Abstract
Calderón-Larrañaga A, Vetrano DL, Ferrucci L, Mercer SW, Marengoni A, Onder G, Eriksdotter M, Fratiglioni L. (Karolinska Institutet-Stockholm University, Stockholm, Sweden; Catholic University of the Sacred Heart, Rome, Italy; National Institutes of Health, Baltimore, MD, USA; University of Glasgow, Glasgow, UK; University of Brescia, Brescia, Italy; Karolinska Institutet, Stockholm, Sweden; Stockholm Gerontology Research Center, Stockholm, Sweden; Fondazione Policlinico Universitario “A. Gemelli” IRCCS, Rome, Italy). Multimorbidity and functional impairment– bidirectional interplay, synergistic effects and common pathways.
This review discusses the interplay between multimorbidity (i.e. co-occurrence of more than one chronic health condition in an individual) and functional impairment (i.e. limitations in mobility, strength or cognition that may eventually hamper a person’s ability to perform everyday tasks). On the one hand, diseases belonging to common patterns of multimorbidity may interact, curtailing compensatory mechanisms and resulting in physical and cognitive decline. On the other hand, physical and cognitive impairment impact the severity and burden of multimorbidity, contributing to the establishment of a vicious circle. The circle may be further exacerbated by people’s reduced ability to cope with treatment and care burden and physicians’ fragmented view of health problems, which cause suboptimal use of health services and reduced quality of life and survival. Thus, the synergistic effects of medical diagnoses and functional status in adults, particularly older adults, emerge as central to assessing their health and care needs. Furthermore, common pathways seem to underlie multimorbidity, functional impairment and their interplay. For example, older age, obesity, involuntary weight loss and sedentarism can accelerate damage accumulation in organs and physiological systems by fostering inflammatory status. Inappropriate use or overuse of specific medications and drug–drug and drug–disease interactions also contribute to the bidirectional association between multimorbidity and functional impairment. Additionally, psychosocial factors such as low socioeconomic status and the direct or indirect effects of negative life events, weak social networks and an external locus of control may underlie the complex interactions between multimorbidity, functional decline and negative outcomes. Identifying modifiable risk factors and pathways common to multimorbidity and functional impairment could aid in the design of interventions to delay, prevent or alleviate age-related health deterioration; this review provides an overview of knowledge gaps and future directions.
Keywords: multimorbidity, physical function, cognitive function, ageing
Introduction
In recent decades, multimorbidity – the co-occurrence of more than one chronic health condition in an individual – has emerged as a major challenge for healthcare systems [1], both in high-income countries and in low- and middle-income countries, where populations are ageing fastest [2]. In high-income countries, up to 20% of the population experiences multimorbidity before the age of 40 years [3]. Prevalence then sharply increases, reaching 75% at 70 years, after which it remains relatively stable, probably because of selective mortality [4]. The absolute number of people affected by multimorbidity is expected to double by 2035, and at least two-thirds of the gain in life expectancy above 65 years will be spent with four or more chronic conditions [5].
Both the number of people with multimorbidity and the prevalence of multimorbidity seem to have increased in recent years [6], and greater increases are documented in more recent birth cohorts [7]. The rise has been mainly driven by population ageing and longer survival of people with chronic conditions. The mortality rate from conditions such as stroke, myocardial infarction and diabetes is lower than in the past. Thus, more diseases accumulate in those who survive. The use of more sensitive diagnostic procedures, better control of risk factors like hypertension and improvements in detecting and recording chronic disorders may also have contributed to the increasing prevalence of multimorbidity [6].
Multimorbidity leads to physical decline, and people with more conditions, more severe disease and specific disease patterns experience steeper deterioration [8]. People with multimorbidity are also more likely to have poorer cognitive status [9] and worse quality of life in both midlife [10] and old age [11] than those without multimorbidity. In a meta-analysis conducted in 2016, older adults with multimorbidity were 44% more likely to die during follow-up than those with no or only one chronic disease [12]. According to a large collaborative study involving 1.2 million participants, any combination of co-occurring cardio-metabolic conditions was associated with a multiplicative mortality risk [13]. The impact of multimorbidity on individuals’ health profiles surpasses the impact we would expect from the summed effect of single conditions. This nonlinear pattern may be further exacerbated by a continuous imbalance between illness and treatment burden and the ability/resources of people with multimorbidity to cope with such burden, which leads to a vicious cycle of breakdowns in care, self-care and health outcomes [14].
Limitations in physical and cognitive function due to multimorbidity decisively affect people’s illness and treatment burden and their response capacity, which may further increase multimorbidity [15, 16]. Thus, assessing their health requires considering not only specific medical diagnoses and functional status, but also the interaction between the two. This approach is likely to provide more information about people’s baseline risk for poor outcomes, responsiveness to treatment and vulnerability to the adverse effects of treatment [17]. Moreover, because the same mechanisms are likely to underlie multimorbidity and functional deficits, the traditional idea of a causal pathway that leads from multimorbidity to functional impairment is somewhat misleading. Instead of a simple linear pathway, a multimorbidity-functional impairment circle is probably a better representation of the health and social care needs of adults, particularly older adults.
The objective of this review was to summarize the scientific evidence on: (i) the interplay between multimorbidity and impairment in both physical and cognitive function, as well as potential synergistic effects of the interplay on health-related outcomes, and (ii) the major risk factors shared by multimorbidity and functional impairment. The ultimate goal is to provide a solid rationale for integrating and measuring both constructs in research and clinical practice.
Methodological considerations
This narrative literature review is based on the authors’ knowledge of the current literature, their expertise in the field and a discussion panel on the latest major findings on multimorbidity and functional decline that took place during a 2-day meeting in Stockholm in May 2018. The meeting included a symposium at which the background to the topic was elucidated and a workshop with 18 multidisciplinary experts from North America and Europe with backgrounds in geriatrics, primary care, public health and health services research, epidemiology, and pharmacy/pharmacology. The authors surveyed the literature published over the last two decades, making sure that key results from population-based longitudinal studies were included. As the aim was to summarize main findings and suggest new research avenues, the definitions of multimorbidity, physical function and cognitive function were not restricted. Table 1 provides an overview of the definitions and measurements of multimorbidity and functional impairment encountered in the cited literature.
Table 1.
Measures, definitions and operationalizations of multimorbidity, physical function and cognitive function in the cited literature
| Measures and definitions | Differences in operationalization |
|---|---|
| Multimorbidity | |
|
(1) Disease counts Number of diseases on a continuous scale or categorized in accordance with specific cut-offs (2) Patterns of diseases Systematic associations amongst diseases defined on the basis of expert criteria or statistical associations (3) Weighted indices Scores derived from disease weights obtained for specific outcomes (e.g. Charlson, CIRS) (4) Speed of disease accumulation Rate of change in the number of diseases in a given observation period (5) Comorbidities of an index disease Combinations of diseases that occur together with the index disorder |
Diseases may include: • Chronic or acute and chronic conditions • Symptoms, syndromes and/or risk factors • Conditions selected from specific existing lists or on the basis of a priori criteria such as the prevalence or predictability of specific outcomes • Conditions that were self-reported, diagnosed by a physician or inferred from medication use |
| Impairment of physical function | |
|
(1) Mobility limitations Limitations in the ability to move around that may eventually impair a person’s ability to accomplish daily activities (2) Strength limitations Limitations in upper or lower limb muscle strength as defined by specific thresholds (3) Limitations in the activities of daily living (ADL) Number of activities that a person can only accomplish with the help of another person |
• Mobility may be assessed with objective tests (e.g. usual walking speed) or via self- report (e.g. SF-36) Strength may be objectively measured or inferred from the self-reported ability to perform specific tasks • ADL may include personal ADL (PADL) and instrumental ADL (IADL), counted sepa rately or grouped in an overall score • Measures of mobility and strength may be embedded in a more comprehensive test (e.g. SPPB, frailty phenotype) |
| Impairment of cognitive function | |
|
(1) Dementia Loss of cognitive function that interferes with daily life and ADL (2) Cognitive impairment Difficulty with global cognitive function or specific domains of cognitive function (e.g. memory, decision-making, concentration, learning) that does not interfere with daily life and ADL (3) Cognitive decline Decline of global or specific domains of cognitive function over time |
• Diagnosis of dementia may be based on neuropsychological assessment by direct clinical examination (e.g. NIA-AA, DSM) or derived from hospital registries • Dementia can be further classified by age of symptom onset (early/late onset), symptom severity (mild/moderate/severe) or demen tia subtype (vascular/mixed/Alzheimer’s/ secondary) • Cognitive impairment and decline may be assessed by face-to-face neuropsychological assessment, by telephone interview (e.g. TICS) or reported by the affected person or a proxy • Cognitive impairment and decline may be assessed with global cognitive measures (e.g. MMSE, MOCA, CDR, GDS) or domain specific tests (e.g. digit symbol test for evaluating attention) |
ADL, activities of daily living; CDR, Clinical Dementia Rating; CIRS, Cumulative Illness Rating Scale; DSM, Diagnostic and Statistical Manual of Mental Disorders; GDS, Global Deterioration Scale; IADL, instrumental activities of daily living; MMSE, Mini-Mental State Examination; MOCA, Montreal Cognitive Assessment; NIA-AA, National Institute on Aging and Alzheimer’s Association; PADL, personal activities of daily living; SF-36, 36-Item Short Form Health Survey; SPBB, Short Physical Performance Battery; TICS, Telephone Interview for Cognitive Status.
Interplay between multimorbidity and function
Multimorbidity and physical function
In the general adult population, multimorbidity often predicts decline in physical function and loss of independence, which suggests a causal link between these phenomena [18]. With few exceptions, both cross-sectional and longitudinal studies have shown that multimorbidity is associated with poor physical function in older adults [19–21]. In the Maastricht Aging Study (MAAS), the poorer physical functioning that accompanied multimorbidity persisted and seemed to increase over time in older adults [22]. The Kungsholmen Project, which included people 78 years and older living in central Stockholm, showed that on average, those with multimorbidity spent 81% of their remaining years of life with disability [23].
Walking speed and handgrip strength, two measures of physical performance commonly used in geriatric medicine, decrease in the presence of multimorbidity, and older age further strengthens this association [24–26]. This finding highlights the reduced resilience to morbidity in older adults. However, multimorbidity does not fully explain observed age-related differences in physical performance, which suggests that morbidity, as described by the current nosological definition of diseases, is not the only determinant of functional decline [27]. Although studies have not found gender differences in walking speed, multimorbidity seems to have a greater effect on muscle strength and number of impaired ADL in women than men [28, 29]. Women’s greater vulnerability to the negative effects of multimorbidity is still not well explained. The results of some studies support the idea that symptom severity and the presence of geriatric syndromes (e.g. falls, urinary incontinence, pain) – independent of multimorbidity – are associated with the development of functional impairment and disability [30, 31]. In fact, multimorbidity may be a mediator between pathophysiological processes and negative health outcomes such as impaired physical function [32].
Rapid accumulation of chronic diseases may be an indicator of accelerated ageing [33]. In line with this finding, analyses of data from the Swedish National Study on Aging and Care in Kungsholmen (SNAC-K) showed that the risk of developing new ADL impairments was more than twice as high in older adults who more rapidly developed multimorbidity than those who accumulated diseases more slowly [34]. Interestingly, women and those with a poorer social network were more susceptible to the detrimental consequences of a fast accumulation of diseases [34].
Diseases that share similar aetiology and pathophysiology can be associated with specific profiles of functional impairment and disability [11, 35]. There is evidence that diseases belonging to common patterns of multimorbidity may interact, curtailing compensatory mechanisms and resulting in more severe functional limitation [36, 37]. According to analyses of data from the SNAC-K population-based study, neuropsychiatric diseases, alone or in association with each other, are major determinants of disability and slow walking speed in older adults, whereas isolated cardiovascular multimorbidity is associated only with a decline in walking speed [38]. Other longitudinal study results support these findings [39–42].
Several parameters of physical function are embedded in the concept of physical frailty, a condition of increased susceptibility to negative events that is characterized by the presence of at least three of the following disorders: unintentional weight loss, self-reported exhaustion, low energy expenditure, slow gait speed and weak grip strength [43]. According to a systematic review and meta-analysis, community- dwelling older adults with multimorbidity have twofold higher odds of being frail than those without multimorbidity [44]. That study also showed that more than two-thirds of older adults with frailty have multimorbidity, but less than one-fifth of those with multimorbidity are frail [44]. Once again, multimorbidity appears to be an important – but not the only – determinant of poor functioning.
Although the majority of studies support the idea that multimorbidity plays a causal role in the development of functional impairment and disability, recent findings suggest that the association could be bidirectional. Unfortunately, only a few studies have specifically addressed this hypothesis. According to findings from the Irish Longitudinal Study on Ageing (TILDA), walking speed and handgrip strength are inversely associated with developing multimorbidity and, in general, with accruing new diseases [45]. The idea that better physical fitness may slow the accumulation of chronic diseases is also supported by studies that have observed a link between physical activity and multimorbidity [46–49]. However, because none of these studies was longitudinal, they may be biased by reverse causality; that is, people with multimorbidity could be less physically active because of low fitness. Nevertheless, secondary analyses of data from the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) showed that a multi-domain intervention that included but was not limited to physical exercise and cognitive training was associated with a reduced risk of developing chronic diseases during a 2-year follow-up [50].
Several studies show that diseases and functional status interact to determine the risk for multiple outcomes. In SNAC-K, the coexistence of multiple cardiovascular, and to a lesser extent, of multiple neuropsychiatric diseases, was associated with an increased risk for all-cause mortality and cardiovascular mortality, but only in older adults with slow walking speed (D. L. Vetrano, D. Rizzuto, A. Calderon-Larranaga, G. Onder, A. K. Welmer, C. Qiu, R. Bernabei, A. Marengoni, & L. Fratiglioni, under review). Similarly, in a study from the United States that included older adults with heart failure, those with both functional limitation and multimorbidity were at higher risk for mortality, emergency department visits, hospitalizations and outpatient visits than people free from these conditions or those who only had multimorbidity [51]. In that study, the association between multimorbidity and number of outpatient visits was stronger than the association between functional impairment and number of outpatient visits [51]. Another study from the United States, which used administrative data, showed that older adults with both multimorbidity and mobility limitations used healthcare resources more frequently and intensively than those with only multimorbidity or only mobility limitations [52]. Three longitudinal studies that examined co-occurring multimorbidity and functional impairment have reported that only functional impairment is associated with higher mortality [20, 53, 54]. However, only one study formally tested and showed that functional decline mediates the impact of multimorbidity on mortality [55]. Finally, two studies suggest that physical activity may mediate the association between multimorbidity and mortality [56, 57].
Multimorbidity and cognitive function
Evidence that multimorbidity can contribute to dementia and cognitive decline has emerged from animal models and from clinical and epidemiological studies [58]. Brain autopsies of people with dementia have identified significant comorbidities [59]. Furthermore, studies show that lower cognitive function is associated with an increased burden of multimorbidity [60, 61], and longitudinal trajectories of cognitive decline and rising multimorbidity seem to co-occur in older adults without dementia [62]. Indeed, multimorbidity and cognition seem to be bidirectionally correlated. The FINGER trial, the first large-scale intervention consisting of diet, physical exercise, cognitive training and vascular risk monitoring to prevent cognitive decline in older adults, was effective not only in preventing cognitive decline in at-risk older adults [63], but also in lowering the risk of developing new chronic diseases during the 2-year follow-up [50].
Observational studies have shown that multimorbidity is associated with all the stages of the cognitive dysfunction continuum, from the preclinical phase [64] to cognitive decline [65], mild cognitive impairment [66] and established dementia [67]. It is even associated with early-onset Alzheimer’s disease [68]. One study has reported a dose-dependent relationship between the number of diseases and subjective cognitive impairment [9], a finding consistent with the hypothesis that multiple aetiologies may contribute to cognitive impairment and dementia [69].
Multimorbidity occurs frequently in people with dementia [70]. Data from more than 30 000 patients included in the Swedish National Quality Registry for Dementia (SveDem) show that several cardiovascular comorbidities are typically present at the time of the dementia diagnosis [71], and that one- quarter of patients with dementia are treated with antidepressants [72]. Interest in understanding the relative contribution of comorbid conditions to the development of cognitive impairment and dementia has increased, as this research has the potential to reveal shared mechanisms and provide insight into dementia pathogenesis [73]. Such research might also contribute to improving the prognosis of people with dementia via adequate treatment of comorbidity [73]. Exploratory analyses using diagnostic register data have shown that dementia is part of a pattern of multimorbidity that includes other neurological disorders (e.g. Parkinson’s disease, insomnia and mental health problems), cardiovascular diseases (e.g. congestive heart failure, cerebrovascular disease, cardiac arrhythmia) and other diseases (e.g. anaemia, chronic skin ulcers, osteoporosis, thyroid disease, retinal disorders, prostatic hypertrophy) [74–76]. Some of these conditions have an aetiological link (e.g. cerebrovascular disease, thyroid disease), others are likely to be complications (e.g. skin ulcers, insomnia), and others, at least apparently, are not pathophysiologically correlated (e.g. osteoporosis, prostatic hypertrophy) [77].
The coexistence of multimorbidity and cognitive impairment makes it particularly challenging to provide health care, as multimorbidity may speed dementia progression. A recent systematic review provided evidence that somatic comorbidities are associated with a worsening of cognitive, functional and neuropsychiatric symptoms in people with late-onset Alzheimer’s disease [78]. At the same time, the presence of dementia may adversely affect and complicate the clinical care of other conditions in several ways. First, detecting comorbid conditions is especially difficult in people with dementia, as they may be less likely to attend regular appointments or to express discomfort sufficiently. Second, clinicians may focus predominantly on the behavioural and psychological symptoms associated with dementia, and by doing so may fail to diagnose other conditions. In fact, there is some evidence that particular conditions are likely to remain untreated or even undiagnosed in people with dementia, such as vision and hearing problems, diseases of the musculoskeletal system, lipoprotein disorders, hypertension and atrial fibrillation [79, 80]. Finally, cognitive decline and dementia may remain undetected in older adults with severe multimorbidity [81].
Cognitive impairment and multimorbidity interact to impact older adults’ health status, quality of life and survival. In a cross-sectional study undertaken in residential care settings in Spain, people with dementia and high comorbidity reported the most compromised health status, especially when vision, oral and genitourinary problems coexisted [82]. The extent of comorbidity was even more important than severity of dementia in precipitating the onset of disability in nursing home residents in western Canada [83].
Additionally, co-occurring multimorbidity and dementia contribute to increased mortality rates. A retrospective population-based cohort study in Canada found that in people with dementia, comorbidity increased the risk for death by 1.5–6.4 [84]. High levels of comorbidity even cancelled the effect of dementia type or severity in predictions of long-term mortality amongst very old patients discharged from a Swiss acute geriatric hospital unit [85].
When multimorbidity and limitations in cognitive function co-occur, people’s self-management abilities, their engagement in health-promoting activities and their use of health care might be suboptimal. In a Canadian home care cohort of over 30 000 people with dementia, the risk for hospitalization and emergency department visits increased as the level of multimorbidity rose [86]. Hospital and emergency department visits are particularly detrimental for people with dementia because they are at heightened risk for cognitive and functional decline during and after hospitalization [87]. Increasing numbers of comorbidities were also associated with more primary care visits and prescriptions in a retrospective cohort of incident dementia patients diagnosed in primary care in the United Kingdom [88]. All of this adds to the cost of health care for older adults with multimorbidity and dementia [89].
Main risk factors common to multimorbidity and functional impairment
Biological factors
Chronological ageing is the major risk factor for multimorbidity; both the prevalence and severity of multimorbidity increase exponentially as the body ages [90, 91]. Epidemiological studies, especially those with a longitudinal design, have identified several risk factors for the development and progression of multimorbidity that are independent of chronological age. For example, studies have shown that obese men and women are more likely than those who are not obese to be affected by multimorbidity and to develop it earlier in life [7, 92]. Overweight and obesity may lead to multimorbidity through multiple mechanisms such as reduced functional capacity and fitness and/or stimulation of inflammation and insulin resistance, all of which are shared risk factors for cardiovascular and non cardiovascular disease and for functional impairment [93, 94]. Counterintuitively, in obese older adults, loss of weight is also associated with increasingly severe multimorbidity [95]. In obese older adults who lose weight, the increase in multimorbidity is mostly accounted for by the development of anaemia and chronic kidney disease [95], which together with weight loss are typical features of the frailty phenotype.
This is consistent with the hypothesis that loss of weight in late life is a consequence and not the cause of quickly deteriorating health status and progressing multimorbidity.
Many studies also report that sedentarism is a risk factor for multimorbidity. The mechanisms behind this association are still uncertain, but sedentarism is a risk factor for all age-associated diseases, from cancer to cardiovascular disease and depression [96, 97]. Whether increasing one’s level of physical activity may inhibit the vicious cycle of disease accumulation and prevent or slow the progression of multimorbidity is unknown and should be tested in appropriately designed clinical trials [98–101].
Insight into the possible biological substrate of multimorbidity is provided by emerging evidence that high circulating levels of inflammatory markers, a condition referred to as ‘inflammaging’, is cross sectionally associated with multimorbidity and predicts steeper rates of multimorbidity development over time [33, 102]. Researchers have proposed that both inflammaging and multimorbidity result from damage that accumulates in organs and physiological systems with age, which challenges organismal reserve capacity and resilience [91, 103–105]. Consistent with this hypothesis, higher resting metabolic rate per kilogram of lean body mass, a recognized biomarker of active resilience and accelerated ageing in animals and in humans, predicts future multimorbidity in older adults [106, 107]. Two additional observations indirectly support this hypothesis: first, weight loss is a strong prognostic factor for mortality in old age [108], and second, the majority of people who die very old experience a substantial decline in weight already in the 3 to 8 years prior to death [109]. The research outlined above suggests that people who develop multimorbidity earlier in life are ageing biologically faster than the general population, a hypothesis that could be tested and may provide information useful to preventing and clinically managing multimorbidity [107].
A small but growing body of evidence suggests that specific mechanisms of biological ageing (i.e. hallmarks of ageing) may impact both global susceptibility to disease and physical functional decline [110–112] (Fig. 1). Recent studies also connect the basic biology of ageing with cognitive decline and late-onset dementia as well as with mobility loss, which suggests that multimorbidity and physical and cognitive decline have a common causal route [113] Common shared mechanisms would be consistent with epidemiological reports suggesting substantial heterogeneity in the rate at which older adults develop and accumulate age-related diseases [114] Thus, some people may develop a susceptibility to multiple diseases and functional impairment earlier than others [90]. Of note, the existence of shared mechanisms is not contrary to but rather complements the traditional view that diseases, and especially coexisting multiple diseases, cause functional impairment and disability, although their effects may be delayed by redundancy and compensatory strategies (see arrows on the right of Fig. 1) [115].The finding that physical activity protects against both multimorbidity and physical and cognitive functional impairment is also consistent with the hypothesis that biological ageing lies at the root of both. In fact, physical activity is the only intervention that has direct beneficial effects on the mechanisms of biological ageing and on the physical and cognitive deterioration that occur with ageing [116, 117].
Fig. 1.
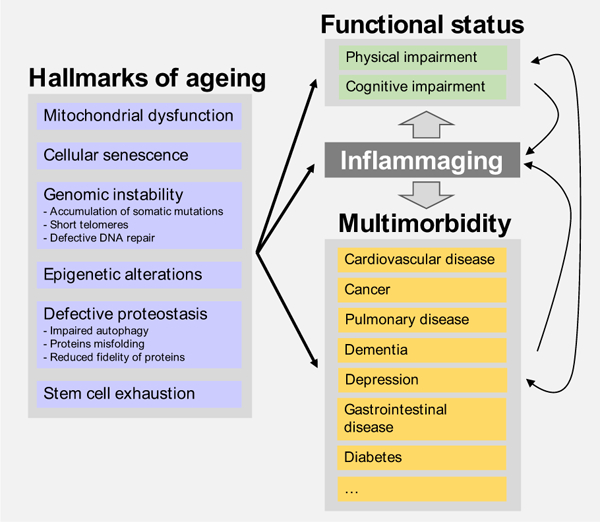
Biological mechanisms underlying the bidirectional association between multimorbidity and functional impairment.
Gathering direct evidence of a link between the biology of ageing and the risk for multimorbidity and functional impairment remains problematic, however. Even if the putative biological mechanisms that underlie the ageing process have been identified, measuring them in humans is challenging. Efforts are underway. Recently, researchers have developed an epigenetic biomarker of phenotypic ageing, the DNAm PhenoAge. This biomarker tracks biological ageing and is sensitive to phenotypic deviation from normal ageing [118]. Similarly, the epigenetic age of the prefrontal cortex has been associated with pathology, cognitive impairment and even dementia [119]. Premature changes in these biomarkers are associated with more severe multimorbidity, although the strength of the association is modest, possibly because the effect of specific methylation sites relevant to this outcome is buried by the complexity of the index [119].
Care-related factors
Despite the acknowledged impact of other care-related factors such as hospitalization on functional decline in people with multimorbidity [120], the following section focuses on drug-related problems, which both result from and lead to multimorbidity.
Medications can help preserve function by treating symptoms (e.g. low back pain [121]) and by preventing, stabilizing or slowing the progress of chronic conditions (e.g. hypertension, Parkinson’s disease, dyspnoea, COPD, ischaemic heart disease, osteoporosis, diabetes [122–125]). However, drugs can also promote multimorbidity and functional impairment. First, the coexistence of multiple diseases and symptoms may lead to polypharmacy – the use of multiple medications. More than 50% of people 65 years or older receive five or more medications concomitantly [126]. Polypharmacy increases the risk for drug-drug interactions, especially in older adults [127]. The more coexisting diseases a person has and the more drugs they are prescribed, the more likely drug-drug interactions are to occur [128]. Such interactions increase the risk for adverse drug reactions, which in turn can lead to worse physical and cognitive function.
Second, multiple medications can also lead to drug–disease interactions, which occur when a medication used to treat a disease or symptom has a negative effect on another coexisting health problem [127]. For example, some beta-blockers prescribed for heart disease or hypertension can worsen asthma and mask hypoglycaemia in people with diabetes. In addition, specific diseases may alter medication metabolism. Typical examples are kidney and liver diseases that lead to reduced drug clearance and therefore to a higher risk for adverse drug reactions. The risk for adverse reactions may be further intensified by changes in body composition that are frequently observed in older people (e.g. sarcopenia, sarcopenic obesity) [129]. Heart failure may also cause changes in pharmacokinetics, including diminished renal and hepatic blood flow, reduced splanchnic blood flow and liver metabolic capacity, hepatic venous congestion, and a reduction in the volume of distribution. A relevant phenomenon related to the complex relationship amongst polypharmacy, adverse drug effects and drug-disease interactions is the prescribing cascade, the process whereby the side effects of medications are misinterpreted as a symptom of a new disease, resulting in further prescriptions [130]. Examples of this phenomenon are the use of anti-Parkinson medications to treat extrapyramidal symptoms caused by antipsychotics and the use of anticholinergic medications to manage urge incontinence due to treatment with cholinesterase inhibitors [131, 132].
Third, several medications can have a direct negative impact on physical or cognitive function, in particular in older adults. The use of anticholinergic drugs is associated with falls, hip fractures, ADL limitations and impaired cognition [133, 134]. Inappropriate medication use is in fact another pathway through which medications might cause functional and cognitive deficits. For example, the inappropriate use of proton pump inhibitors in older adults can cause vitamin B12 deficiency. Vitamin B12 is known to impact cognition, and deficiency reduces calcium absorption, increases fracture risk and is associated with increased mortality [135]. Finally, overtreatment of chronic conditions, often because of excessively strict therapeutic goals, might also lead to negative health outcomes. For example, overtreatment of diabetes in older adults is associated with disability and increased risk for mortality [136].
The relationship between impaired physical and cognitive function and pharmacological treatment of multimorbidity may be bidirectional [137] (Fig. 2). On the one hand, medications might influence the onset of physical and cognitive deficits, as described above; on the other, physical and cognitive function may influence therapeutic choices and medication effects [125]. For example, physical dysfunction can make it hard to manage pill containers, which can lead to reduced adherence to treatment and increased risk for medication errors [138]. Cognitive impairment and dementia can affect decision-making capability, alter treatment benefit and burden, impact medication adherence and cause communication difficulties, including a decreased ability to report adverse effects [139]. Finally, both functional and cognitive impairment are associated with reduced life expectancy, which may limit the beneficial effect of some preventive drugs (e.g. statins, antihypertensives, antiosteoporotics) given that their time-to-benefit might exceed individuals’ actual life expectancy [125].
Fig. 2.
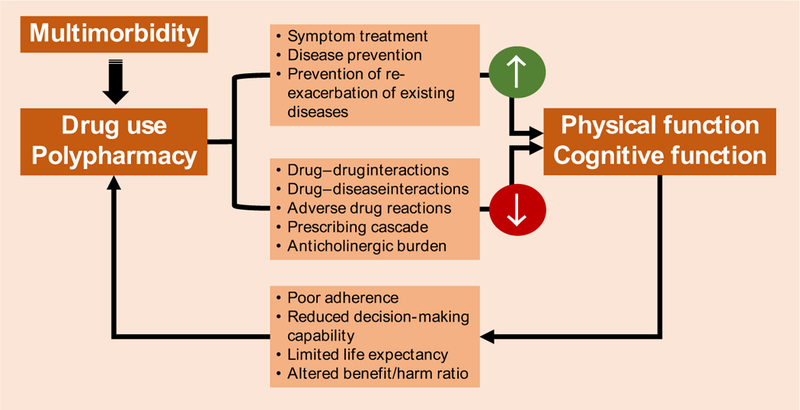
Drug-related problems underlying the bidirectional association between multimorbidity and functional impairment.
Psychosocial factors
The term ‘psychosocial’ refers to the influence of social factors on people’s minds or behaviours and to the relationship between behavioural and social factors.
Multimorbidity is more common and starts some 10–15 years earlier in people who live in areas of high socioeconomic deprivation than in those who live in more affluent communities [140]. The authors of a 2018 systematic review of 24 studies confirmed this effect [141]. In their meta-analysis, they also found that people with a low level of education had 64% higher odds of multimorbidity than those with a high level of education [141]. The social patterning of multimorbidity has several important implications. First, it means that multimorbidity contributes to the broad inequalities that exist in many countries and to the high level of premature mortality in people of lower socioeconomic status (SES) [142]. The high prevalence of multimorbidity also contributes to the high levels of prefrailty and frailty seen in deprived areas [143]. Second, health-related quality of life is worse in people of lower SES who have multimorbidity than those of higher SES who have multimorbidity, and this is apparent at a younger age [142]. Third, healthcare use – and hence, healthcare costs – are higher in people with multimorbidity and low SES [144].
As the number of physical conditions a person has increases, so does the person’s chance of having a common mental health disorder [141, 145]. There is, however, an important interaction between social factors and psychological factors in this association. People of low SES are substantially more likely to have multimorbidity that includes mental and physical conditions than those of higher SES [141, 146]. This is apparent in all age groups but more pronounced in those of younger age [146]. Such mental-physical multimorbidity exacerbates the effect of SES on service use. For example, unplanned and potentially avoidable hospital admissions rise with increasing levels of physical multimorbidity, and low SES intensifies this rise at all levels of multimorbidity. Mental health problems further increase admissions at all levels of multimorbidity [144]. Moreover, the association between mental health and physical multimorbidity is known to be bidirectional [147]. Not only do increasing numbers of somatic health problems raise the risk of developing mental health problems, but people with mental health problems also have a higher risk of developing multimorbidity over time than those without such problems.
Traditional risk factors, such as smoking, alcohol and diet, are of some importance in the aetiology of multimorbidity, but a recent analysis of data from a longitudinal cohort study in Scotland found that traditional risk factors only explained around 40% of the excess multimorbidity in people of low SES [148]. The remaining 60% is still unexplained but might be attributable to the direct or indirect effects of psychosocial factors such as adverse childhood experiences [149]. Factors such as negative life events, weak social networks and an external locus of control also predict the development of multimorbidity [150–152]. Conversely, a high sense of coherence protects against adverse health outcomes in older adults with multimorbidity [153] and can help mitigate the negative effects of low SES on health [154]. SES also affects brain development and function, which may have implications for the future development of multimorbidity and poor outcomes in people in deprived areas [155].
Psychosocial factors such as social connectedness protect against age-related decline in cognitive function, probably through multiple neurobiological mechanisms. In the Rush Memory and Aging Project, an ongoing longitudinal clinical-pathologic cohort study that began in 1997, a wide array of psychosocial behaviours were independently associated with the subsequent rate of cognitive decline and/or risk for incident cognitive diagnoses [156]. These include trait and state measures of psychological distress, social isolation, social capital, emotional isolation and purpose in life. Using data from the MacArthur Study of Successful Aging, researchers found connections between a range of psychosocial factors and decline in physical function in older adults with different chronic diseases [157]. In that study, greater emotional support was associated with less decline in physical function in people with cardiovascular disease, and greater social conflict was associated with greater decline in people with hypertension or diabetes. Interestingly, social and psychological factors were unrelated to changes in functioning in those with no chronic conditions.
Conclusion
To date, most studies of the relationship between multimorbidity and functional impairment have focused on the former as a pre condition for the incidence of the latter. Researchers have aimed at identifying risk or protective factors for either multimorbidity or function, but rarely considering both simultaneously. This review summarizes evidence suggesting that using an integrated model will support better framing open questions about the origin and consequences of multimorbidity. In such a model, lifelong biological, care-related and psychosocial factors operate as determinants of the interaction between multimorbidity and both physical and cognitive function (Fig. 3).
Fig. 3.
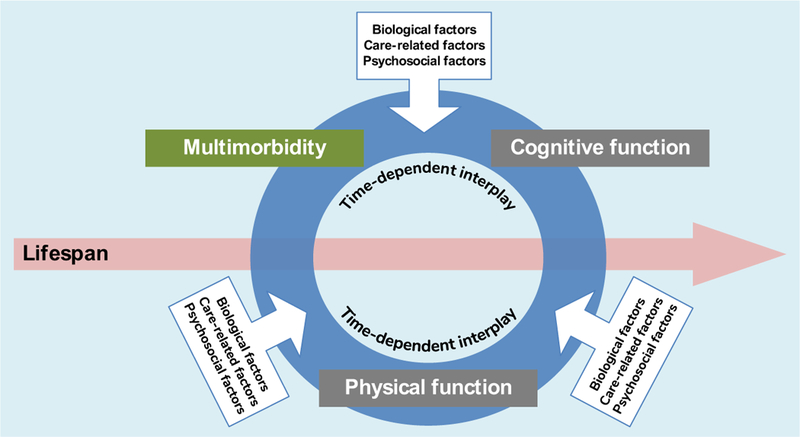
Integrated framework for the development of multimorbidity and functional impairment: a proposal for future research.
Although certain aspects of the topics of multimorbidity and function have been widely investigated (e.g. prevalence, patterns of disease combinations, consequences, effect on healthcare use and costs), information on the common causes of and risk factors for both health constructs is very scarce. Studies that incorporate multimorbidity and function as coexisting and interacting outcomes are even rarer. Thus, we also provide an overview of knowledge gaps and future directions that might help improve our understating of common risk factors upon which interventions can be built (Table 2). We believe the following research questions deserve priority: Is it possible to identify shared modifiable risk factors for multimorbidity and functional impairment? If so, what would be the impact of addressing them in vulnerable people? Should multimorbidity, or a certain level of it, systematically prompt screening for physical and cognitive impairment? What else can we learn from the biology of ageing that may contribute to delaying, preventing, alleviating or reversing age-related multimorbidity and functional impairment?
Table 2.
Pathways and risk factors common to multimorbidity and functional impairment: current knowledge
| What we know | Knowledge gaps |
|---|---|
| Biological factors | |
| • Old age, obesity, involuntary weight loss and sedentarism | • Specific biological mechanisms of ageing in |
| Pathway: All are risk factors for the burden and severity of | single individuals have not yet been identi |
| multimorbidity, probably because of their effect on levels of | fied |
| circulating inflammatory mediators (i.e. inflammaging) and thus | • Criteria to identify those forms of multi |
| on accelerated damage accumulation in organs and physiological | morbidity caused by accelerated biological |
| systems | ageing are lacking, which hampers the |
| • Ageing-related factors | development of targeted therapeutic and |
| Pathway: Intrinsic biological mechanisms of ageing (e.g. mito- | management interventions |
| chondrial dysfunction, cellular senescence, defective proteostasis) | • Specific clinical trials with outcomes related |
| could be the basis of the bidirectional association between | to the development and progression of mul |
| multimorbidity and functional impairment | timorbidity are still rare. An exception is the |
| • Epigenetic markers | future TAME study on chronic use of met |
| Pathway: Premature changes in epigenetic biomarkers (sensitive | formin [160] |
| to phenotypic deviations from normal biological ageing) are | |
| associated with the severity of multimorbidity, providing further | |
| support for the hypothesis that there is a link between the biology | |
| of ageing and the risk for multimorbidity and functional impair- | |
| ment | |
| Care-related factors (i.e. drugs) | |
| • Polypharmacy | • The need for and added value of preventive |
| Pathway: Drug-drug and drug-disease interactions and the pre | medicines in people with shortened life |
| scribing cascade (e.g. use of anti-Parkinson medication to treat | expectancy due to multimorbidity and/or |
| extrapyramidal symptoms caused by antipsychotics) | functional impairment is poorly understood |
| • Inappropriate use (e.g. anticholinergic drugs, proton pump | • The effectiveness of individual drugs in |
| inhibitors) or overuse (e.g. antidiabetics) of specific drugs | people with multimorbidity and/or func |
| • Lack of adherence to drug treatment | tional impairment, based on numbers |
| Pathway: Multimorbidity and functional deficits may limit | needed to treat, is little understood |
| patients’ ability to take medications accurately because of prob | • There is wide uncertainty about the impact |
| lems with pill handling. They may also affect decision-making and | of drug treatment on functional outcomes |
| reporting of adverse effects | (both physical and cognitive) |
| • Few studies have addressed the bidirec | |
| tional association between sarcopenia and | |
| adverse drug events | |
| Psychosocial factors | |
| • Socioeconomic status | • The role of psychosocial factors in multi |
| Pathway: Less than half of the excess multimorbidity in deprived | morbidity, functional impairment and neg |
| populations is explained by lifestyle; the rest may be due to factors | ative outcomes needs to be better |
| such as adverse childhood experiences, negative life events, weak | delineated. Specifically, little is known |
| social networks and an external locus of control | about the direction of the associations and |
| • Psychological distress, social isolation, social conflict, emotional | potential moderating or mediating effects |
| isolation and lack of purpose in life | • Although there is some evidence that social |
| Pathway: The association between these factors and decline in | interventions such as social prescribing |
| physical and cognitive function in old age could be due to poor | may improve anxiety, depression and phys |
| self-management; amotivation; risk factors such as smoking, | ical activity [161], the influence of such |
| alcohol, poor diet and low exercise levels; and/or direct effects on | approaches on functional impairment or |
| inflammation | mortality is unknown |
| • Clinical trials performed specifically on | |
| people living in deprived areas and incor | |
| porating modifiable psychosocial factors are | |
| rare. Recent primary care-based complex | |
| interventions focusing on priority goal set- | |
| ting [162] and patient-centredness [163] | |
| could serve as examples | |
In the meantime, the integrated assessment of multimorbidity and function – already embedded in instruments like the Health Assessment Tool [158] – should remain the basis of the overall clinical decision-making process, allowing physicians to more easily weigh treatment benefits and risks and patients to make properly informed choices. However, both constructs need to be considered as means towards the goal of patient-centred medicine rather than end goals in and of themselves [159]. We should avoid taxonomic simplification and/or routinization whereby both constructs become simple labels added to patients’ medical records, and no subsequent action is taken by healthcare professionals. Instead, such an integrated assessment should lead to measuring each patient’s improvement potential and establishing an individualized therapeutic or palliative plan of social and medical care.
From a health policy perspective, the reorganization and reinforcement of primary care will be essential to optimize health outcomes in people with multimorbidity and functional impairment, as it is person-focused and continuous over time. Primary care physicians are well prepared to appraise the interactions between multimorbidity and both physical and cognitive function whilst considering the lifelong effect of biological, care-related and psychosocial factors, as depicted in our circular diagram in Fig. 3. General practitioners may also play a major role in increasing awareness of the bidirectional interplay and synergistic effects of multimorbidity and functional impairment during their clinical encounters with older patients and their caregivers. Moreover, primary care is ideally suited to orchestrate the multidisciplinary care needed by older people with coexisting multimorbidity and functional limitations, given that it is embedded within local communities, where other social and public health agents coexist.
Footnotes
Content List - Read more articles from the symposium: “Multimorbidity research at the cross-roads: developing the evidence for clinical practice and health policy”.
References
- 1.Valderas JM, Starfield B, Sibbald B, Salisbury C, Roland M. Defining comorbidity: implications for understanding health and health services. Ann Fam Med 2009; 7: 357–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arokiasamy P, Uttamacharya U, Jain K et al. The impact of multimorbidity on adult physical and mental health in low- and middle-income countries: what does the study on global ageing and adult health (SAGE) reveal? BMC Med 2015; 13: 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fortin M, Stewart M, Poitras M, Maddocks H. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med 2012; 2012: 142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Violan C, Foguet-Boreu Q, Flores-Mateo G et al. Prevalence, determinants and patterns of multimorbidity in primary care: a systematic review of observational studies. PLoS ONE 2014; 9:e102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kingston A, Robinson L, Booth H, Knapp M, Jagger C; MODEM project. Projections of multi-morbidity in the older population in England to 2035: estimates from the Population Ageing and Care Simulation (PACSim) model. Age Ageing 2018; 47: 374–80. 10.1093/ageing/afx201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Oostrom SH, Gijsen R, Stirbu I et al. Time trends in prevalence of chronic diseases and multimorbidity not only due to aging: data from general practices and health surveys. PLoS ONE 2016; 11: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canizares M, Hogg-Johnson S, Gignac MAM, Glazier RH, Badley EM. Increasing trajectories of multimorbidity over time: birth cohort differences and the role of changes in obesity and income. J Gerontol B Psychol Sci Soc Sci 2017; 73: 1303–14. [DOI] [PubMed] [Google Scholar]
- 8.Ryan A, Wallace E, O’Hara P, Smith SM. Multimorbidity and functional decline in community-dwelling adults: a systematic review. Health Qual Life Outcomes 2015; 13: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caracciolo B, Gatz M, Xu W, Marengoni A, Pedersen NL, Fratiglioni L. Relationship of subjective cognitive impairment and cognitive impairment no dementia to chronic disease and multimorbidity in a nation-wide twin study. J Alzheimers Dis 2013; 36: 275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanesarajah J, Waller M, Whitty JA, Mishra GD. Multimorbidity and quality of life at mid-life: a systematic review of general population studies. Maturitas 2018; 109: 53–62. [DOI] [PubMed] [Google Scholar]
- 11.Marengoni A, Angleman S, Melis R et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev 2011; 10: 430–9. [DOI] [PubMed] [Google Scholar]
- 12.Nunes BP, Flores TR, Mielke GI, Thume E, Facchini LA. Multimorbidity and mortality in older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr 2016; 67: 130–8. [DOI] [PubMed] [Google Scholar]
- 13.Di Angelantonio E, Kaptoge S, Wormser D et al. Association of cardiometabolic multimorbidity with mortality. JAMA 2015; 314: 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shippee ND, Shah ND, May CR, Mair FS, Montori VM. Cumulative complexity: a functional, patient-centered model of patient complexity can improve research and practice. J Clin Epidemiol 2012; 65: 1041–51. [DOI] [PubMed] [Google Scholar]
- 15.Depp CA, Jeste DV. Definitions and predictors of successful aging: a comprehensive review of larger quantitative studies. Am J Geriatr Psychiatry 2006; 14: 6–20. [DOI] [PubMed] [Google Scholar]
- 16.Parsons S, Gale CR, Kuh D, Elliot J. Physical capability and the advantages and disadvantages of ageing: perceptions of older age by men and women in two British cohorts. Ageing Soc 2014; 34: 452–71. [Google Scholar]
- 17.Richardson WS, Doster LM. Comorbidity and multimorbidity need to be placed in the context of a framework of risk, responsiveness, and vulnerability. J Clin Epidemiol 2014; 67: 244–6. [DOI] [PubMed] [Google Scholar]
- 18.Santoni G, Angleman S, Welmer A-K, Mangialasche F, Marengoni A, Fratiglioni L. Age-related variation in health status after age 60. PLoS ONE 2015; 10: e0120077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayliss EA, Bayliss MS, Ware JE, Steiner JF. Predicting declines in physical function in persons with multiple chronic medical conditions: what we can learn from the medical problem list. Health Qual Life Outcomes 2004; 2: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marengoni A, von Strauss E, Rizzuto D, Winblad B, Fratiglioni L. The impact of chronic multimorbidity and disability on functional decline and survival in elderly persons. A community-based, longitudinal study. J Intern Med 2009; 265: 288–95. [DOI] [PubMed] [Google Scholar]
- 21.Garin N, Olaya B, Moneta MV et al. Impact of multimorbidity on disability and quality of life in the Spanish older population. PLoS ONE 2014; 9: e111498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aarts S, den Akker M, van Bosma H et al. The effect of multimorbidity on health related functioning: temporary or persistent? Results from a longitudinal cohort study. J Psychosom Res 2012; 73: 211–7. [DOI] [PubMed] [Google Scholar]
- 23.Rizzuto D, Melis RJF, Angleman S, Qiu C, Marengoni A. Effect of chronic diseases and multimorbidity on survival and functioning in elderly adults. J Am Geriatr Soc 2017; 65: 1056–60. [DOI] [PubMed] [Google Scholar]
- 24.Cheung C-L, Nguyen U- SDT, Au E, Tan KCB, Kung AWC. Association of handgrip strength with chronic diseases and multimorbidity: a cross-sectional study. Age (Dordr) 2013; 35: 929–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei MY, Kabeto MU, Langa KM, Mukamal KJ. Multimorbidity and physical and cognitive function: performance of a new multimorbidity-weighted index. J Gerontol A Biol Sci Med Sci 2018; 73: 225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falvey JR, Gustavson AM, Price L, Papazian L, Stevens- Lapsley JE. Dementia, comorbidity, and physical function in the program of all-inclusive care for the elderly. J Geriatr Phys Ther 2017. 10.1519/jpt.0000000000000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welmer A-K, Kareholt I, Angleman S, Rydwik E, Fratiglioni L. Can chronic multimorbidity explain the age-related differences in strength, speed and balance in older adults? Aging Clin Exp Res 2012; 24: 480–9. [DOI] [PubMed] [Google Scholar]
- 28.Jindai K, Nielson CM, Vorderstrasse BA, Quinones AR. Multimorbidity and functional limitations among adults 65 or older, NHANES 2005–2012. Prev ChronicDis 2016; 13: E151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volaklis KA, Halle M, Thorand B et al. Handgrip strength is inversely and independently associated with multimorbidity among older women: results from the KORA-Age study. Eur J Intern Med 2016; 31: 35–40. [DOI] [PubMed] [Google Scholar]
- 30.Lu FP, Chang WC, Wu SC. Geriatric conditions, rather than multimorbidity, as predictors of disability and mortality among octogenarians: a population-based cohort study. Geriatr Gerontol Int 2016; 16: 345–51. [DOI] [PubMed] [Google Scholar]
- 31.Portz JD, Kutner JS, Blatchford PJ, Ritchie CS. High symptom burden and low functional status in the setting of multimorbidity. J Am Geriatr Soc 2017; 65: 2285–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yancik R, Ershler W, Satariano W, Hazzard W, Cohen HJ, Ferrucci L. Report of the national institute on aging task force on comorbidity. J Gerontol A Biol Sci Med Sci 2007; 62: 275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fabbri E, An Y, Zoli M et al. Aging and the burden of multimorbidity: associations with inflammatory and anabolic hormonal biomarkers. J Gerontol A Biol Sci Med Sci 2015; 70: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calderon-Larranaga A, Santoni G, Wang HX et al. Rapidly developing multimorbidity and disability in older adults: does social background matter? J Intern Med 2018; 283: 489–99. [DOI] [PubMed] [Google Scholar]
- 35.Marventano S, Ayala A, Gonzalez N, Rodroguez-Blaozquez C, Garcia-Gutierrez S, Forjaz MJ. Multimorbidity and functional status in community-dwelling older adults. Eur J Intern Med 2014; 25: 610–6. [DOI] [PubMed] [Google Scholar]
- 36.Kriegsman DMW, Deeg DJH, Stalman WAB. Comorbidity of somatic chronic diseases and decline in physical functioning:; the Longitudinal Aging Study Amsterdam. J Clin Epidemiol 2004; 57: 55–65. [DOI] [PubMed] [Google Scholar]
- 37.Marengoni A, Angleman S, Fratiglioni L. Prevalence of disability according to multimorbidity and disease clustering: a population-based study. J Comorb 2011; 1: 11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vetrano DL, Rizzuto D, Calderon-Larranaga A et al. Trajectories of functional decline in older adults with neuropsychiatric and cardiovascular multimorbidity: a Swedish cohort study. PLoS Med 2018; 15: e1002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koller D, Schon G, Schafer I, Glaeske G, van den Bussche H, Hansen H. Multimorbidity and long-term care dependency— a five-year follow-up. BMC Geriatr 2014; 14: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quinones AR, Markwardt S, Botoseneanu A. Multimorbidity combinations and disability in older adults. J Gerontol A Biol Sci Med Sci 2016; 71: 823–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quinones AR, Markwardt S, Thielke S, Rostant O, Vasquez E, Botoseneanu A. Prospective disability in different combinations of somatic and mental multimorbidity. J Gerontol A Biol Sci Med Sci 2018; 73: 204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jackson CA, Jones M, Tooth L, Mishra GD, Byles J, Dobson A. Multimorbidity patterns are differentially associated with functional ability and decline in a longitudinal cohort of older women. Age Ageing 2015; 44: 810–6. [DOI] [PubMed] [Google Scholar]
- 43.Fried LP, Tangen CM, Walston J et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–56. [DOI] [PubMed] [Google Scholar]
- 44.Vetrano DL, Palmer K, Marengoni A et al. Frailty and multimorbidity: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci 2018. 10.1093/gerona/gly110. [DOI] [PubMed] [Google Scholar]
- 45.Ryan A, Murphy C, Boland F, Galvin R, Smith SM. What is the impact of physical activity and physical function on the development of Multimorbidity in older adults over time? A population based cohort study. J Gerontol A Biol Sci Med Sci 2018; 73: 1538–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cimarras-Otal C, Calderon-Larranaga A, Poblador-Plou B et al. Association between physical activity, multimorbidity, self-rated health and functional limitation in the Spanish population. BMC Public Health 2014; 14: 1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dankel SJ, Loenneke JP, Loprinzi PD. Participation in muscle-strengthening activities as an alternative method for the prevention of multimorbidity. Prev Med (Baltim) 2015; 81: 54–7. [DOI] [PubMed] [Google Scholar]
- 48.Dankel SJ, Loenneke JP, Loprinzi PD. Combined associations of muscle-strengthening activities and accelerometer- assessed physical activity on multimorbidity: findings from NHANES. Am J Health Promot 2017; 31: 274–7. [DOI] [PubMed] [Google Scholar]
- 49.Dhalwani NN, O’Donovan G, Zaccardi F et al. Long terms trends of multimorbidity and association with physical activity in older English population. Int J Behav Nutr Phys Act 2016; 13: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marengoni A, Rizzuto D, Fratiglioni L et al. The effect of a 2- year intervention consisting of diet, physical exercise, cognitive training, and monitoring of vascular risk on chronic morbidity-the FINGER randomized controlled trial. J Am Med Dir Assoc 2018; 19: e1. [DOI] [PubMed] [Google Scholar]
- 51.Manemann SM, Chamberlain AM, Roger VL et al. Multimorbidity and functional limitation in individuals with heart failure: a prospective community study. J Am Geriatr Soc 2018; 66: 1101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ensrud KE, Lui L-Y, Langsetmo L et al. Effects of mobility and multimorbidity on inpatient and post-acute health care utilization. J Gerontol A Biol Sci Med Sci 2017; 73: 1343–9. 10.1093/gerona/glx128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Landi F, Calvani R, Tosato M et al. Impact of physical function impairment and multimorbidity on mortality among community-living older persons with sarcopaenia: results from the ilSIRENTE prospective cohort study. BMJ Open 2016; 6: e008281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.St John PD, Tyas SL, Menec V, Tate R. Multimorbidity, disability, and mortality in community-dwelling older adults. Can Fam Physician 2014; 60: e272–80. [PMC free article] [PubMed] [Google Scholar]
- 55.Wei MY, Kabeto MU, Galecki AT, Langa KM. Physical functioning decline and mortality in older adults with multimorbidity: joint modeling of longitudinal and survival data. J Gerontol A Biol Sci Med Sci 2018. 10.1093/gerona/gly038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loprinzi PD, Addoh O, Joyner C. Multimorbidity, mortality, and physical activity. Chronic Illn 2016; 12: 272–80. [DOI] [PubMed] [Google Scholar]
- 57.Martinez-Gomez D, Guallar-Castillon P, Garcia-Esquinas E, Bandinelli S, Rodriguez-Artalejo F. Physical activity and the effect of multimorbidity on all-cause mortality in older adults. Mayo Clin Proc 2017; 92: 376–82. [DOI] [PubMed] [Google Scholar]
- 58.Bunn F, Burn A-M, Goodman C et al. Comorbidity and dementia: a scoping review of the literature. BMC Med 2014; 12: 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Magaki S, Yong WH, Khanlou N, Tung S, Vinters HV. Comorbidity in dementia: update of an ongoing autopsy study. J Am Geriatr Soc 2014; 62: 1722–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Melis R, Marengoni A, Angleman S, Fratiglioni L. Incidence and predictors of multimorbidity in the elderly: a population- based longitudinal study. PLoS ONE 2014; 9: e103120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koroukian SM, Schiltz N, Warner DF et al. Combinations of chronic conditions, functional limitations, and geriatric syndromes that predict health outcomes. J Gen Intern Med 2016; 31: 630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fabbri E, An Y, Zoli M et al. Association between accelerated multimorbidity and age-related cognitive decline in older baltimore longitudinal study of aging participants without dementia. J Am Geriatr Soc 2016; 64: 965–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ngandu T, Lehtisalo J, Solomon A et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet (London, England) 2015; 385: 2255–63. [DOI] [PubMed] [Google Scholar]
- 64.Vassilaki M, Aakre JA, Mielke MM et al. Multimorbidity and neuroimaging biomarkers among cognitively normal persons. Neurology 2016; 86: 2077–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aarts S, van den Akker M, Tan FES, Verhey FRJ, Metse- makers JFM, van Boxtel MPJ. Influence of multimorbidity on cognition in a normal aging population: a 12-year follow-up in the Maastricht Aging Study. Int J Geriatr Psychiatry 2011; 26: 1046–53. [DOI] [PubMed] [Google Scholar]
- 66.Vassilaki M, Aakre JA, Cha RH et al. Multimorbidity and risk of mild cognitive impairment. J Am Geriatr Soc 2015; 63: 1783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang J-H, Wu Y- J, Tee BL, Lo RY. Medical comorbidity in Alzheimer’s disease: a nested case-control study. J Alzheimers Dis 2018; 63: 773–81. [DOI] [PubMed] [Google Scholar]
- 68.Gerritsen AAJ, Bakker C, Verhey FRJ et al. Prevalence of comorbidity in patients with young-onset Alzheimer disease compared with late-onset: a comparative cohort study. J Am Med Dir Assoc 2016; 17: 318–23. [DOI] [PubMed] [Google Scholar]
- 69.Winblad B, Amouyel P, Andrieu S et al. Defeating Alzheimer’s disease and other dementias: a priority for European science and society. Lancet Neurol 2016; 15: 455–532. [DOI] [PubMed] [Google Scholar]
- 70.Clague F, Mercer SW, McLean G, Reynish E, Guthrie B. Comorbidity and polypharmacy in people with dementia: insights from a large, population-based cross-sectional analysis of primary care data. Age Ageing 2017; 46: 33–9. [DOI] [PubMed] [Google Scholar]
- 71.Cermakova P, Johnell K, Fastbom J et al. Cardiovascular diseases in ~30,000 patients in the Swedish Dementia Registry. J Alzheimers Dis 2015; 48: 949–58. [DOI] [PubMed] [Google Scholar]
- 72.Enache D, Fereshtehnejad S-M, Kareholt I et al. Antidepressants and mortality risk in a dementia cohort: data from SveDem, the Swedish Dementia Registry. Acta Psychiatr Scand 2016; 134: 430–40. [DOI] [PubMed] [Google Scholar]
- 73.Qiu C, Fratiglioni L. A major role for cardiovascular burden in age-related cognitive decline. Nat Rev Cardiol 2015; 12: 267–77. [DOI] [PubMed] [Google Scholar]
- 74.Poblador-Plou B, Calderon-Larranaga A, Marta-Moreno J et al. Comorbidity of dementia: a cross-sectional study of primary care older patients. BMC Psychiatry 2014; 14: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Newcomer SR, Steiner JF, Bayliss EA. Identifying subgroups of complex patients with cluster analysis. Am J Manag 2011; 17: e324–32. [PubMed] [Google Scholar]
- 76.Schafer I, von Leitner EC, Schon G et al. Multimorbidity patterns in the elderly: a new approach of disease clustering identifies complex interrelations between chronic conditions. PLoSONE 2010; 5: e15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Duthie A, Chew D, Soiza RL. Non-psychiatric comorbidity associated with Alzheimer’s disease. QJM 2011; 104: 913–20. [DOI] [PubMed] [Google Scholar]
- 78.Haaksma MLML, Vilela LRLR, Marengoni A et al. Comorbidity and progression of late onset Alzheimer’s disease: a systematic review. PLoS ONE 2017; 12: e0177044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bauer K, Schwarzkopf L, Graessel E, Holle R. A claims data-based comparison of comorbidity in individuals with and without dementia. BMC Geriatr 2014; 14: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Subic A, Cermakova P, Religa D et al. Treatment of atrial fibrillation in patients with dementia: a cohort study from the Swedish Dementia Registry. J Alzheimers Dis 2018; 61: 1119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ekdahl AW, Odzakovic E, Hellström I. Living unnoticed: cognitive impairment in older people with multimorbidity. J Nutr Health Aging 2016; 20: 275–9. [DOI] [PubMed] [Google Scholar]
- 82.Martin-Garcia S, Rodriguez-Blazquez C, Martinez-Lopez I, Martinez-Martin P, Forjaz MJ. Comorbidity, health status, and quality of life in institutionalized older people with and without dementia. Int Psychogeriatr 2013; 25: 1077–84. [DOI] [PubMed] [Google Scholar]
- 83.Slaughter SE, Hayduk LA. Contributions of environment, comorbidity, and stage of dementia to the onset of walking and eating disability in long-term care residents. J Am Geriatr Soc 2012; 60: 1624–31. [DOI] [PubMed] [Google Scholar]
- 84.Tonelli M, Wiebe N, Straus S et al. Multimorbidity, dementia and health care in older people:a population-based cohort study. CMAJ Open 2017; 5: E623–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zekry D, Herrmann FR, Graf CE et al. High levels of comorbidity and disability cancel out the dementia effect in predictions of long-term mortality after discharge in the very old. Dement Geriatr Cogn Disord 2011; 32: 103–10. [DOI] [PubMed] [Google Scholar]
- 86.Mondor L, Maxwell CJ, Hogan DB et al. Multimorbidity and healthcare utilization among home care clients with dementia in Ontario, Canada: a retrospective analysis of a population-based cohort. PLoS Med 2017; 14: e1002249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Phelan EA, Debnam KJ, Anderson LA, Owens SB. A systematic review of intervention studies to prevent hospitalizations of community-dwelling older adults with dementia. Med Care 2015; 53: 207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Browne J, Edwards DA, Rhodes KM, Brimicombe DJ, Payne RA. Association of comorbidity and health service usage among patients with dementia in the UK: a population-based study. BMJ Open 2017; 7: e012546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kuo T-C, Zhao Y, Weir S, Kramer MS, Ash AS. Implications of comorbidity on costs for patients with Alzheimer disease. Med Care 2008; 46: 839–46. [DOI] [PubMed] [Google Scholar]
- 90.Fabbri E, Zoli M, Gonzalez-Freire M, Salive ME, Studenski SA, Ferrucci L. Aging and multimorbidity: new tasks, priorities, and frontiers for integrated gerontological and clinical research. J Am Med Dir Assoc 2015; 16: 640–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yarnall AJ, Sayer AA, Clegg A, Rockwood K, Parker S, Hindle JV. New horizons in multimorbidity in older adults. Age Ageing 2017; 46: 882–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jovic D, Marinkovic J, Vukovic D. Association between body mass index and prevalence of multimorbidity: a cross-sectional study. Public Health 2016; 139: 103–11. [DOI] [PubMed] [Google Scholar]
- 93.Kivimaki M, Kuosma E, Ferrie JE et al. Overweight, obesity, and risk of cardiometabolic multimorbidity: pooled analysis of individual-level data for 120 813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health 2017; 2: e277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hirani V, Naganathan V, Blyth F et al. Longitudinal associations between body composition, sarcopenic obesity and outcomes of frailty, disability, institutionalisation and mortality in community-dwelling older men: the Concord Health and Ageing in Men Project. Age Ageing 2017; 46: 413–20. [DOI] [PubMed] [Google Scholar]
- 95.Fabbri E, Tanaka T, An Y et al. Loss of weight in obese older adults: A biomarker of impending expansion of multimorbidity? J Am Geriatr Soc 2015; 63: 1791–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kerr J, Anderson C, Lippman SM. Physical activity, sedentary behaviour, diet, and cancer: an update and emerging new evidence. Lancet Oncol 2017; 18: e457–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Endrighi R, Steptoe A, Hamer M. The effect of experimentally induced sedentariness on mood and psychobiological responses to mental stress. Br J Psychiatry 2016; 208: 245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Duffield SJ, Ellis BM, Goodson N et al. The contribution of musculoskeletal disorders in multimorbidity: implications for practice and policy. Best Pract Res Clin Rheumatol 2017; 31: 129–44. [DOI] [PubMed] [Google Scholar]
- 99.Dhalwani NN, Zaccardi F, O’Donovan G et al. Association Between lifestyle factors and the incidence of multimorbidity in an older english population. J Gerontol A Biol Sci Med Sci 2016; 72: 528–34. [DOI] [PubMed] [Google Scholar]
- 100.Loprinzi PD. Associations between bouted and non-bouted physical activity on multimorbidity. Clin Physiol Funct Imaging 2017; 37: 782–4. [DOI] [PubMed] [Google Scholar]
- 101.Volaklis KA, Thorand B, Peters A et al. Physical activity, muscular strength, and polypharmacy among older multimorbid persons: results from the KORA-Age study. Scand J Med Sci Sports 2018; 28: 604–12. [DOI] [PubMed] [Google Scholar]
- 102.Friedman EM, Christ SL, Mroczek DK. Inflammation partially mediates the association of multimorbidity and functional limitations in a national sample of middle-aged and older adults: the MIDUS study. J Aging Health 2015; 27: 843–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bektas A, Schurman SH, Sen R, Ferrucci L. Aging, inflammation and the environment. Exp Gerontol 2018; 105: 10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barnes PJ. Mechanisms of development of multimorbidity in the elderly. Eur Respir J 2015; 45: 790–806. [DOI] [PubMed] [Google Scholar]
- 105.Sturmberg JP, Bennett JM, Martin CM, Picard M. ‘Multimorbidity’ as the manifestation of network disturbances. J Eval Clin Pract 2017; 23: 199–208. [DOI] [PubMed] [Google Scholar]
- 106.Fabbri E, An Y, Schrack JA et al. Energy metabolism and the burden of multimorbidity in older adults: results from the baltimore longitudinal study of aging. J Gerontol A Biol Sci Med Sci 2015; 70: 1297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ruggiero C, Metter EJ, Melenovsky V et al. High basal metabolic rate is a risk factor for mortality: the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci 2008; 63: 698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Haugsgjerd TR, Dierkes J, Vollset SE et al. Association between weight change and mortality in community living older people followed for up to 14 years. The Hordaland Health Study (HUSK). J Nutr Health Aging 2017; 21: 909–17. [DOI] [PubMed] [Google Scholar]
- 109.Alley DE, Metter EJ, Griswold ME et al. Changes in weight at the end of life: characterizing weight loss by time to death in a cohort study of older men. Am J Epidemiol 2010; 172: 558–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gensous N, Bacalini MG, Pirazzini C et al. The epigenetic landscape of age-related diseases: the geroscience perspective. Biogerontology 2017; 18: 549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kohanski RA, Deeks SG, Gravekamp C et al. Reverse geroscience: how does exposure to early diseases accelerate the age-related decline in health? Ann N Y Acad Sci 2016; 1386: 30–44. [DOI] [PubMed] [Google Scholar]
- 112.Ferrucci L Commentary: life course epidemiology embraces geroscience. Int J Epidemiol 2016; 45: 1015–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Martin GM. Geroscience: addressing the mismatch between its exciting research opportunities, its economic imperative and its current funding crisis. Exp Gerontol 2017; 94: 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.St Sauver JL, Boyd CM, Grossardt BR et al. Risk of developing multimorbidity across all ages in an historical cohort study: differences by sex and ethnicity. BMJ Open 2015; 5: e006413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Izaks GJ, Westendorp RGJ. Ill or just old? Towards a conceptual framework of the relation between ageing and disease. BMC Geriatr 2003; 3: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Newman AB, Dodson JA, Church TS et al. Cardiovascular events in a physical activity intervention compared with a successful aging intervention: the LIFE study randomized trial. JAMA Cardiol 2016; 1: 568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pahor M, Guralnik JM, Ambrosius WT et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA 2014; 311: 2387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Levine ME, Lu AT, Quach A et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY) 2018; 10: 573–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Levine ME, Lu AT, Bennett DA, Horvath S. Epigenetic age of the pre-frontal cortex is associated with neuritic plaques, amyloid load, and Alzheimer’s disease related cognitive functioning. Aging (Albany NY) 2015; 7: 1198–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Boyd CM, Landefeld CS, Counsell SR et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am GeriatrSoc 2008; 56: 2171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Enthoven WTM, Roelofs PD, Koes BW. NSAIDs for chronic low back pain. JAMA 2017; 317: 2327–8. [DOI] [PubMed] [Google Scholar]
- 122.Roland KP, Cornett KMD, Theou O, Jakobi JM, Jones GR. Concurrence of frailty and parkinson’s disease. J Frailty Aging 2012; 1: 123–7. [DOI] [PubMed] [Google Scholar]
- 123.Vaz Fragoso CA, Enright PL, McAvay G, Van Ness PH, Gill TM. Frailty and respiratory impairment in older persons. Am J Med 2012; 125: 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Williamson JD, Supiano MA, Applegate WB et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged >75 years: a randomized clinical trial. JAMA 2016; 315: 2673–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Onder G, Vetrano DL, Marengoni A et al. Accounting for frailty when treating chronic diseases. Eur J Intern Med 2018; 56: 49–52. 10.1016/j.ejim.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 126.Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United Statesfrom 1999–2012. JAMA 2015; 314: 1818–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Marengoni A, Onder G. Guidelines, polypharmacy, and drug-drug interactions in patients with multimorbidity. BMJ 2015; 350: h1059. [DOI] [PubMed] [Google Scholar]
- 128.Marengoni A, Pasina L, Concoreggi C et al. Understanding adverse drug reactions in older adults through drug-drug interactions. Eur J Intern Med 2014; 25: 843–6. [DOI] [PubMed] [Google Scholar]
- 129.Baracos VE, Arribas L. Sarcopenic obesity: hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann Oncol 2018; 29: ii1–9. [DOI] [PubMed] [Google Scholar]
- 130.Rochon PA, Gurwitz JH. Optimising drug treatment for elderly people: the prescribing cascade. BMJ 1997; 315: 1096–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Onder G, Bonassi S, Abbatecola AM et al. High prevalence of poor quality drug prescribing in older individuals: a nation wide report from the Italian Medicines Agency (AIFA). J Gerontol A Biol Sci Med Sci 2014; 69: 430–7. [DOI] [PubMed] [Google Scholar]
- 132.Gill SS, Mamdani M, Naglie G et al. A prescribing cascade involving cholinesterase inhibitors and anticholinergic drugs. Arch Intern Med 2005; 165: 808–13. [DOI] [PubMed] [Google Scholar]
- 133.Gutierrez-Valencia M, Martinez-Velilla N, Vetrano DL et al. Anticholinergic burden and health outcomes among older adults discharged from hospital: results from the CRIME study. Eur J Clin Pharmacol 2017; 73: 1467–74. [DOI] [PubMed] [Google Scholar]
- 134.Vetrano DL, Villani ER, Grande G et al. Association of polypharmacy with 1-year trajectories of cognitive and physical function in nursing home residents: results from a multicenter european study. J Am Med Dir Assoc 2018; 19: 710–3. [DOI] [PubMed] [Google Scholar]
- 135.Corsonello A, Lattanzio F, Bustacchini S et al. Adverse events of proton pump inhibitors: potential mechanisms. Curr Drug Metab 2018; 19: 142–54. [DOI] [PubMed] [Google Scholar]
- 136.ACCORD Study Group, Gerstein HC, Miller ME et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 2011; 364: 818–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Palmer K, Marengoni A, Russo P, Mammarella F, Onder G. Frailty and drug use. J Frailty Aging 2016; 5: 100–3. [DOI] [PubMed] [Google Scholar]
- 138.Fialovao D, Onder G. Medication errors in elderly people: contributing factors and future perspectives. Br J Clin Pharmacol 2009; 67: 641–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Brauner DJ, Muir JC, Sachs GA. Treating nondementia illnesses in patients with dementia. JAMA 2000; 283: 3230–5. [DOI] [PubMed] [Google Scholar]
- 140.Barnett K, Mercer S, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012; 380: 37–43. [DOI] [PubMed] [Google Scholar]
- 141.Pathirana TI, Jackson CA. Socioeconomic status and multimorbidity: a systematic review and meta-analysis. Aust NZ J Public Health 2018; 42: 186–94. [DOI] [PubMed] [Google Scholar]
- 142.Lund Jensen N, Pedersen HS, Vestergaard M, Mercer S, Glumer C, Prior A. The impact of socioeconomic status and multimorbidity on mortality: a population-based cohort study. Clin Epidemiol 2017; 9: 279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lawson KD, Mercer SW, Wyke S et al. Double trouble: the impact of multimorbidity and deprivation on preference- weighted health related quality of life a cross sectional analysis of the Scottish Health Survey. Int J Equity Health 2013; 12: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Payne RA, Abel GA, Guthrie B, Mercer SW. The effect of physical multimorbidity, mental health conditions and socioeconomic deprivation on unplanned admissions to hospital: a retrospective cohort study. Can Med Assoc J 2013; 185: E221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gunn JM, Ayton DR, Densley K et al. The association between chronic illness, multimorbidity and depressive symptoms in an Australian primary care cohort. Soc Psychiatry Psychiatr Epidemiol 2012; 47: 175–84. [DOI] [PubMed] [Google Scholar]
- 146.McLean G, Gunn J, Wyke S et al. The influence of socioeconomic deprivation on multimorbidity at different ages: a cross-sectional study. Br J Gen Pract 2014; 64: e440–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ohrnberger J, Fichera E, Sutton M. The relationship between physical and mental health: a mediation analysis. Soc Sci Med 2017; 195: 42–9. [DOI] [PubMed] [Google Scholar]
- 148.Katikireddi SV, Skivington K, Leyland AH, Hunt K, Mercer SW. The contribution of risk factors to socioeconomic inequalities in multimorbidity across the life course: a longitudinal analysis of the Twenty-07 cohort. BMC Med 2017; 15: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sinnott C, Mc Hugh S, Fitzgerald AP, Bradley CP, Kearney PM. Psychosocial complexity in multimorbidity: the legacy of adverse childhood experiences. Fam Pract 2015; 32: 269–75. [DOI] [PubMed] [Google Scholar]
- 150.van den Akker M, Buntinx F, Metsemakers JF, van der Aa M, Knottnerus JA. Psychosocial patient characteristics and GP- registered chronic morbidity: a prospective study. J Psychosom Res 2001; 50: 95–102. [DOI] [PubMed] [Google Scholar]
- 151.van den Akker M, Vos R, Knottnerus JA. In an exploratory prospective study on multimorbidity general and disease- related susceptibility could be distinguished. J Clin Epidemiol 2006; 59: 934–9. [DOI] [PubMed] [Google Scholar]
- 152.Mounce LTA, Campbell JL, Henley WE, Arreal MCT, Porter I, Valderas JM. Predicting incident multimorbidity. Ann Fam Med 2018; 16: 322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Boeckxstaens P, Vaes B, De Sutter A et al. A high sense of coherence as protection against adverse health outcomes in patients aged 80 years and older. Ann Fam Med 2016; 14: 337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Larsson G Sense of coherence, socioeconomic conditions and health. Interrelationships in a nation-wide Swedish sample. Eur J Public Health 1996; 6: 175–80. [Google Scholar]
- 155.Krishnadas R, McLean J, Batty GD et al. Socioeconomic deprivation and cortical morphology: psychological, social, and biological determinants of ill health study. Psychosom Med 2013; 75: 616–23. [DOI] [PubMed] [Google Scholar]
- 156.Wilson RS, Bennett DA. How does psychosocial behavior contribute to cognitive health in old age? Brain Sci 2017; 7 10.3390/brainsci7060056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Seeman T, Chen X. Risk and protective factors for physical functioning in older adults with and without chronic conditions: MacArthur Studies of Successful Aging. J Gerontol B Psychol Sci Soc Sci 2002; 57: S135–44. [DOI] [PubMed] [Google Scholar]
- 158.Santoni G, Marengoni A, Calderon-Larranaga A et al. Defining health trajectories in older adults with five clinical indicators. J Gerontol A Biol Sci Med Sci 2017; 72: 1123–9. 10.1093/gerona/glw204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Heath I, Sweeney K. Medical generalists: connecting the map and the territory. BMJ 2005; 331: 1462–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a tool to target aging. Cell Metab 2016; 23: 1060–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bickerdike L, Booth A, Wilson PM, Farley K, Wright K. Social prescribing: less rhetoric and more reality. A systematic review of the evidence. BMJ Open 2017; 7: e013384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mercer SW, Fitzpatrick B, Guthrie B et al. The CARE Plus study – a whole-system intervention to improve quality of life of primary care patients with multimorbidity in areas of high socioeconomic deprivation: exploratory cluster randomised controlled trial and cost-utility analysis. BMC Med 2016; 14:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Salisbury C, Man M-S, Bower P et al. Articles Management of multimorbidity using a patient-centred care model: a pragmatic cluster-randomised trial of the 3D approach. Lancet 2018; 6736: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]


