Abstract
Cystic fibrosis (CF) is a recessive genetic disease that is characterized by airway mucus plugging and reduced mucus clearance. There are currently alternative hypotheses that attempt to describe the abnormally viscous and elastic mucus that is a hallmark of CF airways disease, including: a) loss of cystic fibrosis transmembrane regulator (CFTR)-dependent airway surface volume (water) secretion, producing mucus hyper-concentration-dependent increased viscosity; and b) impaired bicarbonate secretion by CFTR, producing acidification of airway surfaces and increased mucus viscosity. A series of experiments were conducted to determine the contributions of mucus concentration vs. pH to the rheological properties of airway mucus across length scales from the nanoscopic to macroscopic. Our results showed that, for length scales greater than the nanoscopic, i.e., those relevant to mucociliary clearance, the effect of mucus concentration dominated over the effect of airway acidification. Our data also showed that mucus hydration and chemical reduction of disulfide bonds that connect mucin monomers are more promising therapeutic approaches than alkalization.
Summary Statement:
Over length scales greater than the mesh size of the mucin polymeric network of mucus, concentration, and not pH, dominates the physical properties of mucus that are associated with cystic fibrosis airway disease.
Introduction
Airway surface liquid (ASL) protects the lung from airborne environmental irritants and infections. The ASL consists of the periciliary layer (PCL) and mucus layer. The PCL is a grafted polymeric brush of tethered mucins and other high molecular weight glycoproteins provides: 1) lubrication for ciliary beat and mucus layer transport; and 2) a size exclusion barrier [1]. The mucus layer is a viscoelastic fluid comprised of over 1000 different proteins [2], including two secreted mucins, MUC5B and MUC5AC [3], which give mucus its gel-like properties [3]. Collectively, the two layers of the ASL coordinate to trap and clear inhaled pathogens from the lung via mucociliary transport (MCT).
It is well established that CF airway mucus has abnormal rheological properties, including viscosities and elasticities orders of magnitude higher than normal mucus [1, 4-10]. There are currently alternative hypotheses that describe the genesis of the pathologically viscoelastic mucus in CF. One hypothesis predicts that impaired anion transport by CFTR, coupled to persistent sodium absorption, reduces airway surface ion content [5]. The reduction of Na+ and Cl− on airway surfaces is coupled to reduced water flow to the airway lumen, dehydrating the ASL, concentrating the mucus layer, and producing abnormal mucus viscoelastic properties and osmotic pressures [1, 11]. A competing hypothesis posits that defective CFTR fails to transport HCO3− to the airway surface, acidifying the ASL. The resultant reduced mucus layer pH is predicted to perturb the rheological properties of the mucus layer and impair clearance [12-14].
Mucus is a soft hydrogel composed of high molecular weight mucin polymers in an isotonic solvent. The concentration and molecular weight of mucin in the mucus layer defines the average distance between adjacent polymers in solution, i.e., the correlation length. The evidence that concentration profoundly affects airway mucus biophysical properties has emerged from recent measurements of mucus osmotic pressures and microbead rheology that employed probes with diameters between 200 nm and 2 μm [1, 8, 11, 15]. The evidence that pH has effects on CF mucus gel viscosity arose from fluorescent recovery after photobleaching (FRAP) mucus studies that employed probes < 10 nm in diameter [13]. Due to the length scale-dependent rheological properties of mucus [16-18], it is impossible to compare mucus viscosity measurements directly with probes of differing size. The 5 nm nanoscopic probes employed in FRAP protocols [13] diffuse in the solvent space between mucin molecules, while the larger particles used in microbead rheology assays probe the polymeric mucin network. We hypothesized that the mucus macroscopic rheological properties would correlate better with MCT than nanoscopic measures.
Accordingly, studies were performed to measure the effects of mucus concentration vs. pH on mucus properties and transport at different length scales. Studies of the buffer capacity of mucus as a function of concentration were first made (See Supplemental Materials). Next, pH and mucus concentration-dependent mucus viscosities were measured by FRAP. Biophysical measurements with length scales relevant to mucus gels, e.g., traditional microrheology and microbead rheology with particles larger than the mucus correlation lengths, were performed for comparison to FRAP measurements. Model porcine gastric mucin (PGM), bovine submaxillary mucin (BSM), and human bronchial epithelial (HBE) mucus from both normal and CF cultures were utilized for studies exploring: 1) pH ranges that exceeded that of normal and CF airway mucus; and 2) mucus concentrations that spanned normal vs. CF mucus. The effects of changes in pH vs. concentration on HBE MCT rates were directly compared. To test the relevance of in vitro mucus to disease samples, the concentrations, pHs, and viscoelastic properties of CF sputum were measured and correlations analyzed. Finally, the efficacy of three therapeutic strategies designed to restore mucus viscoelastic properties, e.g., mucus hydration, alkalization, and chemical reduction (i.e., mucolytics), were compared.
Methods
Mucus samples were prepared as previously described [19, 20], with buffer capacity measurements performed as described by Holma [21, 22] and Kim [23]. Spontaneous sputum samples were collected as detailed previously [11, 24]. Detailed methods for the pooling of sputum samples are given in the supplemental materials. Mucus nanorheology was performed with fluorescence recovery after photobleaching (FRAP) assays [24], microrheology assays were performed with particle tracking microrheology of 1μm diameter COOH modified beads [8], and cone and plate macroscopic rheology was performed as previously described [7, 25], ensuring that measurements we taking in the linear viscoelastic regime (See supplemental material). Osmotic pressure, PCL height, and mucociliary transport measurements were performed as previously described [1, 26]. Mucus harvested from human bronchial epithelial cell cultures are considered non-human subject protocol #03-1396. Sputum samples were collected under IRB #15-2431. See supplemental material for detailed methods.
Results
Biophysical Measurements: FRAP
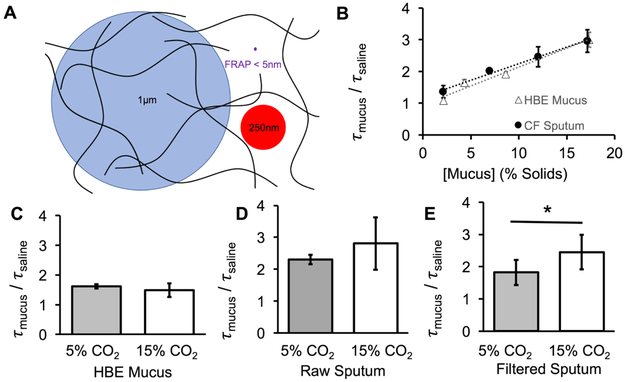
Biophysical measurements of complex fluids such as mucus must be tailored to the relevant biologic questions (See Supplementary Materials for more details). FRAP was measured using ~5 nm dextran molecules in HBE mucus and CF sputum of varying concentrations to measure the nanoscopic, interstitial rheology of mucus (Figure 1A). In both sample types, the diffusion of small molecules was linearly dependent on the concentration of the mucus gels at a constant pH of 7.2 (Figure 1B). An analysis of covariance performed on the regression lines for tau_mucus/tau_solvent (which equates to the ratio of the viscosity of the sample divide by the viscosity of the solvent) vs. % solids in HBE mucus and CF sputum revealed that each sample type exhibited significant (p < 0.01) and similar (p=0.4, NS) correlations with mucus concentration (n = 3 per concentration). These data indicate that HBE mucus is a reliable surrogate for the study of concentration-dependent FRAP-measured viscosity of CF sputum.
Figure 1:
Relevant biophysical size scales in mucus, showing mucin polymers (black lines) with an average correlation length, or mesh size, of 300 to 500 nm. 1-μm diameter beads (Blue) are larger than the correlation length. 250-nm beads (red) are sufficiently near to the mesh size that their diffusive motion reflects both the polymeric mucins network as well as the small proteins and solvent that permeate the intra-mucin space. The 5 nm probes (Purple) that are typically used in FRAP assays report the rheological properties of the solvent / small protein component of mucus. B: Frap recovery time ratio of mucus ratioed to saline recovery time for HBE mucus and CF sputum at various concentrations ranging from 3 to 17% solids. C: Recovery times for HBE mucus (no significance, p = 0.6). D: Recovery times for whole, raw sputum at 5 and 15% CO2, resulting in a change in pH of 7.2 to 6.6 (no significances, p = 0.19). E: Recovery times for filtered, mucin-less solvent component of sputum at the same CO2’s.
A second group of experiments examined the effect of pH on FRAP measured viscosity in HBE mucus and CF sputum at a constant concentration. Little evidence for a pCO2-dependent effect on FRAP measured viscosity was observed in HBE mucus (n = 14). In raw CF sputum (9% solids), a trend towards an increase in FRAP recovery times (proxy for increased viscosity on the nanometer length scale) was observed with acidification produced by 15% CO2 (p = 0.19, n = 7, Figure 1C). To distinguish whether the pH effect on FRAP reflected an effect of the solvent or the mucin polymer matrix, the sputum was filtered to remove the mucin polymer matrix (See supplemental material). The filtered sputum supernatant exhibited a significant (p = 0.013, n = 9) increase in FRAP at 15% CO2, similar in pattern to whole sputum (Figure 1D, E). Thus, the 5-nm FRAP probe reported viscosity of the solvent component of mucus, not of the polymeric gel. From the comparison of the composition of HBE mucus and CF sputum, our results indicate that inflammatory proteins generated in vivo in CF subjects, which are absent in HBE mucus [2], may be responsible for the exhibited pH-dependent changes in viscosity.
Biophysical Measurements: Microbead Rheology
Microbead rheology is a well-established technique capable of probing length scales of polymer networks like mucus gels [18]. In PGM studies, a 2.5-fold increase in mucin concentration produced a 5-fold reduction in bead mean squared displacement (MSD) at 1 sec at each designated pH (Figure 2A). In contrast, changes in pH over a 100-fold range (pH 6-8) produced only a modest (1.5-fold) effect on MSD at 1 second (Figure 2A) at either mucin concentration. Parallel studies of the rheology of BSM showed similar results (Figure 2B). Notably, HBE mucus exhibited the greatest dependence on concentration and least on pH (Figure 2C). The mean squared displacement of 1μm beads embedded in normal HBE mucus exhibited a 10 × reduction in MSD in response to a 2.5-fold increase in concentration compared to a 1.5 × reduction in MSD to a 100-fold increase in proton concentration. A nearly identical result was observed in CF HBE mucus (Figure 2D). Two-way ANOVA analysis of the MSD at 1 sec revealed a highly significant relationship between microbead rheology with respect to concentration (p < 0.01 for all samples, n = 3 per mucus type) while statistical significance was absent over the pH range investigated (p > 0.05).
Figure 2:
Microbead Rheology: A: PGM (20mg/ml (Blue) and 50mg/ml (Red)) MSD curves at pH 6, 7, and 8. B: BSM (20mg/ml (Blue) and 50mg/ml (Brown)) MSD curves at pH 6, 7, and 8. C: HBE mucus 2% solids (Blue), and 4% solids (Red) MSD curves at pH 6, 7, 8. D: CF HBE mucus 2% solids (Blue) and 4% solids (Red). In each case, the results of two-way ANOVA showed significant dependence of MSD on concentration (p << 0.01) and no significance on pH (P > 0.05).
Biophysical Measurements: Macroscopic Rheology
The macroscopic, or bulk, rheological properties of mucus are most often related to clearance of mucus from the lung [27-29]. Both concentration and pH have been shown to affect the rheological properties of mucus at these largest length scales [20, 30-32]. Figure 3 shows the relative effects of mucus concentration vs. pH on the complex viscosity of PGM, BSM, and HBE mucus. PGM at 50 mg/ml exhibited a viscosity that was more than 10-fold higher than at 20 mg/ml. In contrast, PGM at 20 mg/ml exhibited a complex viscosity that did not vary systematically between pH 6-8 (Figure 3A). BSM also exhibited large concentration-dependent viscosities, e.g., 20 mg/ml vs. 50 mg/ml, with little pH dependence (Figure 3B). With respect to airway mucus, the complex viscosity of a 4% solids HBE mucus was 6 to 10-fold higher than 2% solids HBE mucus with again little systematic pH dependence (Figure 3C). A similar pattern was also found in CF HBE mucus (Figure 3D). Two-way ANOVA revealed that concentration significantly altered the macroscopic rheology of all mucus types (p < 0.05), while pH showed no effect (p >> 0.05, n = 3 per mucus type, concentration, and pH). Thus, the result across all mucus samples was that varying the concentration of mucus altered the complex viscosity dramatically whereas variations in pH did not. It is noteworthy that it is only at pH < 5 that significant changes in the viscosity of 20 mg/ml PGM mucus were observed, consistent with the previous findings of Celli [20] (See supplemental materials).
Figure 3:
Macroscopic Rheology of two concentrations of mucus at pH 6,7, and 8. A: Complex viscosity, (η*), of 20 (white) and 50 mg/ml (grey) PGM. B: Complex viscosity of 20 (white) and 50 mg/ml (grey) BSM. C: Complex viscosity of 2.0% (white), and 4% (grey) normal HBE mucus. D: Complex viscosity of 2.0% (white), and 4% (grey) CF HBE mucus. Two-way ANOVA showed that the complex viscosities of PGM and normal and CF HBE were significantly dependent on concentration (p = 0.031 and p = 0.038), while BSM was of borderline significance (p = 0.05). In no case was there a significant correlation between pH and complex viscosity (p = 0.46 for PGM, p = 0.77 for BSM, and p = 0.83 for HBE). For reference, the viscosity of water is ~ 0.001 Pa·s.
Osmotic Pressure and PCL Height
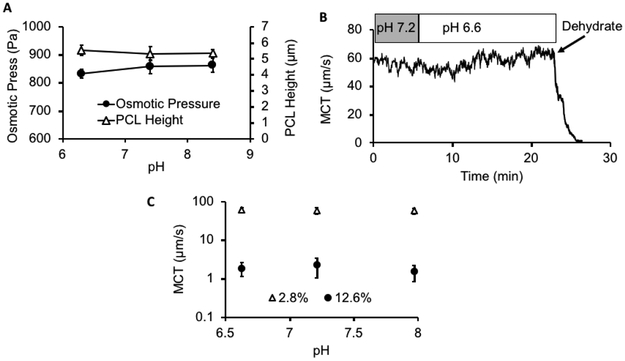
The airway surface is lined by two apposing layers: 1) a mucus layer containing the secreted MUC5AC and MUC5B mucins and 2) a brush-like periciliary layer (PCL) containing tethered mucin (e.g., MUC1, MUC4, and MUC16) and other glycoconjugates [1]. We have previously reported a HBE mucus concentration/osmotic pressure-dependent reduction in PCL height and MCT [24]. We selected a mucus concentration for study that produced a reduction in PCL height to test whether mucus osmotic pressure and PCL height reduction were modified by pH. Changing mucus pH over a range from pH8 to pH6 did not alter osmotic pressure of a concentrated (6.5% solids) mucus layer (Figure 4A; p ≥ 0.14 between each pH by paired t test). Consistent with the absence of a pH effect on osmotic pressure, PCL height was also not affected by varying pH over this range (Figure 4A; p ≥ 0.11 between each pH by paired t test). Note, PCL height with normal mucus concentrations is 7 μm.
Figure 4:
Effect of pH on MCT, osmotic pressure, and PCL height A: Effect of pH on mucus osmotic pressure (●) and PCL height (△) of 6.5% solids mucus on HBE culture. Note, the PCL height is normally ~ 7μm with a normal 2% solids (100 Pa) mucus layer apposed to it. B: Example trace of the effects of acidification (5% CO2, pH 7.2, to 15% CO2, pH 6.6) compared to changes in mucus concentration (by dehumidification) on MCT rate during a single experiment. C: Summary of the MCT rates comparing changes in concentration, 2.8% solids (△) vs. 12.6% solids (●) vs. pH (6.6, 7.2, and 7.9).
Mucociliary Transport
Mucociliary transport (MCT) measures the integrated activities of cilia beat and mucus properties. Measurements of MCT in HBE cultures identified that the baseline rate of MCT in these cultures over the initial portion of the experiment was ~ 60 μm/sec. A representative experiment demonstrated that increasing the concentration of CO2 from 5 to 15% in the luminal environment produced a decrease in the pH of the mucus layer from 7.2 to 6.6 over 5 minutes (Figure 4B). However, this reduction in pH did not produce a significant change in mucus transport velocity. In contrast, when the concentration of mucus was raised from 2.8 to 12.6% by removal of humidity from the environmental chamber, MCT ceased. The mean values for the effect of pH vs. concentration on MCT (Figure 4C) demonstrated, like micro and macrorheology measurements, mucus concentration (p << 0.05), not pH (p >> 0.05, n = 4 – 12), dominated MCT rates.
Relationships Between Mucus Concentration, pH, and Viscoelastic Properties in CF Sputum
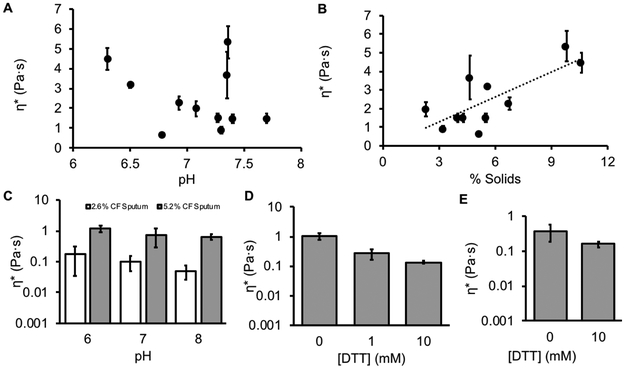
The concentration, pH, and rheology of 11 CF sputum samples were measured (Table 1). The average concentration of these samples was 5.2 ± 2.3 %, the average pH 7.2 ± 0.22, and the average complex viscosity 2.2 ± 1.6 Pa·s. A significant correlation was observed between concentration and complex viscosity (p = 0.006, Figure 5A). In contrast, no correlation was observed between the pH of sputum samples and complex viscosity (p = 0.42, Figure 5B). Thus, our data from cultured normal and CF HBE mucus appears to predict data from CF sputum.
Table 1. Demographics of patients from whom sputum was obtained.
Demographics of CF sputum donors. In total, 11 sputum samples were collected from 7 female and 4 male patients, one of whom was African American. The remaining 10 were Caucasian. Mean patient age was 28.5 years, ranging from 18-46 years of age. Spirometry data were obtained for each patient at the time of sample collection. The mean FEV1 % predicted value was 48.8 ranging from normal (90% predicted) to severely impaired (24% predicted) lung function. Additionally mean FVC was 2.7 L, and mean FEF25-75 was 0.96. Values of pH, % solids, and mean complex viscosity (η*) were also obtained for each sputum sample. Mean pH was 7.1 within a narrow range (6.3-7.7), mean % solids was 5.6 and the mean η* was 2.5 Pa·s. Sputum was collected from patients with CF according to UNC IRB protocol #15-2431.
| Category | Mean or Count (Std. Dev.) |
Range |
|---|---|---|
| # Patients (male) | 11 (4) | - |
| Age (years) | 28.5 (9.6) | 18-46 |
| FEV1 % Predicted | 48.8 (22.0) | 24-90 |
| FVC (L) | 2.7 (1.0) | 1.7-4.3 |
| FEF25-75 | 0.96 (0.82) | 0.3-2.7 |
| pH | 7.1 (0.42) | 6.3-7.7 |
| % solids | 5.6 (2.5) | 2.3-10.6 |
| η* (Pa·s) | 2.5 (1.5) | 0.65-4.5 |
Figure 5:
CF sputum targets and candidate muco-corrective therapeutic strategies. A: Complex viscosity vs. % solids for CF sputum exhibited a significant relationship (n = 11, p = 0.006, r2 = 0.58). B: Complex viscosity (η*) vs. pH for CF sputum samples exhibited no significant correlation (p = 0.42). C: Complex viscosity of pooled, homogenized CF sputum at 5.2 (grey) and 2.6% (white) solids, pH 6, 7, and 8. Two-way ANOVA indicated that complex viscosity was correlated to concentration (p = 0.013), but not pH (p = 0.10). D: Reduction of normal HBE mucus by DTT. The reduction of mucus by 10 mM DTT was found to be significantly different than control (p = 0.027). E: Reduction of 5% pooled CF sputum shows a similar reduction when treated with 10mM DTT (p = 0.008). For reference, the viscosity of water is ~ 0.001 Pa·s.
Testing Therapeutic Strategies to Normalize CF Sputum Viscoelastic Properties
To generate sufficient material for systematic studies of therapeutic agents, four sputum samples were pooled and homogenized in the presence of protease inhibitors to blunt proteolysis. This pooled stock was similar in concentration, pH, and rheology to sample group as a whole (See supplemental materials). The concentration of the pooled samples, 5.2%, matched the average concentration of all CF sputum samples. Aliquots of the pooled sputum were hydrated to 2.6% to mimic the effect of a mucus hydrator [33, 34], and then aliquots of sputum at both 2.6% and 5.2% concentrations were adjusted to pH 6, 7, and 8 to test the effects of modulating pH on CF sputum.
Diluting (hydrating) mucus by a factor of 2 from 5.2 to 2.6% decreased the complex viscosity by a factor of 8. In contrast, the effects of a 100-fold range in pH were small, i.e., ~ a 2-fold change in complex viscosity. Importantly, increasing the pH of 5.2% CF sputum from 6 to 8 resulted in a complex viscosity greater than the most acidic 2.6% concentration sputum sample (Figure 5C). Thus, therapies directed at hydration will likely produce more favorable changes in CF sputum viscosity than modulation of pH.
Finally, the effect of reduction of mucin disulfide bonds was tested. We chose to reduce mucus with DTT rather than DNAse since it has previously been shown to have a greater effect on sputum rheology [35]. Normal HBE mucus was prepared to 5% solids to mimic CF sputum and reduced with 1 and 10mM dithiothreitol (DTT) vs PBS control. These data (Figure 5D) showed that reduction of the disulfide bounds that link mucin monomers into polymeric macromolecules had a greater effect on the rheological properties of mucus than pH, i.e., exhibiting an effect similar to reducing concentration. This result was mimicked by the reduction of 5% pooled CF sputum with 10 mM DTT (Figure 5E).
Discussion
CF airways disease is characterized by a highly viscous airway mucus that leads to mucus stasis, inflammation, chronic infection, and damage to airway walls. To test the current hypotheses that may account for the increased viscosity of CF mucus, i.e., mucus acidification [13] vs. concentration [1, 8, 11, 15], the relative effects of each variable were measured in model mucus systems (PGM, BSM), both normal and CF HBE mucus, and CF sputum.
The pH ranges observed in human airway mucus [36, 37] are much narrower than mucus in other organs that are typically studied to describe pH effects on mucus properties, e.g., the endocervix [32] or stomach [20]. The pH range of normal human airway mucus is ~ 7.0-7.2. It is not clear how much lower the pH of mucus in CF airways may be. CF airway epithelial cultures and CF animal models exhibit a pH that may be ~ 0.4 pH lower than normal airway mucus, i.e., ~ pH 6.8 [36, 38]. The lowest pH reported for CF airway mucus emanated from studies of pH of mucopurulent material contained in excised CF lungs and was 6.6 [37]. However, recent in vivo data have described little difference in large airway pH in CF vs. normal neonates [39]. Our strategy to address all possible CF mucus pH vs. concentration scenarios was to develop protocols that spanned: 1) pH ranges relevant to both CF (pH 6-8) and mucus from other organs (pH 4-8); and 2) mucus concentrations from normals (2% solids) and CF subjects (>5% solids) [11].
The mucus gel is composed of high molecular weight mucin polymers dissolved in an airway surface liquid (ASL) solvent. In the concentrations reported for normal and diseased mucus, the mucin polymers are in “overlap” concentrations, meaning the mucin polymers interpenetrate. Mucin polymers in overlap conditions typically exhibit viscoelastic properties (e.g., viscosity, elasticity [40]) that scale geometrically with concentration. The correlation length, ξ, i.e., mesh size produced by interpenetrating mucins, also scales to a higher order power of concentration (ξ ~ kT / [concentration]3) and has been reported to be ~ 100-500 nm in normal mucus [15, 41]. The ASL solvent for mucus gels on airway surfaces has been characterized as an isotonic liquid [42, 43].
The smallest nanoscopic rheological length scales of mucus scale to its solvent, which can be explored by fluorescence recovery after photobleaching (FRAP). Recent FRAP studies by Tang et al., employing FITC labeled 70 kDa dextran (radius of gyration 6.6 nm [44]), demonstrated modest pH-dependent changes in the diffusion of these small molecules in mucus. Our experiments with CF sputum, but not HBE mucus, are in agreement with the FRAP data obtained by Tang et al. in isolated CF piglet mucus (Figures 3 and 5) [13]. We interpret these data as indicating that the low molecular weight (potentially inflammatory) proteins dissolved in the isotonic mucus solvent generated pH-dependent changes in viscosity that exceeded that of water. Notably, the FRAP viscosity valves measured in this study (0.001 – 0.003 Pa·s) and that of Tang et al. were orders of magnitude lower than the viscosity values of mucus (0.1 – 10 Pa·s) as measured by macrorheology (see below and Figure 3). The disparity in FRAP vs. macrorheology measurements, coupled with the mucin matrix-solvent separation studies (Figure 2B), indicate that the small molecule FITC-labeled dextrans utilized in FRAP studies measured the pH sensitive properties of the low viscosity mucus solvent, and not the mucin structure/function that dominates mucus clearance.
Microrheology protocols were performed with 1 μm beads which probe mucus at length scales relevant to the mucin mesh. Our microrheology studies of both PGM and BSM mucus showed a strong (geometric) dependence of mucus complex viscosity on mucus concentration. In contrast, pH effects on PGM complex viscosity were much smaller and only significant at pH < 5. These findings are in agreement with data of Wang et al. in MUC5B-dominated endocervical mucus [32]. Importantly, the effects of pH on HBE mucus complex viscosity over pH ranges that span those of airway mucus in health or disease, pH 6 – 8, were minimal compared to the effects of concentration on mucus harvested from both normal and CF cultures.
Conventional cone and plate studies probe mucus rheology at macroscopic length scales. With this approach, similar directional and quantitative data were observed as for microrheology (Figure 3). Namely, pH had little effect on complex viscosity over airway pH-relevant ranges. In contrast, strong concentration-dependent relationships were observed, consistent with data of Thornton and Waigh [31]. Although it has typically been difficult to reconcile findings probing mucus rheological properties at different length scales, our findings at all length scales are consistent with mucus concentration, not pH, dominating mucus mesh dependent rheological properties relevant to MCT.
Studies of CF sputum were performed for comparison to our mucus model systems. In raw sputum from CF patients, like our model systems, a strong correlation between mucus concentration (p = <0.05), but not pH (p = >0.3), and rheology was observed (Figure 5A and B).
The water drawing power of a polymer gel is described by its osmotic pressure. Like rheologic properties, osmotic pressures of polymer gels scale to higher order powers of polymer (mucus) concentration. Under normal conditions, the PCL is the more concentrated gel and is fully hydrated, permitting the cilia to beat in a well-lubricated PCL environment. However, our data have shown that even minor osmotic compression of the PCL by a modestly hyperconcentrated mucus layer (i.e., 4-5% solids), produces sufficient compression of the cilia to slow mucus transport. Data presented in Figure 4A demonstrate that altering the pH across acidic vs. alkaline ranges did not affect the osmotic properties of the concentrated mucus layer. In contrast, modest mucus hyperconcentration produced the predicted modest PCL compression (Figure 4A).
To directly compare the effects of pH vs. concentration on muco-ciliary tansport (MCT) rates, the effects of mucus pH vs. concentration on MCT were measured in HBE cultures (Figure 4B & C). Consistent with the micro- and macrorheology. MCT was governed by mucus concentration and not pH. These findings are consistent with previous reports that showed MCT is dependent on mucus concentration, with MCT slowing at 4 - 5% solids and ceasing at 8 - 10% solids [24, 45].
Therapeutic strategies to normalize CF mucus biophysical properties focused on concentration (hydration), pH, and disulfide bond reduction. Studies of CF sputum samples exposed to two-fold variations in concentration vs 100-fold variations in pH demonstrated that concentration, not pH, normalized sputum complex viscosity. Chemical reduction of mucus by cleaving disulfide bonds with DTT decreased the complex viscosity of mucus by similar fold values as hydration in both HBE mucus (Figure 5D) and pooled CF sputum (Figure 5E). These data indicate that both hydration and chemical reduction may be a more effective muco-corrective treatment than alkalization.
The importance of concentration for the biophysical properties of mucus is rooted in the polymeric nature of the mucin macromolecules that are the key gel forming molecules in mucus. Increasing the concentration of mucus and mucins in disease states [8, 11, 24] geometrically alters the viscoelastic and osmotic properties of mucus [40], leading to a collapsed PCL [1], decreased mucociliary clearance [24], reduced neutrophil penetration and bacteria killing [45], biofilm formation [15], and decreased pulmonary function [24, 41, 46]. Collectively, increased mucus concentration can generate much of the muco-obstructive pathophysiology typical of CF [8, 11, 24, 41].
The role of pH in airway disease states is less clear, with studies differing on the magnitude of the change in pH across nasal models [47], early piglets [13], human cell cultures [36, 38, 39] and early in CF patients in vivo [39]. Our data strongly argue that pH has little effect on the biophysical properties of mucus relevant to basal mucus transport. It is possible that there is a role for acidification in bacterial killing [48] that may add to failed mucus clearance in CF disease pathogenesis. We speculate, though, that pathophysiologically relevant CF defects in airway pH regulation may be more important during gastric aspiration. After aspiration, the pH of the airway surface is acutely acidified and takes longer to return to baseline in CF than normal cultures [38]. Previous macro and microscopic rheology studies found that a reduction to pH 1 - 3 was required to produce the magnitude of mucus rheologic abnormalities produced by CF-relevant increases in mucus concentration [20, 30-32]. Thus, gastric aspiration may be combined with increased mucus concentration to trigger early CF events and/or spread of CF lung disease.
Conclusions
Over the micron to millimeter length scales that probe the micro- and macrorheological properties of mucin structure, mucus concentration, not pH, governed the biophysical properties of mucus important for clearance from the lung. In contrast, FRAP studies that probe the solvent properties of the gel are not relevant to the mucus mesh. Data from the larger length scale biophysical assays, but not FRAP, were congruent with data from HBE MCT assays that also demonstrated the dominance of concentration in controlling MCT. Mucus pH may have effects on antimicrobial killing in the CF lung. However, our findings indicate that therapies designed to restore transport properties of mucus should focus on reducing mucus concentration and/or mucolytic reduction rather than raising pH.
Supplementary Material
Acknowledgements
The authors wish to thank Erik Perou, Matthew Combs, and Jessica Malachowski for their experimental efforts on this project. The authors would like to thank Dr. Scott Randell and the UNC Tissue Core for HBE cells and Mr. Eric Roe for editorial assistance. This work was funding by the National Science Foundation (DMS 1462992), the National Institutes of Health (R01HL125280, 5P30DK065988, and 4P50HL120100) and Cystic Fibrosis Foundation (Hill16XX0, Ramsey16I0, Bouche15R0, and Button07XX0).
Footnotes
Conflict of Interest:
The authors have declared that no conflicts exist.
References
- 1.Button B, Cai LH, Ehre C, Kesimer M, Hill DB, Sheehan JK, Boucher RC, Rubinstein M. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science 2012: 337(6097): 937–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kesimer M, Kirkham S, Pickles RJ, Henderson AG, Alexis NE, Demaria G, Knight D, Thornton DJ, Sheehan JK. Tracheobronchial air-liquid interface cell culture: a model for innate mucosal defense of the upper airways? Am J Physiol Lung Cell Mol Physiol 2009: 296(1): L92–L100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol 2008: 70: 459–486. [DOI] [PubMed] [Google Scholar]
- 4.Boucher RC. An overview of the pathogenesis of c1ystic fibrosis lung disease. Adv Drug Deliv Rev 2002; 54(11): 1359–1371. [DOI] [PubMed] [Google Scholar]
- 5.Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Resp J 2004: 23(1): 146–158. [DOI] [PubMed] [Google Scholar]
- 6.Dawson M, Wirtz D, Hanes J. Enhanced viscoelasticity of human cystic fibrotic sputum correlates with increasing microheterogeneity in particle transport. J Biol Chem 2003: 278(50): 50393–50401. [DOI] [PubMed] [Google Scholar]
- 7.Hill DB, Button B. Establishment of Respiratory Air-Liquid Interface Cultures and their Use in Studying Mucin Production, Secretion, and Function In: Thornton DJ, ed. Mucins. Springer, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Hill DB, Vasquez PA, Mellnik J, McKinley SA, Vose A, Mu F, Henderson AG, Donaldson SH, Alexis NE, Boucher RC. A Biophysical Basis for Mucus Solids Concentration as a Candidate Biomarker for Airways Disease. Plos One 2014: 9(2): e87681 %@ 81932-86203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubin BK. Mucus structure and properties in cystic fibrosis. Paediatric Respiratory Reviews 2007: 8(1): 4–7. [DOI] [PubMed] [Google Scholar]
- 10.Williams HD, Behrends V, Bundy JG, Ryall B, Zlosnik JEA. Hypertonic Saline Therapy in Cystic Fibrosis: Do Population Shifts Caused by the Osmotic Sensitivity of Infecting Bacteria Explain the Effectiveness of this Treatment? Frontiers in Microbiology 2010: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henderson AG, Ehre C, Button B, Abdullah LH, Cai LH, Leigh MW, DeMaria GC, Matsui H, Donaldson SH, Davis CW, Sheehan JK, Boucher RC, Kesimer M. Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J Clin Invest 2014: 124(7): 3047–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birket SE, Davis JM, Fernandez CM, Tuggle KL, Oden AM, Chu KK, Tearney GJ, Fanucchi MV, Sorscher EJ, Rowe SM. Development of an airway mucus defect in the cystic fibrosis rat. JCI Insight 2018: 3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang XX, Ostedgaard LS, Hoegger MJ, Moninger TO, Karp PH, McMenimen JD, Choudhury B, Varki A, Stoltz DA, Welsh MJ. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. The Journal of clinical investigation 2016: 126(3): 879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer H, Widdicombe JH. Mechanisms of acid and base secretion by the airway epithelium. J Membr Biol 2006; 211(3): 139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsui H, Wagner VE, Hill DB, Schwab UE, Rogers TD, Button B, Taylor RM 2nd, Superfine R, Rubinstein M, Iglewski BH, Boucher RC. A physical linkage between cystic fibrosis airway surface dehydration and Pseudomonas aeruginosa biofilms. Proc Natl Acad Sci U S A 2006; 103(48): 18131–18136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witten J, Ribbeck K. The particle in the spider’s web: transport through biological hydrogels. Nanoscale 2017: 9(24): 8080–8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newby JM, Seim I, Lysy M, Ling Y, Huckaby J, Lai SK, Forest MG. Technological strategies to estimate and control diffusive passage times through the mucus barrier in mucosal drug delivery. Adv Drug Deliv Rev 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai SK, Wang YY, Wirtz D, Hanes J. Micro- and macrorheology of mucus. Adv Drug Deliv Rev 2009: 61(2): 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fulcher ML, Randell SH. Human nasal and tracheo-bronchial respiratory epithelial cell culture. Methods Mol Biol 2013: 945: 109–121. [DOI] [PubMed] [Google Scholar]
- 20.Celli JP, Turner BS, Afdhal NH, Ewoldt RH, McKinley GH, Bansil R, Erramilli S. Rheology of gastric mucin exhibits a pH-dependent sol-gel transition. Biomacromolecules 2007: 8(5): 1580–1586. [DOI] [PubMed] [Google Scholar]
- 21.Holma B Influence of buffer capacity and pH-dependent rheological properties of respiratory mucus on health effects due to acidic pollution. Science of the total environment 1985: 41(2): 101–123. [DOI] [PubMed] [Google Scholar]
- 22.Holma B, Hegg PO. pH- and protein-dependent buffer capacity and viscosity of respiratory mucus. Their interrelationships and influence on health. Sci Total Environ 1989: 84: 71–82. [DOI] [PubMed] [Google Scholar]
- 23.Kim D, Liao J, Hanrahan JW. The buffer capacity of airway epithelial secretions. Frontiers in physiology 2014: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson WH, Coakley RD, Button B, Henderson AG, Zeman KL, Alexis NE, Peden DB, Lazarowski ER, Davis CW, Bailey S, Fuller F, Almond M, Qaqish B, Bordonali E, Rubinstein M, Bennett WD, Kesimer M, Boucher RC. The Relationship of Mucus Concentration (Hydration) to Mucus Osmotic Pressure and Transport in Chronic Bronchitis. Am J Respir Crit Care Med 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seagrave J, Albrecht HH, Hill DB, Rogers DF, Solomon G. Effects of guaifenesin, N-acetylcysteine, and ambroxol on MUC5AC and mucociliary transport in primary differentiated human tracheal-bronchial cells. Respir Res 2012: 13: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Button B, Okada SF, Frederick CB, Thelin WR, Boucher RC. Mechanosensitive ATP Release Maintains Proper Mucus Hydration of Airways. Science signaling 2013: 6(279): ra46–ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shih CK, Litt M, Khan MA, Wolf DP. Effect of nondialyzable solids concentration and viscoelasticity on ciliary transport of tracheal mucus. Am Rev Respir Dis 1977: 115(6): 989–995. [DOI] [PubMed] [Google Scholar]
- 28.Winet H The role of the periciliary fluid in mucociliary flows: flow velocity profiles in frog palate mucus. Biorheology 1987: 24(6): 635–642. [DOI] [PubMed] [Google Scholar]
- 29.Winet H, Yates GT, Wu TY, Head J. On the mechanics of mucociliary flows. III. Flow-velocity profiles in frog palate mucus. J Appl Physiol 1984: 56(3): 785–794. [DOI] [PubMed] [Google Scholar]
- 30.Wagner CE, Turner BS, Rubinstein M, McKinley GH, Ribbeck K. A Rheological Study of the Association and Dynamics of MUC5AC Gels. Biomacromolecules 2017: 18(11): 3654–3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Georgiades P, Pudney PD, Thornton DJ, Waigh TA. Particle tracking microrheology of purified gastrointestinal mucins. Biopolymers 2014; 101(4): 366–377. [DOI] [PubMed] [Google Scholar]
- 32.Wang YY, Lai SK, Ensign LM, Zhong W, Cone R, Hanes J. The microstructure and bulk rheology of human cervicovaginal mucus are remarkably resistant to changes in pH. Biomacromolecules 2013: 14(12): 4429–4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goralski JL, Wu D, Thelin WR, Boucher RC, Button B. The In Vitro Effect of Nebulised Hypertonic Saline on Human Bronchial Epithelium. Eur Respir J 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donaldson SH. Hydrator therapies for cystic fibrosis lung disease. Pediatr Pulmonol 2008: 43(9): S18–S23. [Google Scholar]
- 35.Horsley A, Rousseau K, Ridley C, Flight W, Jones A, Waigh TA, Thornton DJ. Reassessment of the importance of mucins in determining sputum properties in cystic fibrosis. J Cyst Fibros 2014; 13(3): 260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coakley RD, Boucher RC. Regulation and functional significance of airway surface liquid pH. JOP 2001: 2(4 Suppl): 294–300. [PubMed] [Google Scholar]
- 37.Garland AL, Walton WG, Coakley RD, Tan CD, Gilmore RC, Hobbs CA, Tripathy A, Clunes LA, Bencharit S, Stutts MJ, Betts L, Redinbo MR, Tarran R. Molecular basis for pH-dependent mucosal dehydration in cystic fibrosis airways. Proc Natl Acad Sci U S A 2013; 110(40): 15973–15978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coakley RD, Grubb BR, Paradiso AM, Gatzy JT, Johnson LG, Kreda SM, O’Neal WK, Boucher RC. Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc Natl Acad Sci U S A 2003; 100(26): 16083–16088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schultz A, Puvvadi R, Borisov SM, Shaw NC, Klimant I, Berry LJ, Montgomery ST, Nguyen T, Kreda SM, Kicic A, Noble PB, Button B, Stick SM. Airway surface liquid pH is not acidic in children with cystic fibrosis. Nature Communications 2017; 8(1): 1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubinstein M, Colby RH. Polymer Physics. Oxford University Press, Oxford, 2003. [Google Scholar]
- 41.Duncan GA, Jung J, Joseph A, Thaxton AL, West NE, Boyle MP, Hanes J, Suk JS. Microstructural alterations of sputum in cystic fibrosis lung disease. JCI insight 2016: 1(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsui H, Davis CW, Tarran R, Boucher RC. Osmotic water permeabilities of cultured, well-differentiated normal and cystic fibrosis airway epithelia. J Clin Invest 2000: 105(10): 1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RC. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 1998: 95(7): 1005–1015. [DOI] [PubMed] [Google Scholar]
- 44.Armstrong J, Wenby R, Meiselman H, Fisher T. The hydrodynamic radii of macromolecules and their effect on red blood cell aggregation. Biophys J 2004: 87(6): 4259–4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsui H, Verghese MW, Kesimer M, Schwab UE, Randell SH, Sheehan JK, Grubb BR, Boucher RC. Reduced three-dimensional motility in dehydrated airway mucus prevents neutrophil capture and killing bacteria on airway epithelial surfaces. J Immunol 2005: 175(2): 1090–1099. [DOI] [PubMed] [Google Scholar]
- 46.Ma JT, Tang C, Kang L, Voynow JA, Rubin BK. Cystic fibrosis sputum rheology correlates with both acute and longitudinal changes in lung function. Chest 2018. [DOI] [PubMed] [Google Scholar]
- 47.Song Y, Salinas D, Nielson DW, Verkman A. Hyperacidity of secreted fluid from submucosal glands in early cystic fibrosis. Am J Physiol-Cell Physiol 2006: 290(3): C741–C749. [DOI] [PubMed] [Google Scholar]
- 48.Pezzulo AA, Tang XX, Hoegger MJ, Alaiwa MH, Ramachandran S, Moninger TO, Karp PH, Wohlford-Lenane CL, Haagsman HP, van Eijk M, Banfi B, Horswill AR, Stoltz DA, McCray PB Jr., Welsh MJ, Zabner J. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 2012: 487(7405): 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.