Abstract
Background: Acute kidney injury (AKI) is associated with the increased short-term mortality of critically ill patients on continuous renal replacement therapy (CRRT). The aim of this research was to evaluate the association of kidney function at discharge with the long-term renal and overall survival of critically ill patients with AKI who were on CRRT in an intensive care unit (ICU).
Methods: We retrospectively collected data for critically ill patients with AKI who were admitted to ICU on CRRT at a tertiary metropolitan hospital in China between 2008 and 2013. The patients were followed up to their death or to 30 September 2016 by telephone.
Results: A total of 403 patients were enrolled in this study. The 1-, 3- and 5-year patient survival rates were 64.3 ± 2.4, 55.8 ± 2.5 and 46.3 ± 2.7%, respectively. In multivariate analysis, age, sepsis, decreased renal perfusion (including volume contraction, congestive heart failure, hypotension and cardiac arrest), preexisting kidney disease, Apache II score, Saps II score, vasopressors and eGFR <45 mL/min/1.73 m2 at discharge were independent factors for worse long-term patient survival. And age, preexisting kidney disease, Apache II score, mechanical ventilation (MV) and eGFR <45 mL/min/1.73 m2 at discharge were also associated with worse renal survival.
Conclusions: This study showed that impaired kidney function at discharge was shown to be an important risk factor affecting the long-term renal survival rates of critically ill patients with AKI. An eGFR <45 mL/min/1.73 m2 was an independent risk factor for decreased overall survival and renal survival.
Keywords: Acute kidney injury, continuous renal replacement therapy, critically ill, long-term survival
Introduction
Acute kidney injury (AKI) is characterized by the rapid loss of renal function, resulting in a number of complications, including fluid imbalance, metabolic acidosis and uremia [1], and it occurs quite commonly in hospitalized patients. AKI is also a common clinical problem encountered with critically ill patients and is generally related to an increase in morbidity and mortality [2]. According to epidemiological investigations, AKI affects an estimated 13–18% of hospitalized patients [3]. However, the incidence of AKI in critically ill patients varies from 30 to 60%, and it is associated with increasing in-hospital mortality in critically ill patients requiring renal replacement therapy (RRT) [4–6]. Numerous clinical studies indicate the relationships between AKI and long-term mortality, the development of chronic kidney disease (CKD), as well as the eventual progression to end-stage renal disease (ESRD) [7–12]. However, there are also some patients with a complete recovery from AKI may be confronted with adverse long-term outcomes [13–16], and those with little recovery or partial recovery can be faced with an aggravation of predisposition [17–20].
Being a worldwide public health problem, it is indicated that AKI can lead to CKD in 10% of critically ill patients [21]. In addition, it is declared that need for RRT increased the likelihood of the progression to CKD by 500-fold [22]. Wu et al. revealed the long-term survival of 4393 AKI patients after surgery (follow-up of 4.76-years) and found hazard ratios (HRs) of 1.94, 2.64 and 3.28 for patient mortality associated with AKI, CKD and AKI on CKD, respectively [17].
Existing analyses primarily focused on assessing in-hospital mortality in patients with AKI demanding continuous renal replacement therapy (CRRT) [23]. Meanwhile, renal function at discharge may be associated with increased mortality at follow-up, which has not been concerned much [24]. The aim of this study was to research on the relation between renal function at discharge and long-term renal survival and overall long-term mortality of critically ill patients diagnosed with AKI and treated with CRRT in the intensive care unit (ICU).
Patients and methods
Patients
This study was conducted retrospectively in ICU at the First Affiliated Hospital, Medical College, Zhejiang University from 1 January 2008 to 31 December 2013. The patients were survivors at discharge among those in the ICU, who diagnosed with AKI and accepted CRRT. The patients were excluded for the following reasons: on RRT or kidney transplantation before ICU admission; died in hospital; incomplete data; lack of follow-up.
AKI criteria
In this study, the AKI diagnostic criteria referred to KDIGO AKI Guideline Work Group: an absolute increase in serum creatinine of ≥0.3 mg/dl (≥26.4 µmol/L), a percentage increase in serum creatinine of ≥50% (1.5-fold from baseline), or a reduction in urine output (documented oliguria of <0.5 mL/kg/h for >6 h) [25].
Data collection
Demographic data and clinical data
Age, gender, weight, urine output before CRRT, medical history, causes of AKI, presence of comorbidities, the use of vasopressors, requirements for mechanical ventilation (MV), acute physiology and chronic health evaluation (APACHE) II score [26], simplified acute physiology score (SAPS II) [27], length of hospital stay, length of ICU stay, length of CRRT stay and CRRT therapeutic dose of all patients.
Laboratory data
Routine blood parameters, liver function, kidney function, blood gases and blood electrolytes before CRRT, the liver function and kidney function at discharge.
Follow-up information
The patients were followed up to their death or to 30 September 2016 by telephone. The renal survival was considered as the patients without RRT or transplantation.
Groups
According to the modification of diet in renal disease (MDRD) formula: estimated glomerular filtration rate (eGFR) (mL/min1.73 m2) = 186 * (Scr) − 1.154 * (age) − 0.203* (0.742 female), the kidney function at discharge was arbitrarily defined with per eGFR category for the estimation of GFR. As the eGFR levels at discharge, patients were categorized into five groups: the first group was defined as eGFR ≥60 mL/min per 1.73 m2, the second category was defined as eGFR of 45–59 mL/min per 1.73 m2, the third category was defined as eGFR of 30–44 mL/min per 1.73 m2, the fourth category was defined as eGFR of 15–29 mL/min per 1.73 m2 and the last category was defined as eGFR <15 mL/min per 1.73 m2 (including RRT) at discharge.
Statistical analyses
SPSS version 22.0 (SPSS Inc., Chicago, IL) was used for all statistical analyses. Continuous normal data were expressed as the mean ± standard deviation (SD). Non-normal distribution of measurement data were expressed as the median and range. Categorical data were expressed as the number of cases and percentages. Patient survival curves and renal survival curves were generated according to each eGFR category by the Kaplan–Meier method and a log-rank test was employed to analyze statistical differences among groups. Cox proportional hazard survival model was adopted to evaluate independent predictors for long-term mortality. When constructing the Cox multivariate model, univariate with p values <.2 were used [28]. Statistical significance was defined as a p values <.05. Data were presented as HRs with 95% confidence intervals (CI).
Results
Baseline patient characteristics
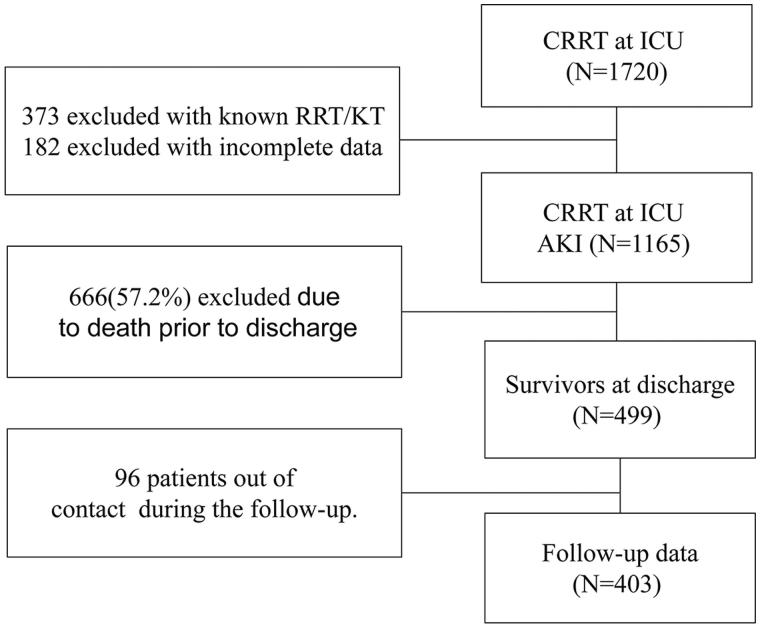
There were 1720 patients who received CRRT in the ICU between 2008 and 2013 (Figure 1). Among those, 373 patients were excluded because they were on RRT or had received a kidney transplant before ICU admission and 182 patients were excluded because of incomplete data. Of the 1165 patients who were diagnosed with AKI and were treated with CRRT in the ICU, 666 patients (57.2%) died in the hospital and the remaining 499 patients were alive at discharge. Among the 499 patients, 96 patients were lack of follow-up. The remaining 403 patients in total were enrolled in this study (Figure 1).
Figure 1.
Patients selection scheme.
The main characteristics of the 403 patients analyzed are summarized in Table 1. Among those, 66.5% (268/403) was male and the age was 60.8 ± 17.8 (17–95)years old. Median length of CRRT was 6 d. The median Apache II score was 23, and the median length of survival was 1133 (1–2975) d (Table 1).
Table 1.
Baseline characteristics at discharge of the 403 patients.
| Variable | Mean ± SD/median(range) |
|---|---|
| Age (years) | 60.8 ± 17.8 |
| Gender (male, %) | 268 (66.5%) |
| Length of hospital stay (days) | 26 (2–181) |
| Length of ICU stay (days) | 10 (1–161) |
| Length of CRRT (days) | 6 (1–149) |
| Serum albumin (g/L) | 31.1 ± 6.3 |
| Total bilirubin (µmol/L) | 23.0 (3.0–638.0) |
| Serum creatinine (µmol/L) | 270.0 (40.0–2069.0) |
| Urea (mmol/L) | 20.6 (3.4–70.7) |
| Hemoglobin (g/L) | 97.3 ± 27.9 |
| Bicarbonate (mmol/L) | 19.8 ± 6.7 |
| Lactate level (mmol/L) | 1.7 (0.3–15) |
| 24 h urine output (mL) | 100 (0–1000) |
| Apache II score | 23 (12–63) |
| Saps II score | 49.3 ± 15.5 |
| Serum albumin at discharge (g/L) | 33.7 (20.4–55.0) |
| Serum creatinine at discharge (µmol/L) | 134.0 (38.0–1256.0) |
| Urea at discharge (mmol/L) | 10.6 (2.1–55.8) |
| Vasopressors (n, %) | 175 (43.4%) |
| Mechanical ventilation (n, %) | 207 (51.4%) |
| Dose of CRRT (mL/kg.h) | 50.0 ± 13.7 |
| Length of survival (days) | 1133 (1–2975) |
Causes of AKI
The causes of AKI for these 403 cases are shown in Table 2. Sepsis (38.5%) was the primary cause of AKI, followed by decreased renal perfusion (28.8%) and surgical cause (15.9%) (Table 2).
Table 2.
The causes of AKI (n = 403).
| Cause of AKI | N (%) |
|---|---|
| Decreased renal perfusion (including volume contraction, congestive heart failure, hypotension and cardiac arrest) | 116 (28.8%) |
| Sepsis | 155 (38.5%) |
| Surgical | 64 (15.9%) |
| Others | 68 (16.9%) |
The most common comorbidities of 403 patients are summarized in Table 3. Respiratory diseases, hypertension and cardiovascular diseases occupy the greatest proportion. A percentage of 26.6 (107/403) was diagnosed with preexisting CKD (Table 3).
Table 3.
The comorbidities of patients (n = 403).
| Co-morbidities | N (%) |
|---|---|
| Respiratory disease | 134 (33.3%) |
| Hypertension | 124 (30.8%) |
| Cardiovascular disease | 113 (28.0%) |
| Preexisting CKD | 107 (26.6%) |
| Diabetes mellitus | 69 (17.1%) |
| Neoplasm | 62 (15.4%) |
| Liver disease | 55 (13.6%) |
| Neurological disease | 26 (6.5%) |
| Peripheral vascular disease | 21 (5.2%) |
Groups
The patients were categorized into five groups according to the eGFR level. There were 135(33.5%), 32(7.9%), 51(12.7%), 56(13.9%) and 129(32.0%) patients in the group defined as eGFR ≥60 mL/min, 45–59 mL/min, 30–44 mL/min, 15–29 mL/min and <15 mL/min, respectively (Table 4).
Table 4.
Groups according to eGFR levels at discharge (n = 403).
| Group | N (%) |
|---|---|
| eGFR ≥60 mL/min | 135 (33.5%) |
| eGFR45–59 mL/min | 32 (7.9%) |
| eGFR30–44 mL/min | 51 (12.7%) |
| eGFR15–29 mL/min | 56 (13.9%) |
| eGFR <15 mL/min | 129 (32.0%) |
Long-term patient survival
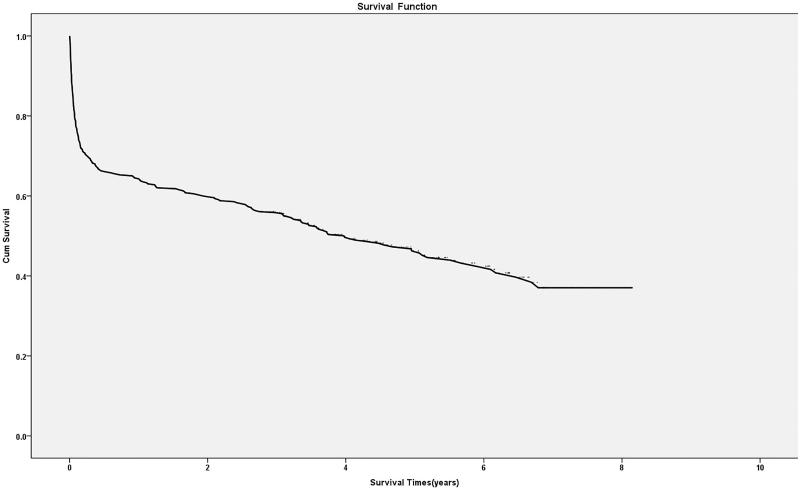
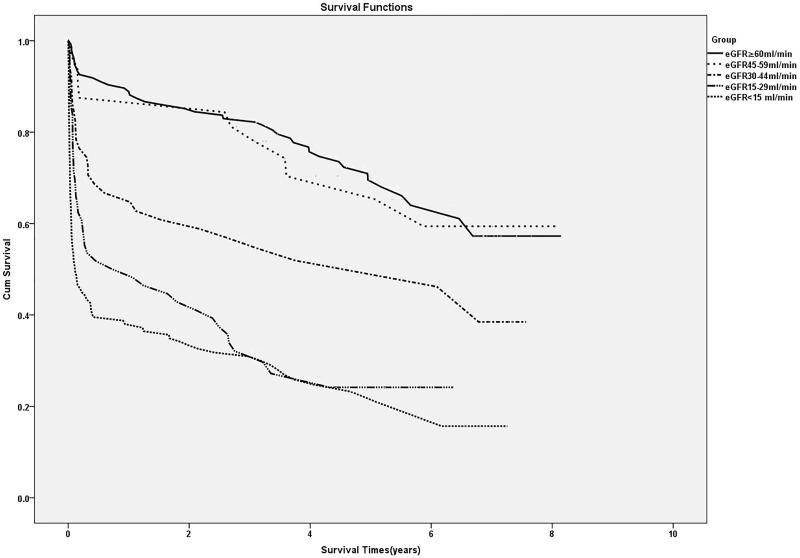
By 30 September 2016, 53.8% (217/403) patients had died, and the rest of them were still alive. The cumulative survival rates were 64.3 ± 2.4% in the first year, 55.8 ± 2.5% in the third years and 46.3 ± 2.7% in the fifth years (Figure 2). Kaplan–Meier curves for survival indicated that long-term survival was tightly relevant to the degree of kidney dysfunction at discharge (Figure 3). The long-term survival of patients with eGFR30–44 mL/min/1.73 m2, with eGFR 15–29 mL/min/1.73 m2 and those with eGFR <15 mL/min/1.73 m2 at discharge were much lower compared with that of the patients with eGFR ≥60 mL/min/1.73m2, according to a log-rank test (p = .001, p < .001 and p < .001, respectively).
Figure 2.
Kaplan–Meier curves for overall survival after hospital discharge.
Figure 3.
Kaplan–Meier curves for survival after hospital discharge according to renal function.
Multivariate proportional hazard regression analysis identified that age, the causes of AKI (sepsis and decreased renal perfusion), a preexisting kidney disease, Apache II score, Saps II score, vasopressors and the eGFR <45 mL/min/1.73 m2 groups at discharge were all associated with decreased long-term patient survival (eGFR 30–44 mL/min/1.73m2, HR 2.26 [95% CI, 1.36–3.74]; eGFR 15–29 mL/min/1.73 m2, HR 4.89 [95% CI, 3.03–7.89]; eGFR <15 mL/min/1.73 m2 and HR 5.67 [95% CI, 3.70–8.68]) (Table 5).
Table 5.
Multivariate analysis of the variables associated with overall survival (n = 403).
| Variable | HR (95% CI) | p |
|---|---|---|
| Age | 1.03 (1.02–1.04) | <.001* |
| Gender | 0.80 (0.60–1.07) | .135 |
| The cause of AKI | ||
| Sepsis | 1.70 (1.05–2.73) | .030* |
| Decreased renal perfusion | 1.67 (1.01–2.73) | .044* |
| Surgical | 1.27 (0.73–2.21) | .395 |
| Others | – | – |
| Co-morbidities | ||
| Neoplasm | 1.04 (0.71–1.53) | .836 |
| Diabetes | 1.44 (0.98–2.14) | .066 |
| Hypertension | 1.38 (0.99–1.92) | .058 |
| Pre-CKD | 1.49 (1.10–2.03) | .010* |
| Apache II score | 1.03 (1.01–1.06) | .005* |
| Saps II score | 1.03 (1.01–1.04) | <.001* |
| Vasopressors | 1.75 (1.30–2.35) | <.001* |
| Mechanical ventilation | 1.04 (0.99–1.08) | .079 |
| Renal function at hospital discharge | ||
| eGFR ≥60 mL/min | – | – |
| eGFR45–59 mL/min | 1.06 (0.52–2.14) | .874 |
| eGFR30–44 mL/min | 2.26 (1.36–3.74) | .002* |
| eGFR15–29 mL/min | 4.89 (3.03–7.89) | <.001* |
| eGFR <15 mL/min | 5.67 (3.70–8.68) | <.001* |
*Significant at p < .05 level.
Renal survival
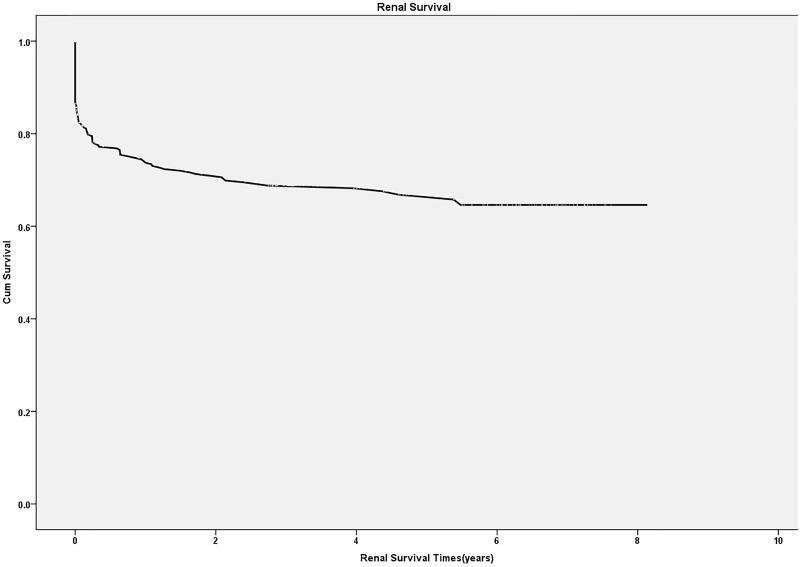
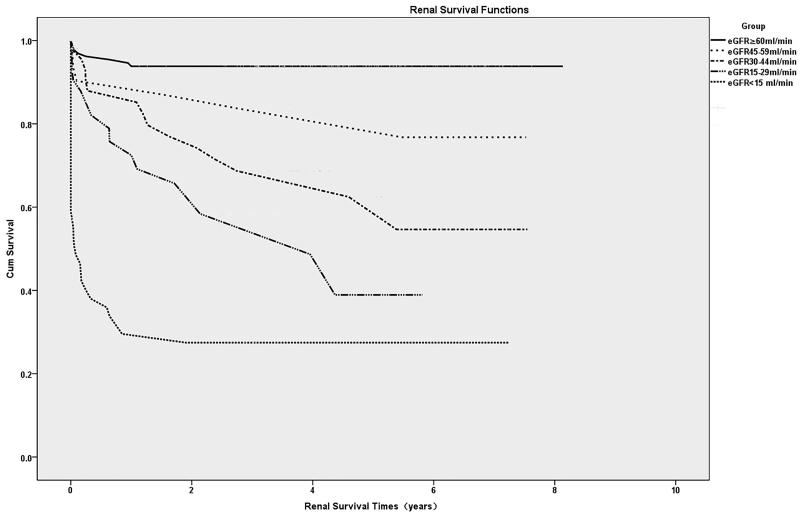
Renal survival rates after hospital discharge in the first, third and fifth year were 74.4 ± 2.3%, 68.8 ± 2.6% and 66.8 ± 2.7%, respectively (Figure 4). A Kaplan–Meier analysis for renal survival after hospital discharge revealed that the renal survival rates of patients with eGFR30–44 mL/min/1.73 m2, eGFR 15–29 mL/min/1.73 m2 and those with eGFR <15 mL/min/1.73 m2 at discharge were lower than that of patients with eGFR ≥60 mL/min/1.73 m2 (p < .001, p < .001 and p < .001, respectively, Figure 5).
Figure 4.
Kaplan–Meier curves for renal survival after hospital discharge.
Figure 5.
Kaplan–Meier curves for renal survival after hospital discharge according to renal function.
Multivariate proportional hazard regression analysis identified that age, a preexisting kidney disease, Apache II score, MV, eGFR30–44 mL/min/1.73 m2 (HR = 5.46, p < .001) and eGFR15–29 mL/min/1.73 m2 (HR = 6.76, p < .001) and eGFR <15 mL/min/1.73 m2 (HR = 23.88, p < .001) at discharge were all associated with decreased renal survival (Table 6).
Table 6.
Multivariate analysis of the variables associated with renal survival (n = 403).
| Variable | HR (95% CI) | p |
|---|---|---|
| Age | 1.02 (1.00–1.03) | .041* |
| Gender | 0.72 (0.47–1.09) | .115 |
| The cause of AKI | ||
| Sepsis | 1.63 (0.87–3.06) | .129 |
| Decreased renal perfusion | 1.44 (0.75–2.76) | .275 |
| Surgical | 1.40 (0.70–2.78) | .338 |
| Others | – | – |
| Co-morbidities | ||
| Neoplasm | 0.97 (0.56–1.68) | .916 |
| Diabetes | 1.56 (0.92–2.64) | .100 |
| Hypertension | 0.80 (0.51–1.25) | .326 |
| Pre-CKD | 2.07 (1.38–3.11) | <.001* |
| Apache II score | 1.05 (1.02–1.08) | .002* |
| Saps II score | 0.99 (0.98–1.02) | .740 |
| Vasopressors | 0.78 (0.51–1.19) | .250 |
| Mechanical ventilation | 1.07 (1.03–1.12) | .001* |
| Renal function at hospital discharge | ||
| eGFR ≥60 mL/min | – | – |
| eGFR45–59 mL/min | 2.64 (0.85–8.15) | .093 |
| eGFR30–44 mL/min | 5.46 (2.24–13.29) | <.001* |
| eGFR15–29 mL/min | 6.76 (2.81–16.30) | <.001* |
| eGFR <15 mL/min | 23.88 (10.54–54.11) | <.001* |
*Significant at p < .05 level.
Discussion
AKI is the most common complication of critically ill patients and obviously increases rates of in-hospital mortality. This study showed that the in-hospital mortality was 57.2%, coinciding with earlier studies, which reported that an estimated 30–70% of ICU patients suffered from AKI and it resulted in increased in-hospital mortality in critically ill patients requiring dialysis, which accounted for 50–60% [29–31].
Meanwhile, the recent studies indicated that AKI was relevant to long-term outcomes [9–12,32–34]. This study indicated that the 5 year survival rate was 46.3 ± 2.7%, which is similar to the study that the 5 year survival rate was 47% in the followed 226 survivors of RRT in the ICU [35], but is low to the survival rate in the sixth years was 62% in the followed 475 survivors of RRT in the ICU [24].
Recently, some studies have also revealed the relationship between impaired kidney function at discharge and the long-term survival of patients who received RRT in the ICU. In the study conducted by Susanne Stads et al., multivariate analysis demonstrated that an eGFR <30 mL/min/1.73 m2 (including patients receiving long-term RRT) at discharge was closely connected with the long-term mortality in 1220 patients [24]. However, in this study eGFR <45 mL/min/1.73 m2 was associated with the long-term mortality. The patients with eGFR between 30 and 45 mL/min/1.73 m2 also should be paid more attention to. Low eGFR is an important independent risk factor for mortality. This may contribute to the increased inflammation and oxidative stress related to reduced renal function. Besides, kidney dysfunction may be associated with many other physical changes including high levels of homocysteine, hyperuricemia, hypercalcemia and uremia, all of which have detrimental cardiovascular effects [36].
This study also suggested that the long-term renal survival rate was much lower in eGFR <45 mL/min/1.73 m2 groups and those groups also had a higher risk of requesting long-term RRT. AKI is now recognized as being in a bidirectional relationship with CKD in hospitalized patients. Preexisting CKD increases the susceptibility for the development of AKI and likewise, exposure to AKI would also accelerate the development of CKD compared with its occurrence on those without AKI [37]. Moreover, AKI in concurrent with CKD leads to ESRD at a higher frequency than AKI alone [38–40]. Bagshaw stated that 10% of critically ill patients with AKI end up with CKD [21]. In another study, the need for RRT increased the likelihood of progression to CKD by 500-fold [22]. Lo et al. [39] researched on 3773 AKI patients (follow-up of 8-years) and found that the proportion of patients with AKI who developed CKD4 and ESRD was 47.9%, which was significantly higher than that in non-AKI group (1.7%). Other studies have also confirmed that AKI is an independent risk factor for the onset of CKD [37–41]. Pre-clinical studies have suggested a multitude of possible mechanisms: acute endothelial injury leading to vascular dropout, nephron loss followed by glomerular hypertrophy or development of fibrosis after sustaining AKI [42].
AKI is not only closely associated with CKD, but also an important cause of CKD, and CKD is also an independent risk factor for AKI patients. Besides, impaired kidney function at discharge is an important risk factor affecting the long-term renal and overall survival of critically ill patients. Therefore, long-term follow-up in the department of nephrology is necessary for patients with an episode of AKI, especially for patients discharged with eGFR <45 mL/min/1.73 m2.
This study also indicated that age, causes of AKI (sepsis and decreased renal perfusion), vasopressors were independent risk factors of increased mortality of the objects. This is similar to many studies that have consistently shown that patients who develop ESRD are at higher risk of sepsis, cardiovascular events and surgical operation, and also have a higher mortality risk [43–45]. Moreover, many studies indicated that AKI in association with sepsis was particularly hazardous [46,47]. The mechanism may be that the persistent proinflammatory milieu with an episode of sepsis was amplified by a decline in the ability of the kidneys to clear these toxic molecules. Alternatively, the inflammatory injury to the kidneys may persist and feed a persistent systemic inflammatory phenotype.
This study is a retrospective and observational database study and surely has some limitations. To begin with, objects admitted are restricted to a single center. Furthermore, due to the disadvantage of telephone follow-up, there is no obvious data of renal function. Accepting RRT or kidney transplantation is considered as the only index of renal failure. Some patients who had worse renal outcome without RRT may be missed out during follow-up. Lastly, there were 96 (19.2%) survivors that were out of contact during the follow-up. However, the limitations mentioned are believed to have minimal influence on the results.
Conclusions
This study showed that impaired kidney function at discharge was shown to be an important risk factor affecting the long-term renal survival rates of critically ill patients with AKI, and a low GFR was an independent risk factor for decreased overall survival.
Disclosure statement
The authors declare that they have no conflict of interest. This study was approved by the Ethics Committee of The First Affiliated Hospital, Medical College, Zhejiang University.
Funding Statement
This study was supported by project grants from the National Science & Technology Pillar Program in the twelfth Five-year Plan Period [grant numbers 2011BA160B07].
References
- 1.Hsing-Shan T, Yung-Chang C, Pao-Hsien C.. The influence of acute kidney injury on acute cardiovascular disease. Acta Cardiol Sin. 2014;30:93–97. [PMC free article] [PubMed] [Google Scholar]
- 2.Bagshaw SM, George C, Bellomo R, et al. A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23:1569–1574. [DOI] [PubMed] [Google Scholar]
- 3.Ftough S, Lewington A. Guideline development group convened by the National Clinical Guidelines Centre and commissioned by the National Institute for Health and Care Excellence in association with The Royal College of Physicians’ Clinical Effectiveness and Evaluation Unit . Prevention, detection and management of acute kidney injury. Clin Med. 2014;14:61–65. [Google Scholar]
- 4.Kidney Disease: improving global outcomes (KDIGO) acute kidney injury work group KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Inter Suppl. 2012;2:1–138. [Google Scholar]
- 5.Fang Y, Ding X, Zhong Y, et al. Acute kidney injury in a Chinese hospitalized population. Blood Purif. 2010;30:120–126. [DOI] [PubMed] [Google Scholar]
- 6.Schetz M, Darmon M.. Measuring acute kidney injury around the world: are we using the right thermometer (and adequately)? Intensive Care Med. 2015;41:1857–1859. [DOI] [PubMed] [Google Scholar]
- 7.Cohen SD, Kimmel P.. Long-term sequelae of acute kidney injury in the ICU. Curr Opin Crit Care. 2012;18:623–628. [DOI] [PubMed] [Google Scholar]
- 8.Chawla LS, Eggers PW, Star RA, et al. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lafrance JP, Miller DR. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol. 2010;21:345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olsson D, Sartipy U, Braunschweig F, et al. Acute kidney injury following coronary artery bypass surgery and long-term risk of heart failure. Circ Heart Fail. 2013;6:83–90. [DOI] [PubMed] [Google Scholar]
- 11.Coca SG, Yusuf B, Shlipak MG, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heung M, Chawla LS.. Predicting progression to chronic kidney disease after recovery from acute kidney injury. Curr Opin Nephrol Hypertens. 2012;21:628–634. [DOI] [PubMed] [Google Scholar]
- 13.Bucaloiu ID, Kirchner HL, Norfolk ER, et al. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int. 2012;81:477–485. [DOI] [PubMed] [Google Scholar]
- 14.Lafrance JP, Djurdjev O, Levin A.. Incidence and outcomes of acute kidney injury in a referred chronic kidney disease cohort. Nephrol Dial Transplant. 2010;25:2203–2209. [DOI] [PubMed] [Google Scholar]
- 15.Jones J, Holmen J, De Graauw J, et al. Association of complete recovery from acute kidney injury with incident CKD stage 3 and all-cause mortality. Am J Kidney Dis. 2012;60:402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang AY, Bellomo R, Cass A, et al. Health-related quality of life in survivors of acute kidney injury: the prolonged outcomes study of the randomized evaluation of normal versus augmented level replacement therapy study outcomes. Nephrology (Carlton). 2015;20:492–498. [DOI] [PubMed] [Google Scholar]
- 17.Wu VC, Huang TM, Lai CF, et al. Acute-on-chronic kidney injury at hospital discharge is associated with long-term dialysis and mortality. Kidney Int. 2011;80:1222–1230. [DOI] [PubMed] [Google Scholar]
- 18.Pannu N, James M, Hemmelgarn B, et al. Association between AKI, recovery of renal function, and longterm outcomes after hospital discharge. Clin J Am Soc Nephrol. 2013;8:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talabani B, Zouwail S, Pyart RD, et al. Epidemiology and outcome of community-acquired acute kidney injury. Nephrology (Carlton). 2014;19:282–287. [DOI] [PubMed] [Google Scholar]
- 20.Sawhney S, Mitchell M, Marks A, et al. Long-term prognosis after acute kidney injury (AKI): what is the role of baseline kidney function andrecovery? A systematic review. BMJ Open. 2015;5:e006497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bagshaw SM.The long-term outcome after acute renal failure. Curr Opin Crit Care. 2006;12:561–566. [DOI] [PubMed] [Google Scholar]
- 22.Chawla LS, Amdur RL, Amodeo S, et al. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011;79:1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allegretti AS, Steele DJR, Ann David-Kasdan J, et al. Continuous renal replacement therapy outcomes in acute kidney injury and end-stage renal disease: a cohort study. Crit Care. 2013;17:R109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stads S, Fortrie G, van Bommel J, et al. Impaired kidney function at hospital discharge and long-term renal and overall survival in patients who received CRRT. Clin J Am Soc Nephrol. 2013;8:1284–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kellum JA, Lameire N. KDIGO AKI guideline work group . Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part1). Crit Care. 2013;17:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 27.Le Gall JR, Lemeshow S, Saulnier F.. A new simplified acute physiology score (SAPS II) based on a European/North American multicentre study. JAMA. 1993;270:2957–2963. [DOI] [PubMed] [Google Scholar]
- 28.ZhangYang PY, Lv R, et al. Effect of the intensity of continuous renal replacement therapy in patients with sepsis and acute kidney injury: a single-center randomized clinical trial. Nephrol Dial Trantsplant. 2012;27:967–973. [DOI] [PubMed] [Google Scholar]
- 29.Abdulaziz A.Outcome and prognostic factors of critically ill patients with acute renal failure requiring continuous renal replacement therapy. Saudi J Kidney Dis Transplant. 2010;21:1106–1110. [PubMed] [Google Scholar]
- 30.Lewington AJ, Cerdá J, Mehta RL.. Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney Int. 2013;84:457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yasuda H, Kato A, Fujigaki Y, The Shizuoka Kidney Disease Study Group, et al. Incidence and clinical outcomes of acute kidney injury requiring renal replacement therapy in Japan. Ther Apher Dial. 2010;14:541–546. [DOI] [PubMed] [Google Scholar]
- 32.Coca SG.Long-term outcomes of acute kidney injury. Curr Opin Nephrol Hypertens. 2010;19:266–272. [DOI] [PubMed] [Google Scholar]
- 33.Dasta JF, Kane-Gill SL, Durtschi AJ, et al. Costs and outcomes of acute kidney injury following cardiac surgery. Nephrol Dial Trantsplant. 2008;23:1970–1974. [DOI] [PubMed] [Google Scholar]
- 34.Welten GM, Schouten O, Chonchol M, et al. Temporary worsening of renal function after aortic surgery is associated with higher long-term mortality. Am J Kidney Dis. 2007;50:219–228. [DOI] [PubMed] [Google Scholar]
- 35.Schiffl H, Fischer R.. Five-year outcomes of severe acute kidney injury requiring renal replacement therapy. Nephrol Dial Transplant. 2008;23:2235–2241. [DOI] [PubMed] [Google Scholar]
- 36.Domoto S, Tagusari Nakamura OY, et al. Preoperative estimated glomerular filtration rate as a significant predictor of long-term outcomes after coronary artery bypass grafting in Japanese patients. Gen Thorac Cardiovasc Surg. 2014;62:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishani A, Xue JL, Himmelfarb J, et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ali T, Khan I, Simpson W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007;18:1292–1298. [DOI] [PubMed] [Google Scholar]
- 39.Amdur RL, Chawla LS, Amodeo S, et al. Outcomes following diagnosis of acute renal failure in, U. S veterans: focus on acute tubular necrosis. Kidney Int. 2009;76:1089–1097. [DOI] [PubMed] [Google Scholar]
- 40.Lo LJ, Go AS, Chertow GM, et al. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009;76:893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wald R, Quinn RR, Luo J, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302:1179–1185. [DOI] [PubMed] [Google Scholar]
- 42.Wang Z, Holthoff JH, Seely KA, et al. Development of oxidative stress in the peritubular capillary microenvironment mediates sepsis-induced renal microcirculatory failure and acute kidney injury. Am J Pathol. 2012;180:505–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singbartl K, Kellum JA.. AKI in the ICU: definition, epidemiology, risk stratification, and outcomes. Kidney Int. 2012;81:819–825. [DOI] [PubMed] [Google Scholar]
- 44.Tsai TT, Patel UD, Chang TI, et al. Contemporary incidence, predictors, and outcomes of acute kideney injury in patients undergoing percutaneous coronary interventions:insights from the NCDR Cath-PCI registry. JACC Cardiovasc Interv. 2014;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bihorac A, Yavas S, Subbiah S, et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249:851–858. [DOI] [PubMed] [Google Scholar]
- 46.Zarjou A, Agarwal A.. Sepsis and acute kidney injury. J Am Soc Nephrol. 2011;22:999–1006. [DOI] [PubMed] [Google Scholar]
- 47.Linder A, Fjell C, Levin A, et al. Small acute increases in serum creatinine are associated with decreased long-term survival in the critically ill. Am J Respir Crit Care Med. 2014;189:1075–1081. [DOI] [PubMed] [Google Scholar]