Abstract
Background
Data on survival and prognosis factors in incident cohorts are scarce in systemic sclerosis (SStc). To describe survival, standardized mortality ratio (SMR), and prognosis factors in systemic sclerosis (SSc), we analyzed a multicenter French cohort of incident patients and performed a systematic review of the literature and meta-analysis.
Methods
A multicenter, French cohort study was conducted between January 1, 2000, and December 31, 2013. Patients were followed-up until July 1, 2016.
A systematic review of the literature was carried out in MEDLINE and EMBASE up to July 2017. Meta-analysis was performed using all available data on SMR and hazard ratios of prognosis factors.
Results
A total of 625 patients (493 females, 446 lcSSc) were included. During the study period, 104 deaths (16.6%) were recorded and 133 patients were lost to follow-up. Overall survival rates at 1, 3, 5, and 10 years from diagnosis were 98.0%, 92.5%, 85.9%, and 71.7% respectively in the French cohort. Overall SMR was 5.73 (95% CI 4.68–6.94). Age at diagnosis > 60 years, diffuse cutaneous SSc, scleroderma renal crisis, dyspnea, 6-min walking distance (6MWD), forced vital capacity < 70%, diffusing capacity of the lungs for carbon monoxide < 70%, pulmonary hypertension (PH), telangiectasia, valvular disease, malignancy, anemia, and CRP > 8 mg/l were associated with a poorer survival after adjustment.
Eighteen studies (11,719 patients) were included in the SMR meta-analysis and 36 studies (26,187 patients) in the prognosis factor analysis. Pooled SMR was 3.45 (95%CI 3.03–3.94). Age at disease onset, male sex, African origin, diffuse cutaneous SSc, anti-Scl70 antibodies, cardiac and renal involvement, interstitial lung disease, PH, and malignancy were significantly associated with a worse prognosis. Anti-centromere antibodies were associated with a better survival.
Conclusions
Overall, our study highlights a high mortality rate in SSc patients and confirms previously described prognosis factors related to skin extension and organ involvement while identifying additional prognosis factors such as autoantibody status, telangiectasia, 6MWD, and valvular disease.
Electronic supplementary material
The online version of this article (10.1186/s13075-019-1867-1) contains supplementary material, which is available to authorized users.
Keywords: Systemic sclerosis, Prognosis factors, Survival, Meta-analysis
Background
Systemic sclerosis (SSc) is an autoimmune disease, characterized by microvascular damage, dysregulation of both innate and adaptative immunity, and fibrosis of multiple organs. The causes of SSc-related deaths evolved over the last decades, with cardiac and respiratory complications currently being the leading causes of death [1, 2].
Prior cohort studies comparing contemporary and historical cohort have suggested an improvement of survival rates over time [1, 3, 4]. Yet, two recent meta-analyses have reported that standardized mortality ratio (SMR) was stable over time [5, 6]. Moreover, most of the observational studies investigating mortality in SSc included prevalent cases, which may result in an underestimation of mortality due to a survivor bias. Data on survival in incident cohorts are scarce in SSc [7–12].
Previous studies [7, 13–26] reported risk factors for poor survival in SSc such as male sex, diffuse cutaneous subtype, and specific organ involvement. Recently, Elhai et al. developed a prognostic score from the large EUSTAR database, which accurately predicts 3-year mortality [2]. To our knowledge, two meta-analyses combined the results of the available literature to assess prognosis factors [5, 27]. However, these meta-analyses did not assess prognosis factors such as auto-antibody profile, genetic background, and cancer and did not assess the influence of prevalent versus incident cases.
The aim of the present study was to fill these gaps by assessing survival and prognosis factors in a multicenter French cohort of incident SSc patients and by performing a systematic review of the literature and meta-analysis including all available prognosis factors and SMR.
Methods
Population
The French National Scleroderma Cohort includes 42 centers. The present analysis was restricted to five university hospitals, Lille, Paris (two centers), Nantes, and Lyon to ensure better quality of data, especially on survival data. These five centers participated in recruiting about two thirds of the National Cohort. Data were retrospectively collected before 2010 and then prospectively collected.
Patients were included between January 1, 2000, and December 31, 2013, if they met the following inclusion criteria: (i) be aged over 18, (ii) fulfill the ACR 1980 preliminary classification criteria [28] or ACR/EULAR 2013 classification criteria [29] for SSc, (iii) have at least one additional visit after the inclusion visit, and (iv) be incident cases, defined as patients having disease duration from time of diagnosis to enrolment in the study of less than 3 years. Patients were followed-up until July 1, 2016. Patients were considered as lost to follow-up if the vital status could not be ascertained. When possible, the vital status was ascertained by querying death registers at birth town councils.
Collected data and variable definition
Data collected at the inclusion visit were patient demographics, history of Raynaud phenomenon (RP) and first non-RP symptom, SSc subtype and modified Rodnan skin score (mRSS), auto-antibody profile, and organ involvement.
Disease onset was defined as the time of onset of first non-RP symptom. Interstitial lung disease was diagnosed on HRCT or chest x-ray. Pulmonary function tests including forced vital capacity (FVC) and diffusing capacity of the lungs for carbon monoxide (DLCO) were collected. Six-minute walking distance (6MWD) was collected. Pulmonary hypertension (PH) was suspected on a Doppler echocardiogram when systolic pulmonary arterial pressure (PAP) was estimated to be > 35 mmHg or maximum tricuspid regurgitant jet velocity > 2.8 m/s. Pulmonary arterial hypertension (PAH) was confirmed by right heart catheterization (RHC) when mean PAP was found to be ≥ 25 mmHg at rest, with mean pulmonary arterial wedge pressure ≤ 15 mmHg. EKG alterations, left ventricular ejection fraction (LVEF), diastolic dysfunction [30], valvular disease (excluding tricuspid valve regurgitation to avoid confounding with PH), and pericarditis were recorded according to the American Society of Echocardiography and European Association of Cardiovascular Imaging guidelines [30]. Scleroderma renal crisis was defined as new onset hypertension > 150/85 mmHg associated with a decrease in renal function or manifestations of malignant hypertension. Gastrointestinal tract involvement included reflux, dysmotility, constipation, or diarrhea; signs of bacterial overgrowth and/or malabsorption; and abnormal manometry and/or endoscopy test. Muscle involvement included myalgia and/or muscle weakness and/or elevation of creatinine kinase (CPK). Joint involvement included arthralgia, synovitis, and/or tendon friction rubs. Anemia was defined as a hemoglobin level < 12 g/dl. Smoking included self-reported current or former cigarette smoking.
Systematic review and meta-analysis
The meta-analysis was conducted according to MOOSE guidelines [31]. MEDLINE and EMBASE databases were queried by two of the authors (MRP and DL) using the following search terms: ((systemic sclerosis [Title]) OR (scleroderma, systemic[Title])) AND ((death) OR (mortality) OR (prognosis) OR (survival)). Cochrane did not retrieve additional abstracts. All records published before July 1, 2017, were included in the search. Language was restricted to English or French. Reference list of selected studies was hand-searched for additional relevant studies to be included in the meta-analysis.
Two of the authors (MRP and DL) independently screened the titles and abstracts of the retrieved records to identify eligible articles. The full text of eligible articles was read for inclusion in the meta-analysis. Selected articles were compared, and in case of disagreement, decisions were made by consensus.
Cohort studies of unselected adult SSc patients assessing routine clinical and laboratory prognosis factors and SMR were included. Studies which included patients diagnosed with SSc overlap with other connective tissue diseases were excluded.
Studies from the same centers were included if their respective study periods were different. If, for the same center, two studies covered an overlapping study period, data from the largest cohort were kept in the analysis. A study was recorded as an incident according to the authors’ definition.
Quality of the studies was assessed using the Newcastle-Ottawa scale [32].
Data were extracted and entered into a predefined spreadsheet table which included the following items: study design, length of follow-up, definition of disease onset, disease duration, SMR, and adjusted or, if unavailable, unadjusted hazard ratios (HR) for each studied prognosis factor.
Data analysis
Characteristics of the population were described using mean ± standard deviation or median (interquartile range (IQR)) in case of non-normality, for quantitative variables, and number (percentage) for qualitative variables. Comparisons between limited cutaneous SSc (lcSSc) and diffuse cutaneous SSc (dcSSc) patients were conducted using the Student t test or Wilcoxon test in case of non-normality for quantitative variables and Fisher’s exact test for qualitative variables.
Survival was estimated from diagnosis using the Kaplan-Meier method. Prognosis factors were assessed by Cox regression analysis in the non-adjusted analysis and subsequently adjusted for age, sex, and SSc subtype. The assumption that hazard ratios were constant over time was verified. SMR was calculated as the ratio of observed death in the cohort to the number of death of the French age/sex-matched population in 2014.
We calculated weighted pooled summary estimates of SMR and HR of prognosis factors. For each meta-analysis, we used the DerSimonian and Laird method. Accordingly, studies were considered to be a random sample from a population of studies. Heterogeneity was assessed using an I2 statistic and a chi-square heterogeneity statistic. A random-effects model was used to combine data. The overall effect was estimated using a weighted average of individual effects, with weights inversely proportional to variance in observed effects. Publication bias was evaluated with a funnel plot and Egger’s test. The pooled SMR and HR were estimated with 95% confidence interval (CI). Meta-regression was used to assess the impact of mid-cohort year, the proportion of males, the proportion of diffuse cutaneous forms, and the prevalence of anti-Scl70 antibodies on SMR. The impact of diagnosis of PH by RHC on the association of PH with mortality was evaluated. Separate analyses were performed for (i) SMR according to whether a given study included incident cases only and (ii) HR of PH diagnosed by either echocardiography and/or RHC and PH diagnosed by RHC.
All analyses were performed using R software with the survival and metafor packages. p values less than 0.05 were considered significant.
Results
French cohort study
Baseline characteristics
A total of 625 patients (493 females, 446 lcSSc) were included. Mean age at disease onset was 52.7 ± 14.9 years. The median disease duration from disease onset was 0.8 (IQR 2.2) years. Median follow-up time was 4.4 (IQR 5.3) years. The baseline characteristics are shown in Table 1.
Table 1.
Demographics and clinical characteristics of 625 patients with SSc at baseline
| N (N for dcSSc) | Total | dcSSc | lcSSc | p | |
|---|---|---|---|---|---|
| Demographics | |||||
| Female sex | 625/179 | 493 (79) | 124 (69) | 369 (83) | < 0.001 |
| Age at first RP (years) | 554/155 | 45.4 ± 15.7 | 45.8 ± 15.7 | 45.3 ± 15.8 | 0.736 |
| Age at first non-RP symptom (years) | 502/160 | 50.6 ± 14.5 | 48.5 ± 14.4 | 51.5 ± 14.4 | 0.031 |
| Age at diagnosis (years) | 625/179 | 52.7 ± 14.9 | 49.5 ± 14.5 | 53.9 ± 14.9 | < 0.001 |
| Disease duration from first non-RP symptom to diagnosis (years) | 499/160 | 0.8 [2.2] | 0.7 [1.4] | 0.9 [2.7] | 0.042 |
| Follow-up time from inclusion to death or last visit (years) | 625/179 | 4.4 [5.3] | 4.0 [5.2] | 4.8 [5.3] | 0.023 |
| Genetic background | |||||
| European | 503/147 | 453 (90) | 118 (80) | 335 (94) | < 0.001 |
| African | 503/147 | 50 (10) | 29 (20) | 21 (6) | < 0.001 |
| Skin involvement | |||||
| lcSSc | 625/179 | 446 (71) | – | – | |
| mRSS | 342/123 | 9.2 ± 10.2 | 19.6 ± 10.1 | 3.5 ± 3.6 | < 0.001 |
| Telangiectasia | 572/160 | 264 (46) | 65 (41) | 199 (48) | 0.112 |
| Calcinosis | 549/152 | 64 (12) | 7 (5) | 57 (14) | < 0.001 |
| Digital ulcers (past or active) | 538/145 | 161 (30) | 66 (46) | 95 (24) | < 0.001 |
| Pulmonary involvement | |||||
| NYHA | 0.702 | ||||
| Classes I–II | 515/150 | 425 (83) | 122 (81) | 303 (83) | |
| Classes III–IV | 515/150 | 90 (17) | 28 (19) | 62 (17) | |
| 6MWD (meters) | 274/61 | 427 ± 127 | 432 ± 135 | 425 ± 125 | 0.705 |
| TLC < 70% predicted | 472/145 | 64 (14) | 33 (23) | 31 (9) | < 0.001 |
| FVC < 70% predicted | 475/148 | 82 (17) | 44 (30) | 38 (12) | < 0.001 |
| DLCO < 70% predicted | 471/141 | 249 (53) | 102 (72) | 147 (45) | < 0.001 |
| Interstitial lung disease | 582/166 | 262 (45) | 115 (69) | 147 (35) | < 0.001 |
| PH (echo. and/or RHC) | 547/157 | 67 (12) | 18 (11) | 49 (13) | 0.775 |
| sPAP (echo.) | 0.004 | ||||
| < 35 mmHg | 397/118 | 307 (77) | 89 (75) | 218 (78) | |
| 35–46 mmHg | 397/118 | 43 (11) | 21 (18) | 22 (8) | |
| > 46 mmHg | 397/118 | 47 (12) | 8 (7) | 39 (14) | |
| PAH (RHC) | 490/116 | 40 (8) | 4 (3) | 36 (10) | 0.033 |
| Heart involvement | |||||
| Arrhythmia | 519/150 | 17 (3) | 5 (3) | 12 (3) | 1.000 |
| AV block | 512/146 | 7 (1) | 4 (3) | 3 (1) | 0.106 |
| BB block | 479/128 | 16 (3) | 6 (5) | 10 (3) | 0.388 |
| LVEF (%) | 402/102 | 64.9 ± 7.1 | 65.5 ± 8.6 | 64.7 ± 6.6 | 0.251 |
| Diastolic dysfunction | 423/110 | 20 (5) | 6 (5) | 14 (4) | 0.613 |
| Pericarditis | 478/136 | 32 (7) | 14 (10) | 18 (5) | 0.066 |
| Valvular disease | 430/111 | 29 (7) | 5 (5) | 24 (8) | 0.380 |
| Renal involvement | |||||
| GFR < 80 ml/min | 459/136 | 179 (39) | 38 (28) | 141 (44) | 0.002 |
| Scleroderma renal crisis | 428/139 | 44 (10) | 31 (22) | 13 (5) | < 0.001 |
| Gastrointestinal involvement | 611/172 | 429 (70) | 135 (78) | 294 (67) | 0.006 |
| BMI (kg/m2) | 514/159 | 24.4 ± 5.0 | 23.6 ± 4.0 | 24.7 ± 5.3 | 0.016 |
| Albuminemia < 35 g/l | 331/108 | 52 (16) | 28 (26) | 24 (11) | < 0.001 |
| Muscular involvement | 604/172 | 137 (23) | 71 (41) | 66 (15) | < 0.001 |
| CPK > 200 IU/l | 250/82 | 66 (26) | 33 (40) | 33 (20) | < 0.001 |
| Joint involvement | 598/172 | 291 (49) | 127 (74) | 164 (39) | < 0.001 |
| Cancer | 625/179 | 49 (8) | 17 (10) | 32 (7) | 0.327 |
| Hemoglobin | 559/163 | 13.0 ± 1.6 | 12.5 ± 1.6 | 13.1 ± 1.5 | < 0.001 |
| Anemia | 559/163 | 127 (23) | 52 (32) | 75 (19) | 0.001 |
| CRP > 8 mg/l | 470/136 | 118 (25) | 57 (42) | 61 (18) | < 0.001 |
| Serologic features | |||||
| ACA | 557/151 | 221 (40) | 6 (4) | 215 (53) | < 0.001 |
| Anti-Scl70 antibodies | 504/149 | 177 (35) | 90 (60) | 87 (25) | < 0.001 |
| Anti-U1RNP antibodies | 342/63 | 15 (4) | 4 (6) | 11 (4) | 0.492 |
| Anti-RNAP3 antibodies | 345/72 | 18 (5) | 13 (18) | 5 (2) | < 0.001 |
| Anti-PMScl antibodies | 343/62 | 16 (5) | 3 (5) | 13 (5) | 1.000 |
| Anti-SSa antibodies | 387/79 | 60 (16) | 20 (25) | 40 (13) | 0.014 |
| Anti-SSb antibodies | 338/62 | 11 (3) | 3 (5) | 8 (3) | 0.431 |
| APL antibodies | 441/129 | 31 (7) | 16 (12) | 15 (5) | 0.007 |
| Low complement | 482/130 | 18 (4) | 6 (5) | 12 (3) | 0.589 |
| Smoking | 572/158 | 215 (38) | 69 (44) | 146 (35) | 0.067 |
Results are expressed as n (%) for qualitative variables and mean ± SD or median [IQR] for quantitative variables
N number of patients with available data, lcSSc limited cutaneous systemic sclerosis, mRSS modified Rodnan score, GFR glomerular filtration rate, AV block atrioventricular block, BB block bundle branch block, LVEF left ventricular ejection fraction, PH pulmonary hypertension, PAH pulmonary arterial hypertension, echo echocardiography, RHC right heart catheterization, 6MWD 6-min walking distance, sPAP systolic pulmonary arterial pressure, TLC total lung capacity, FVC forced vital capacity, DLCO diffusing capacity of the lungs for carbon monoxide, CRP C reactive protein, BMI body mass index, ACA anti-centromere antibodies, APL antiphospholipid antibodies
Survival and standardized mortality ratio
During the study period, 104 deaths (16.6%) were recorded and 133 patients were lost to follow-up. Overall survival rates at 1, 3, 5, 10, and 15 years from diagnosis were 98.0% (95% CI 96.9–99.1%), 92.5% (90.4–94.7%), 85.9% (82.8–89.1%), 71.7% (66.3–77.5%), and 53% (33.8–83.4%) respectively. Survival rates for the diffuse and limited cutaneous subtypes are shown in Fig. 1 and in Additional file 1: Table S1. Overall SMR was 5.73 (95% CI 4.68–6.94).
Fig. 1.
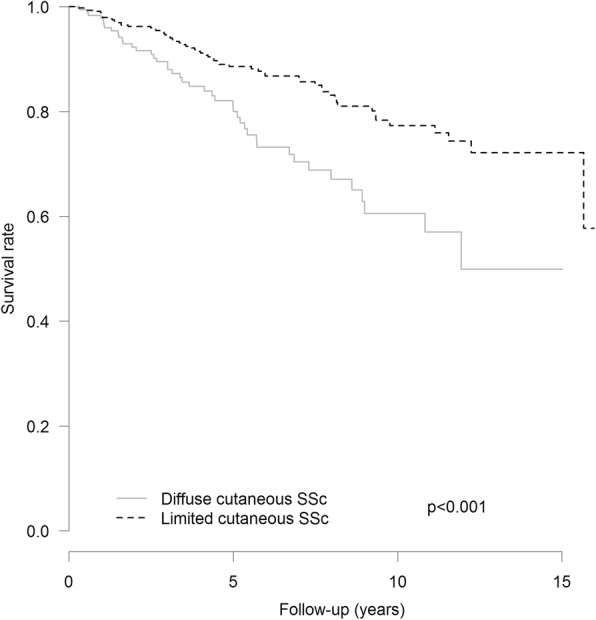
Kaplan-Meier survival curves from diagnosis for lcSSc and dcSSc patients in the French cohort
Prognosis factors
Age of diagnosis > 60 years, dcSSc subtype, telangiectasia, scleroderma renal crisis, severe dyspnea NYHA functional classes III and IV, a shorter distance at the 6MWD, FVC < 70%, DLCO < 70%, PH, valvular disease, anemia, CRP > 8 mg/l, and cancer were associated with a worse prognosis (Table 2).
Table 2.
Prognosis factors: non-adjusted and adjusted analysis on age at diagnosis, sex, and SSc subtype in the French cohort
| Non-adjusted HR | p | Adjusted HR | p | |
|---|---|---|---|---|
| Demographics | ||||
| Male sex | 2.00 (1.31–3.05) | 0.001 | 1.53 (0.98–2.39) | 0.060 |
| Age at diagnosis (per 1 year) | 1.05 (1.04–1.07) | < 0.001 | 1.08 (1.04–1.12) | < 0.001 |
| Age at diagnostic > 60 years | 4.97 (2.53–9.78) | < 0.001 | 5.79 (2.92–11.49) | < 0.001 |
| Disease duration at time of diagnosis (per 1 year) | 1.02 (0.97–1.07) | 0.542 | 1.01 (0.96–1.06) | 0.763 |
| African origin (vs. European) | 0.79 (0.38–1.62) | 0.516 | 0.93 (0.43–2.03) | 0.864 |
| Skin involvement | ||||
| dcSSc subtype (vs. lcSSc) | 2.06 (1.39–3.05) | < 0.001 | 2.40 (1.58–3.64) | < 0.001 |
| mRSS > 5 | 1.24 (1.12–1.38) | < 0.001 | 1.21 (1.03–1.43) | 0.022 |
| Past and/or active digital ulcers | 1.22 (0.79–1.90) | 0.371 | 1.29 (0.81–2.04) | 0.277 |
| Telangiectasia | 1.64 (1.08–2.48) | 0.019 | 1.55 (1.02–2.35) | 0.039 |
| Calcinosis | 1.37 (0.79–2.36) | 0.260 | 1.22 (0.69–2.16) | 0.503 |
| Lung involvement | ||||
| NYHA class I | – | – | ||
| NYHA class II | 2.68 (1.46–4.92) | 0.001 | 2.37 (1.29–4.36) | 0.006 |
| NYHA class III | 17.53 (3.97–14.27) | < 0.001 | 6.74 (3.53–12.88) | < 0.001 |
| NYHA class IV | 25.76 (10.55–62.92) | < 0.001 | 16.61 (6.68–41.26) | < 0.001 |
| NYHA classes III–IV (vs. class I) | 4.68 (3.07–7.13) | < 0.001 | 4.33 (2.82–6.66) | < 0.001 |
| 6MWD (per 100 m) | 0.46 (0.36–0.58) | < 0.001 | 0.51 (0.39–0.67) | < 0.001 |
| TLC < 70% predicted | 3.87 (2.36–6.35) | < 0.001 | 3.38 (1.96–5.82) | < 0.001 |
| FVC < 70% predicted | 3.11 (1.92–5.02) | < 0.001 | 2.79 (1.62–4.80) | < 0.001 |
| DLCO < 70% predicted | 4.01 (2.33–6.89) | < 0.001 | 3.31 (1.87–5.88) | < 0.001 |
| Interstitial lung disease | 1.99 (1.32–2.99) | < 0.001 | 1.50 (0.96–2.34) | 0.072 |
| PH (echo. and/or RHC) | 5.01 (3.18–7.89) | < 0.001 | 4.15 (2.59–6.65) | < 0.001 |
| sPAP < 35 mmHg | – | – | ||
| 35–46 mmHg | 2.05 (0.98–4.28) | 0.056 | 1.26 (0.58–2.70) | 0.559 |
| > 46 mmHg | 6.44 (3.69–11.22) | < 0.001 | 5.94 (3.30–10.72) | < 0.001 |
| PAH (RHC) | 4.96 (2.82–8.72) | < 0.001 | 4.39 (2.43–7.93) | < 0.001 |
| Heart involvement | ||||
| Arrhythmia | 2.44 (0.98–6.02) | 0.054 | 1.31 (0.52–3.32) | 0.569 |
| AV block | 0.95 (0.13–6.80) | 0.956 | 1.15 (0.15–8.58) | 0.890 |
| BB block | 1.26 (0.31–5.15) | 0.748 | 1.37 (0.33–5.67) | 0.661 |
| LVEF < 50% | 1.82 (0.25–13.24) | 0.555 | 0.92 (0.12–6.84) | 0.938 |
| Diastolic dysfunction | 1.36 (0.43–4.35) | 0.603 | 0.97 (0.30–3.13) | 0.953 |
| Pericarditis | 1.74 (0.84–3.61) | 0.139 | 1.07 (0.50–2.26) | 0.864 |
| Valvular disease | 4.03 (1.97–8.25) | < 0.001 | 2.20 (1.05–4.60) | 0.037 |
| Renal involvement | ||||
| Scleroderma renal crisis | 3.44 (2.01–5.89) | < 0.001 | 2.95 (1.61–5.40) | < 0.001 |
| GFR < 80 ml/min | 1.64 (1.06–2.52) | 0.025 | 1.37 (0.85–2.21) | 0.199 |
| Gastrointestinal involvement | 1.07 (0.68–1.69) | 0.756 | 1.02 (0.65–1.62) | 0.916 |
| BMI < 18.5 kg/m2 | 1.10 (0.45–2.74) | 0.831 | 1.79 (0.71–4.51) | 0.220 |
| Albuminemia < 35 g/l | 2.30 (1.24–4.30) | 0.009 | 1.45 (0.75–2.82) | 0.270 |
| Muscular involvement | 1.66 (1.10–2.51) | 0.016 | 1.46 (0.92–2.31) | 0.106 |
| CPK > 200 IU/L | 1.27 (0.58–2.76) | 0.550 | 1.15 (0.50–2.64) | 0.740 |
| Joint involvement | 1.22 (0.82–1.80) | 0.329 | 1.08 (0.70–1.66) | 0.720 |
| Cancer | 2.44 (1.41–4.21) | 0.001 | 1.86 (1.07–3.26) | 0.029 |
| Anemia | 2.66 (1.75–4.06) | < 0.001 | 2.37 (1.54–3.66) | < 0.001 |
| CRP > 8 mg/l | 2.05 (1.28–3.27) | 0.003 | 1.70 (1.02–2.82) | 0.041 |
| Serologic features | ||||
| ACA | 0.95 (0.62–1.44) | 0.795 | 0.85 (0.55–1.31) | 0.459 |
| Anti-Scl70 antibodies | 0.87 (0.55–1.36) | 0.534 | 0.82 (0.51–1.30) | 0.390 |
| Anti-U1RNP antibodies | 1.41 (0.51–3.93) | 0.506 | 1.32 (0.44–3.92) | 0.616 |
| Anti-RNAP3 antibodies | 0.96 (0.23–3.94) | 0.949 | 1.32 (0.44–3.92) | 0.616 |
| Anti-PMScl antibodies | 0.33 (0.05–2.41) | 0.277 | 0.49 (0.07–3.54) | 0.476 |
| APL antibodies | 1.54 (0.71–3.35) | 0.280 | 1.18 (0.53–2.63) | 0.679 |
| Low complement | 2.40 (0.97–5.95) | 0.059 | 2.38 (0.95–5.95) | 0.063 |
| Smoking | 1.06 (0.69–1.62) | 0.795 | 0.97 (0.59–1.59) | 0.901 |
Results are expressed as hazard ratios and 95% confidence interval
lcSSc limited cutaneous systemic sclerosis, dcSSc diffuse cutaneous systemic sclerosis, mRSS modified Rodnan score, GFR glomerular filtration rate, AV block atrioventricular block, BB block bundle branch block, LVEF left ventricular ejection fraction, PH pulmonary hypertension, PAH pulmonary arterial hypertension, echo echocardiography, RHC right heart catheterization, 6MWD 6-min walking distance, sPAP systolic pulmonary arterial pressure, HRCT high-resolution computer tomography, TLC total lung capacity, FVC forced vital capacity, DLCO diffusing capacity of the lungs for carbon monoxide, CRP C reactive protein, BMI body mass index, ACA anti-centromere antibodies, APL antiphospholipid antibodies
No association was found for digital ulcers, gastrointestinal, articular, muscular involvement, and specific auto-antibodies after adjustment.
Male sex showed a trend towards worse outcome, but without reaching statistical significance (HR = 1.53; 95% CI 0.98–2.39; p = 0.06).
Meta-analysis: study selection
A total of 4128 citations were assessed for inclusion. After screening, 244 abstracts were deemed potentially relevant and the full-text copies were obtained. Of these articles, 44 studies, including our cohort, were included in the meta-analysis (Fig. 2). Eighteen articles were included in the SMR analysis, representing a total population of 11,719 patients [7–12, 15, 22–24, 26, 33–38]. Thirty-six studies were included in the prognosis factor analysis, representing a total of 26,187 patients [3, 7, 10–18, 20, 22–26, 34, 39–55]. No study was excluded based on poor quality. The main characteristics of the studies are summarized in Additional file 1: Table S2 and Table S3.
Fig. 2.
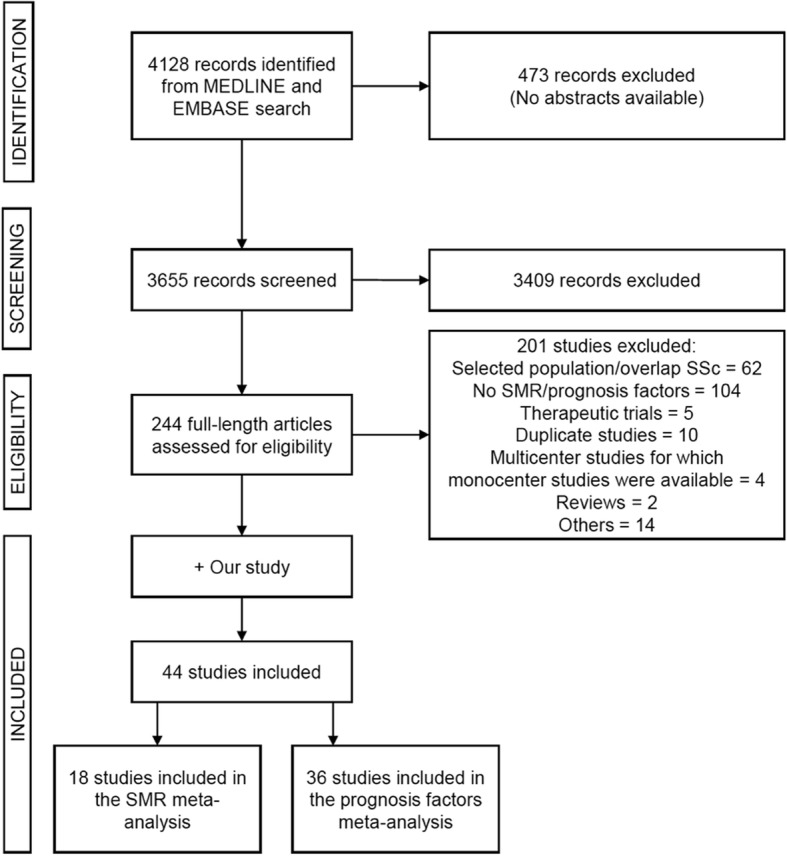
Flow chart showing search strategy to identify studies in the meta-analysis
SMR meta-analysis
The pooled SMR for all studies was 3.45 (95% CI 3.03–3.94; I2 = 88.8%; p(het) < 0.001). The pooled SMR for studies including only incident patients was 3.64 (95% CI 3.06–4.34; I2 = 82.0%; p(het) < 0.001), and the pooled SMR for studies including prevalent patients was 3.28 (95% CI 2.69–3.99; I2 = 91.6%; p(het) < 0.001). There was no funnel plot asymmetry, and Egger’s test failed to provide any evidence for small study effect, making publication bias unlikely. Meta-regression stratified by study type (incident or prevalent) did not show any association with SMR (p = 0.461), meaning no statistical difference between pooled SMR of incident and prevalent studies. Subsequent analyses were therefore conducted on all studies. Meta-regression analysis revealed a significant increase of SMR with proportion of dcSSc (p < 0.001) and prevalence of anti-Scl70 antibodies (p = 0.021). There was no association with male sex (p = 0.130). There was no significant association between SMR and mid-cohort year (p = 0.656) (Additional file 2: Figure S1. Prognosis factors meta-analysis).
Prognosis factor meta-analysis
Table 3 shows the results of the meta-analysis of prognosis factors. Age at disease onset, age at diagnosis, male sex, African origin, dcSSc, anti-Scl70 antibodies, renal involvement, scleroderma renal crisis, ILD, cardiac involvement, PH, and cancer were significantly associated with a worse prognosis. The presence of PH, diagnosed by Doppler echocardiography and/or RHC, was associated with a poor outcome (pooled HR = 3.44; 95% CI 2.59–4.58; I2 = 61.5%; p(het) = 0.002). Meta-analysis of the five studies with PH defined by RHC revealed a pooled HR of 5.27 (95% CI 2.98–9.31; I2 = 63.7%; p(het) = 0.027) for mortality. Heterogeneity could not be fully explained by the use of either echocardiography or RHC alone in defining PH as revealed by meta-regression stratified by the PH diagnosis method (p for residual heterogeneity = 0.012). The presence of ACA was associated with a better survival, while the presence of joint involvement was not associated with prognosis (Additional file 2: Figure S2).
Table 3.
Results of the meta-analysis of prognosis factors in SSc
| Number of cohorts | HR | 95% CI | I2 (%) | p(het) | Egger’s test | |
|---|---|---|---|---|---|---|
| Age at disease onset (per 1 year) | 6 | 1.05 | (1.04–1.07) | 68.6 | 0.007 | 0.783 |
| Age at diagnosis (per 1 year) | 5 | 1.04 | (1.04–1.05) | 71.2 | 0.008 | 0.025 |
| Male sex | 21 | 1.87 | (1.61–2.18) | 50.9 | 0.004 | < 0.001 |
| African origin | 5 | 1.38 | (1.15–1.66) | 25.0 | 0.255 | 0.774 |
| dcSSc | 23 | 1.90 | (1.62–2.23) | 58.3 | < 0.001 | < 0.001 |
| Anti-Scl70 autoantibodies | 13 | 1.38 | (1.09–1.74) | 49.6 | 0.022 | 0.024 |
| ACA | 8 | 0.62 | (0.47–0.82) | 56.4 | 0.025 | 0.590 |
| Joint involvement | 4 | 1.32 | (0.82–2.12) | 54.0 | 0.089 | 0.508 |
| Renal involvement | 9 | 2.79 | (1.95–3.99) | 50.9 | 0.039 | 0.512 |
| Scleroderma renal crisis | 10 | 3.89 | (2.38–6.36) | 75.6 | < 0.001 | 0.097 |
| ILD | 14 | 2.34 | (1.78–3.08) | 69.5 | < 0.001 | < 0.001 |
| Cardiac involvement | 7 | 4.35 | (2.28–8.29) | 89.9 | < 0.001 | 0.077 |
| PH (echocardiography or RHC) |
13 | 3.44 | (2.59–4.58) | 61.5 | 0.002 | 0.057 |
| PH (RHC) | 5 | 5.27 | (2.98–9.31) | 63.7 | 0.027 | 0.761 |
| Cancer | 6 | 2.11 | (1.27–3.50) | 76.2 | < 0.001 | 0.016 |
Results are expressed as hazard ratios with 95% confidence interval. The I2 statistics describes the percentage of variation across studies that is due to heterogeneity rather than chance. p(het) is the p value for the 휒2 test for heterogeneity. Egger’s test checks for funnel plot asymmetry
dcSSc diffuse cutaneous systemic sclerosis, ILD interstitial lung disease, ACA anti-centromere antibodies, PH pulmonary hypertension, RHC right heart catheterization
Discussion
The main results of our study are (i) a high risk of mortality in our cohort of incident patients, as shown by a high SMR of 5.73; (ii) the identification of age > 60 years, dcSSc, dyspnea, PH, low FVC, low DLCO, kidney involvement, valvular disease, cancer, telangiectasia, shorter 6MWD, anemia, and inflammation as prognosis factors in our cohort; (iii) a high pooled SMR of 3.45 in the meta-analysis of the literature, including our new cohort; and (iv) the additional identification of male sex, African origin, ILD, cardiac involvement, and anti-Scl-70 antibodies as associated with worse prognosis in our meta-analysis, while ACA were associated with better prognosis.
Survival and SMR
With a mid-cohort year of 2008, our study population is the largest multicenter incident and well-phenotyped cohort study of SSc patients in France and is among the most recent published to date in the literature. The overall survival rates at 5 and 10 years from diagnosis were 85.9% and 71.7%, respectively, and are lower than those reported in other recent cohorts [7, 22, 24, 44, 54]. We also report one of the highest SMR of 5.73. These differences could be explained by a high heterogeneity between studies as well as methodological issues such as the inclusion of prevalent cases in many studies or differences in time origin from which survival time is calculated (from disease onset, diagnosis, or enrolment). It is usually admitted that studies including prevalent cases underestimate mortality and that better survival is observed in prevalent patients with longer disease duration prior to inclusion. Yet, our meta-analysis did not show a significant difference between pooled SMR of studies that included prevalent cases and those restricted to incident according to the authors’ definition. The high heterogeneity observed within studies with incident patients could be due to the definition of incidence and the proportion of males and patients with anti-Scl70 antibodies. This high heterogeneity could explain the lack of difference between incident and prevalent cohorts. Meta-regression showed a significant association between SMR and proportion of dcSSc (p < 0.001) and prevalence of anti-Scl70 antibodies (p = 0.021). Our high SMR of 5.73 could therefore be partly explained by the high proportion of anti-Scl70 antibodies (35%) in our population. Interestingly, there has been a debate whether or not the survival could have improved over time in SSc. Our study did not show any improvement of SMR over time, which is in line with the study of Elhai et al. [6]. However, considering life expectancy in OECD (Organisation for Economic Co-operation and Development) countries increased by 12 years from 1960 to 2014 [56], and SMR being the ratio of mortality in SSc cohorts to that of the general population, this suggests that all-cause mortality has decreased proportionately to the general population in SSc cohorts.
Prognosis factors
Prognosis factors have been assessed in many observational studies [7, 13–26] and have been recently reviewed. Our systematic review and meta-analysis, as well as two prior meta-analyses [5, 27] and a recent EUSTAR study [2], have identified the following characteristics as consistently associated with a worse prognosis: male gender; older age; dcSSc; lung and cardiac involvement, including PH and ILD; kidney involvement; and inflammation. These robust factors are included in a recent prognosis score [2] as well as in older ones [57, 58].
Besides these well-known prognosis factors, our cohort study identifies new ones: telangiectasia, 6MWD, valvular disease, cancer, and autoantibody status.
Telangiectasia was slightly associated with a higher mortality in our study population. In contrast, Poormoghim et al. [51] reported a non-significant, yet elevated HR of 1.44 in a smaller cohort of Iranian patients. An increased number of telangiectasia has been suggested to be a clinical marker of microvascular disease in SSc and is associated with an increased risk of PAH [59].
The 6MWD is a simple tool used to assess submaximal functional capacity. It is influenced by various disease parameters during SSc and lacks organ specificity [60]. While the 6MWD has been shown to be an independent predictor of mortality in idiopathic PAH, its prognosis value in SSc-PAH is less clear [61]. To our knowledge, we are the first to report a negative association of the 6MWD with survival (HR = 0.51) in SSc patients. This can be at least partly explained by the association between the 6MWD and PAH [60].
While cardiac involvement in SSc patients is robustly associated with a poor prognosis, conferring a nearly fivefold increased risk of mortality in our meta-analysis, no study had yet focused on the valvular manifestations of SSc. A recent article comparing echocardiography in SSc patients and a matched control population showed a greater frequency of valvular regurgitation and valvular replacement due to regurgitation [62]. Moreover, in a large multicenter French cohort, De Groote et al. [63] reported 6.7% mitral regurgitation and 2.5% aortic regurgitations. To our knowledge, we are the first to describe an association between valvular disease and survival in SSc. These data indicate that more attention should be paid to valvular disease in SSc patients and further studies are needed to confirm its prognostic significance.
An increased incidence of malignancy has been reported during SSc, especially lung and hematological cancer [64]. Cancer has also been described among the leading cause of non-SSc-related deaths [7, 11, 15, 65], and a temporal relation has been reported between the onset of cancer and SSc [66]. As expected, in our cohort as well as in the meta-analysis, malignancy was significantly associated with shorter survival.
In our cohort, we did not observe any association between anti-Scl70 antibodies or ACA and survival. Interestingly, the absence of association of anti-Scl70 antibodies with survival has been suggested by numerous studies [7, 17, 18, 23, 25] while the protective role of anti-centromere antibodies is better established [7, 18, 22, 25, 48]. Our meta-analysis confirms that the presence of ACA is associated with better survival (pooled HR = 0.58). Moreover, our meta-analysis suggests that the presence of anti-Scl70 antibodies could be indeed a predictor of mortality with a pooled HR of 1.38. Our analysis also highlights a probable publication bias. Small studies reporting a negative association of anti-Scl70 antibodies with death are notably lacking. Therefore, it is difficult to draw a firm conclusion on the role of anti-Scl70 antibodies as a prognosis factor in SSc.
The major strength of our study is the availability of detailed clinical and laboratory characteristics in a multicenter cohort of incident patients. The major strengths of our meta-analysis include (i) the first analysis of pooled HR of anti-Scl70/ACA antibodies, (ii) the separate analysis of pooled SMR in incident cohorts of SSc, and (iii) the separate analysis of pooled HR of PH diagnosed by RHC only.
The main limitation is a proportion of loss to follow-up of around 20% in our cohort, despite our attempts to collect information on participants who dropped out. These patients lost to follow-up had a higher prevalence of ILD and lower prevalence of ACA at baseline, leading to potential underestimation of mortality. A second limitation is the variable definition of incident patients among studies. Although we defined incident patients as newly diagnosed ones, these patients had relatively short disease duration from first non-RP symptom diagnosis, thus minimizing survivor bias. Because of missing values, the effect of specific treatments and other data such as type and stage of cancer could not be studied, and no multivariate analysis could be performed. In addition, multiple univariate tests are responsible for an inflation of the alpha risk. However, multiple test adjustments in such exploratory study, in addition for a rare disease, are not strictly required [67]. Finally, our study was performed in five selected referral centers and may therefore have focused on a subset of patients with more severe disease, which could limit the representativeness of our findings.
Conclusions
Our results show that mortality is still high in SSc. Strong prognosis factors identified at baseline are age at diagnosis > 60 years, dcSSc subtype, scleroderma renal crisis, severe dyspnea, FVC and DLCO < 70%, PH, anemia, and CRP > 8 mg/l. Our study also suggests the prognosis value of telangiectasia, 6MWD, valvular disease, cancer, and autoantibody status.
Additional files
Table S1. Survival rates and survival curve for lcSSc and dcSSc. Table S2. Main characteristics of studies in the SMR meta-analysis. Table S3. Main characteristics of studies in the prognosis factors meta-analysis. (DOCX 51 kb)
Figure S1. Funnel plots, forest plots, and meta-regression for the meta-analysis of SMR. Figure S2. Funnel plots and forest plots of the risk factors related with mortality. (PDF 948 kb)
Acknowledgements
French National Scleroderma Cohort coauthors: Zahir AMOURA Z, Olivier AUMAITRE, Eric AUXENFANTS, Marie-Hélène BALQUET, Cristina BELIZNA, Alice BEREZNE A, Bernard BONNOTTE, Pascal CATHEBRAS, Emmanuel CHATELUS, Joël CONSTANS, Vincent COTTIN V, Gonzalo DE LUNA G, Robin DHOTE, Elisabeth DIOT, Olivier FAIN, Anne-Laure FAUCHAIS, Yves FRANCES, Jean-Gabriel FUZIBET, Jean-Baptiste GAULTIER, Tiphaine GOULENOK, Brigitte GRANEL, Jean-Robert HARLE, Pierre-Yves HATRON, Arnaud HOT, Bernard IMBERT, Jean-Emmanuel KAHN, Gilles KAPLANSKI, Pierre KIEFFER, Noémie LE GOUELLEC, Alain LE QUELLEC, Olivier LIDOVE, Nadine MAGY-BERTRAND, François MAURIER, Sylvain PALAT, Thomas PAPO, Jean-Louis PENNAFORTE, Jacques POUCHOT, Grégory PUGNET, Thomas QUEMENEUR, Viviane QUEYREL, Laurent SAILLER, Thierry SCHAEVERBEKE, Marie-Elise TRUCHETET, Denis WAHL.
Funding
Grants or financial supporters: none.
Financial support or other benefits from commercial sources: none.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due data protection regulations but are available from the corresponding author on reasonable request.
Abbreviations
- 6MWD
6-min walking distance
- ACA
Anti-centromere antibodies
- APL
Antiphospholipid antibodies
- AV block
Atrioventricular block
- BB block
Bundle branch block
- BMI
Body mass index
- CPK
Creatinine kinase
- CRP
C reactive protein
- dcSSc
Diffuse cutaneous systemic sclerosis
- DLCO
Diffusing capacity of the lungs for carbon monoxide
- FVC
Forced vital capacity
- ILD
Interstitial lung disease
- lcSSc
Limited cutaneous systemic sclerosis
- LVEF
Left ventricular ejection fraction
- mRSS
Modified Rodnan skin score
- OECD
Organisation for Economic Co-operation and Development
- PAH
Pulmonary arterial hypertension
- PAP
Pulmonary arterial pressure
- PH
Pulmonary hypertension
- RHC
Right heart catheterization
- RP
Raynaud phenomenon
- SMR
Standardized mortality ratio
- SSc
Systemic sclerosis
- TLC
Total lung capacity
Authors’ contributions
MRP performed the systematic review of the literature and was a major contributor in interpreting the cohort data and meta-analysis and in writing the manuscript. JG was a major contributor in the design of the cohort study, performed data management, analyzed the cohort data, and was a major contributor in the interpretation of data. LD performed the statistical tests of the meta-analysis and was a major contributor in the interpretation of data. LM, CA, JCL, YA, BB, SB, and AM were significant contributors in the acquisition of data and in the critical review of the manuscript. EH was a major contributor in the design of the study, in the interpretation of data, and in the critical review of the manuscript. DL was a major contributor in the design of the study, in the systematic review of the literature, in the interpretation of data, and in writing the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was authorized by the French Ministry of Research. The authorization number is 13145. To issue such authorization, the Ministry of Research has sought the advice of an independent ethics committee, namely the “Comité consultatif sur le traitement de l’information en matière de recherche (CCTIRS),” which voted positively. French legislation on non-interventional studies requires collecting the non-opposition of patients but does not require written consent. As such, non-opposition was obtained from each patient included in the study for the use of their de-identified medical record data.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
M. R. Pokeerbux, Email: r.pokeerbux@gmail.com
J. Giovannelli, Email: jonathan.giovannelli@gmail.com
L. Dauchet, Email: ldauchet@gmail.com
L. Mouthon, Email: luc.mouthon@aphp.fr
C. Agard, Email: christian.agard@chu-nantes.fr
J. C. Lega, Email: jean-christophe.lega@chu-lyon.fr
Y. Allanore, Email: yannick.allanore@me.com
P. Jego, Email: patrick.jego@chu-rennes.fr
B. Bienvenu, Email: bbienvenu@hopital-saint-joseph.fr
S. Berthier, Email: sabine.berthier@chu-dijon.fr
A. Mekinian, Email: arsene.mekinian@aphp.fr
E. Hachulla, Email: eric.hachulla@chru-lille.fr
D. Launay, Phone: +33320444433, Email: launayd@gmail.com, Email: david.launay@univ-lille.fr
References
- 1.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972-2002. Ann Rheum Dis. 2007;66:940–944. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elhai M, Meune C, Boubaya M, Avouac J, Hachulla E, Balbir-Gurman A, et AL. Mapping and predicting mortality from systemic sclerosis. Ann Rheum Dis. 2017;76:1897-1905. [DOI] [PubMed]
- 3.Al-Dhaher FF, Pope JE, Ouimet JM. Determinants of morbidity and mortality of systemic sclerosis in Canada. Semin Arthritis Rheum. 2010;39:269–277. doi: 10.1016/j.semarthrit.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Nihtyanova SI, Tang EC, Coghlan JG, Wells AU, Black CM, Denton CP. Improved survival in systemic sclerosis is associated with better ascertainment of internal organ disease: a retrospective cohort study. QJM. 2010;103:109–115. doi: 10.1093/qjmed/hcp174. [DOI] [PubMed] [Google Scholar]
- 5.Rubio-Rivas M, Royo C, Simeón CP, Corbella X, Fonollosa V. Mortality and survival in systemic sclerosis: systematic review and meta-analysis. Semin Arthritis Rheum. 2014;44:208-19. [DOI] [PubMed]
- 6.Elhai M, Meune C, Avouac J, Kahan A, Allanore Y. Trends in mortality in patients with systemic sclerosis over 40 years: a systematic review and meta-analysis of cohort studies. Rheumatology. 2012;51:1017–1026. doi: 10.1093/rheumatology/ker269. [DOI] [PubMed] [Google Scholar]
- 7.Hao Y, Hudson M, Baron M, Carreira P, Stevens W, Rabusa C, et al. Early mortality in a multinational systemic sclerosis inception cohort. Arthritis Rheumatol. 2017;69:1067-77. [DOI] [PubMed]
- 8.Alamanos Y, Tsifetaki N, Voulgari P, Siozos C, Tsamandouraki K, Alexiou G, et al. Epidemiology of systemic sclerosis in northwest Greece 1981 to 2002. Semin Arthritis Rheum. 2005;34:714–720. doi: 10.1016/j.semarthrit.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Bryan C, Howard Y, Brennan P, Black CM, Silman A. Survival following the onset of scleroderma: results from a retrospective inception cohort study of the UK patient population. Rheumatology. 1996;35:1122–1126. doi: 10.1093/rheumatology/35.11.1122. [DOI] [PubMed] [Google Scholar]
- 10.Ioannidis JPA, Vlachoyiannopoulos PG, Haidich A-B, Medsger TA, Lucas M, Michet CJ, et al. Mortality in systemic sclerosis: an international meta-analysis of individual patient data. Am J Med. 2005;118:2–10. doi: 10.1016/j.amjmed.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 11.Jacobsen S, Halberg P, Ullman S. Mortality and causes of death of 344 Danish patients with systemic sclerosis (scleroderma) Rheumatology. 1998;37:750–755. doi: 10.1093/rheumatology/37.7.750. [DOI] [PubMed] [Google Scholar]
- 12.Kuo C-F, See L-C, Yu K-H, Chou I-J, Tseng W-Y, Chang H-C, et al. Epidemiology and mortality of systemic sclerosis: a nationwide population study in Taiwan. Scand J Rheumatol. 2011;40:373–378. doi: 10.3109/03009742.2011.553736. [DOI] [PubMed] [Google Scholar]
- 13.Lee P, Langevitz P, Alderdice C, Aubrey M, Baer P, Baron M, et al. Mortality in systemic sclerosis (scleroderma) QJM. 1992;82:139–148. [PubMed] [Google Scholar]
- 14.Ferri C, Valentini G, Cozzi F, Sebastiani M, Michelassi C, La Montagna G, et al. Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 2002;81:139–153. doi: 10.1097/00005792-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Scussel-Lonzetti L, Joyal F, Raynauld J-P, Roussin A, Rich E, Goulet J-R, et al. Predicting mortality in systemic sclerosis: analysis of a cohort of 309 French Canadian patients with emphasis on features at diagnosis as predictive factors for survival. Medicine (Baltimore) 2002;81:154–167. doi: 10.1097/00005792-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Czirjak L, Kumanovics G, Varju C, Nagy Z, Pakozdi A, Szekanecz Z, et al. Survival and causes of death in 366 Hungarian patients with systemic sclerosis. Ann Rheum Dis. 2008;67:59–63. doi: 10.1136/ard.2006.066340. [DOI] [PubMed] [Google Scholar]
- 17.Hachulla E, Carpentier P, Gressin V, Diot E, Allanore Y, Sibilia J, et al. Risk factors for death and the 3-year survival of patients with systemic sclerosis: the French ItinerAIR-Sclerodermie study. Rheumatology. 2008;48:304–308. doi: 10.1093/rheumatology/ken488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ssassi S, del Junco D, Sutter K, McNearney TA, Reveille JD, Karnavas A, et al. Clinical and genetic factors predictive of mortality in early systemic sclerosis. Arthritis Rheum. 2009;61:1403–11. [DOI] [PMC free article] [PubMed]
- 19.Joven BE, Almodovar R, Carmona L, Carreira PE. Survival, causes of death, and risk factors associated with mortality in Spanish systemic sclerosis patients: results from a single university hospital. Semin Arthritis Rheum. 2010;39:285–293. doi: 10.1016/j.semarthrit.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Kim J, Park SK, Moon KW, Lee EY, Lee YJ, Song YW, et al. The prognostic factors of systemic sclerosis for survival among Koreans. Clin Rheumatol. 2010;29:297–302. doi: 10.1007/s10067-009-1324-7. [DOI] [PubMed] [Google Scholar]
- 21.Sampaio-Barros PD, Bortoluzzo AB, Marangoni RG, Rocha LF, Del Rio APT, Samara AM, et al. Survival, causes of death, and prognostic factors in systemic sclerosis: analysis of 947 Brazilian patients. J Rheumatol. 2012;39:1971–1978. doi: 10.3899/jrheum.111582. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann-Vold A-M, Molberg Ø, Midtvedt Ø, Garen T, Gran JT. Survival and causes of death in an unselected and complete cohort of Norwegian patients with systemic sclerosis. J Rheumatol. 2013;40:1127–1133. doi: 10.3899/jrheum.121390. [DOI] [PubMed] [Google Scholar]
- 23.Strickland G, Pauling J, Cavill C, Shaddick G, McHugh N. Mortality in systemic sclerosis—a single centre study from the UK. Clin Rheumatol. 2013;32:1533–1539. doi: 10.1007/s10067-013-2289-0. [DOI] [PubMed] [Google Scholar]
- 24.Alba MA, Velasco C, Simeón CP, Fonollosa V, Trapiella L, Egurbide MV, et al. Early- versus late-onset systemic sclerosis: differences in clinical presentation and outcome in 1037 patients. Medicine (Baltimore) 2014;93:73–81. doi: 10.1097/MD.0000000000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cottrell TR, Wise RA, Wigley FM, Boin F. The degree of skin involvement identifies distinct lung disease outcomes and survival in systemic sclerosis. Ann Rheum Dis. 2014;73:1060–1066. doi: 10.1136/annrheumdis-2012-202849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nihtyanova SI, Schreiber BE, Ong VH, Rosenberg D, Moinzadeh P, Coghlan JG, et al. Prediction of pulmonary complications and long-term survival in systemic sclerosis: pulmonary complications and survival in SSc. Arthritis Rheumatol. 2014;66:1625–1635. doi: 10.1002/art.38390. [DOI] [PubMed] [Google Scholar]
- 27.Komócsi A, Vorobcsuk A, Faludi R, Pintér T, Lenkey Z, Költő G, et al. The impact of cardiopulmonary manifestations on the mortality of SSc: a systematic review and meta-analysis of observational studies. Rheumatology. 2012;51:1027–1036. doi: 10.1093/rheumatology/ker357. [DOI] [PubMed] [Google Scholar]
- 28.Preliminary criteria for the classification of systemic sclerosis (scleroderma) Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23:581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 29.van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against rheumatism collaborative initiative: ACR/EULAR classification criteria for SSc. Arthritis Rheum. 2013;65:2737–2747. doi: 10.1002/art.38098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 32.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis [internet]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 33.Abu-Shakra M, Lee P. Mortality in systemic sclerosis: a comparison with the general population. J Rheumatol. 1995;22:2100–2102. [PubMed] [Google Scholar]
- 34.Cruz-Domínguez MP, García-Collinot G, Saavedra MA, Montes-Cortes DH, Morales-Aguilar R, Carranza-Muleiro RA, et al. Malnutrition is an independent risk factor for mortality in Mexican patients with systemic sclerosis: a cohort study. Rheumatol Int. 2017;37:1101–1109. doi: 10.1007/s00296-017-3753-y. [DOI] [PubMed] [Google Scholar]
- 35.Hesselstrand R, Scheja A, Akesson A. Mortality and causes of death in a Swedish series of systemic sclerosis patients. Ann Rheum Dis. 1998;57:682–686. doi: 10.1136/ard.57.11.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mok CC, Kwok CL, Ho LY, Chan PT, Yip SF. Life expectancy, standardized mortality ratios, and causes of death in six rheumatic diseases in Hong Kong, China. Arthritis Rheum. 2011;63:1182–1189. doi: 10.1002/art.30277. [DOI] [PubMed] [Google Scholar]
- 37.Perez-Bocanegra C, Solans-Laque R, Simeon-Aznar CP, Campillo M, Fonollosa-Pla V, Vilardell-Tarres M. Age-related survival and clinical features in systemic sclerosis patients older or younger than 65 at diagnosis. Rheumatology. 2010;49:1112–1117. doi: 10.1093/rheumatology/keq046. [DOI] [PubMed] [Google Scholar]
- 38.Zarafonetis CJ, Dabich L, Negri D, Skovronski JJ, Devol EB, Wolfe R. Retrospective studies in scleroderma: effect of potassium para-aminobenzoate on survival. J Clin Epidemiol. 1988;41:193–205. doi: 10.1016/0895-4356(88)90094-7. [DOI] [PubMed] [Google Scholar]
- 39.Beretta L, Santaniello A, Cappiello F, Chawla NV, Vonk MC, Carreira PE, et al. Development of a five-year mortality model in systemic sclerosis patients by different analytical approaches. Clin Exp Rheumatol. 2010;28:S18. [PubMed] [Google Scholar]
- 40.Beretta L, Cappiello F, Barili M, Scorza R. Proximal interleukin-10 gene polymorphisms in Italian patients with systemic sclerosis. Tissue Antigens. 2007;69:305–312. doi: 10.1111/j.1399-0039.2007.00811.x. [DOI] [PubMed] [Google Scholar]
- 41.Bernal-Bello D, de Tena JG, Guillén-del Castillo A, Selva-O’Callaghan A, Callejas-Moraga EL, Marín-Sánchez AM, et al. Novel risk factors related to cancer in scleroderma. Autoimmun Rev. 2017;16:461–468. doi: 10.1016/j.autrev.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 42.Codullo V, Cereda E, Klersy C, Cavazzana I, Alpini C, Bonardi C, et al. Serum prealbumin is an independent predictor of mortality in systemic sclerosis outpatients. Rheumatology. 2016;55:315–319. doi: 10.1093/rheumatology/kev322. [DOI] [PubMed] [Google Scholar]
- 43.Fernández-Codina A, Simeón-Aznar CP, Pinal-Fernandez I, Rodríguez-Palomares J, Pizzi MN, Hidalgo CE, et al. Cardiac involvement in systemic sclerosis: differences between clinical subsets and influence on survival. Rheumatol Int. 2017;37:75–84. doi: 10.1007/s00296-015-3382-2. [DOI] [PubMed] [Google Scholar]
- 44.Ferri C, Sebastiani M, Lo Monaco A, Iudici M, Giuggioli D, Furini F, et al. Systemic sclerosis evolution of disease pathomorphosis and survival. Our experience on Italian patients’ population and review of the literature. Autoimmun Rev. 2014;13:1026–1034. doi: 10.1016/j.autrev.2014.08.029. [DOI] [PubMed] [Google Scholar]
- 45.Gelber AC, Manno RL, Shah AA, Woods A, Le EN, Boin F, et al. Race and association with disease manifestations and mortality in scleroderma. Medicine (Baltimore) 2013;92:191–205. doi: 10.1097/MD.0b013e31829be125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hesselstrand R. The association of antinuclear antibodies with organ involvement and survival in systemic sclerosis. Rheumatology. 2003;42:534–540. doi: 10.1093/rheumatology/keg170. [DOI] [PubMed] [Google Scholar]
- 47.Hinchcliff M, Desai CS, Varga J, Shah SJ. Prevalence, progn,osis, and factors associated with left ventricular diastolic dysfunction in systemic sclerosis. Clin Exp Rheumatol. 2012;30:S30. [PMC free article] [PubMed] [Google Scholar]
- 48.Hussein H, Lee P, Chau C, Johnson SR. The effect of male sex on survival in systemic sclerosis. J Rheumatol. 2014;41:2193–2200. doi: 10.3899/jrheum.140006. [DOI] [PubMed] [Google Scholar]
- 49.Költő G, Faludi R, Aradi D, Bartos B, Kumánovics G, Minier T, et al. Impact of cardiac involvement on the risk of mortality among patients with systemic sclerosis: a 5-year follow-up of a single-center cohort. Clin Rheumatol. 2014;33:197–205. doi: 10.1007/s10067-013-2358-4. [DOI] [PubMed] [Google Scholar]
- 50.Mayes MD, Lacey JV, Beebe-Dimmer J, Gillespie BW, Cooper B, Laing TJ, et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 2003;48:2246–2255. doi: 10.1002/art.11073. [DOI] [PubMed] [Google Scholar]
- 51.Poormoghim H, Andalib E, Jalali A, Ghaderi A, Ghorbannia A, Mojtabavi N. Survival and causes of death in systemic sclerosis patients: a single center registry report from Iran. Rheumatol Int. 2016;36:925–934. doi: 10.1007/s00296-016-3475-6. [DOI] [PubMed] [Google Scholar]
- 52.Ruangjutipopan S, Kasitanon N, Louthrenoo W, Sukitawut W, Wichainun R. Causes of death and poor survival prognostic factors in thai patients with systemic sclerosis. J Med Assoc Thail Chotmaihet Thangphaet. 2002;85:1204–1209. [PubMed] [Google Scholar]
- 53.Simeon CP. Mortality and prognostic factors in Spanish patients with systemic sclerosis. Rheumatology. 2003;42:71–75. doi: 10.1093/rheumatology/keg033. [DOI] [PubMed] [Google Scholar]
- 54.Simeón-Aznar CP, Fonollosa-Plá V, Tolosa-Vilella C, Espinosa-Garriga G, Campillo-Grau M, Ramos-Casals M, et al. Registry of the Spanish network for systemic sclerosis: survival, prognostic factors, and causes of death. Medicine (Baltimore) 2015;94:e1728. doi: 10.1097/MD.0000000000001728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steen V, Domsic RT, Lucas M, Fertig N, Medsger TA. A clinical and serologic comparison of African American and Caucasian patients with systemic sclerosis. Arthritis Rheum. 2012;64:2986–2994. doi: 10.1002/art.34482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.OECD. “Life expectancy at birth”, in Health at a Glance 2017: OECD Indicators [Internet]. OECD Publishing (Paris); Available from: 10.1787/health_glance-2017-6-en
- 57.Fransen J, Popa-Diaconu D, Hesselstrand R, Carreira P, Valentini G, Beretta L, et al. Clinical prediction of 5-year survival in systemic sclerosis: validation of a simple prognostic model in EUSTAR centres. Ann Rheum Dis. 2011;70:1788–1792. doi: 10.1136/ard.2010.144360. [DOI] [PubMed] [Google Scholar]
- 58.Bryan C, Knight C, Black CM, Silman AJ. Prediction of five-year survival following presentation with scleroderma: development of a simple model using three disease factors at first visit. Arthritis Rheum. 1999;42:2660–2665. doi: 10.1002/1529-0131(199912)42:12<2660::AID-ANR23>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 59.Jouvray M, Launay D, Dubucquoi S, Sobanski V, Podevin C, Lambert M, et al. Whole-body distribution and clinical association of Telangiectases in systemic sclerosis. JAMA Dermatol. 2018;154:796-805. [DOI] [PMC free article] [PubMed]
- 60.Sanges S, Giovannelli J, Sobanski V, Morell-Dubois S, Maillard H, Lambert M, et al. Factors associated with the 6-minute walk distance in patients with systemic sclerosis. Arthritis Res Ther. 2017;19:279-90. [DOI] [PMC free article] [PubMed]
- 61.Lefèvre G, Dauchet L, Hachulla E, Montani D, Sobanski V, Lambert M, et al. Survival and prognostic factors in systemic sclerosis-associated pulmonary hypertension: a systematic review and meta-analysis. Arthritis Rheum. 2013;65:2412–2423. doi: 10.1002/art.38029. [DOI] [PubMed] [Google Scholar]
- 62.Nordin A, Svenungsson E, Björnådal L, Elvin K, Larsson A, Jensen-Urstad K. Troponin I and echocardiography in patients with systemic sclerosis and matched population controls. Scand J Rheumatol. 2017;46:226–235. doi: 10.1080/03009742.2016.1192217. [DOI] [PubMed] [Google Scholar]
- 63.de GP, Gressin V, Hachulla E, Carpentier P, Guillevin L, Kahan A, et al. Evaluation of cardiac abnormalities by Doppler echocardiography in a large nationwide multicentric cohort of patients with systemic sclerosis. Ann Rheum Dis. 2008;67:31–36. doi: 10.1136/ard.2006.057760. [DOI] [PubMed] [Google Scholar]
- 64.Onishi A, Sugiyama D, Kumagai S, Morinobu A. Cancer incidence in systemic sclerosis: meta-analysis of population-based cohort studies. Arthritis Rheum. 2013;65:1913–1921. doi: 10.1002/art.37969. [DOI] [PubMed] [Google Scholar]
- 65.Tyndall AJ, Bannert B, Vonk M, Airo P, Cozzi F, Carreira PE, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69:1809–1815. doi: 10.1136/ard.2009.114264. [DOI] [PubMed] [Google Scholar]
- 66.Shah AA, Rosen A, Hummers L, Wigley F, Casciola-Rosen L. Close temporal relationship between onset of cancer and scleroderma in patients with RNA polymerase I/III antibodies. Arthritis Rheum. 2010;62:2787–2795. doi: 10.1002/art.27549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bender R, Lange S. Adjusting for multiple testing—when and how? J Clin Epidemiol. 2001;54:343–349. doi: 10.1016/S0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Survival rates and survival curve for lcSSc and dcSSc. Table S2. Main characteristics of studies in the SMR meta-analysis. Table S3. Main characteristics of studies in the prognosis factors meta-analysis. (DOCX 51 kb)
Figure S1. Funnel plots, forest plots, and meta-regression for the meta-analysis of SMR. Figure S2. Funnel plots and forest plots of the risk factors related with mortality. (PDF 948 kb)
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due data protection regulations but are available from the corresponding author on reasonable request.


