Abstract
Background. Although obesity is a commonly discussed issue in the medical management of children with Down syndrome, there have been no large studies published on its prevalence in the United States or associations with other common comorbidities in this population. Methods. Using a database of children from a single medical center Down syndrome specialty clinic and the standard Centers for Disease Control and Prevention definitions, we calculated rates of obesity and overweight by age group and examined possible associations with common comorbidities including cardiac disease, thyroid disease, sleep apnea, autism, and visual and hearing impairment. We also examined mean body mass index (BMI) percentile and change in BMI percentile by age. Results. Data were obtained from 823 visits from 412 unique patients ranging in age from 2 years to 23 years of age. A total of 1.2% were underweight, 55.2% were normal weight, 23% were overweight, and 20.6% were obese. BMI percentile increased with female gender, age, and height percentile for age. Sleep apnea was associated with higher BMI percentile, while autism was associated with lower BMI percentile. Conclusions. Children with Down syndrome have higher rates of obesity than the general population, with especially high risk for girls. Much of the increase in obesity occurs between ages 2 and 6 years. Further research needs to target interventions for prevention in this vulnerable population, particularly in young girls.
Keywords: down syndrome, obesity, growth charts
Background
The risk for obesity is mentioned on 5 separate occasions in the Clinical Report on Health Supervision for Children with Down syndrome published by the American Academy of Pediatrics in 2011.1 Small studies have shown that the rates of overweight and obesity in adolescent and adult populations with Down syndrome (DS) are higher than average.1-4 However, few of these studies have provided estimates of the prevalence of obesity in the pediatric population of patients with DS. In the general population, it is well established that obesity is a risk factor for multiple adverse health outcomes including sleep apnea and subclinical hypothyroidism, both of which have high prevalence in DS.5 In 2012, a Dutch study concluded that rates of overweight and obesity among healthy patients with DS were twice those of the general population.3 However, this study’s findings may not be comparable to the patient population in the United States as the rates of obesity in the general US population are much higher than in the Dutch (17% vs 2%).3,6 In the most recent release of growth charts for pediatric patients with DS, body mass index (BMI) charts were published, though they were not formally compared with those for children without DS.7 A recent follow-up made this comparison, and using DEXA (dual-energy X-ray absorptiometry) scan evaluation of body composition reported that use of the Centers for Disease Control and Prevention (CDC) growth charts and definitions for overweight and obesity were appropriate for the population with DS.8
This study seeks to characterize the rates of overweight and obesity in a representative sample of pediatric patients with DS and to assess associations with common DS comorbidities.
Methods
Data were collected from the electronic medical records (EMRs) of eligible children who were seen in an outpatient regional referral clinic in Oregon for patients with DS. Records were available from the establishment of the clinic in November 2007 through January 2015.
Data included demographic data such as gender, ethnicity, and age at the visit. Race was not collected as it was found to be poorly tracked in the EMR. Anthropometric data included height, weight, and a calculated BMI. Age- and gender-appropriate percentiles were available for height and weight based on the published DS growth charts from 1988.9 BMI percentiles were calculated from the CDC BMI growth chart.10 Underweight is defined as BMI percentile less than fifth percentile; normal weight is fifth percentile to less than 85th percentile, overweight is 85th percentile to less than the 95th percentile, and obesity is 95th percentile or higher.11
Presence of common comorbidities was determined including cardiac disease (and need for surgical intervention), thyroid disease, sleep apnea, visual impairment, hearing impairment, celiac disease, type 1 diabetes, and autism. The clinic follows a well-defined screening template at visits, and diagnoses were confirmed with external medical records when available.
A mixed-effects regression model, with a random-effects model for the individual child to account for multiple visits for the same individual, was used to determine the associations between BMI percentile and gender, with age, ethnicity, cardiac status, and presence of hypothyroidism as risk factors. A standard linear regression was used to compare variables with the presence of the above-mentioned comorbidities, as the age at comorbidity diagnosis was not always clear in the medical record. Age was sometimes treated as a continuous variable and sometimes categorical; categories were chosen to balance group size and age interval size, as well as to capture the observed shape of the relationship between age and BMI. The Wald test was used to compare individual variables for significant associations.
For our binary outcome of obese versus nonobese, we chose a modified Poisson approach with robust variance estimates to model the relative risk of obesity directly, rather than odds ratios, because odds ratios tend to overstate risk when outcomes are common. When modeling mean BMI percentile by age, we found significant departures from linearity and used restricted cubic splines to capture the relationship between BMI and age.
To examine patterns in weight change related to age, we restricted our analysis to children with BMI percentile available from more than 1 clinic visit (n = 199), we calculated the change in BMI percentile points from the previous visit divided by elapsed time, excluding 2 observations where visits occurred less than 6 months apart. We categorized these changes as large (>10 absolute points change), moderate (5-10 points), or weight maintenance (within ±5 points), and calculated the distribution of these categories by age groups of approximately equal size. Visual confirmation of linear trends in the growth charts was done for all patients to insure relative accuracy and uniformity of measurements.
Ethical Approval and Informed Consent
The study was approved by the Institutional Review Board of Oregon Health and Science University, the original data repository was created under IRB #00010774, and this study was further approved for data analysis under IRB#00016783. A waiver of consent was granted for the collection of de-identified patient data obtained as part of routine medical care through the Down Syndrome Clinic at the associated children’s hospital. Oregon law allows for “opt-out” status for any potential collection of genetic information, and absence of opt-out status was confirmed through the EMR for every patient in the database.
Results
Data were available from 823 visits from 412 unique patients ranging in age from 2 years to 18 years (see Table 1). Children had a mean age of 5 years 5 months at the time of their visit, with a mean of 2 visits per individual child (range 1-7). Children were on average taller and heavier than seen in the 1988 DS growth charts, with median height percentile near the 75th percentile and weight percentile near the 60th percentile.9 This is similar to what has been reported in the updated 2015 DS growth charts.7
Table 1.
Demographic Data for Patients Seen in the Down Syndrome Clinic.
| Demographic Data | Age Categories (Years) |
|||||
|---|---|---|---|---|---|---|
| 2 to 4 | 5 to 7 | 8 to 10 | 11 to 13 | 14 to 18 | Total | |
| Number of children | 166 | 85 | 72 | 42 | 47 | 412 |
| Total visits | 363 | 189 | 112 | 86 | 73 | 823 |
| Visits per child (%) | ||||||
| 1 | 65.1 | 38.8 | 38.9 | 50 | 44.7 | 51.2 |
| 2 | 25.9 | 20 | 20.8 | 28.6 | 12.8 | 22.6 |
| 3 | 9 | 18.8 | 12.5 | 11.9 | 17 | 12.9 |
| 4 | 0 | 11.8 | 12.5 | 7.1 | 10.6 | 6.6 |
| 5 to 7 | 0 | 10.6 | 15.3 | 2.4 | 14.9 | 6.8 |
| Gender (%) | ||||||
| Male | 52.6 | 55.6 | 55.4 | 41.9 | 60.3 | 53.2 |
| Female | 47.4 | 44.4 | 44.6 | 58.1 | 39.7 | 46.8 |
| Ethnicity (%) | ||||||
| Non-Hispanic/Latino | 66.6 | 72 | 69.7 | 68.6 | 77.8 | 69.5 |
| Hispanic/Latino | 33.4 | 28 | 30.3 | 31.4 | 22.2 | 30.5 |
| Cardiac defect/abnormality (%) | ||||||
| No | 28.4 | 32.3 | 33 | 40.7 | 31.5 | 31.5 |
| Yes | 71.6 | 67.7 | 67 | 59.3 | 68.5 | 68.5 |
| Thyroid disease (%) | ||||||
| No | 82.4 | 71.4 | 65.2 | 73.3 | 60.3 | 74.6 |
| Yes | 17.6 | 28.6 | 34.8 | 26.7 | 39.7 | 25.4 |
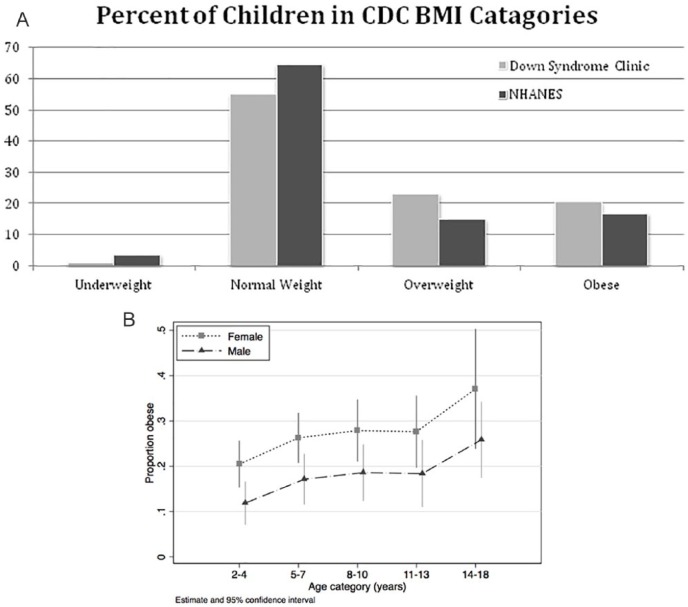
As seen in Figure 1, 1.2% were underweight, 55.2% were normal weight, 23% were overweight, and 20.5% were obese (over half of whom have a BMI-for-age greater than the 99th percentile, or over 3 SD above the mean). Rates of overweight and obesity were higher for girls compared with boys regardless of ethnicity (25% in non-Hispanic/Latina and 26% for Hispanic/Latina girls, compared with 21.5% and 16%, respectively, for boys). The highest rate of overweight (33%) was in Latina girls and for obesity (27%) was in non-Latina girls. A further breakdown by age can be found in Table 2. A complete table of rates of comorbidities can be found in the supplemental materials (available online). Because of concerns regarding the metabolic effects of thyroid disease and early cardiac defects, analysis was repeated for the 196 visits in patients without cardiac defect or thyroid disease. In these patients, there was a higher percentage of obese patients (30% obese, with 45% normal weight) but similar rate of overweight.
Figure 1.
(A) Categories of body mass index of children seen in the Down syndrome compared with those in the National Health and Nutrition Statistics 2010. (B) Percent of Down syndrome clinic patients who are obese by age category and sex.
Table 2.
Body Mass Index (BMI).
| Age Categories (Years) |
||||||
|---|---|---|---|---|---|---|
| 2 to 4 | 5 to 7 | 8 to 10 | 11 to 13 | 14 to 18 | Total | |
| BMI category (%) | ||||||
| Underweight | 1.7 | 1.6 | 0 | 0 | 0 | 1.1 |
| Normal | 62 | 51.3 | 53.6 | 47.7 | 46.6 | 55.5 |
| Overweight | 20.7 | 25.9 | 23.2 | 24.4 | 27.4 | 23.2 |
| Obese | 15.7 | 21.1 | 23.2 | 27.9 | 26.1 | 20.2 |
| >99 percentile | 8.8 | 11.6 | 14.3 | 2.3 | 15.1 | 10.1 |
| Median height percentile (Down syndrome charts, 1988) | 77 | 69 | 47 | 76 | 76 | 73 |
| Median weight percentile (Down syndrome charts, 1988) | 62 | 55 | 51 | 67 | 62 | 59 |
| Median BMI percentile (CDC charts, 2010) | 73 | 83 | 81 | 85 | 86 | 79 |
As shown in Figure 1B, risk of obesity increased with age and female gender, as well as with height percentile (not pictured). Looking only at children with DS and using 2 to 3 year olds as the reference groups, children aged 4 to 6 years were 61% more likely to be obese, and risk increased ~20% in each successive 3-year age group (risk ratio [RR] = 1.82 in 7-9 year olds, 1.97 in 10-13 year olds, and 2.27 in 14-18 year olds). Girls were almost twice as likely to be obese as boys (RR = 1.94). On the other hand, both cardiac disease and hypothyroidism (congenital or acquired) treated with levothyroxine were associated with significantly lower risk (RR = 0.69 and 0.65, respectively) of obesity.
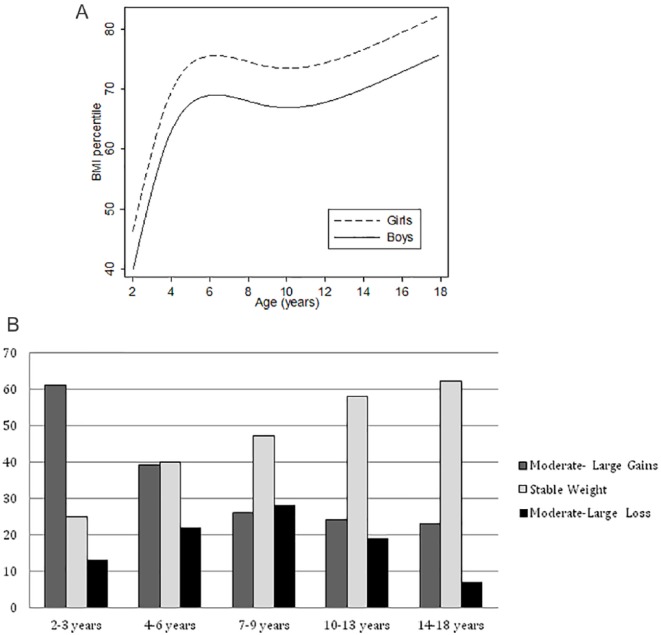
The greatest gains in average weight (as percentile of BMI for age) occurred between the ages of 2 and 6 years (Figure 2). The curve of mean BMI percentile-for-age increases sharply from near 40th for boys and 50th for girls at age 2, to between the 70th and 80th percentile around age 6, where the curve mostly levels off apart from a slight dip in proportion of patients who are obese in the 10- to 12-year age range. Although BMI curves are variable by age and gender, the plotting of BMI percentile illustrates the relative difference between the DS and general populations.
Figure 2.
Change in body mass index (BMI) percentile for age for boys and girls with Down syndrome (n = 412 children at 823 clinical visits), adjusted for presence of hypothyroidism, cardiac disease, and height-for-age percentile. (A) Mean BMI percentile for age and (B) change in BMI for age from previous clinical visit (at least 6 months apart) classified as moderate-large gain, stable, or moderate-large loss. The largest gains are seen in the youngest age groups (2-6 years) with weight stabilizing in the majority of older patients.
As illustrated in Figure 2A, our clinic population shows a relatively rapid rise in BMI percentile-for-age in early childhood, followed by a period of relatively stable BMI percentile in the school years and a further slow increase in BMI during adolescence. Figure 2B represents these data longitudinally as well: when looking at weight change for an individual patient from the previous clinical visit in terms of (change in percentile)/(years elapsed since previous visit) and classifying those changes as large (|change| > 10 points), moderate (5-10 points), or essentially unchanged (<5 points), we see that more than half of the youngest cohort (53% of those 2-3 years) experienced large gains in BMI percentile for age and a further 26% have large gains at ages 4 to 6 years. After age 7, the proportions of weight gains balance weight losses, and an increased percentage (47% to 62%) are at a stable, but still elevated, BMI for age.
Looking only at the first visit to the DS clinic for 398 unique patients who had complete comorbidity data available, linear regression showed positive associations between BMI percentile and female gender and between BMI percentile and the presence of sleep apnea and a negative association with autism. When controlling for age, gender, height percentile, and presence of other comorbidities, those with autism have a mean BMI percentile 15% (95% confidence interval = 5.5-25) lower than those without. Sleep apnea is associated with higher BMI percentile.
When this analysis is further stratified for gender, the association between height percentile and BMI percentile is very strong in females, though only trends toward statistical significance in males. However, both males and females show a strong association between autism and lower BMI percentile, though the decrease is larger in females (−34% points vs −14% points). The association between BMI percentile and sleep apnea is only statistically significant in females.
Discussion
This study provides one of the largest and most comprehensive evaluations of overweight and obesity in children with DS in the United States. It utilizes provider measurements, as opposed to patient reporting, and controls for possible associations with common comorbidities. We found 20% higher rates of obesity in our DS population (23% overweight, 20.6% obese), compared with the general US population where 16.2% of children were overweight and 17% were obese.6 This is in contrast to the Dutch population with DS, where 25.5% to 32.0% of children were overweight and only 4.2% to 5.1% were obese, rates that are close to twice those in the general Dutch population.3
The rates of overweight and obesity increase with increasing age, particularly in very early childhood, in both boys and girls as well as with increased height percentile on the DS growth charts, particularly for girls. This is in contrast to newer studies in the general population, which do not show increasing risk of obesity with age but is evident from visual inspection of the recently published DS growth curves, which show a widening of the weight curves with age.7 It also confirms the findings of Hatch-Stein et al who noted higher rates of obesity in girls with DS compared with age-matched boys with DS and age-matched girls without DS.8 There is emerging evidence that this type of early “adiposity rebound” may increase risks for metabolic syndrome and type 2 diabetes in older age.12
A primary limitation of this study is its comparison to national rates of obesity, rather than a local control population without DS. However, the DS population at our center is diverse, and rates of common comorbidities are similar to national estimates for children with DS; therefore, it is likely a representative sample for comparison. It is also a retrospective study that required the use of heights collected in a clinical, not research, setting and plotted on the 1988 DS growth charts as these are the charts currently available in the clinical EMR. All percentiles were accurately calculated through the computerized system, and it is not currently feasible to update historical medical record information in the EMR.
We feel it is important to emphasize the discrepancy between the DS growth curves and the CDC BMI curves for DS. If a child with DS were to remain at the 75th percentile for both height and weight, as they grow older, their BMI percentile will increase dramatically, and although the height and weight would seem proportionate, the child has actually become obese. This is well demonstrated in Table 2, which shows that although the median weight on the DS growth charts tends to be around the 60th percentile in our population, these same children have median BMI percentile that are much higher, around the 80th percentile or higher. Thus, following BMI percentile on the CDC growth curves is recommended for children with DS to better appreciate progression to overweight and obesity. This will allow implementation of clinical interventions that may ultimately lead to less obesity and better health.
Supplemental Material
Supplemental material, Supplemental_Table for Trends in Obesity and Overweight in Oregon Children With Down Syndrome by Melinda Pierce, Katrina Ramsey and Joseph Pinter in Global Pediatric Health
Footnotes
Author Contributions: MP: conceptualized the study, completed the data collection and data analysis and wrote the draft of the manuscript.
JP: participated in the design of the study, data interpretation, and manuscript completion.
KR: assisted in data analysis and presentation.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Melinda Pierce  https://orcid.org/0000-0002-9908-9409
https://orcid.org/0000-0002-9908-9409
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Bull MJ; Committee on Genetics. Health supervision for children with Down syndrome. Pediatrics. 2011;128:393-406. [DOI] [PubMed] [Google Scholar]
- 2. Rubin SS, Rimmer JH, Chicoine B, Braddock D, McGuire DE. Overweight prevalence in persons with Down syndrome. Ment Retard. 1998;36:175-181. [DOI] [PubMed] [Google Scholar]
- 3. van Gameren-Oosterom HB, van Dommelen P, Schönbeck Y, Oudesluys-Murphy AM, van Wouwe JP, Buitendijk SE. Prevalence of overweight in Dutch children with Down syndrome. Pediatrics. 2012;130:e1520-e1526. [DOI] [PubMed] [Google Scholar]
- 4. Rimmer JH, Yamaki K, Lowry BM, Wang E, Vogel LC. Obesity and obesity-related secondary conditions in adolescents with intellectual/developmental disabilities. J Intellect Disabil Res. 2010;54:787-794. [DOI] [PubMed] [Google Scholar]
- 5. Reinehr T. Obesity and thyroid function. Mol Cell Endocrinol. 2010;316:165-171. [DOI] [PubMed] [Google Scholar]
- 6. Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA. 2016;315:2292-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zemel BS, Pipan M, Stallings VA, et al. Growth charts for children with Down syndrome in the United States. Pediatrics. 2015;136:e1204-e1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hatch-Stein JA, Zemel BS, Prasad D, et al. Body composition and BMI growth charts in children with Down syndrome. Pediatrics. 2016;138:e20160541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cronk C, Crocker AC, Pueschel SM, et al. Growth charts for children with Down syndrome: 1 month to 18 years of age. Pediatrics. 1988;81:102-110. [PubMed] [Google Scholar]
- 10. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;(314):1-27. [PubMed] [Google Scholar]
- 11. Ogden CL, Flegal KM. Changes in terminology for childhood overweight and obesity. Natl Health Stat Report. 2010;(25):1-5. [PubMed] [Google Scholar]
- 12. Koyama S, Ichikawa G, Kojima M, Shimura N, Sairenchi Y, Arisaka O. Adiposity rebound and the development of metabolic syndrome. Pediatrics. 2014;133:e114-e119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Table for Trends in Obesity and Overweight in Oregon Children With Down Syndrome by Melinda Pierce, Katrina Ramsey and Joseph Pinter in Global Pediatric Health