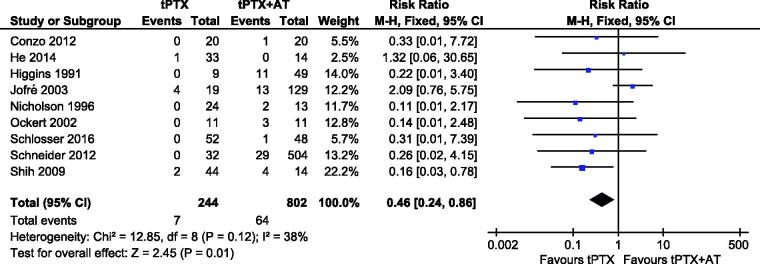
There was no substantial heterogeneity among the studies (p = .12, I2 = 38%), so finally we accepted a fixed-effects model for the meta-analysis. Nine studies [12–16,18–21] reported data about reoperation, 244 patients in the tPTX group and 802 patients in the tPTX + AT group. The tPTX group had 7 events (2.87%), whereas another group had 64 events (8.0%). The tPTX + AT group had a higher prevalence of reoperation and simultaneously there was a significant difference (RR, 0.46; 95% CI, 0.24–0.86; p = .01) (Figure 4).
Figure 4.
Forest plot for reoperation because of persistence or recurrence of sHPT.
Persistence of sHPT
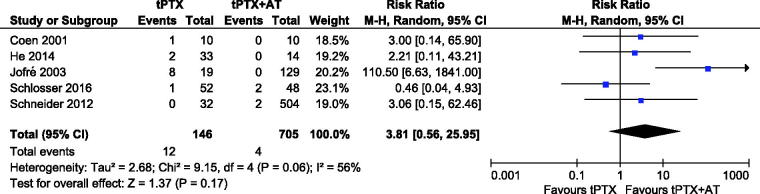
Data about persistence of the disease were reported in five articles [13,14,18,20,22]. Twelve events were noted in the tPTX group, whereas four events were observed in the tPTX + AT group. The meta-analysis showed no significant difference (RR, 3.81; 95% CI, 0.56–25.95; p = .17). The heterogeneity between the two groups was substantial (p = .06, I2 = 56%), so we accepted a random-effects model for the analysis (Figure 5).
Figure 5.
Forest plot for persistence of sHPT.
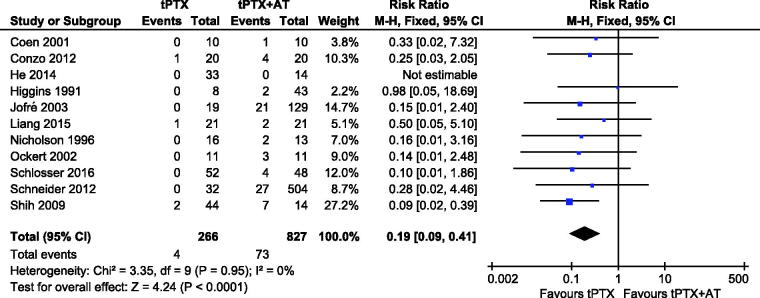
sHPT recurrence
A meta-analysis on 11 studies [12–22] showed that patients in the tPTX group had a significantly lower risk of disease recurrence compared with the tPTX + AT group (RR, 0.19; 95% CI, 0.09–0.41; p < .0001) (Figure 6). There was no substantial heterogeneity among studies (p = .95, I2 = 0), so a fixed-effects model was used for the meta-analysis.
Figure 6.
Forest plot for sHPT recurrence.
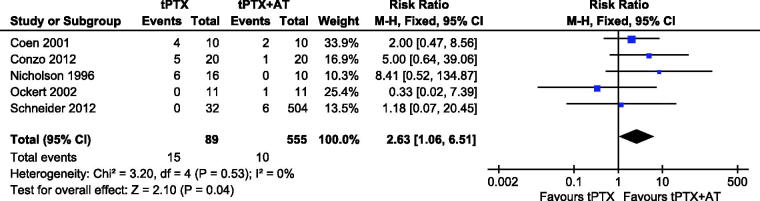
Hypoparathyroidism
Five studies [13,15,16,21,22] reported on hypoparathyroidism. There was no heterogeneity (p = .53, I2 = 0), so a fixed-effects model was used for this meta-analysis. Results showed no significant difference between studies (RR, 2.63; 95% CI, 1.06–6.51; p = .04) (Figure 7).
Figure 7.
Forest plot for hypoparathyroidism.
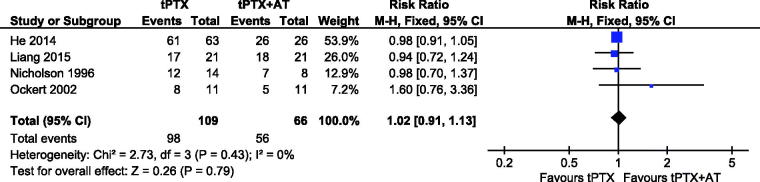
Symptomatic improvement
Four studies [15–17,20] reported on symptomatic improvement. A fixed-effects model was used for the analysis because heterogeneity was absent (p = .43, I2 = 0). Results showed no significant difference between tPTX and tPTX + AT (RR, 1.02; 95% CI, 0.91–1.13; p = .79) (Figure 8).
Figure 8.
Forest plot for symptomatic improvement.
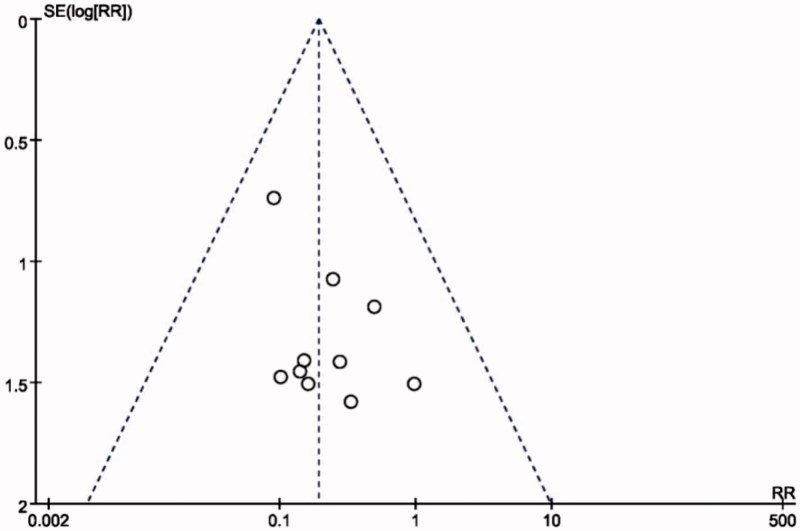
Publication bias
A funnel plot was used to assess publication bias. There was no evidence of publication bias because the funnel plot of standard error by effect estimate of recurrence showed all studies lay within the limits (Figure 9).
Figure 9.
Funnel plot for sHPT recurrence.
Sensitivity analyses
A sensitivity analysis for disease persistence were used to determine the dependability of our results by accepting the leave-one-out approach. The heterogeneity of all five studies was significant (p = .06, I2 = 56%), and the heterogeneity disappeared (p = .70, I2 = 0) when the study by Jofré et al. [18] was excluded. The result continued to show no significant difference. When we removed each studies back, the pooled result indicating that no study dominated the analysis.
Discussion
The relationship between sHPT and CKD has been well established [23]. sHPT is a common sequel of CKD that causes electrolyte disturbance and results in pathological fractures, cardiovascular disease and even death [24,25]. Despite recent advances in drug therapy, many patients still require surgery. The prevalence of surgery to treat sHPT with maintenance dialysis over 10–20 years is ≈15–38% [13]. PTX can improve the survival from sHPT as well as symptomatology and QoL [26,27].
Our systematic review was carried out by a comprehensive search for studies to appraise the safety and efficacy of two surgical approaches (tPTX and tPTX + AT) in sHPT. Finally, 11 studies involving 1108 patients with sHPT met the inclusion criteria and were enrolled in our meta-analysis. Our findings showed that both approaches were equally effective with regard to avoidance of surgical complications (bleeding and voice hoarseness), all-cause mortality, persistence of disease and symptomatic improvement. However, there were significantly fewer episodes of recurrence and reoperation because of recurrence/persistence of disease in the tPTX group compared with the tPTX + AT group. The tPTX group had an increased risk of hypoparathyroidism according to our meta-analysis.
The results of this meta-analysis showed no difference in the prevalence of surgical complications (bleeding and voice hoarseness) between the two groups. Both surgical procedures included removal of all parathyroid glands. After resection, tPTX + AT required another procedure: AT. The sites used for AT were the sternocleidomastoid muscle, brachioradialis muscle and tibialis anterior muscle [20,28,29]. Most surgeons chose the brachioradialis muscle for AT because this site is simple for reoperation. The AT site chosen would not increase the prevalence of bleeding and voice hoarseness.
The aim of every treatment is to improve survival or QoL. These two approaches had an identical effect on symptomatic improvement. Almost all patients suffered from bone pain and pruritus before surgery. Some studies indicated that these symptoms were relieved or disappeared the day after surgery regardless of the surgical approach. Also, the level of PTH in serum decreased dramatically after surgery. When the PTH level was >495 pg/mL, the mortality risk was increased by 25% [30]. PTX can reduce the prevalence of mortality in renal hyperparathyroidism [31]. However, the evidence from our study does not explain which one was better.
Persistent hyperparathyroidism is a common complication of surgery. There was no difference in persistence of hyperparathyroidism in our meta-analysis. The reasons for persistence can be ectopic and supernumerary parathyroid glands. Identification of ectopic parathyroid gland is challenging because parathyroid glands are not always in the same anatomic position. Gomes et al. [32] described the most common sites to be thyroid parenchyma (33.3%), thyroid-thymus conduit (18.5%) and thymus (14.8%).The prevalence of supernumerary parathyroid glands by random autopsies was ≈13% [33]. Akerstrom et al. [34] reported that 3% of patients had three parathyroid glands, 84% had four parathyroid glands and 13% had supernumerary glands. Many supernumerary glands were located in thymus tissue. The prevalence of persistent hyperparathyroidism could not be improved by surgical methods alone.
Recurrent hyperparathyroidism was a serious complication after PTX. Results showed that tPTX could reduce the risk of recurrence better than tPTX + AT. Recurrent hyperparathyroidism could not be avoided in patients undergoing maintenance dialysis with time. In 80% of patients after TPTX + AT, disease recurrence was in the graft, and the other 20% was in the neck [35]. Zhong et al. [36] reported that tPTX + AT is associated with sHPT recurrence. Melck et al. [37] suggested that tPTX + AT should be abandoned as sHPT treatment because of frequent recurrence. Tominaga et al. [38] stated that graft-dependent recurrence could be controlled by removing the autograft.
The pooled result of included studies suggested that tPTX could increase the risk of hypoparathyroidism. However, adynamic bone disease or hypocalcemia were absent. This procedure was conducted by removal of all parathyroid glands. However, PTH levels could be measured in many patients after tPTX because PTH may be secreted from the thymus gland [39]. Conzo et al. [40] suggested that hypoparathyroidism could be treated by pharmacological means.
Strengths and limitations of our meta-analysis
The strengths of our meta-analysis were explicit inclusion criteria, broad search strategy and independent assessment of eligibility by two reviewers. This meta-analysis had three main limitations. First, most of the included studies had small sample-sized cohort studies, so the results of our study may not be adequate to judge the safety and efficacy of tPTX and tPTX + AT. Second, some studies reported recurrent and persistent hyperparathyroidism, but the definitions of recurrence and persistence were not mentioned in most of them. Third, some studies described long-term serum levels of calcium, phosphate, alkaline phosphatase and PTH, but the authors presented them in different ways (histogram, line graphs), so we could not draw conclusions on changes in biochemistry.
Conclusions
Our meta-analysis suggests that tPTX and tPTX + AT are effective surgical approaches to the treatment of sHTP. They also show the apparent benefits of tPTX on sHPT recurrence and reoperation. Hence, tPTX could be the best way to treat uncontrolled sHPT. Nevertheless, tPTX increases the risk of hypoparathyroidism compared with tPTX + AT. Surgeons counseling patients should consider the benefits and disadvantages of tPTX versus tPTX + AT. To guide future work in this area, large multicenter RCTs comparing the two approaches are necessary.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (grant numbers 81270449 and 81572314), the Natural Science Foundation of Shandong Province, China (ZR2012HM046), Qingdao Minsheng Science and Technology Foundation, Shandong, China (14-2-3-5-nsh) and the Qingdao Science and Technology Plan Project (13-1-4-220-jch).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Eknoyan G, Lameire N, Barsoum R, et al. The burden of kidney disease: improving global outcomes. Kidney Int. 2004;66:1310–1314. [DOI] [PubMed] [Google Scholar]
- 2.Riss P, Asari R, Scheuba C, et al. Current trends in surgery for renal hyperparathyroidism (RHPT) – an international survey. Langenbecks Arch Surg. 2013;398:121–130. [DOI] [PubMed] [Google Scholar]
- 3.Komaba H, Kakuta T, Fukagawa M.. Diseases of the parathyroid gland in chronic kidney disease. Clin Exp Nephrol. 2011;15:797–809. [DOI] [PubMed] [Google Scholar]
- 4.Kim WY, Lee JB, Kim HY.. Efficacy of intraoperative parathyroid hormone monitoring to predict success of parathyroidectomy for secondary hyperparathyroidism. J Korean Surg Soc. 2012;83:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneider R, Kolios G, Koch BM, et al. An economic comparison of surgical and medical therapy in patients with secondary hyperparathyroidism – the German perspective. Surgery. 2010;148:1091–1099. [DOI] [PubMed] [Google Scholar]
- 6.Lorenz K, Bartsch DK, Sancho JJ, et al. Surgical management of secondary hyperparathyroidism in chronic kidney disease – a consensus report of the European Society of Endocrine Surgeons. Langenbecks Arch Surg. 2015;400:907–927. [DOI] [PubMed] [Google Scholar]
- 7.National Kidney Foundation K/DOQIK/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(4 Suppl 3):S1–S201. [PubMed] [Google Scholar]
- 8.Fukagawa M, Yokoyama K, Koiwa F, et al. Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial. 2013;17:247–288. [DOI] [PubMed] [Google Scholar]
- 9.Jia X, Wang R, Zhang C, et al. Long-term outcomes of total parathyroidectomy with or without autoimplantation for hyperparathyroidism in chronic kidney disease: a meta-analysis. Ther Apher Dial. 2015;19:477–485. [DOI] [PubMed] [Google Scholar]
- 10.Lau J, Ioannidis JP, Schmid CH.. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JP, Thompson SG.. Quantifying heterogeneity in a meta-analysis. Statist Med. 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 12.Shih ML, Duh QY, Hsieh CB, et al. Total parathyroidectomy without autotransplantation for secondary hyperparathyroidism. World J Surg. 2009;33:248–254. [DOI] [PubMed] [Google Scholar]
- 13.Schneider R, Slater EP, Karakas E, et al. Initial parathyroid surgery in 606 patients with renal hyperparathyroidism. World J Surg. 2012;36:318–326. [DOI] [PubMed] [Google Scholar]
- 14.Schlosser K, Bartsch DK, Diener MK, et al. Total parathyroidectomy with routine thymectomy and autotransplantation versus total parathyroidectomy alone for secondary hyperparathyroidism: results of a nonconfirmatory multicenter prospective randomized controlled pilot trial. Ann Surg. 2016;264:745–753. [DOI] [PubMed] [Google Scholar]
- 15.Ockert S, Willeke F, Richter A, et al. Total parathyroidectomy without autotransplantation as a standard procedure in the treatment of secondary hyperparathyroidism. Langenbecks Arch Surg. 2002;387:204–209. [DOI] [PubMed] [Google Scholar]
- 16.Nicholson ML, Veitch PS, Feehally J.. Parathyroidectomy in chronic renal failure: comparison of three operative strategies. J R Coll Surg Edinb. 1996;41:382–387. [PubMed] [Google Scholar]
- 17.Liang Y, Sun Y, Ren L, et al. Short-term efficacy of surgical treatment of secondary hyperparathyroidism. Eur Rev Med Pharmacol Sci. 2015;19:3904–3909. [PubMed] [Google Scholar]
- 18.Jofré R, Lòpez Gòmez JM, Menárguez J, et al. Parathyroidectomy: whom and when? Kidney Int Suppl. 2003;63:S97–S100. [DOI] [PubMed] [Google Scholar]
- 19.Higgins RM, Richardson AJ, Ratcliffe PJ, et al. Total parathyroidectomy alone or with autograft for renal hyperparathyroidism? Q J Med. 1991;79:323–332. [PubMed] [Google Scholar]
- 20.He Q, Zhuang D, Zheng L, et al. Total parathyroidectomy with trace amounts of parathyroid tissue autotransplantation as the treatment of choice for secondary hyperparathyroidism: a single-center experience. BMC Surg. 2014;14:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conzo G, Perna AF, Sinisi AA, et al. Total parathyroidectomy without autotransplantation in the surgical treatment of secondary hyperparathyroidism of chronic kidney disease. J Endocrinol Invest 2012;35:8–13. [DOI] [PubMed] [Google Scholar]
- 22.Coen G, Calabria S, Bellinghieri G, et al. Parathyroidectomy in chronic renal failure: short- and long-term results on parathyroid function, blood pressure and anemia. Nephron. 2001;88:149–155. [DOI] [PubMed] [Google Scholar]
- 23.Pitt SC, Sippel RS, Chen H.. Secondary and tertiary hyperparathyroidism, state of the art surgical management. Surg Clin North Am. 2009;89:1227–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the dialysis outcomes and practice patterns study (DOPPS). Am J Kidney Dis. 2008;52:519–530. [DOI] [PubMed] [Google Scholar]
- 25.Slinin Y, Foley RN, Collins AJ.. Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: the USRDS waves 1, 3, and 4 study. J Am Soc Nephrol. 2005;16:1788–1793. [DOI] [PubMed] [Google Scholar]
- 26.Iwamoto N, Sato N, Nishida M, et al. Total parathyroidectomy improves survival of hemodialysis patients with secondary hyperparathyroidism. J Nephrol. 2012;25:755–763. [DOI] [PubMed] [Google Scholar]
- 27.Cheng SP, Lee JJ, Liu TP, et al. Parathyroidectomy improves symptomatology and quality of life in patients with secondary hyperparathyroidism. Surgery. 2014;155:320–328. [DOI] [PubMed] [Google Scholar]
- 28.Sicard GA, Anderson CB, Hruska KA, et al. Parathormone levels after subtotal and total (autotransplantation) parathyroidectomy for secondary hyperparathyroidism. J Surg Res. 1980;29:541–545. [DOI] [PubMed] [Google Scholar]
- 29.Anamaterou C, Lang M, Schimmack S, et al. Autotransplantation of parathyroid grafts into the tibialis anterior muscle after parathyroidectomy: a novel autotransplantation site. BMC Surg. 2015;15:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richards ML, Wormuth J, Bingener J, et al. Parathyroidectomy in secondary hyperparathyroidism: is there an optimal operative management? Surgery. 2006;139:174–180. [DOI] [PubMed] [Google Scholar]
- 31.Costa-Hong V, Jorgetti V, Gowdak LH, et al. Parathyroidectomy reduces cardiovascular events and mortality in renal hyperparathyroidism. Surgery. 2007;142:699–703. [DOI] [PubMed] [Google Scholar]
- 32.Gomes EM, Nunes RC, Lacativa PG, et al. Ectopic and extranumerary parathyroid glands location in patients with hyperparathyroidism secondary to end stage renal disease. Acta Cir Bras. 2007;22:105–109. [DOI] [PubMed] [Google Scholar]
- 33.Pattou FN, Pellissier LC, Noel C, et al. Supernumerary parathyroid glands: frequency and surgical significance in treatment of renal hyperparathyroidism. World J Surg. 2000;24:1330–1334. [DOI] [PubMed] [Google Scholar]
- 34.Akerström G, Malmaeus J, Bergström R.. Surgical anatomy of human parathyroid glands. Surgery. 1984;95:14–21. [PubMed] [Google Scholar]
- 35.Tominaga Y, Uchida K, Haba T, et al. More than 1,000 cases of total parathyroidectomy with forearm autograft for renal hyperparathyroidism. Am J Kidney Dis. 2001;38:S168–S171. [DOI] [PubMed] [Google Scholar]
- 36.Zhong A, Billa V, Rotstein LE, et al. Recurrence of hyperparathyroidism after total parathyroidectomy and autotransplantation in peritoneal dialysis patients. Perit Dial Int. 2000;20:200–208. [PubMed] [Google Scholar]
- 37.Melck AL, Carty SE, Seethala RR, et al. Recurrent hyperparathyroidism and forearm parathyromatosis after total parathyroidectomy. Surgery. 2010;148:867–873. [DOI] [PubMed] [Google Scholar]
- 38.Tominaga Y, Matsuoka S, Uno N, et al. Removal of autografted parathyroid tissue for recurrent renal hyperparathyroidism in hemodialysis patients. World J Surg. 2010;34:1312–1317. [DOI] [PubMed] [Google Scholar]
- 39.Günther T, Chen ZF, Kim J, et al. Genetic ablation of parathyroid glands reveals another source of parathyroid hormone. Nature. 2000;406:199–203. [DOI] [PubMed] [Google Scholar]
- 40.Conzo G, Perna A, Candela G, et al. Long-term outcomes following “presumed” total parathyroidectomy for secondary hyperparathyroidism of chronic kidney disease. G Chir. 2012;33:379–382. [PubMed] [Google Scholar]