Abstract
Background
Metabolic syndrome (MS) is prevalent in chronic kidney disease (CKD). Klotho, a protein linked to aging, is closely associated with CKD. Each component of MS and klotho has an association. However, little is known about the association between klotho and MS per se. We investigated the association between serum klotho levels and MS using baseline cross-sectional data obtained from a large Korean CKD cohort.
Methods
Of the 2238 subjects recruited in the KoreaN Cohort Study for Outcome in Patients With Chronic Kidney Disease (KNOW-CKD) between 2011 and 2016, 484 patients with missing data on serum klotho and extreme klotho values (values lower than the detectable range or > 6000 pg/mL) or with autosomal dominant polycystic kidney disease patients were excluded. The data of the remaining 1754 subjects were included in the present study. MS was defined using the revised National Cholesterol Education Program Adult Treatment Panel (NCEP-ATP) III criteria. Serum klotho levels were measured using an enzyme-linked immunosorbent assay.
Results
Mean patient age was 54.9 ± 12.1 years and 1110 (63.3%) were male. The prevalence of MS among all study subjects was 63.7% (n = 1118). The median serum klotho level was 527 pg/mL (interquartile range [IQR]: 418–656 pg/mL). Serum klotho level was significantly lower in MS patients than patients without MS (Median [IQR]; 521 pg/mL [413, 651] vs. 541 pg/mL [427, 676], respectively; P = 0.012). After adjusting for age, sex, estimated glomerular filtration rate, and overt proteinuria, serum klotho was independently associated with MS (adjusted odds ratio [OR], 0.44; 95% confidence interval, 0.23–0.82; P = 0.010). Furthermore, the adjusted OR for MS was found to be significantly increased at serum klotho levels of < 518 pg/mL (receiver operating characteristic curve cut-off value).
Conclusions
Serum klotho was inversely associated with the presence of MS in patients with CKD.
Trial registration
This trial was registered on ClinicalTrials.gov on 26 June 2012 (https://clinicaltrials.gov;NCT01630486).
Keywords: Chronic kidney disease, Klotho, Metabolic syndrome
Background
Metabolic syndrome (MS) is a cluster of risk factors for diabetes and cardiovascular (CV) disease and is characterized by hypertension, hyperglycemia, hypertriglyceridemia, decreased high-density lipoprotein (HDL) cholesterol, and abdominal obesity [1, 2]. Furthermore, MS is common in chronic kidney disease (CKD) patients [3]. Previous studies showed that MS was independently associated with an increased risk for CKD and vice versa [4, 5].
Klotho, a protein linked to aging, is closely associated with CKD. In a previous study, klotho knock-out mice exhibited similarities with CKD patients, such as, hyperphosphatemia, ectopic soft tissue calcification, and arteriosclerosis [6], which suggested CKD might result from a state of klotho deficiency. Thus, in addition to serving as a biomarker for CKD, klotho deficiency is also viewed as a pathogenetic indicator of renal and extra-renal complications in CKD [7]. Recent reports indicated klotho is associated with each component of MS. Arking et al. [8] showed that a functional variant of the Klotho gene was associated with increased systolic blood pressure and decreased HDL-cholesterol levels in 525 Jewish subjects. In experimental studies, klotho has been shown to ameliorate vascular endothelial dysfunction, to increase nitric oxide production, and to reduce elevated blood pressure in an animal models of MS [9, 10]. Klotho polymorphism was significantly associated with glucose metabolism in apparently healthy Korean females [11]. In addition, genetic variants of Klotho have been associated with insulin resistance and hypertriglyceridemia [12]. Accordingly, it was suggested in all of these studies that klotho be considered a candidate molecule in MS. However, little is known about the association between klotho and MS per se in CKD patients. In the present study, we investigated the metabolic profile characteristics of Korean CKD patients and evaluated the association between serum klotho and MS using baseline cross-sectional data obtained from a large Korean CKD cohort.
Methods
Study population
We conducted a cross-sectional analysis of clinical profiles obtained at enrollment for the KoreaN Cohort Study for Outcome in Patients With Chronic Kidney Disease (KNOW-CKD) study. KNOW-CKD is a nationwide prospective cohort study, including predialysis subjects with CKD from stage 1 to 5, aged between 20 and 75 years and recruited from nine clinical centers of major university-affiliated Korean hospitals. Details of the rationale and study design of the KNOW-CKD have been described elsewhere [13]. Of 2238 CKD patients recruited between June 2011 and January 2016, 484 were excluded for the following reasons: 125 for missing serum klotho data; 9 for a serum klotho level lower than the detectable range; 3 for an extremely higher klotho level (> 6000 pg/mL); and 347 for autosomal dominant polycystic kidney disease patients because large cystic masses in their kidneys and liver can cause an overestimation of abdominal obesity. Extreme klotho values were not influence value by Cook’s distance analysis. Finally, 1754 CKD patients were included in the cross-sectional analysis. Klotho expression is known to decline in cases of acute kidney injury, regardless of whether it is caused by ischemia, infection, a toxin, or ureteral obstruction [14–16]. However, in the present study, we did not enroll patients with acute illness at the time of initial enrollment. Therefore, it is unlikely that initial klotho levels were influenced by acute illness. The study protocol was approved by the ethical committees of participating clinical centers, that is, by the Institutional Review Boards of Seoul National University Hospital (1104–089-359), Seoul National University Bundang Hospital (B-1106/129–008), Yonsei University Severance Hospital (4–2011-0163), Kangbuk Samsung Medical Center (2011–01-076), Seoul St. Mary’s Hospital (KC11OIMI0441), Gil Hospital (GIRBA2553), Eulji General Hospital (201105–01), Chonnam National University Hospital (CNUH-2011-092), and Pusan Paik Hospital (11–091) in 2011. All study subjects provided written informed consent. This study was performed in accordance with the principles of the Declaration of Helsinki.
Clinical data collection and laboratory analyses
Laboratory values and demographic characteristics at enrollment were extracted from an electronic case-reporting form (http://www.phactax.org) developed by the assistance of the Division of Data Management at Seoul National University Medical Research Collaborating Center. Waist circumferences were measured midway between the lower part of the lowest rib and the upper iliac crest using a standardized protocol at clinics, as recommended by the World Health Organization (WHO) [17]. Brachial systolic blood pressure (SBP) and diastolic blood pressure (DBP) were obtained by averaging two measurements (separated by a minimum rest of 5 min in the sitting position) obtained using a calibrated oscillometric device (BP-203RV III; Omron Co., Kyoto, Japan). Serum creatinine levels were measured by an isotope dilution mass spectrometry (IDMS)-traceable method [18] at a central laboratory. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation [19]. Fasting blood glucose and triglyceride levels were measured in the hospital laboratories of participating center according to a standardized protocol. Overt proteinuria was defined as a 24-h urine protein result of > 500 mg/day. Serum α-klotho level was measured using an enzyme linked immunosorbent assay (ELISA) kit (Immuno-Biological Laboratories Co., Gunma, Japan) according to the manufacturer’s instructions [20]. The intra- and inter-assay coefficients of variation of this kit were 2.7–3.5% (klotho levels 186.64–2968.78 pg/mL) and 2.9–11.4% (klotho levels 165.47–2903.01 pg/mL), respectively. The data for intra- and inter-assay coefficients of variations were validated in our central laboratory by measurements of a serum control in 20 repeats on each ELISA plate. The intra- and inter-assay coefficients of variations were 0.57–1.78% and 3.01–6.12% (klotho levels 120.30–4468.50 pg/mL), respectively. The standard curve was shown to be linear up to 4000 pg/mL. C-terminal FGF23 was measured using a second generation human FGF23 ELISA kit (Immutopics, San Clemente, California, USA) according to the manufacturer’s instructions. The intra- and inter-assay coefficients of variation as informed by the manufacturer were 1.4–2.4% (FGF23 levels 33.7–302 RU/mL) and 2.4–4.7% (FGF23 levels 33.6–293 RU/mL), respectively.
Definition of metabolic syndrome
MS was defined using the modified National Cholesterol Education Program Adult Treatment Panel (NCEP-ATP) III criteria according to American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement, which adopted the Asian-Pacific waist circumference threshold by WHO. Subjects with three or more of the following 5 metabolic components were defined as having MS [2, 21]: 1) history of hypertension, elevated blood pressure (systolic blood pressure ≥ 130 mmHg, or diastolic blood pressure ≥ 85 mmHg), or taking anti-hypertensive medication; 2) history of diabetes mellitus, elevated fasting plasma glucose (≥100 mg/dL), or the use of anti-diabetes medication; 3) elevated triglycerides (≥150 mg/dL), or on drug treatment for elevated triglycerides; 4) reduced HDL cholesterol (< 40 mg/dL for men and < 50 mg/dL for women), or on drug treatment for reduced HDL cholesterol; and 5) elevated waist circumference (≥90 cm for men and ≥ 80 cm for women) according to the Asia-Pacific criteria [21, 22].
Statistical analysis
Categorical variables were evaluated using the Chi-square test or Fisher’s exact test and presented as frequencies and percentages. Continuous variables were analyzed with independent sample t-tests or the Mann-Whitney U test. Results are presented as the mean ± standard deviation (SD) for normally distributed variables and the median (interquartile range [IQR]) for variables with skewed distributions. Log transformation was used to normalize klotho variability. We performed binominal logistic regression model analysis with adjustments (the Enter method), including variables that were significant in a univariate analysis or other clinically relevant variables to investigate the independent risk factors related to MS. To explore the association between MS and eGFR, or between MS and serum klotho considering the possible non-linearity, we adopted 3-knots restricted cubic splines with 95% confidence interval (CI) which described odds ratios (ORs) of MS according to the eGFR or klotho level. Knots were chosen using SAS LGTPHCURV9 Macro by Li et al. at the eGFR of 13.4, 42.7, and 106 ml/min/1.73m2; 257, 526, and 946 pg/mL for klotho, respectively. An eGFR of 60 ml/min/1.73m2, as a reference value of decreased renal function, and a serum klotho of 518 pg/mL (ROC [receiver operating characteristic] curve cut-off value) were taken as the reference point (OR = 1.00). The final model was adjusted for confounders. In subgroup analyses using an adjusted binominal logistic regression model (adjusted for age, sex, eGFR, overt proteinuria), we categorized patients according to age (aged < 50 vs. ≥50 years), sex, and CKD stages. P-values of < 0.05 were considered statistically significant. The SPSS statistical software (SPSS version 18.0, Chicago, IL, USA) and SAS (Version 9.4, SAS Institute Inc., Cary, NC, USA) were used for all descriptive and outcome analyses.
Results
Baseline clinical characteristics of subjects according to the presence of metabolic syndrome
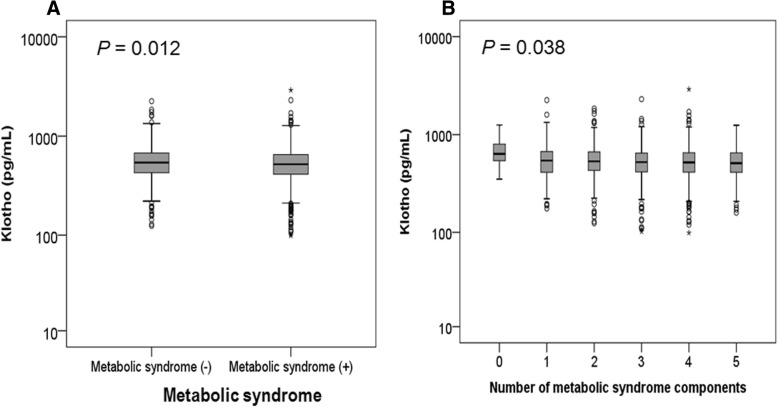
The clinical characteristics of patients at enrollment are shown in Table 1. Mean age of the 1754 study subjects was 54.9 ± 12.1 years, and 1110 (63.3%) were male. Median serum klotho level was 527 pg/mL (IQR: 418–656 pg/mL). Among them, 1118 (63.7%) patients exhibited MS. The frequencies of the five MS component in the study subjects are shown in Fig. 1. High blood pressure (95.8%) was the most prevalent component, followed by high fasting glucose (63.7%) and abdominal obesity (53.8%). Figure 2 presents the prevalence of MS across CKD stages. The prevalence of MS was > 50% even for early stage CKD. The prevalence of MS was higher in advanced stages of CKD (P < 0.001, P for linear trend < 0.001). As show in Fig. 3, the adjusted OR (adjusted for age, sex, eGFR, and overt proteinuria) of MS was significantly increased at eGFR levels of < 60 ml/min/1.73m2. Subjects were divided into two groups according to the presence of MS. Patients with MS were older (P < 0.001, Table 1) and had higher blood pressure (P < 0.001), body mass index (P < 0.001), and waist circumference (P < 0.001). At enrollment, diabetes mellitus (DM) (P < 0.001), hypertension (P < 0.001), and preexisting CV disease (P < 0.001) were more prevalent among subjects with MS. Mean eGFR (P < 0.001) was lower and uric acid (P = 0.025) level was higher in MS patients. Serum klotho was significantly lower in MS patients compared with patients without MS (Median [IQR]; 521 pg/mL [413, 651] vs. 541 pg/mL [427, 676], respectively; P = 0.012; Fig. 4a). In addition, klotho levels tended to decrease as numbers of MS components increased (Fig. 4b; P = 0.038).
Table 1.
The clinical characteristics of study subjects at enrollment with respect to the presence of metabolic syndrome
| Total (N = 1754) |
Subjects without MS (n = 636) |
Subjects with MS (n = 1118) |
P–value | |
|---|---|---|---|---|
| Age (years) | 54.9 ± 12.1 | 52.3 ± 12.8 | 56.3 ± 11.4 | < 0.001 |
| Sex, male, n (%) | 1110 (63.3) | 387 (60.8) | 723 (64.7) | 0.111 |
| SBP (mmHg) | 128.5 ± 17.0 | 124.9 ± 16.4 | 130.6 ± 17.0 | < 0.001 |
| DBP (mmHg) | 76.0 ± 11.2 | 75.5 ± 10.9 | 76.5 ± 11.4 | 0.059 |
| BMI (kg/m2) | 24.7 ± 3.4 | 22.9 ± 2.9 | 25.8 ± 3.2 | < 0.001 |
| Waist circumference (cm) | 88.1 ± 9.8 | 81.4 ± 8.4 | 91.7 ± 8.5 | < 0.001 |
| DM, n (%) | 700 (39.9) | 110 (17.3) | 590 (52.8) | < 0.001 |
| Hypertension, n (%) | 1719 (98.0) | 606 (95.3) | 1113 (99.6) | < 0.001 |
| Preexisting CV disease, n (%) | 298 (17.0) | 72 (11.3) | 226 (20.2) | < 0.001 |
| CAD, n (%) | 110 (6.3) | 21 (3.3) | 89 (8.0) | < 0.001 |
| PVD, n (%) | 76 (4.3) | 22 (3.5) | 54 (4.8) | 0.175 |
| Cerebrovascular disease, n (%) | 110 (6.3) | 24 (3.8) | 86 (7.7) | 0.001 |
| HF, n (%) | 28 (1.6) | 9 (1.4) | 19 (1.7) | 0.648 |
| Arrhythmia, n (%) | 52 (3.0) | 19 (3.0) | 33 (3.0) | 0.966 |
| Cause of CKD, n (%) | < 0.001 | |||
| GN, n (%) | 746 (42.5) | 387 (60.8) | 359 (32.1) | |
| Diabetic nephropathy, n (%) | 491 (28.0) | 72 (11.3) | 419 (37.5) | |
| Hypertension, n (%) | 389 (22.2) | 142 (22.3) | 247 (22.1) | |
| Others, n (%) | 128 (7.3) | 35 (5.5) | 93 (8.3) | |
| Laboratory findings | ||||
| eGFR (mL/min/1.73m2) | 49.1 ± 28.6 | 53.1 ± 29.6 | 46.8 ± 27.9 | < 0.001 |
| Hemoglobin (g/dL) | 12.7 ± 2.1 | 12.8 ± 2.0 | 12.7 ± 2.1 | 0.798 |
| Albumin (g/dL) | 4.1 ± 0.4 | 4.1 ± 0.4 | 4.1 ± 0.5 | 0.357 |
| Uric acid (mg/dL) | 7.2 ± 1.9 | 7.1 ± 1.9 | 7.3 ± 1.9 | 0.025 |
| Creatinine (mg/dL) | 1.9 ± 1.2 | 1.8 ± 1.1 | 2.0 ± 1.2 | 0.002 |
| hsCRP, median, (IQR) (mg/dL) | 0.07 (0.03, 0.17) | 0.04 (0.02, 0.11) | 0.08 (0.03, 0.21) | 0.005 |
| Fasting blood sugar (mg/dL) | 113.9 ± 42.7 | 100.4 ± 28.5 | 121.5 ± 47.2 | < 0.001 |
| Total cholesterol (mg/dL) | 173.4 ± 40.4 | 173.5 ± 36.2 | 173.3 ± 42.5 | 0.917 |
| Triglyceride (mg/dL) | 164.1 ± 103.1 | 106.3 ± 52.0 | 196.0 ± 110.2 | < 0.001 |
| LDL cholesterol (mg/dL) | 95.0 ± 32.7 | 96.5 ± 30.1 | 95.7 ± 34.1 | 0.609 |
| HDL cholesterol (mg/dL) | 48.2 ± 15.5 | 56.5 ± 15.0 | 43.7 ± 13.8 | < 0.001 |
| Overt proteinuriaa, n (%) | 942 (61.0) | 319 (58.3) | 623 (62.5) | 0.108 |
| Medication, n (%) | ||||
| ACEi or ARB, n (%) | 1521 (86.9) | 526 (83.0) | 995 (89.1) | < 0.001 |
| Diuretics, n (%) | 443 (25.3) | 99 (15.6) | 344 (25.3) | < 0.001 |
| Statin, n (%) | 998 (56.9) | 323 (50.8) | 675 (60.4) | < 0.001 |
| Ca-based P binders, n (%) | 161 (9.2) | 67 (10.5) | 94 (8.4) | 0.179 |
a24-h urine protein > 500 mg/day
MS metabolic syndrome, SBP systolic blood pressure, DBP diastolic blood pressure, BMI body mass index, DM diabetes mellitus, CV cardiovascular, CAD coronary artery disease, HF heart failure, PVD peripheral vascular disease, CKD chronic kidney disease, GN glomerulonephritis, eGFR estimated glomerular filtration rate by CKD-EPI creatinine equation, hsCRP high sensitivity C-reactive protein, IQR interquartile range, LDL low-density lipoprotein, HDL high-density lipoprotein, ACEi angiotensin converting enzyme inhibitor, ARB angiotensin II receptor blocker, Ca calcium, P phosphorus
Fig. 1.
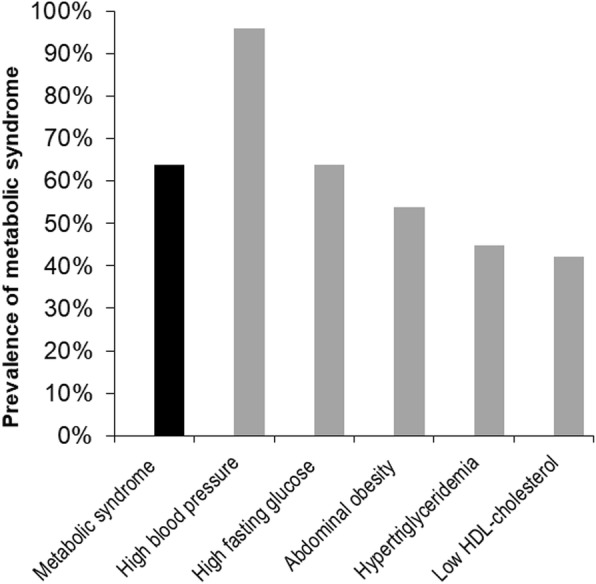
Prevalence of the metabolic syndrome and components of metabolic syndrome in the study subjects. Sixty four percent of patients had MS. Of the components of MS, high blood pressure (95.8%) was the most common, followed by high fasting glucose (63.7%) and abdominal obesity (53.8%). MS, metabolic syndrome; HDL, high-density lipoprotein
Fig. 2.
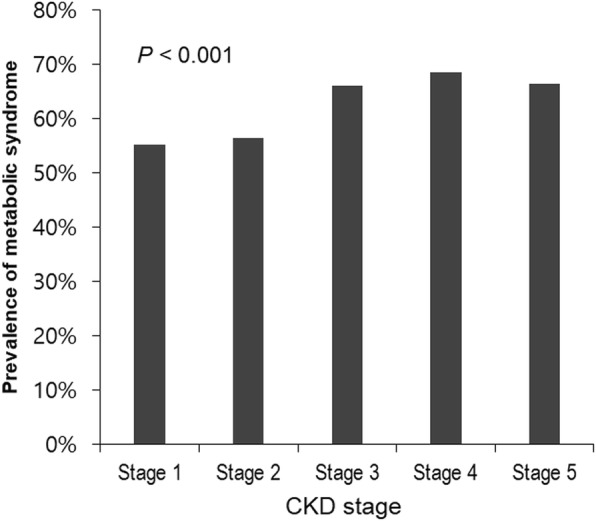
Prevalence of metabolic syndrome across CKD stages. The prevalence of MS was > 50% even for early stage CKD. The prevalence of MS was higher in advanced stages of CKD (P < 0.001). MS, metabolic syndrome
Fig. 3.
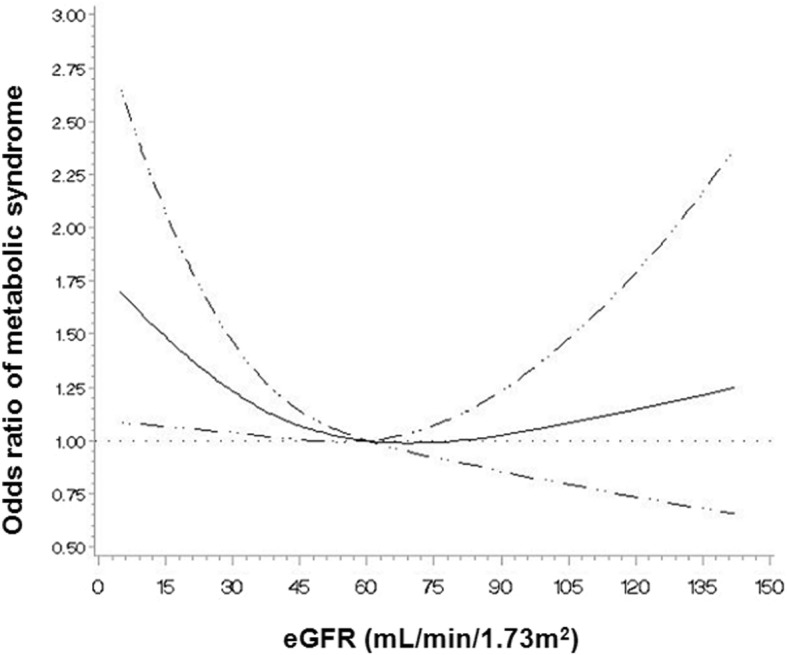
Multivariable-adjusted odds ratios of metabolic syndrome according to levels of estimated glomerular filtration rate. An eGFR of 60 ml/min/1.73m2, as a reference value of decreased renal function, was taken as the reference point (OR = 1.00). The adjusted OR of MS was significantly increased at eGFR levels of < 60 ml/min/1.73m2. The model was adjusted for age, sex, eGFR, and overt proteinuria. The solid line represents the multivariable-adjusted ORs of MS according to levels of eGFR. The dashed lines indicate 95% confidence intervals. eGFR, estimated glomerular filtration rate; OR, odds ratio; MS, metabolic syndrome
Fig. 4.
Serum klotho level according to metabolic syndrome and numbers of metabolic syndrome components. a Serum klotho was significantly lower in MS patients compared with patients without MS (median [interquartile range]; 521 pg/mL [413, 651] vs. 541 pg/mL [427, 676], respectively; P = 0.012). b Klotho levels tended to decrease as numbers of MS components increased (P = 0.038). MS, metabolic syndrome
Clinical characteristics of subjects stratified by serum klotho level
The clinical characteristics of patients dichotomized by serum klotho level (lower than median vs. equal to or higher than median) are shown in Table 2. Age age, sex, blood pressure, and underlying comorbidities were similar in these two groups. However, patients with a lower klotho level had a higher mean uric acid level (P < 0.001) and a higher C-reactive protein (CRP) level (P = 0.005). Mean eGFR (P < 0.001) and hemoglobin (P < 0.001) were lower in the low serum klotho level group. Serum klotho levels across CKD stages are shown in Fig. 5. Advanced CKD stages were associated with lower serum klotho levels (P < 0.001).
Table 2.
Baseline characteristics of subjects stratified by serum klotho level
| Klotho groups | P–value | ||
|---|---|---|---|
| Lower than median (n = 877) (99–526 pg/mL) |
Equal to or higher than median (n = 877) (527–2909 pg/mL) |
||
| Age (years) | 55.3 ± 11.9 | 54.5 ± 12.3 | 0.152 |
| Sex, male, n (%) | 556 (63.4) | 554 (63.2) | 0.921 |
| SBP (mmHg) | 128.9 ± 16.7 | 128.2 ± 17.2 | 0.387 |
| DBP (mmHg) | 76.3 ± 11.5 | 76.0 ± 10.9 | 0.568 |
| BMI (kg/m2) | 24.9 ± 3.4 | 24.6 ± 3.4 | 0.060 |
| Waist circumference (cm) | 88.4 ± 9.8 | 87.8 ± 9.8 | 0.203 |
| DM, n (%) | 348 (39.7) | 352(40.1) | 0.845 |
| Hypertension, n (%) | 866 (98.7) | 853 (97.3) | 0.026 |
| Preexisting CV disease, n (%) | 144 (16.4) | 154 (17.6) | 0.525 |
| CAD, n (%) | 46 (5.2) | 64 (7.3) | 0.076 |
| PVD, n (%) | 40 (4.6) | 36 (4.1) | 0.639 |
| Cerebrovascular disease, n (%) | 50 (5.7) | 60 (6.8) | 0.325 |
| HF, n (%) | 11 (1.3) | 17 (1.9) | 0.253 |
| Arrhythmia, n (%) | 23 (2.6) | 29 (3.3) | 0.398 |
| Cause of CKD, n (%) | 0.012 | ||
| GN, n (%) | 358 (40.8) | 388 (44.2) | |
| Diabetic nephropathy, n (%) | 253 (28.8) | 238 (27.1) | |
| Hypertension, n (%) | 214 (24.4) | 175 (20.0) | |
| Others, n (%) | 52 (5.9) | 73 (8.7) | |
| Laboratory findings | |||
| eGFR (mL/min/1.73m2) | 44.9 ± 26.0 | 53.6 ± 30.6 | < 0.001 |
| Hemoglobin (g/dL) | 12.5 ± 2.0 | 13.0 ± 2.1 | < 0.001 |
| Albumin (g/dL) | 4.1 ± 0.4 | 4.1 ± 0.5 | 0.953 |
| Uric acid (mg/dL) | 7.5 ± 1.9 | 7.0 ± 1.9 | < 0.001 |
| Creatinine (mg/dL) | 2.0 ± 1.2 | 1.8 ± 1.1 | < 0.001 |
| hsCRP, median, (IQR) (mg/dL) | 0.07 (0.03, 0.18) | 0.06 (0.02, 0.15) | 0.005 |
| Fasting blood sugar (mg/dL) | 110.9 ± 34.9 | 117.0 ± 49.1 | 0.003 |
| Total cholesterol (mg/dL) | 172.8 ± 39.7 | 174.0 ± 41.0 | 0.531 |
| Triglyceride (mg/dL) | 168.6 ± 107.0 | 159.7 ± 98.9 | 0.072 |
| LDL cholesterol (mg/dL) | 95.0 ± 32.3 | 97.0 ± 33.1 | 0.205 |
| HDL cholesterol (mg/dL) | 47.9 ± 15.9 | 48.6 ± 15.1 | 0.356 |
| Overt proteinuriaa, n (%) | 475 (61.3) | 467 (60.7) | 0.821 |
| Klotho, median, (IQR) (pg/mL) | 418 (337, 475) | 656 (583, 774) | < 0.001 |
| Medication, n (%) | |||
| ACEi or ARB, n (%) | 773 (88.2) | 748 (85.5) | 0.088 |
| Diuretics, n (%) | 342 (39.0) | 295 (33.6) | 0.058 |
| Statin, n (%) | 514 (58.6) | 484 (55.2) | 0.309 |
| Ca-based P binders, n (%) | 88 (10.0) | 73 (8.3) | 0.396 |
| Metabolic syndrome, n (%) | 574 (65.5) | 544 (62.0) | 0.136 |
a24-hour urine protein > 500 mg/day
MS metabolic syndrome, SBP systolic blood pressure, DBP diastolic blood pressure, BMI body mass index, DM diabetes mellitus, CV cardiovascular, CAD coronary artery disease, HF heart failure, PVD peripheral vascular disease, CKD chronic kidney disease, GN glomerulonephritis, eGFR estimated glomerular filtration rate by CKD-EPI creatinine equation, hsCRP high sensitivity C-reactive protein, IQR interquartile range, LDL low-density lipoprotein, HDL high-density lipoprotein, ACEi angiotensin converting enzyme inhibitor, ARB angiotensin II receptor blocker, Ca calcium, P phosphorus
Fig. 5.
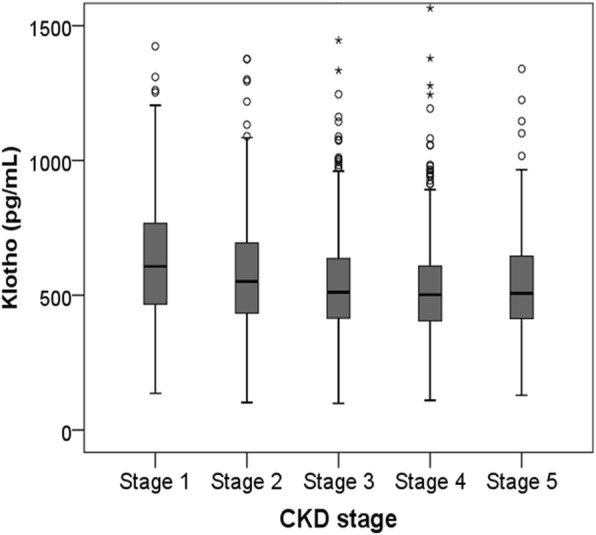
Serum klotho levels across CKD stages. Advanced CKD stages were associated with lower serum klotho levels (P < 0.001). CKD, chronic kidney disease
Associations of metabolic syndrome and each metabolic syndrome component with serum klotho
In the univariate analysis, serum log klotho (OR, 0.40; 95% confidence interval [CI], 0.23–0.72; P = 0.002) and eGFR (OR, 0.99; 95% CI, 0.98–0.99; P < 0.001) were inversely associated with the presence of MS. On the other hand, age (OR, 1.03; 95% CI, 1.02–1.04; P < 0.001) was positively associated with MS. Binominal logistic regression analysis, adjusted for age, sex, eGFR, and overt proteinuria, showed that log klotho was independently associated with the presence of MS (adjusted OR, 0.44; 95% CI, 0.23–0.82; P = 0.010; model B in Table 3). As shown in Fig. 6, the adjusted OR of MS was significantly increased at serum klotho levels of < 518 pg/mL (the ROC cut-off value). Furthermore, adjusted for age, sex, eGFR, overt proteinuria, and drug information (ACEi or ARB, diuretics, statin), log klotho was still independently associated with the presence of MS (adjusted OR, 0.51; 95% CI, 0.27–0.98; P = 0.045).
Table 3.
Multivariable logistic regression analysis presenting associations between log klotho and metabolic syndrome
| Variable | Model Aa | Model Bb |
|---|---|---|
| Adjusted OR (95% CI) | Adjusted OR (95% CI) | |
| Log klotho | 0.44 (0.25–0.80)d | 0.44 (0.23–0.82)d |
| Age (per year) | 1.03 (1.02–1.04)d | 1.03 (1.02–1.04)d |
| Sex (male vs. female) | 1.15 (0.94–1.41) | 1.13 (0.91–1.40) |
| eGFR (per mL/min/1.73m2) | 1.00 (0.99–1.00) | |
| Overt proteinuriac | 1.26 (1.01–1.58)d |
OR Odds ratio, CI confidence interval, eGFR estimated glomerular filtration rate by CKD-EPI creatinine equation
aModel A adjusted for log klotho, age, and sex
bModel B adjusted for covariates in model A plus eGFR, and overt proteinuria
c24-hour urine protein > 500 mg/day
dsignificant association with MS (P < 0.05). P < 0.05 was considered significant
Fig. 6.
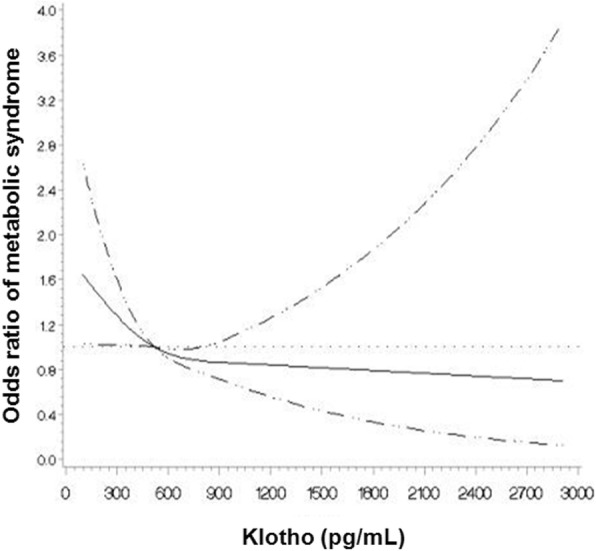
Multivariable-adjusted odds ratio of metabolic syndrome according to levels of serum klotho. A serum klotho level of 518 pg/mL (ROC curve cut-off value) was taken as the reference point (OR = 1.00). The adjusted OR of MS was significantly increased at serum klotho levels of < 518 pg/mL. The model was adjusted for age, sex, eGFR, and overt proteinuria. The solid line represents the multivariable-adjusted ORs of MS according to levels of eGFR. The dashed lines indicate 95% confidence intervals. Overt proteinuria, 24-hour urine protein > 500 mg/day; ROC, receiver operating characteristic; eGFR, estimated glomerular filtration rate; OR, odds ratio; MS, metabolic syndrome
ORs of relations between each MS component and serum klotho are shown in Table 4. Only high blood pressure (adjusted OR, 0.05; 95% CI, 0.01–0.29; P = 0.001) and hypertriglyceridemia (adjusted OR, 0.48; 95% CI, 0.27–0.87; P = 0.016) were found to be independently associated with log klotho. High fasting glucose, abdominal obesity, and low HDL cholesterol were not significantly associated with serum klotho.
Table 4.
Multivariable logistic regression analysis presenting associations between log klotho and each component of metabolic syndrome
| Variable | Adjusted OR (95% CI) | ||||
|---|---|---|---|---|---|
| High blood pressure | High fasting glucose | Abdominal obesity | Hypertriglyceridemia | Low HDL-cholesterol | |
| Log klotho | 0.05 (0.01–0.29) | 1.32 (0.70–2.51) | 0.68 (0.36–1.28) | 0.48 (0.27–0.87) | 0.71 (0.38–1.31) |
| P-value | 0.001 | 0.389 | 0.235 | 0.016 | 0.271 |
Adjusted for covariates log klotho, age, sex, eGFR, and overt proteinuria
OR Odds ratio, CI confidence interval, HDL high-density lipoprotein, eGFR estimated glomerular filtration rate by CKD-EPI creatinine equation
Subgroup analysis
We further analyzed the relationship between klotho and MS in several groups. Significant interaction was found in age group (aged < 50 vs. ≥50 years), suggesting that the association between klotho and MS was particularly evident in subjects aged < 50 years (adjusted OR, 0.18; 95% CI, 0.06–0.56; P = 0.003 vs. adjusted OR, 0.72; 95% CI, 0.33–1.57; P = 0.412 in aged ≥50 years). Klotho levels tended to decrease as age increased (Pearson’s correlation coefficient r = − 0.083, P < 0.001), but the value was not large. Klotho levels were not significantly different in aged < 50 and ≥ 50 years (Median [IQR]; 535 pg/mL [418, 668] vs. 525 pg/mL [418, 655], respectively; P = 0.465). There were no statistical interactions with sex group (interaction P = 0.605) and CKD stages (interaction P = 0.425) for the association between klotho and MS.
Discussion
MS, a common clinical phenotype demonstrating as a combination of metabolic abnormalities including, hypertension, hyperglycemia, hypertriglyceridemia, decreased high-density lipoprotein cholesterol, and central obesity, is common in CKD [23]. In the present study, 63.7% of the 1754 CKD patients enrolled exhibited MS. The prevalence of MS exceeded 50% even in patients with early stage CKD and its prevalence was higher in advanced CKD stages. In the univariate analysis, serum klotho was significantly lower in patients with MS. After adjusting for various factors, such as, age, sex, eGFR, and overt proteinuria, serum klotho level remained an independent factor associated with MS in CKD.
In previous studies, genetic variants of Klotho increased the risk of MS which could be related to their susceptibility to high fasting glucose, high blood pressure, hypertriglyceridemia, and decreased HDL cholesterol [8, 12]. Klotho has a role in the regulation of vascular tone through homeostatic interplay between the renin-angiotensin system and nitric oxide [24], which might explain the association between klotho and blood pressure. Furthermore, the Klotho has been shown to be a target gene for PPARγ, a key transcription factor controlling lipid metabolism and insulin sensitivity [25]. Thus, we speculated these factors might play major roles in the association of klotho with glucose and lipid metabolism.
MS is a cluster of risk factors of diabetes and CV disease. Subjects with CKD present higher incidence of CV disease and higher CV mortality than the general population [26, 27]. Novel risk factors such as persistent inflammation, oxidative stress, and endothelial dysfunction can be linked to CV disease in CKD patients [28]. Fundamental manifestations of MS include insulin resistance and adipose tissue expansion, and the latter leads to oxidative stress and chronic inflammation, which aggravate insulin resistance [29–31]. Furthermore, these pro-inflammatory manifestations of MS might play roles in the pathogenesis of endothelial dysfunction and atherosclerosis [32]. Klotho is an anti-aging gene that extends life span when overexpressed and accelerates aging when disrupted [6]. Aging which reflects cellular senescence is closely associated with inflammatory reactions, oxidative damage, and endothelial dysfunction [33–35]. Klotho has also been shown to be an anti-inflammatory modulator [36, 37], to be closely associated with oxidative stress [38, 39]. Klotho plays a role in the protection against endothelial dysfunction [10]. The kidney is the principal organ responsible for the production of klotho, and CKD is known to be a klotho deficient state. Accordingly, the effects of klotho mentioned above suggest an association between klotho and MS beyond individual components of MS.
The present study is the first study, to our knowledge, to report the independent association between serum klotho level and MS per se especially in CKD patients. In previous studies, there were not many human studies, and most of studies were related to the association between genetic variants of Klotho and individual components of MS. In addition, few studies have been performed in patients with CKD. Furthermore, it was the strength of our study that we included a large-scale CKD cohort patients and obtained serum klotho level from a large number of our CKD patients. However, the study has several limitations that warrant consideration. First, because it was based on cross-sectional analysis, it could not demonstrate causality between klotho and MS. It could not provide detailed mechanistic links between klotho and MS. We just described the association between klotho and MS, not the causality. Second, serum klotho exhibits circadian variations [40]. They showed that midnight serum klotho level was an approximately 40% reduction with gradual return to near baseline values in the early morning hours. Serum klotho level decreased less than 20% compared with morning klotho level until 6 p.m. In the present study, blood samples were not obtained at any fixed time of the day. However, circadian variation of serum klotho may have been less in our study because blood sampling was done before 6 p.m.
Conclusions
Serum klotho levels were found to be independently and inversely associated with the presence of MS. Further studies are warranted to elucidate the nature of the mechanistic link between klotho and MS in CKD patients.
Acknowledgments
The authors gratefully thank to the clinical research staffs and nurses of KNOW-CKD study.
Funding
This study was supported by the research program funded by the Korea Center for Disease Control and Prevention (2011E3300300, 2012E3301100, 2013E3301600, 2013E3301601, 2013E3301602, and 2016E3300200).
Availability of data and materials
The data set that support the findings is not publicly available. Limited de-identified data sets may be available from the authors upon reasonable request and with permission of the study sponsor.
Abbreviations
- ACEi
Angiotensin converting enzyme inhibitor
- ARB
Angiotensin II receptor blocker
- Ca
Calcium
- CI
Confidence interval
- CKD
Chronic kidney disease
- CKD-EPI
Chronic kidney Disease Epidemiology Collaboration
- CRP
C-reactive protein
- CV
Cardiovascular
- DBP
Diastolic blood pressure
- DM
Diabetes mellitus
- eGFR
Estimated glomerular filtration rate
- ELISA
Enzyme-linked immunosorbent assay
- HDL
High-density lipoprotein
- IDMS
Isotope dilution mass spectrometry
- IQR
Interquartile range
- KNOW-CKD
KoreaN Cohort Study for Outcome in Patients With Chronic Kidney Disease
- MS
Metabolic syndrome
- NCEP-ATP
National Cholesterol Education Program Adult Treatment Panel
- OR
Odds ratio
- P
Phosphorus
- ROC
Receiver operating characteristic
- SBP
Systolic blood pressure
- SD
Standard deviation
- WHO
World Health Organization
Authors’ contributions
HJK and K-HO conceived and designed the study, analyzed and interpreted the data, wrote the manuscript, contributed to discussions, and reviewed and edited the manuscript. JL made substantial contributions to the analysis of data and drafted the article. D-WC, K-BL, SAS, T-HY, SHH, and CA contributed to the study conception and design, contributed to data interpretation and discussion, and reviewed and edited the manuscript. All authors approved the final manuscript. K-HO was the principal investigator and is responsible for the integrity of the work as a whole. All authors revised the manuscript content and approved the final version.
Ethics approval and consent to participate
The study protocol was approved by the ethical committee of each participating clinical center, including the Institutional Review Boards of Seoul National University Hospital, Severance Hospital, Kangbuk Samsung Medical Center, Seoul St. Mary’s Hospital, Gil Hospital, Eulji General Hospital, Chonnam National University Hospital, and Busan Paik Hospital. All participating patients provided written informed consent. The study protocol was in accordance with the principles of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hyo Jin Kim, Email: kimhj923@gmail.com.
Joongyub Lee, Email: denver261@gmail.com.
Dong-Wan Chae, Email: cdw1302@snubh.org.
Kyu-Beck Lee, Email: kyubeck.lee@samsung.com.
Su Ah Sung, Email: sophi35@naver.com.
Tae-Hyun Yoo, Email: yoosy0316@yuhs.ac.
Seung Hyeok Han, Email: hansh@yuhs.ac.
Curie Ahn, Email: curie@snu.ac.kr.
Kook-Hwan Oh, Phone: +82 2 2072 0776, Email: khoh@snu.ac.kr.
References
- 1.Laaksonen DE, Lakka HM, Niskanen LK, Kaplan GA, Salonen JT, Lakka TA. Metabolic syndrome and development of diabetes mellitus: application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. Am J Epidemiol. 2002;156:1070–1077. doi: 10.1093/aje/kwf145. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 3.Thomas G, Sehgal AR, Kashyap SR, Srinivas TR, Kirwan JP, Navaneethan SD. Metabolic syndrome and kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2011;6:2364–2373. doi: 10.2215/CJN.02180311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurella M, Lo JC, Chertow GM. Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol. 2005;16:2134–2140. doi: 10.1681/ASN.2005010106. [DOI] [PubMed] [Google Scholar]
- 5.Nashar K, Egan BM. Relationship between chronic kidney disease and metabolic syndrome: current perspectives. Diabetes Metab Syndr Obes. 2014;7:421–435. doi: 10.2147/DMSO.S45183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 7.Hu MC, Kuro-o M, Moe OW. Klotho and chronic kidney disease. Contrib Nephrol. 2013;180:47–63. doi: 10.1159/000346778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arking DE, Atzmon G, Arking A, Barzilai N, Dietz HC. Association between a functional variant of the KLOTHO gene and high-density lipoprotein cholesterol, blood pressure, stroke, and longevity. Circ Res. 2005;96:412–418. doi: 10.1161/01.RES.0000157171.04054.30. [DOI] [PubMed] [Google Scholar]
- 9.Yamagishi T, Saito Y, Nakamura T, Takeda S, Kanai H, Sumino H, et al. Troglitazone improves endothelial function and augments renal klotho mRNA expression in Otsuka Long-Evans Tokushima Fatty (OLETF) rats with multiple atherogenic risk factors. Hypertens Res. 2001;24:705–709. doi: 10.1291/hypres.24.705. [DOI] [PubMed] [Google Scholar]
- 10.Saito Y, Nakamura T, Ohyama Y, Suzuki T, Iida A, Shiraki-Iida T, et al. In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Commun. 2000;276:767–772. doi: 10.1006/bbrc.2000.3470. [DOI] [PubMed] [Google Scholar]
- 11.Rhee EJ, Oh KW, Yun EJ, Jung CH, Lee WY, Kim SW, et al. Relationship between polymorphisms G395A in promoter and C1818T in exon 4 of the KLOTHO gene with glucose metabolism and cardiovascular risk factors in Korean women. J Endocrinol Investig. 2006;29:613–618. doi: 10.1007/BF03344160. [DOI] [PubMed] [Google Scholar]
- 12.Majumdar V, Christopher R. Association of exonic variants of Klotho with metabolic syndrome in Asian Indians. Clin Chim Acta. 2011;412:1116–1121. doi: 10.1016/j.cca.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 13.Oh KH, Park SK, Park HC, Chin HJ, Chae DW, Choi KH, et al. KNOW-CKD (KoreaN cohort study for outcome in patients with chronic kidney disease): design and methods. BMC Nephrol. 2014;15:80. doi: 10.1186/1471-2369-15-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuro-o M. Klotho in health and disease. Curr Opin Nephrol Hypertens. 2012;21:362–368. doi: 10.1097/MNH.0b013e32835422ad. [DOI] [PubMed] [Google Scholar]
- 15.Hu MC, Moe OW. Klotho as a potential biomarker and therapy for acute kidney injury. Nat Rev Nephrol. 2012;8:423–429. doi: 10.1038/nrneph.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seo MY, Yang J, Lee JY, Kim K, Kim SC, Chang H, et al. Renal Klotho expression in patients with acute kidney injury is associated with the severity of the injury. Korean J Intern Med. 2015;30:489–495. doi: 10.3904/kjim.2015.30.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO expert committee. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1-452. [PubMed]
- 18.Siekmann L. Determination of creatinine in human serum by isotope dilution-mass spectrometry. Definitive methods in clinical chemistry, IV. J Clin Chem Clin Biochem. 1985;23:137–144. [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamazaki Y, Imura A, Urakawa I, Shimada T, Murakami J, Aono Y, et al. Establishment of sandwich ELISA for soluble alpha-Klotho measurement: age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem Biophys Res Commun. 2010;398:513–518. doi: 10.1016/j.bbrc.2010.06.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 22.Hara K, Matsushita Y, Horikoshi M, Yoshiike N, Yokoyama T, Tanaka H, et al. A proposal for the cutoff point of waist circumference for the diagnosis of metabolic syndrome in the Japanese population. Diabetes Care. 2006;29:1123–1124. doi: 10.2337/dc05-2540. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, et al. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med. 2004;140:167–174. doi: 10.7326/0003-4819-140-3-200402030-00007. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, Matsukawa N, Rakugi H, Imai M, Kida I, Nagai M, et al. Upregulation of cAMP is a new functional signal pathway of Klotho in endothelial cells. Biochem Biophys Res Commun. 2003;301:424–429. doi: 10.1016/S0006-291X(02)03056-5. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Li Y, Fan Y, Wu J, Zhao B, Guan Y, et al. Klotho is a target gene of PPAR-gamma. Kidney Int. 2008;74:732–739. doi: 10.1038/ki.2008.244. [DOI] [PubMed] [Google Scholar]
- 26.Causes of death United States renal data system. Am J Kidney Dis. 1998;32:S81–S88. doi: 10.1053/ajkd.1998.v32.pm9713410. [DOI] [PubMed] [Google Scholar]
- 27.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 28.Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimburger O, Massy Z. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: how do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol. 2008;3:505–521. doi: 10.2215/CJN.03670807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Lerman LO. The metabolic syndrome and chronic kidney disease. Transl Res. 2017;183:14-25. [DOI] [PMC free article] [PubMed]
- 30.Wahba IM, Mak RH. Obesity and obesity-initiated metabolic syndrome: mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:550–562. doi: 10.2215/CJN.04071206. [DOI] [PubMed] [Google Scholar]
- 31.Spahis S, Borys JM, Levy E. Metabolic syndrome as a multifaceted risk factor for oxidative stress. Antioxid Redox Signal. 2016. [DOI] [PubMed]
- 32.Tziomalos K, Athyros VG, Karagiannis A, Mikhailidis DP. Endothelial dysfunction in metabolic syndrome: prevalence, pathogenesis and management. Nutr Metab Cardiovasc Dis. 2010;20:140–146. doi: 10.1016/j.numecd.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Rangel-Zuniga OA, Corina A, Lucena-Porras B, Cruz-Teno C, Gomez-Delgado F, Jimenez-Lucena R, et al. TNFA gene variants related to the inflammatory status and its association with cellular aging: from the CORDIOPREV study. Exp Gerontol. 2016;83:56–62. doi: 10.1016/j.exger.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 34.Hamilton ML, Van Remmen H, Drake JA, Yang H, Guo ZM, Kewitt K, et al. Does oxidative damage to DNA increase with age? Proc Natl Acad Sci U S A. 2001;98:10469–10474. doi: 10.1073/pnas.171202698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salmon AB. Beyond diabetes: does obesity-induced oxidative stress drive the aging process? Antioxidants (Basel). 2016;5:24. [DOI] [PMC free article] [PubMed]
- 36.Izquierdo MC, Perez-Gomez MV, Sanchez-Nino MD, Sanz AB, Ruiz-Andres O, Poveda J, et al. Klotho, phosphate and inflammation/ageing in chronic kidney disease. Nephrol Dial Transplant. 2012;27(Suppl 4):iv6–i10. doi: 10.1093/ndt/gfs426. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y, Banerjee S, Dey N, LeJeune WS, Sarkar PS, Brobey R, et al. Klotho depletion contributes to increased inflammation in kidney of the db/db mouse model of diabetes via RelA (serine)536 phosphorylation. Diabetes. 2011;60:1907–1916. doi: 10.2337/db10-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oh HJ, Nam BY, Lee MJ, Kim CH, Koo HM, Doh FM, et al. Decreased circulating klotho levels in patients undergoing dialysis and relationship to oxidative stress and inflammation. Perit Dial Int. 2015;35:43–51. doi: 10.3747/pdi.2013.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, et al. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem. 2005;280:38029–38034. doi: 10.1074/jbc.M509039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carpenter TO. Insogna KL, Zhang JH, Ellis B, Nieman S, Simpson C, et al. Circulating levels of soluble klotho and FGF23 in X-linked hypophosphatemia: circadian variance, effects of treatment, and relationship to parathyroid status. J Clin Endocrinol Metab. 2010;95:E352–E357. doi: 10.1210/jc.2010-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data set that support the findings is not publicly available. Limited de-identified data sets may be available from the authors upon reasonable request and with permission of the study sponsor.